Abstract
Natural interferon-producing cells (NIPC), also referred to as immature plasmacytoid dendritic cells (PDC), constitute a small population of leucocytes secreting high levels of type I interferons in response to certain danger signals. Amongst these signals are those from DNA containing unmethylated CpG motifs. The present work demonstrated that the CpG oligonucleotides (CpG-ODN) 2216, D32 and D19 induce high amounts of interferon-α (IFN-α), tumour-necrosis factor-α (TNF-α) and interleukin (IL)-12 in porcine peripheral blood mononuclear cells (PBMCs). Swine workshop cluster 3 (SWC3)1ow CD4high cells, with high IL-3-binding activity, representing NIPC, were the exclusive cytokine-producing cells responding to the CpG-ODN. These cells did not express CD6, CD8 or CD45RA. Importantly, monocyte-derived DC did not respond to CpG-ODN by secretion of IFN-α or TNF-α or by the up-regulation of costimulatory molecule expression. CpG-ODN up-regulated MHC class II and CD80/86 expression on the NIPC, but were unable to promote NIPC survival. Interestingly, certain CpG-ODN, incapable of inducing NIPC to secrete IFN-α or up-regulate MHC class II and CD80/86, did promote NIPC viability. Taken together, the influence of CpG-ODN on porcine NIPC, monocytes and myeloid DCs relates to that observed with their human equivalents. These results represent an important basis for the application of CpG-ODN as adjuvants for the formulation of novel vaccines and demonstrate the importance of the pig as an alternative animal model for this approach.
Keywords: adjuvants, CpG DNA, animal models: studies, plasmacytoid dendritic cells
Introduction
In humans, natural interferon-producing cells (NIPCs) are phenotypically characterized by high CD4 and interleukin (IL)-3 receptor expression, are major histocompatibility complex (MHC) class II positive, but negative for the lineage markers CD3, CD19, CD56 and CD14, as well as CD11c.1 It was only recently demonstrated that NIPC could differentiate into dendritic cells (DCs) in vitro, corresponding to the plasmacytoid DC (PDCs).2 Nevertheless, their contribution to antigen presentation in vivo is still unclear. In contrast, their function as the major systemic source of interferon-α (IFN-α), during acute virus infection, has been demonstrated in vivo.3,4 Porcine NIPCs5 are functionally and phenotypically similar to their human counterparts. They express high levels of CD4 and IL-3 receptor, low levels of swine cluster 3 [SWC3, a signal regulatory protein (SIRP) family member], MHC class II and CD80/86, and are negative for lineage markers, including CD3, CD8, CD14 and CD21.
Cells of the innate immune system contain a set of molecular pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) present on various pathogens. One example of PRRs is the toll like receptor (TLR) family. Activation of PRRs represents a crucial signal indicating the presence of infection to DCs and other cells of the innate immune responses. One such PAMP is present on microbial DNA containing unmethylated CpG motifs, and is recognized by TLR9.6 CpG oligonucleotides (CpG-ODN), which induce high levels of IFN-α, have a chimeric backbone in which the 5′ and 3′ ends are phosphorothiate-modified and the centre portion contains phosphodiester motifs.7 These CpG-ODNs share the following three features: a poly G sequence at both ends; a central palindromic sequence; and CG dinucleotides within the palindrome.
With human DCs, TLR9 expression is restricted to NIPCs. As a consequence, NIPCs are the exclusive DC subset possessing the capacity to respond directly to immunostimulatory CpG motifs.8 In contrast, mouse monocytes, myeloid DCs and macrophages all express TLR9, and can be directly activated by CpG.8 The response of human NIPCs to CpG-ODN is characterized by the production of large quantities of IFN-α and IL-12, as well as the induction of DC maturation, possibly through an indirect effect of the cytokines. Owing to this extraordinarily strong cytokine boost induced by CpG exclusively in NIPCs, these cells represent the key to the immunostimulatory activity of CpG-ODN in humans.
For the above reasons, caution is required when extrapolating in vivo data on CpG-ODN effects in mouse models to the human situation. Clearly, an alternative animal model displaying a cellular responsiveness to CpG equivalent to that of the human NIPC would be a major advantage for preclinical studies using CpG-ODNs as vaccine adjuvants.
Consequently, the present study analysed the capacity of different CpG-ODNs to stimulate porcine NIPCs. It was demonstrated that the porcine DC system relates closely to that of the human. Only porcine NIPCs responded to CpG-ODNs by secreting large quantities of cytokines, particularly IFN-α.
Materials and methods
Isolation and culture of cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the citrated blood of specific pathogen-free pigs using Ficoll–Paque (1·077 g/l) (Amersham Pharmacia Biotech AG, Dubendorf, Switzerland) density-gradient centrifugation.9 Cells were cultured in phenol red-free Dulbecco's modified Eagle's minimal essential medium (DMEM) (Invitrogen, Basel, Switzerland), supplemented with porcine serum (10% v/v) (Sigma Chemicals, Buchs, Switzerland).
For enrichment of NIPCs, PBMCs were separated into SWC3+ cells using the Miltenyi magnetic antibody cell sorting (MACS) system and LD columns (Miltenyi Biotec GMbH, Bergish Gladbach, Germany). These permit the enrichment of cells that have a low expression of SWC3. Purity for SWC3 expression was >80%. Separation of NIPCs from monocytes was based on the lack of CD4 expression on monocytes and the lack of CD14 expression on NIPCs. PBMCs were labelled with either CD4 monoclonal antibody (mAb) (PT90A; VMRD, Pullmann, WA) or CD14 mAb (CAM36A; VMRD), and then separated into CD4+ CD4− or CD14+ CD14− fractions using MACS (Miltenyi Biotec).
Monocyte-derived DCs (MoDCs) were prepared as previously described.10 Briefly, monocytes were purified by SWC3+ separation, using MACS (LS columns; Miltenyi Biotec GmbH), and cultured in DMEM containing porcine serum (10% v/v), recombinant porcine (rp) granulocyte–macrophage colony-stimulating factor (GM-CSF) (150 ng/ml) and rpIL-4 (100 U/ml). IL-4 and GM-CSF were prepared in our laboratory as described previously.10,11 After 5 days of culture, the non-adherent cells, representing MoDCs, were harvested.
