Abstract
The ability to expand tumour-infiltrating lymphocytes in vitro has been greatly enhanced by the use of antigen-independent mechanisms of immune cell costimulation. We have produced human, using the K562 cell line, and murine, using YAC-1 cells, artificial antigen presenting cells (aAPC) and demonstrate that these cell types stimulate murine lymphocyte populations in distinct ways. Using aAPC that have been transfected with CD137L (4-1BBL) and CD32 (FcRγII), as a means to bind anti-CD3 and anti-CD28 antibody, we found that CD4 cells preferentially expanded in vitro with K562 aAPC, while CD8 cells expanded with both K562 and YAC-1 aAPC. Co-stimulation mediated by CD137L on aAPC was superior to that mediated by anti-CD28 antibody. This was seen in both long and short-term expansion assays, and by the rapid induction of a CD8+ DX5+ population. DX5 serves, under these in vitro conditions, as a general marker for lymphocyte activation. In vivo, the superiority of CD137L was demonstrated by the induction of T helper 1 effectors seen in freshly isolated splenocytes from mice immunized with CD137L-expressing neuroblastoma tumour vaccines. The ability to stimulate a strong CD8 CTL response in vivo correlated with the induction of a DX5+ cell population in splenocytes with a memory-effector phenotype. The presence of this unique DX5+ cell population, phenotypically distinct with regards to CD69 and CD62L expression from DX5+ cells induced by aAPC in vitro, may be associated with the ability of CD137L to induce strong anti-tumour immunity.
Keywords: cancer vaccines, co-stimulation: co-stimulatory molecules, cytotoxic T cells (CTL)
Introduction
Advanced stage or recurrent neuroblastoma remains a serious childhood malignancy for which few effective treatment options exist.1 The use of tumour cell-based vaccines is an attractive mechanism for the generation of antineuroblastoma immunity and in principle represents a treatment modality that does not add toxicity to concurrent radio- or chemotherapy. Clinical evidence suggests that the generation of an immune response to neuroblastoma is possible. A discrete survival advantage is conferred to patients with advanced disease when treatment includes bone marrow transplantation, arguing in favour of an immune contribution to long-term survival.2,3 In a recent published report evaluating tumour contamination in autologous stem cell grafts of patients with high-risk neuroblastoma, a surprising conclusion was that the presence of more than 2000 tumour cells in the graft was associated with a higher probability of survival, which also argues that an antitumour immune response is generated post-transplantation.4 Thus, the time period immediately following stem cell grafting may be ideal for introducing cell-based tumour vaccines.4 Human immunotherapy trials for neuroblastoma have included high-dose interleukin-2 (IL-2), combinations of IL-2 and antiganglioside GD2 antibody, and allogeneic tumour cells doubly transfected with IL-2 and lymphotactin-encoding vectors.5–9 Some antitumour benefit has been demonstrated, but results have yet to match the number of successful strategies shown to protect mice from succumbing to the standard transplantable neuroblastoma cell line, Neuro-2a.
In animal models, both CD8 and natural killer (NK) cells participate in antineuroblastoma immunity when using a wide variety of modified tumour cells, including those modified to produce IL-2, granulocyte–macrophage colony-stimulating factor (GM-CSF) or interferon-γ (IFN-γ), or modified to express intercellular adhesion molecule-1 (ICAM-1) or xenogeneic class II major histocompatibility complex (MHC).10–14 Our previous work demonstrated that an aggressive subclone of Neuro-2a (AGN2a) generated by in vivo passage of the tumour required transfection with both CD80 (B7.1) and CD86 (B7.2) in order to serve as an effective tumour vaccine.15 The effect of dual-expression of CD80 and CD86 was not due to simple additive strength of T-cell signalling, as Scatchard analysis of costimulatory antigen expression on permanently transfected AGN2a lines showed that the combined total of CD80 and CD86 molecules on the surface of these permanent cell lines was approximately equal to the level of CD86 alone.15 Although CD8 cells were primarily responsible for antitumour immunity, lysis of tumour by CD8 TIL exhibited non-classic kinetics. The mechanism of tumour cell kill was non-Fas dependent and full lytic activity, as judged by chromium release, was not seen until 20 hr of coincubation with effector cells. This may be due to a process that requires up-regulation of cell surface molecules on the tumour in response to IFN-γ. In order to explore a potentially more direct pathway of CD8 immune effector cell generation, we combined the direct costimulatory signals afforded by the CD80/86–CD28 ligand system and the CD137–CD137L system, which has been shown to direct immune responses towards T helper 1 (Th1) immunity.