Phenotyping of NIPCs
NIPCs were identified, as described by Summerfield et al.,5 as SWC3low CD4high cells. mAbs against the following cell-surface molecules were used for phenotyping NIPCs: SWC3 (mAb 74-22-15A), CD4 (mAbs 74-12-4 and PT90A), CD8 (mAb 76-2-11), CD45RA (mAb MIL13) and CD6 (mAb a38b2). Hybridomas for mAbs 74-22-15A, 74-12-4 and a38b2 were kindly provided by Dr A. Saalmuller (BFAV, Tubingen, Germany), and for MIL13 by Dr K. Haverson (University of Bristol, Bristol, UK). PT90A was purchased from VMRD. Fluorescein isothiocyanate (FITC), R-phycoerythrin (PE) or biotin-conjugated anti-mouse isotype-specific conjugates were obtained from Southern Biotechnology Associates (Birmingham, AL). Acquisition of data was performed with a FACSCalibur flow cytometer and analysed using cellquest software (both Becton Dickinson, Basel, Switzerland).
Cytokine induction
A panel of 10 modified nuclease-resistant phosphorothiate and unmodified phosphodiester ODNs (Table 1) were tested. These ODNs were synthesized by Biosource International (Camarillo, CA). ODNs were solubilized in sterile endotoxin-free Tris–EDTA. For screening of ODNs, PBMCs (final concentration 3 × 106 cells/ml) were incubated with different concentrations of ODNs for 24 hr.
Table 1.
Synthetic oligonucleotides (ODNs) used in the study
| ODN | Sequence (5′−3′) | Species | References |
|---|---|---|---|
| ODN1 | TTT TCA ATT CGA AGA TGA AT | Human, in vitro | Magnusson, 200120 |
| ODN2 | TT CAT CTT GCA ATT GAA AA | Human, in vitro | Magnusson, 200120 |
| 2006 | TCG TCG TTT TGT CGT TTT GTC GTT | Human, in vitro | Krug, 200321 |
| 1585 | ggG GTC AAC GTT Gag ggg gG | Human, in vitro | Krug, 20017 |
| 2216 | ggG GGA CGA TCG TCg ggg gG | Human, in vitro | Krug, 200321 |
| 2135 | TCG TCG TTT GTC GTT TTG TCG TT | Bovine, in vitro | Pontarollo, 200217 |
| ODN3 | GCT AGA CGT TAG CGT | Pigs, in vivo | Van der Stede, 200219 |
| ODN4 | GCT AGA GCT TAG GCT | Pigs, in vivo | Van der Stede, 200219 |
| D19 | ggT GCA TCG ATG CAG ggg gg | Pigs, in vitro | Kamstrup, 200118 |
| D32 | ggT GCG TCG ACG CAG ggg gg | Pigs, in vitro | Kamstrup, 200118 |
Lower case letters: phosphorothiate linkages; upper case letters: phosphodiester linkage 3′ of the base.
Transmissible gastroenteritis virus (TGEV) (strain Purdue 115) was used as a positive control for IFN-α induction.12 The virus was propagated in PK15 cells. For induction of IFN-α in PBMC populations, virus preparations were UV-inactivated under controlled conditions.13 Optimal concentrations of virus preparations for IFN-α induction were determined by titration on PBMCs.
Alternatively, cells were stimulated with 1 µg/ml lipopolysaccharide (LPS) (Sigma Chemicals) or 10 µg/ml poly I : poly C (pIC) (Sigma), also for 24 hr.
Detection of cytokines in supernatant
IFN type I in the supernatants was quantified using a bioassay based on the antiviral effect of IFN type I against vesicular stomatitis virus (VSV) in PK-15 cells. Recombinant porcine IFN-α (R & D Systems, Baston Lane, UK) was employed as a reference standard, as previously described.14 The presence of IFN type I was controlled by the addition of a neutralizing IFN-α polyclonal antiserum (R & D Systems).
Alternatively, an IFN-α enzyme-linked immunosorbent assay (ELISA) was used.15 ELISA microplates were coated with anti-pig IFN-α mAb K9 (5 µg/ml in PBS; R & D Systems) overnight at 37°. The plates were then washed and incubated with PBS containing 0·05% Tween-20 (v/v) and 0·5% (w/v) bovine serum albumin (PBS/T20/BSA). Test supernatants were diluted to 1 : 2 in PBS/T20/BSA, added to the plates and incubated for 5 hr at 37°. After washing, biotinylated pig IFN-α mAb F17 (kindly donated by Dr B. Charley, INRA, Jouy-en-Josas, France), was added to the wells and incubated overnight at 4°. Following washing, 100 µl/well of peroxidase-conjugated streptavidin (Dako, Zug, Switzerland) was added for 1 hr at 37°. Positive reactions were revealed using the o-phenyl dimethyl substrate (Sigma), at 450 nm.
IL-10 was quantified using a commercial kit from Biosource Int., and tumour necrosis factor-α (TNF-α) was quantified using a kit from Perbioscience (Lausanne, Switzerland).
IL-12 was detected by ELISA. To achieve this, we used microplates coated overnight at 4° with the IL-12 mAb, MP121 (Perbioscience). The plates were washed with PBS/T20/BSA and then blocked with PBS containing 5% (w/v) sucrose and 1% (w/v) BSA. The test supernatants were diluted 1 : 2 in PBS/T20/BSA, added to the ELISA plate and incubated for 2 hr at 37°. After washing, the wells were incubated with the IL-12 detection antibody, PP121 (Perbioscience), for 1 hr at 37°. Then, 100 µl/well of anti-rabbit peroxidase-conjugated antibody (Jackson Immunoresearch, West Grove, PA) was added for 1 hr at 37 °C. Positive reactions were revealed using the o-phenyl dimethyl substrate (Sigma), at 450 nm.