CD137 expression is induced on the surface of both activated T cells and NK cells, and it is a member of the tumour necrosis factor receptor (TNFR) family of cell surface proteins.16 Its receptor, CD137L, is expressed on activated antigen-presenting cells (APC). Tumour cells engineered to over-express the receptor for CD137, CD137L, have been shown to produce tumour immunity due to, at least in part, the stimulation of CD8+ CTL.17 CD137 signalling is independent of, yet often found to work in concert with, the CD28 signalling system as has been demonstrated by the requirement of both signals for the expansion of human CD8 cells in vitro using artificial APC (aAPC).18 Here, we report the optimal configuration for mouse aAPC designed to expand CD8 lymphocytes. We took advantage of CD137L-mediated T-cell expansion both in vitro and in vivo in order to expand murine CD8 cells and potentially other mediators of Th1 immunity. In the in vivo race between tumour growth and immune effectors cells that are either too rare to have an antitumour effect, or which may not be able to expand in response to tumour-specific antigens due to lack of Th1-like signals, CD137L represents a direct means to increase CD8 and NK cell numbers to levels that can mediate antitumour immunity when used in combination with CD28-mediated signalling.19 We also show that induction of a unique CD8+ DX5+ cell population correlates with the induction of tumour immunity by CD137L-bearing tumour-cell vaccines.
Materials and methods
Antibodies, mice, cell lines
The following antibodies, with or without fluorescent label, were obtained from BD Biosciences (BD Biosciences Pharmingen, San Diego, CA): anti-CD16/CD32 (clone 2.4G2), anti-4-1BBL (clone TKS-1), anti-CD3 (clone 145-2C11), anti-CD28 (clone 37.51), anti-CD62L (MEL-14), anti-CD44 (IM7), anti-CD49b (VLA-2 alpha chain, pan-NK, clone DX5), anti-CD69 (H1.2F3), and anti-rat immunoglobulin G2a (IgG2a, clone RG7/1.30). Isotype controls included purified mouse IgG2b and rat IgG2b (BD Biosciences). Anti-B220-, -CD4- and -CD8-conjugated immunomagnetic beads used for automagnetic-activated cell separation (AutoMACS) were purchased from Miltenyi Biotec (Miltenyi Biotec, Auburn, CA). Male A/J mice were purchased from Jackson laboratory (Bar Harbor, ME) and were used at 4–6 weeks old. Mice were housed under AAALAC guidelines at the Medical College of Wisconsin animal resource centre according to institutional guidelines, and experiments were performed under approved protocols. The Neuro-2a cell line was from ATCC (Manassas, VA), and production of the aggressive subclone, AGN2a, was described previously.15
Artificial antigen presenting cell (aAPC) production
The YAC-1 and K562 cells were transfected by electroporation with linearized pcDNA3·1-Hygro plasmid vector (Invitrogen, Carlsbad, CA) encoding CD32 (FcγRII) and the pCI-Neo vector (Promega, Madison, WI) encoding 4-1BBL (CD137-ligand). cDNA for CD32 and 4-1BBL was generated from mouse splenic B cells (selected with anti-B220 microbeads, Miltenyi Biotec), and polymerase chain reaction (PCR) amplified with the following primers for CD32 (restriction sites in bold): forward, 5′-GCTGGCTAGCTCGCTCCAGAGCTGATGGGAAT, reverse, 5′-AATCGCGGCCGCTACAGCATCCCTTGGACCAG; and for 4-1BBL, forward, 5′-GATCCTCGAGATGGACCAGCACACACTTGATGT, reverse, 5′-AATCGCGGCCGCAAAACATAGCAGCTTGAGG. Transduced cells were cultured under drug selection (400 µg/ml hygromycin and/or 800 µg/ml genetecin), cloned by limiting dilution, and subclones selected for high levels of CD137L and CD32 cell surface expression by flow cytometry. Clones were periodically monitored by flow cytometry to ensure high levels of antigen expression. Transduced YAC-1 or K562 aAPC were analyzed for Fc-mediated binding of anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) by incubating the cells with fluoroscein isothiocyanate (FITC)-anti-CD3 and/or phycoerythrin (PE)-anti-CD28 for 30 min on ice, followed by washing in phosphate-buffered saline (PBS)-0·5% bovine serum albumin and detection of bound antibody by flow cytometry (FACScan, Becton-Dickinson). The anti-CD3 concentration used for coating K562 aAPC was 32·0 µg/ml and for YAC-1 it was 2·0 µg/ml at a cell density of 3 × 106/ml. The anti-CD28 concentration used for coating K562 aAPC was 2·0 µg/ml and 32·0 µg/ml for YAC-1 aAPC, at the same cell density. These saturating concentrations were defined by serial dilution of labelled antibody and determination of the concentration at which further increases in concentration did not increase the fluorescence intensity.