Intracellular cytokine staining
SWC3+ PBMCs, sorted using MACS (Miltenyi Biotec), were stimulated with UV-inactivated TGEV, or the CpG-ODNs, at concentrations giving optimal IFN responses, and cultured for 6 hr in growth medium. MoDCs were stimulated overnight with CpG-ODNs at optimal concentrations, or with LPS (1 µg/ml). After staining of cell-surface molecules, the cells were fixed and permeabilized (Fix & Perm, Caltag, CA) for staining with IFN-α mAbs F17 and K9 (10 µg/ml; R & D Systems) or TNF-α mAbs (Perbioscience). For flow cytometry detection, isotype-specific FITC, R-PE (Southern Biotechnology Associates) and R-PE-Cy5 (Dako) conjugates were used.
Maturation of cells
SWC3+ cells were cultured with UV-TGEV, CpG-ODNs (optimal concentration), or 100 U/ml of rpIL-3,5 for 1 or 3 days at 39°. NIPCs and monocytes were identified through the double labelling for CD4 (mAb PT90A) and SWC3 (mAb 74-22-15A). Maturation of the cells was determined by the expression of MHC class II (MSA3 mAb, kindly donated by Dr A. Saalmuller), or CD80/86 expression [detected using a human cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)–mouse immunoglobulin fusion protein] (Alexis Corporation, Lausen, Switzerland).10 To test whether CpG-ODNs induce maturation, MoDCs were stimulated overnight with CpG-ODNs at optimal concentrations. As a positive control of DC maturation, these cells were stimulated with a combination of TNF-α (10 ng/ml) and IFN-α (1000 U/ml), or with LPS (1 µg/ml). The expression of CD80/86 and MHC class II was measured by flow cytometry.
Results
IFN type I-inducing activity of the CpG-ODNs
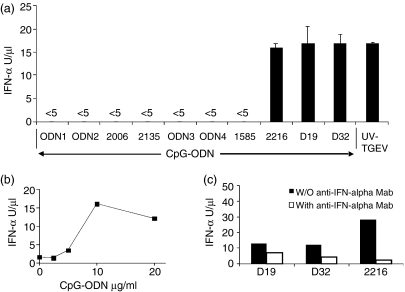
Amongst the CpG-ODNs employed (Table 1), some had been shown to have stimulatory effects in vitro or in vivo in mice,16 humans,7 cows17 or pigs.18,19 ODN1 and ODN2 (inversion of the CpG motif in a complementary strand of ODN1), described as inducers of IFN-α in human PBMCs, did not induce IFN-α in pig PBMCs (Fig. 1a). This was not surprising, in view of the report by Magnusson et al.20 that IFN-α induction by this ODN required lipofection. An objective of the present work was to analyse the immunomodulatory effects of CpG motifs without the added complication of lipofection. Consequently, two other ODNs, ODN3 (CpG ODN) and ODN4 (control GpC), were chosen as a result of their reported effects in vivo in swine.19 Neither of these ODNs induced IFN type I in PBMCs. The CpG-ODN 213517 and the prototype type-B CpG-ODN 2006,21 known to promote B-cell proliferation, also had no effect on IFN-α secretion by porcine PBMCs. In contrast, the CpG-ODNs 2216, D19 and D32 induced the production of IFN-α. Of the CpG-ODNs (1585 and 2216) containing a mixed phosphorothiate-phosphodiester backbone,7 only the type-A CpG-ODN, 2216, induced high levels of IFN-α. The reference control inducer, UV- inactivated TGEV,22 showed that high levels of IFN-α could be induced in porcine PBMCs (Fig. 1a). The CpG-ODN-stimulated IFN-α production occurred in a concentration-dependent manner, with 10 µg/ml giving the maximum responses (Fig. 1b). Addition of neutralizing IFN-α mAb to the VSV bioassay blocked between 50 and 95% of the antiviral activity, demonstrating a major role for IFN-α (Fig. 1c).
Figure 1.
CpG-oligonucleotide (ODN)-mediated stimulation of interferon-α (IFN-α) production by peripheral blood mononuclear cells (PBMCs). PBMCs (3 × 106 cells/ml) were incubated with CpG-ODN (10 µg/ml) or a UV-inactivated transmissible gastroenteritis virus (UV-TGEV) [at a multiplicity of infection of 1 tissue culture infective dose 50% (TCID50)/ml] positive control, for 24 hr. IFN-α was measured in the supernatant by the vesicular-stomatitis virus (VSV) infectivity reduction bioassay. (a) IFN-α response following treatment with different CpG-ODNs and UV-TGEV. (b) Dose dependency of the CpG-ODN D32-induced synthesis of IFN-α. (c) PBMCs stimulated with CpG-ODN D19, D32 or 2216 in the presence of blocking antibodies against IFN-α, with IFN-α measurement at 24 hr (black bars, blocking antibodies absent; white bars, blocking antibodies present).
CpG-ODN induction of SWC3+ cells to produce both IFN-α and TNF-α
In order to characterize further this IFN-α induction, in particular the cells responsible for its production, the SWC3+ populations of PBMCs were analysed. Sorting SWC3+ enriches for the presence of monocytes, DCs and NIPCs. High levels of IFN-α were induced by the CpG-ODNs D32 and 2216 (Table 2). In addition, a high level of TNF-α (relative to LPS induction) was measured. The level of IFN-α induced by CpG-ODNs was 200 times higher than that induced by LPS, contrasting with the levels of TNF-α, which were similar in the CpG-ODN- and LPS-treated cultures. CpG-ODN 2006 induced little or any of these cytokines. pIC induced IFN-α at levels similar to those induced by LPS but, in contrast to LPS and CpG-ODN, pIC did not induce TNF-α (Table 2).
Table 2.