In vitro proliferation assays
Splenocytes were harvested from A/J mice (host strain for Neuro-2a), red blood cells removed by hypotonic lysis, and washed cells incubated with either anti-CD4 or anti-CD8 immunomagnetic beads (Miltenyi Biotec). Cells were then positively selected by AutoMACS separation (Miltenyi Biotec), coincubated with aAPC in Dulbecco's modified Eagle's minimal essential medium (DMEM) at the indicated cell densities, and T-cell numbers counted on day 5 of culture. The initial T-cell number used for coculture with aAPC was 0·5 × 106. The T cell : aAPC ratio was 2 : 1 and the T cell : bead (Dynal beads; Dynal Biotech Inc., Lake Success, NY) coated with a 1 : 50 ratio of anti-CD3 and anti-CD28 antibodies) ratio was 1 : 3. For aAPC preparation, aAPC were exposed to 3000 rad gamma-irradiation, coated with non-conjugated antibodies for 1 hr at room temperature using the concentrations listed above, washed in cDMEM (DMEM supplemented with 10% fetal bovine serum (FBS), 1 mm MEM sodium pyruvate, 69 mm l-arginine, 0·6 mg/ml folic acid, 3·6 mg/ml l-asparagine, 10 µm HEPES, 2 mm l-glutamine, 50 µmβ-mercaptoethanol, and Pen-Strep, all from Invitrogen), and then used in cellular assays.
For the analysis of DX5 expression on immune cell subsets, CD4+ and CD8+ populations were purified from A/J splenocytes by immunomagentic bead selection, and cultured (same aAPC : T cell ratios as above) with irradiated K562-based aAPC loaded with anti-CD3, anti-CD28 or with irradiated K562/CD137L-aAPC loaded with anti-CD3 and anti-CD28 as indicated. A portion of the cultured lymphocytes were harvested from 24-well plates and stained for the expression of CD4 or CD8 (according to the population isolated), CD44, CD62L, CD69, and DX5 on days 0,3,5, and 8 of culture. Lymphocytes were analysed for coexpression of immune activation markers by three-colour flow cytometric analysis using CyChrome labelled anti-CD4 or anti-CD8, PE-labelled anti-DX5, and FITC labelled anti-CD44, -CD62L, or -CD69.
To determine expression of the DX5 antigen on T-cell populations induced by tumour vaccination, strain A/J mice were immunized two times with 2 × 106 viable AGN2a-CD80/CD137L cells (one week interval). Splenocytes were isolated 5 days following the second immunization and analysed by three-colour flow cytometric analysis using CyChrome-labelled anti-CD8, PE-anti-DX5, and FITC-labelled anti-CD62L, CD44 or CD69. Control populations included splenocytes from naive mice with no in vitro culture or stimulation, and naïve splenocytes cultured for 8 days with irradiated K562/CD137L-based aAPC loaded with anti-CD3 and anti-CD28.