Cytokine responses by SWC3+ cells after different stimulations
| IFN-α (U/ml) | TNF-α (pg/ml) | |
|---|---|---|
| Medium | <3 | <5 |
| CpG-ODN D32 | 19 032 ± 840 | 841 ± 82 |
| CpG-ODN 2216 | 12 568 ± 890 | 585 ± 84 |
| CpG ODN 2006 | 123 ± 9 | <5 |
| LPS | 106 ± 4 | 415 ± 44 |
| pIC | 99 ± 2 | <5 |
The SWC3+-sorted cells were stimulated with CpG-oligonucleotides (ODNs) (10 µg/ml), lipopolysaccharide (LPS) (1 µg/ml) or Poly I : C (pIC) (10 µg/ml). The cell supernatants were tested for the presence of interferon-α(IFN-α)and tumour necrosis factor-α(TNF-α) by enzyme-linked immunosorbent assay (ELISA) at 24 hr poststimulation.
Cytokine responses in CD4- and CD14-sorted PBMC subpopulations
Within the SWC3+ population, it has been demonstrated that the phenotypically defined CD4+ CD14− subset contains the NIPCs.5
In order to determine whether cytokine induction by CpG-ODNs was essentially caused by the NIPC subpopulation, PBMCs were separated by MACS using mAbs against CD4 and CD14. Both positive and negative fractions, as well as unsorted PBMCs, were stimulated with several CpG-ODNs, LPS and pIC. The cytokine profiling analysed IFN-α, TNF-α, IL-10 and IL-12 secretion. Table 3 shows that CpG-ODN 2216 induced a high level of IFN-α in the CD4+ and CD14− subpopulations wherein NIPC would be enriched. The other sorted fractions produced up to 10-fold less IFN-α. In these experiments, CpG-ODN 2006 and pIC, and also LPS, were incapable of inducing IFN-α.
Table 3.
Cytokine profiles induced in peripheral blood mononuclear cells (PBMCs), and in CD4+, CD4−, CD14+ and CD14− subpopulations, in response to different stimulations
| Medium | 2006 | 2216 | LPS | pIC | % Purity | |
|---|---|---|---|---|---|---|
| IFN-α (U/ml) | ||||||
| PBMC | <3 | <3 | 6325 ± 370 | <3 | <3 | |
| CD4+ | <3 | <3 | 20 070 ± 1840 | <3 | <3 | 87 |
| CD4− | <3 | <3 | 1505 ± 230 | <3 | <3 | 6 |
| CD14+ | <3 | <3 | 2290 ± 20 | <3 | <3 | 96 |
| CD14− | <3 | <3 | 23 940 ± 650 | <3 | <3 | 1 |
| TNF-α (pg/ml) | ||||||
| PBMC | <5 | <5 | 419 ± 44 | ND | ND | |
| CD4+ | <5 | <5 | 137 ± 33 | ND | ND | 87 |
| CD4− | <5 | <5 | 97 ± 21 | ND | ND | 6 |
| CD14+ | <5 | <5 | 12 ± 6 | ND | ND | 96 |
| CD14− | <5 | <5 | 236 ± 22 | ND | ND | 1 |
| IL-10 (pg/ml) | ||||||
| CD4+ | <8 | <8 | <8 | <8 | <8 | 87 |
| CD4− | <8 | <8 | <8 | 473 ± 11 | 382 ± 4 | 6 |
| CD14+ | <8 | <8 | <8 | 469 ± 10 | 198 ± 3 | 96 |
| CD14− | <8 | <8 | <8 | 371 ± 32 | 405 ± 23 | 1 |
| IL-12 (pg/ml) | ||||||
| CD4+ | <15 | <15 | 191 ± 18 | <15 | <15 | 87 |
| CD4− | <15 | <15 | <15 | <15 | <15 | 6 |
| CD14+ | <15 | <15 | <15 | <15 | <15 | 96 |
| CD14− | <15 | <15 | 30 ± 4 | <15 | <15 | 1 |
PBMCs were separated into CD4 and CD14 fractions by magnetic-activated cell (MACS) sorting. Fractions were then stimulated with the CpG-ODNs 2006 or 2216 (10 µg/ml), lipopolysaccharide (LPS) (1 µg/ml) or Poly I : C (pIC) (10 µg/ml). The cell supernatants were harvested after 24 hr and tested, by enzyme-linked immunosorbent assay (ELISA), for the presence of interferon-α (IFN-α), tumour necrosis factor-α(TNF-α), interleukin (IL)-10 and IL-12. One representative experiment out of two is shown. ND, not determined.
IL-12 was induced only by CpG-ODN 2216, in both the CD14− and CD4+ fractions wherein NIPC are enriched. In contrast to IFN-α, the highest levels of TNF-α were found in the unsorted PBMC after CpG-ODN stimulation. Nevertheless, again the CD4+ and CD14− fractions produced more IFN-α than the other fractions. No IL-10 was induced in any fractions after CpG-ODN stimulation. As expected, LPS induced IL-10 in all fractions, except for the CD4+ fraction. pIC was similar to LPS in terms of IL-10 induction. This was the only cytokine, of those tested, which was induced by pIC.
Role of NIPC in inflammatory cytokine induction by type-A CpG-ODNs
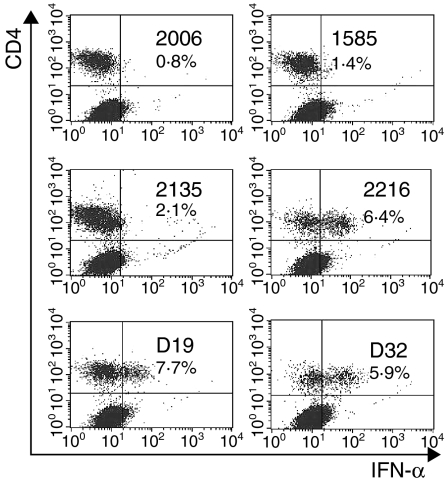
The above experiments indicated that type A CpG-ODNs induce cell fractions containing porcine NIPCs to produce IFN-α and IL-12. High TNF-α values were only obtained after stimulation of total PBMCs (Table 3). Consequently, cytokine induction was monitored by intracellular cytokine detection. As with cytokine detection in the culture supernatants, the type A CpG-ODNs 2216, D19 and D32 were seen to induce IFN-α accumulation within the cells (Fig. 2). Double staining for CD4 and IFN-α expression demonstrated that only the CD4+ cells accumulated IFN-α after stimulation with the CpG-ODN.