In vivo tumour vaccination
AGN2a was stably transfected with cDNA expression vectors for CD137L as above, or CD80 and CD86 as described previously.15 Tumour cells were inactivated by pretreatment with mitomycin-C (75 µg/ml) (Sigma Chemicals, St Louis, MO) for 30 min in PBS at 37°. Mitomycin-C-treated or viable AGN2a cells were washed twice in cDMEM, once in PBS, and 2 × 106 cells re-suspended in 200 µl PBS for s.c. injection. For the tumour challenge group, 5 days following the second vaccination, mice were challenged with 1 × 105 or 1 × 104 wild type AGN2a cells. Tumour evidence was monitored two to three times per week. Tumour area was calculated by length × width square millimetres. Mice with tumour were killed when tumours exceeded 250 mm.2 Long-term tumour challenge experiments were also carried out with A/J mice were immunized s.c with 2 × 106 mitomycin C-treated wild-type AGN2a, AGN2a-CD80, AGN2a-CD80/86 or AGN2a-CD80/137 L twice at weekly intervals. One week after the second immunization, wild-type AGN2a cells were injected s.c and tumour growth rate monitored.
Cytokine assay
RBC-depleted splenocytes from either naive mice or AGN2a-CD80, AGN2a-CD80/86 or AGN2a-CD80/137 L vaccinated mice were incubated in triplicate with 2 × 104 mitomycin-C-treated tumour cells (same transfectants as those used for vaccine) in 96-well plates. After 3 days incubation, 50 µl supernatant was collected and IFN-γ secretion was measured by the BD mouse Th1/Th2 cytokine cytometric bead array kit (BD Biosciences Pharmingen). Briefly, 50 µl of sample or diluted standard was transferred to 50 µl of cytometric beads, and 50 µl PE-conjugated detection reagent was added afterwards. Samples were incubated at room temperature for 2 hr in the dark and washed once. The bead/antibody sandwich complex was then resuspended in 300 µl for analysis. Two-colour flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). Data were acquired and analysed using the Becton Dickinson Cytometric Bead Array (CBA) software.
CTL functional assays
Purified lymphocyte subsets were tested for the ability to lyse tumour cells in standard 51Cr-release assays. Briefly, tumour cell targets were incubated in 100 µCi 51Cr (sodium chromate, Amersham Biosciences, Piscataway, NJ) per million cells for 90 min at 37°, washed extensively, and then coincubated with effector cell populations at the indicated effector to target (E : T) ratio in a final volume of 200 µl. At the indicated times 50 µl of supernatant was collected from individual wells and added to Lumaplate-96 microplates (Packard Instrument Co., Meriden, CT), and radioactivity measured on a Packard TopCount. Percent lysis is reported as the ratio of experimental to total release, with the spontaneous release subtracted from each. Enzyme-linked immunospot (ELISPOT) analysis of IFN-γ-secreting cells was carried out according to the manufacturer's directions. Capture anti-mouse mAb (BD Biosciences Pharmingen) was coated overnight on 96-well hydrophobic PVDF membrane plate (Millipore, Bedford, MA.). On the following day, 2-fold dilutions of CD8 splenocytes – starting at 7·5 × 104 per well were coincubated with 2 × 104 mitomycin-C treated tumour cells at 37° for 30–35 hr. After washing, the plates were incubated with matched biotinylated detection mAb (BD Pharmingen) for 2 hr at room temperature, washed again, and incubated with extravidin alkaline phosphatase conjugate (Sigma-Aldrich). Spots were developed by adding BCIP/NBT substrate (Sigma-Aldrich). The number of spots corresponding to IFN-γ-secreting cells was determined by Immunospot plate scanning services, CTL Analyzers LLC (Cleveland, OH).