Figure 2.
Intracellular detection of interferon-α (IFN-α) in SWC3+ subsets after stimulation with different CpG-oligonucleotides (ODNs). The SWC3+ cells were enriched by magnetic-activated cell sorter (MACS) separation. After 6 hr of incubation with CpG-ODNs (10 µg/ml), the cells were harvested, stained for cell-surface CD4 and SWC3 antigen, fixed, permeabilized and then stained for IFN-α. The cells were gated for the SWC3+ population to give the dot-plots presented for CD4/IFN-α staining.
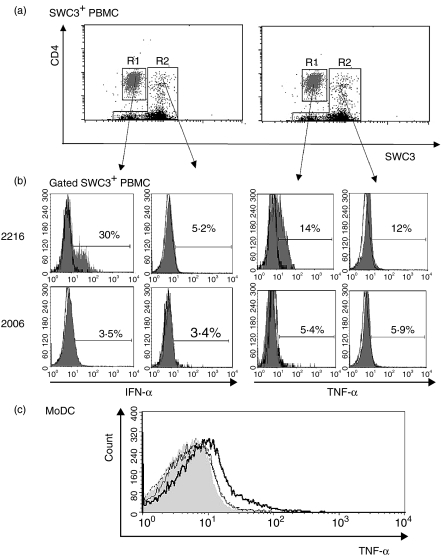
Further characterization of the cell population responsive to CpG-ODN stimulation employed SWC3/CD4/IFN-α, SWC3/CD4/TNF-α triple labelling. Both IFN-α and TNF-α were exclusively detected in the SWC3low CD4high subset (Fig. 3a), corresponding to NIPCs.5 The type A CpG-ODN 2216, but not the type B CpG ODN 2006, induced these responses (Fig. 3b). Similar results were obtained after stimulation with the type A CpG-ODNs, D32 and D19 (data not shown), relating to the reported stimulation with UV-inactivated TGEV.5 In contrast to the SWC3low CD4high subset in R1, the remaining SWC3+ cells, defined in R2 of Fig. 3(a), containing the SWC3+ CD4− monocytes, the SWC3low CD4− myeloid blood DC5 and an unknown SWC3+ CD4+ subset, did not produce detectable intracellular IFN-α and TNF-α after stimulation with CpG-ODNs (Fig. 3b). This was the case with all CpG-ODNs tested, as well as after stimulation with UV-inactivated TGEV (data not shown). MoDCs did not respond to any CpG-ODN tested in this report. However, LPS stimulation induced intracellular TNF-α, but not IFN-α (data not shown), after overnight stimulation (Fig. 3c).
Figure 3.
Detection of interferon-α (IFN-α) and tumour necrosis factor-α (TNF-α) in natural interferon-producing cells (NIPCs) and other SWC3+ subsets after stimulation with CpG-oligonucleotides (ODNs). (a) CD4/SWC3 labelling of sorted SWC3+ cells. This labelling permits the distinction of SWC3low CD4high NIPCs (R1: grey) and of other SWC3+ cells (R2: black). (b) IFN-α and TNF-α staining of NIPCs (R1) and other SWC3+ cells (R2) after incubation with the IFN-α-inducing CpG-ODN 2216 or the non-IFN-α-inducing CpG-ODN 2006, for 6 hr (c) TNF-α expression of monocyte-derived DC (MoDC) treated with medium (grey-filled histogram), lipopolysaccharide (LPS) (bold-line histogram), CpG-ODN 2006 (thin-line histogram) or CpG-ODN D32 (dotted-line histogram).
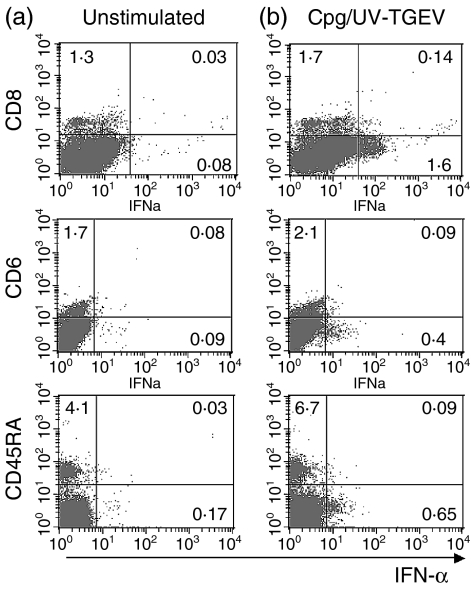
Additional phenotyping experiments demonstrated that the CpG-responsive NIPCs did not express CD8, CD6 or CD45RA (Fig. 4). The figure shows results with CpG-ODNs plus UV-inactivated TGEV to increase the probability of detecting responsive cells. Similar results were obtained with the CpG-ODN or UV-inactivated TGEV alone. The image seen with CpG-ODN relates to that identifying the NIPC by stimulation with UV-inactivated TGEV.5
Figure 4.
Cell-surface expression of SWC3+ cells activated by a mixture of CpG-oligonucleotide (ODN) 2216 and UV-inactivated transmissible gastroenteritis virus (UV-TGEV) positive control. SWC3+ sorted cells were stimulated for 6 hr with either (a) medium alone or (b) a mixture CpG-ODN 2216 and UV-TGEV. The expression of CD8, CD6 and CD45RA and intracellular interferon-α (IFN-α) are shown.