Results
Generation of CD137L (4-1BBL)-expressing aAPC
To determine the ability of 4-1BBL to stimulate lymphocyte expansion, aAPC were generated using the murine YAC-1 cell line and the human K562 cell line. aAPC cell lines were generated by transfection with mammalian expression vectors encoding cloned cDNA for murine CD137L and murine CD32 (see Materials and methods). Upon cloning by limiting dilution culture, clones with the highest levels of CD137L and CD32 were selected (Fig. 1a). Saturation of CD32 molecules on aAPC with anti-CD3 and anti-CD28 mAb was demonstrated by incubating aAPC with increasing concentrations of fluorescently labelled antibody followed by flow cytometric analysis (Fig. 1). Incubating aAPC at near-saturation levels of antibody insured maximal binding of each mAb. For lymphocyte expansion experiments, YAC-1 based aAPC, at a density of 3 × 106/ml, were coated with 1 µg/ml anti-CD3 and 16 µg/ml anti-CD28. K562 based aAPC were coated at the same cell density using 16 µg/ml anti-CD3 and 1–2 µg/ml anti-CD28. Saturation with 16 µg/ml was nearly identical to that at 32 µg/ml, and was used in the expansion experiments below. These are the lowest concentrations at which saturation in a single antibody binding assay was reached, as determined by flow cytometry (not shown). Figure 1 shows dual binding of anti-CD3 and anti-CD28 on aAPC. The labelled antibodies used for these studies and the unlabeled antibody used to load aAPC for lymphocyte expansion were from identical hybridoma clones.
Figure 1.
Flow cytometric profile of aAPC. Artificial antigen presenting cells (aAPC) were produced using K562 and YAC-1 cells. CD32 and CD137L cDNA expression vectors were used to produce stable cell lines under drug selection (see Materials and methods). Cell lines expressing only CD32, or CD32 and CD137L, were produced. (a) Expression of CD32 in YAC-1 (YCD32, column 1) and K562 (KCD32, column 2), and dual expression of CD32 and CD137L in YAC-1 (YCD32/CD137L, column 3) and K562 (KCD32/CD137L, column 4). Below each flow profile is a control profile showing the staining seen with isotypic control antibody. (b) Flow cytometric profile of antibody-loaded aAPCs used in costimulation studies. Saturating levels of antibody were bound to CD32 expressed on aAPC (FITC-anti-CD3 at 2·0 µg/ml and 32·0 µg/ml, and PE-anti-CD28 at 32·0 µg/ml and 2·0 µg/ml for YAC-1 and K562 aAPC, respectively, at 3·0 × 106 cells/ml). Column 1 shows the lack of antibody binding to the parental cells lines, column 2 shows YAC-1 and K562 cell lines that express only CD32, and column 3 shows antibody binding to CD32/CD137L dual-expressing cells.
Short-term expansion of mouse splenocytes by aAPC
aAPC were used to expand naïve mouse splenocytes in vitro. Spleens were harvested from A/J mice and immunomagnetic beads used to isolate CD4+ and CD8+ T-cell populations. A/J mice were used throughout as they are the strain of origin for the Neuro-2a cell line. Purified lymphocytes were cocultured with irradiated aAPC and viable cells counted on day 5 of culture (Fig. 2). Upon cocultivation with aAPC, both CD8 and CD4 cells showed robust expansion with K562-based aAPC. Maximal expansion in this assay was seen when both CD137L was present and exogenous rIL-2 was added. CD137L was clearly superior to the costimulatory effect of anti-CD28 in these assays, either with or without the addition of rIL-2. When YAC-1 aAPC were used for CD4+ cell activation, costimulation was poor (Fig. 2b). CD4 cells expanded somewhat, but did not surpass the expansion seen with anti-CD3/anti-CD28-coated Dynal beads. The response of murine CD8 cells was surprisingly different. As with CD4 cells, superior expansion of CD8 cells was seen with CD137L costimulation, which again was superior to that conferred by anti-CD28 on the surface of aAPC. However, YAC-1 aAPC proved to be equally effective for CD8 cell expansion as compared to K562 aAPC. Minimal expansion was seen when only anti-CD3 was coated on the surface of the aAPC, and no expansion was seen with uncoated aAPC (not shown). These results demonstrate that with aAPC, CD137L-mediated stimulation of mouse T cells is superior to anti-CD28-mediated costimulation. With both aAPC types, IL-2 increased the activation conferred by CD137L, demonstrating the additive effects of these signals.
Figure 2.