Phenotypic maturation of NIPCs following CpG-ODN treatment
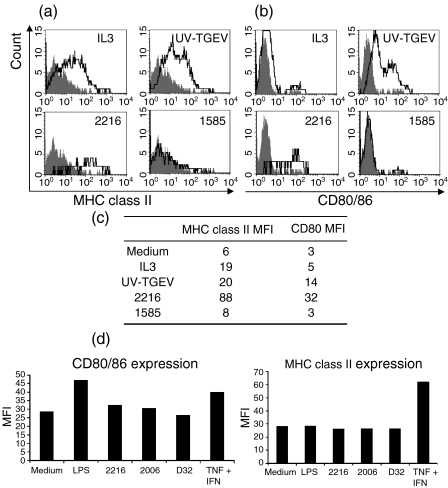
SWC3+ cells were stimulated with different CpG-ODNs for 1 or 3 days before analysing CD80/86 and MHC class II expression. As shown in Fig. 5(a,b,c), CD80/86 and MHC class II up-regulation was observed on the SWC3low CD4high NIPCs after 1 day of stimulation with IL-3 and UV-inactivated TGEV, compared to unstimulated controls. Expression of CD80/86 and MHC class II was also up-regulated by the IFN-α-inducing CpG-ODN 2216, but not by the non-IFN-α-inducing CpG-ODN 1585 (Fig. 5a,b,c). Similar results were observed at 3 days poststimulation (data not shown).
Figure 5.
In vitro maturation of natural interferon-producing cells (NIPCs) (a) SWC3+ sorted cells were incubated for 1 day with interleukin-3 (IL-3) (100 U/ml), UV-inactivated transmissible gastroenteritis virus (UV-TGEV) [at a multiplicity of infection of 1 tissue culture infective dose 50% (TCID50)/ml] positive control, CpG-oligonucleotide (ODN) 2216 (IFN-α inducer) or CpG-ODN 1585 (non-inducer of IFN-α) (10 µg/ml). The maturation of NIPCs (defined as SWC3low CD4high) was assessed by analysis of major histocompatibility complex (MHC) class II and CD80/86 expression. Filled histograms are the untreated ‘medium alone’ controls. (b) The results shown in (a) are represented as mean fluorescence intensity. (c) Monocyte-derived DCs (MoDCs) were stimulated overnight with lipopolysaccharide (LPS), CpG-ODN 2216, CpG-ODN 2006, CpG-ODN D32 or a cocktail of tumour necrosis factor-α (TNF-α) and IFN-α overnight. Then, expression of CD80/86 and MHC class II was analysed. One of three representative experiments is shown.
In contrast to the NIPC, no increase in CD80/86 or MHC class II expression was detected after stimulation of MoDC with the CpG-ODNs (Fig. 5d). Only a ‘maturation cocktail’ of TNF-α and IFN-α induced an increase in the maturation phenotype (Fig. 5d). LPS induced an up-regulation of CD80/86, but not of MHC class II.
Influence of CpG-ODN on the in vitro survival of SWC3low CD4+ cells
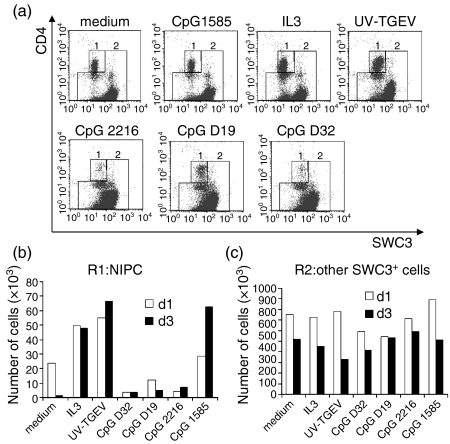
NIPCs have a low survival capacity in vitro without appropriate stimulation, an observation reported also for porcine NIPCs.5 Consequently, the capacity of CpG-ODNs to promote the survival of NIPCs in culture was analysed using SWC3+-sorted cells. The CpG-ODNs inducing a high level of IFN-α (D32, D19 and 2216) did not increase the survival of SWC3low CD4high cells (Fig. 6a,b). This contrasted with the survival-promoting effects of IL-3 and UV-inactivated TGEV (Fig. 6a,b). In fact, these CpG-ODNs appeared to be detrimental to NIPC survival (Fig. 6b). Interestingly, CpG-ODN 1585, which neither induced IFN-α, nor promoted CD80/86 or MHC class II up-regulation, enhanced the survival of NIPC, particularly after 3 days of culture. These effects were restricted to the SWC3low CD4high cells. No effect of the CpG-ODNs, IL-3 or UV-inactivated TGEV on the survival of the remaining SWC3+ cells, defined in R2 of Fig. 6(a), was observed (Fig. 6c). This population is composed mainly of monocytes and myeloid DCs.5
Figure 6.
Effect of CpG-oligonucleotide (ODN) stimulation on the survival of natural interferon-producing cells (NIPCs). SWC3+ cells were treated for 1 or 3 days with medium (negative control), interleukin-3 (IL-3), UV-inactivated transmissible gastroenteritis virus (UV-TGEV) positive control, or different CpG-ODNs (D32, D19, 2216 or 1585). (a) SWC3/CD4 phenotype of the cells at 1 day post-treatment and region (R1 = 1 and R2 = 2) definition. (b) Number of viable cells in R1 (SWC3low CD4high NIPC) at 1 and 3 days post-treatment. (c) Number of viable cells in R2 (other SWC3+ cells) at 1 and 3 days post-treatment.
Discussion
The generation of antigen-specific acquired immune responses against foreign antigens depends upon the concomitant activation of DCs through PRRs, such as TLRs. DNA containing CpG motifs, especially in the unmethylated form, is a particularly potent ‘danger’ signal, inducing strong antiviral and T helper (Th1) profile cytokine responses. In the human immune system, NIPCs are responsible for these responses and therefore represent the main cellular target for CpG-ODN, especially in the context of a vaccine adjuvant. Indeed, the potent IFN-α responses are attractive for many aspects of vaccinology. For this reason, a number of CpG-ODNs were analysed for their immunodulatory capacity.
The CpG-ODNs D19, D32 and 2216, induced high levels of IFN-α production by porcine PBMCs. These CpG-ODNs possess polyG sequences at both 5′ and 3′ ends, with a central palindromic sequence containing the CG dinucleotides. The results suggest that these structural elements are important for the IFN-α-inducing capacity of CpG-ODN. Indeed, CpG-ODNs without polyG sequences at both ends were inactive. It has been proposed that such polyG stretches contribute to CpG-ODN activity by increasing the binding of the ODN to scavenger receptors, mediating cellular uptake.23,24 As for the palindrome, the CpG-ODN 2006, with a central non-palindromic sequence, did not induce IFN-α responses, either in PBMCs or in subpopulations enriched for NIPCs. This relates to the report demonstrating that palindromic unmethylated CpG motifs are required for the IFN-α-inducing activity of ODN on both human and murine spleen PBMCs.25
It has recently been shown that the GTCGTT motif present in CpG-ODN 2135 induces lymphocyte proliferation in several species, including pigs.17,26 CpG-ODN 2135 did not activate porcine PBMCs to produce IFN-α, indicating that the induction of proliferation is IFN-α independent. The CpG-ODN3 contains a GACGTT motif active in mice26 and can promote antibody responses when used as an adjuvant in pigs.19 Yet, this CpG-ODN was unable to induce IFN-α production, suggesting that the adjuvant effect was mediated by targeting another population of leucocytes.
In order to understand the varying biological effects of different CpG-ODNs, it is necessary to determine their target cell populations. This is essential for a rational approach to CpG-adjuvanted vaccine design. The present work demonstrated that IFN-α induced by CpG-ODNs 2216, D19 and D32, is exclusively produced by the porcine equivalent of NIPCs. Intracellular cytokine staining clearly demonstrated that only the SWC3low CD4high population, representing porcine NIPCs,5 expressed IFN-α and TNF-α following CpG-ODN stimulation. These cells are also IL-3 receptor+, CD6–, CD8– and CD45RA–, and are the IFN-α-producing population responding to inactivated virus (UV-TGEV) stimulation.5 The importance of the NIPC was confirmed with the higher IFN-α responses obtained when PBMCs were sorted into CD4+ and CD14− fractions, reflecting the NIPC enrichment therein. This was not the case for TNF-α, even though intracellular cytokine staining clearly demonstrated NIPCs to be the main or only source of TNF-α after stimulation with CpG-ODNs. An explanation for this could be that NIPCs require bystander help from another cell population to produce high amounts of TNF-α in response to CpG. This is not the case for IFN-α production. Nor is there any apparent difficulty for porcine NIPC to produce IL-12 in response to CpG-ODN. Murine PDCs/NIPCs are also capable of producing IL-12 in response to CpG-ODNs,27 although IL-12 production by human PDCs/NIPCs requires costimulation with CpG-ODN and CD40L.28 With porcine PBMCs, efficient production of IL-12 was found with the CD4+ and CD14− fractions of separated PBMCs. The absence of IL-12 induction by CpG-ODNs in the other cell fractions points to the importance of the NIPCs as source of this cytokine. Unfortunately, intracellular cytokine staining was not possible for IL-12.
CpG-containing DNA has been described to induce the differentiation of NIPCs into mature PDCs, thereby promoting an up-regulation of costimulatory molecules such as CD80 and CD86, as well as MHC class II.8 Porcine NIPCs are also induced by CpG-ODN into a high expression of MHC class II and CD80/86. Interestingly, this up-regulation was superior to that induced by virus or IL-3. It is possible that this maturation was secondary, in response to the IFN-α and TNF-α induced by the CpG-ODN. It is known that these cytokines can induce the maturation of DCs in general.4,29–32 There was no direct effect of CpG-ODNs on MoDCs in terms of cytokine secretion or maturation phenotype. This could be explained by the absence of TLR9 on the porcine MoDCs, relating to human MoDCs.
Another characteristic of NIPCs is their low survival rate in the absence of growth factor or stimulation. IL-3 is a key survival factor for both human33,34 and porcine5 NIPCs. Virus (inactivated TGEV) stimulation also induced NIPC survival.5 In contrast, CpG-ODNs with a strong capacity to induce cytokine production by a mature phenotype in NIPC, did not promote survival. It was CpG-ODN 1585, which induced neither cytokines nor a mature phenotype, that promoted NIPC survival. This was a surprising observation considering that the virus stimulation which induced high IFN-α and TNF-α levels did promote NIPC survival. Furthermore, endogenous IFN-α is known to promote NIPC survival.35 One explanation could be that CpG-ODNs and inactivated viruses, such as UV-TGEV, induce different signalling pathways for NIPCs. While CpG-ODN activity is TLR9 mediated, the stimulatory effect of the UV-TGEV is preserved with nucleic acid-free viral pseudoparticles36 and is dependent on the presence of glycosylated viral envelope proteins.37 Although both signalling events would result in IFN-α secretion, this alone is inadequate to maintain NIPC viability. Alternatively, IFN-inducing CpG-ODNs may be modulating the NIPC phenotype such that they lose CD4 expression. However, both flow cytometry and confocal microscopy showed that only CD4+ cells can produce IFN-α.5
It can be concluded that CpG-ODNs, with poly G sequences at both the 5′ and 3′ ends and central CG dinucleotides, target porcine NIPCs, but not other myeloid DCs or monocytes. Although CpG-ODN receptor expression on porcine cells is unknown, the response characteristic suggests a similar distribution compared to human cells. CpG-induced IFN-α would play a key role through its potent immunomodulatory activities, including DC activation, natural killer (NK) cell activation, B-cell isotype switching and induction of Th1 responses.38–41 Of course, CpG-ODNs are known to stimulate monocytes/macrophages, NK cells and other DC subsets, but this is caused by indirect effects of the cytokines released by the activated NIPCs. It is on this point that the human and porcine immune systems differ from those of the mouse, where direct effects of CpG-ODNs on macrophages and various DC subsets have been described.42–44 Such differences must be considered when the effects of CpG-ODNs on the immune response are extrapolated between species. The present report not only elucidates certain critical immunological characteristics important for the development of CpG-ODN-based adjuvants for the pig, but also demonstrates the value of the pig as an alternative relevant animal model for human vaccine development.
Acknowledgments
This work was supported by the Swiss Federal Office for education and science (Project 02·003) and is linked to EU project (QLTR-2001-00825).
References
- 1.Svensson H, Cederblad B, Lindahl M, Alm G. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus. J Interferon Cytokine Res. 1996;16:7–16. doi: 10.1089/jir.1996.16.7. [DOI] [PubMed] [Google Scholar]
- 2.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 3.Dalod M, Salazar-Mather TP, Malmgaard L, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–28. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda K, Sakaguchi S, Nakajima C, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–7. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summerfield A, Guzylack-Piriou L, Schaub A, Carrasco CP, Tache V, Charley B, McCullough KC. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110:440–9. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5:62–9. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 7.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Rothenfusser S, Tuma E, Endres S, Hartmann G. Plasmacytoid dendritic cells: the key to CpG(1) Hum Immunol. 2002;63:1111–9. doi: 10.1016/s0198-8859(02)00749-8. [DOI] [PubMed] [Google Scholar]
- 9.McCullough KC, Basta S, Knotig S, et al. Intermediate stages in monocyte–macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98:203–12. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco CP, Rigden RC, Schaffner R, et al. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–84. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summerfield A, Horn MP, Lozano G, Carrasco CP, Atze K, McCullough K. C-kit positive porcine bone marrow progenitor cells identified and enriched using recombinant stem cell factor. J Immunol Methods. 2003;280:113–23. doi: 10.1016/s0022-1759(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 12.Nowacki W, Cederblad B, Renard C, La Bonnardiere C, Charley B. Age-related increase of porcine natural interferon alpha producing cell frequency and of interferon yield per cell. Vet Immunol Immunopathol. 1993;37:113–22. doi: 10.1016/0165-2427(93)90059-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charley B, Lavenant L, Lefevre F. Coronavirus transmissible gastroenteritis virus-mediated induction of IFN alpha-mRNA in porcine leukocytes requires prior synthesis of soluble proteins. Vet Res. 1994;25:29–36. [PubMed] [Google Scholar]
- 14.Ruggli N, Tratschin JD, Schweizer M, McCullough KC, Hofmann MA, Summerfield A. Classical swine fever virus interferes with cellular antiviral defense: evidence for a novel function of N(pro) J Virol. 2003;77:7645–54. doi: 10.1128/JVI.77.13.7645-7654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz d A, Artursson K, L'Haridon R, Perers A, La Bonnardiere C, Alm GV. A sensitive immunoassay for porcine interferon-alpha. Vet Immunol Immunopathol. 1992;30:319–27. doi: 10.1016/0165-2427(92)90102-v. [DOI] [PubMed] [Google Scholar]
- 16.Klinman DM, Conover J, Coban C. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect Immun. 1999;67:5658–63. doi: 10.1128/iai.67.11.5658-5663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pontarollo RA, Rankin R, Babiuk LA, et al. Monocytes are required for optimum in vitro stimulation of bovine peripheral blood mononuclear cells by non-methylated CpG motifs. Vet Immunol Immunopathol. 2002;84:43–59. doi: 10.1016/s0165-2427(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 18.Kamstrup S, Verthelyi D, Klinman DM. Response of porcine peripheral blood mononuclear cells to CpG-containing oligodeoxynucleotides. Vet Microbiol. 2001;78:353–62. doi: 10.1016/s0378-1135(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 19.Van der Stede Y, Verdonck F, Vancaeneghem S, Cox E, Goddeeris BM. CpG-oligodinucleotides as an effective adjuvant in pigs for intramuscular immunizations. Vet Immunol Immunopathol. 2002;86:31–41. doi: 10.1016/s0165-2427(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson M, Magnusson S, Vallin H, Ronnblom L, Alm GV. Importance of CpG dinucleotides in activation of natural IFN-alpha-producing cells by a lupus-related oligodeoxynucleotide. Scand J Immunol. 2001;54:543–50. doi: 10.1046/j.1365-3083.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 21.Krug A, Rothenfusser S, Selinger S, et al. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol. 2003;170:3468–77. doi: 10.4049/jimmunol.170.7.3468. [DOI] [PubMed] [Google Scholar]
- 22.Charley B, Lavenant L. Characterization of blood mononuclear cells producing IFN alpha following induction by coronavirus-infected cells (porcine transmissible gastroenteritis virus) Res Immunol. 1990;141:141–51. doi: 10.1016/0923-2494(90)90133-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson AM, Rich A, Krieger M. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet-stabilized four-stranded helices. J Biol Chem. 1993;268:3546–54. [PubMed] [Google Scholar]
- 24.Prasad V, Hashim S, Mukhopadhyay A, Basu SK, Roy RP. Oligonucleotides tethered to a short polyguanylic acid stretch are targeted to macrophages: enhanced antiviral activity of a vesicular stomatitis virus-specific antisense oligonucleotide. Antimicrob Agents Chemother. 1999;43:2689–96. doi: 10.1128/aac.43.11.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–6. [PubMed] [Google Scholar]
- 26.Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucl Acid Drug Dev. 2001;11:333–40. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- 27.Gilliet M, Boonstra A, Paturel C, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–8. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Kohrgruber N, Halanek N, Groger M, et al. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–9. [PubMed] [Google Scholar]
- 30.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63:1126–32. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 31.Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J Infect Dis. 2003;187:1126–36. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]
- 32.Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol. 2002;32:2617–22. doi: 10.1002/1521-4141(200209)32:9<2617::AID-IMMU2617>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–9. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 36.Baudoux P, Carrat C, Besnardeau L, Charley B, Laude H. Coronavirus pseudoparticles formed with recombinant M and E proteins induce alpha interferon synthesis by leukocytes. J Virol. 1998;72:8636–43. doi: 10.1128/jvi.72.11.8636-8643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laude H, Gelfi J, Lavenant L, Charley B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J Virol. 1992;66:743–9. doi: 10.1128/jvi.66.2.743-749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–90. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 39.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–24. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald-Bocarsly P. Natural interferon-alpha producing cells: the plasmacytoid dendritic cells. Biotechniques (Suppl) 2002;33:16–9. [PubMed] [Google Scholar]
- 41.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmi H, Kaisho T, Takeda K, Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol. 2003;170:3059–64. doi: 10.4049/jimmunol.170.6.3059. [DOI] [PubMed] [Google Scholar]
- 43.Maurer T, Heit A, Hochrein H, et al. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol. 2002;32:2356–64. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 44.Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]