Short-term expansion of naïve splenocytes using aAPC. Spleens were harvested from A/J mice, and (a) CD8 or (b) CD4 subsets purified by immunomagnetic bead separation. The purity of each population exceeded 93%. CD4 and CD8 cell subsets were coincubated with the indicated irradiated aAPC and cell numbers counted on day 5 of culture. The initial T- cell number used for coculture with aAPC was 0·5 × 106. The T cell : aAPC ratio was 2 : 1, and the T cell : bead (Dynabeads, Dynal, coated with anti-CD3 and anti-CD28 at a ratio of 50 : 1) ratio was 1 : 3. CD32+ K562 aAPC were loaded with anti-CD3 alone (K-CD3) or with anti-CD3 and anti-CD28 (K-CD3/28). Double transfected aAPC (CD32+ and CD137L+), were loaded with anti-CD3 alone (K137L-CD3) or with anti-CD3 and anti-CD28 (K137L-CD3/28). ‘K’ indicates K562 aAPC and ‘Y’ indicates YAC-1 aAPC. Antibody concentrations used for coating aAPC were based on Fig. 1(b). Data is representative of three separate expansion experiments.
Long-term expansion of CD4 and CD8 lymphocytes by aAPC in vitro
To test the ability of aAPC to support long-term expansion of naïve splenic T cells, purified CD4 or CD8 cells were coincubated with irradiated aAPC. Expanding cultures were re-stimulated every 7–10 days with CD137L-expressing aAPC loaded with anti-CD3 alone, or a combination of anti-CD3 and anti-CD28 antibodies. As with the short-term expansion, the use of CD137L-expressing aAPC was not only superior, but was required to see large-scale increases in cell number (Fig. 3). As can be clearly seen with the expansion of CD4 cells, the increase in cell number reached a plateau without the inclusion of CD137L, and YAC-1-based aAPC did not strongly stimulate cell expansion. CD8 expansion likewise required CD137L to reach maximal increase in cell number. These data stand in contrast to reports for human CD4 cell expansion where CD28-mediated expansion was sufficient for long-term expansion.19
Figure 3.
Long-term expansion of murine lymphocytes. The culture conditions for the indicated combinations of purified T cells, aAPCs, and beads were as in Fig. 2. CD137L-expressing and non-CD137L-expressing aAPC were loaded with anti-CD3 and anti-CD28 (K-CD3/28, K137L-CD3/28, Y-CD3/28, or Y137L-CD3/28). (a) For CD8 expansion, K137L-CD3/28 (solid triangles) and Y137L-CD3/28 (solid circles) cocultures were stimulated on days 0, 8, and 16, while CD3/28 beads (open squares), K-CD3/28 (open triangles), and Y-CD3/28 (open circles) were stimulated on days 0 and 8 only. (b) For CD4 expansion, K137L-CD3/28 cocultures were stimulated on days 0, 10, 19, and 27, while all others were stimulated on days 0 and 10. Data is representative of three separate expansion experiments.
Effect of CD137-expressing tumour vaccines in vivo
To examine the effects of CD137L expression on generation of a tumour vaccine in vivo, we initiated a number of immunization trials with an aggressive subclone of the Neuro-2a neuroblastoma cell line, designated AGN2a, generated by serial in vivo passage. Previous work in this tumour system demonstrated that induction of protective immunity to AGN2a requires the dual expression of CD80 and CD86, and that CD80 expression alone is not able to generate a protective immune response.15 In initial studies, the LD50 of AGN2a expressing CD137L, CD80, and a combination of CD80 and CD86 were compared. As the LD50 of AGN2a-CD137L was similar to AGN2a-CD80, we reasoned that as with CD80 or CD86 alone, sole expression of CD137L would not be sufficient to protect mice from tumour challenge (not shown). Therefore, we compared AGN2a transfected with cDNA expression vectors encoding both CD137L and CD80, both CD80 and CD86, or CD80 alone. A/J mice were injected s.c. with 2 × 106 mitomycin C-treated or non-treated, viable tumour cells twice at weekly intervals. We explored the use of viable tumour vaccine constructs in order to demonstrate the strong immune responses generated in our initial live tumour vaccine challenge experiments, where live tumour cell vaccines did not only fail to grow in mice, but resulted in robust immunity. Five days following the second immunization spleen cells were collected and the cells used in functional assays and analysed for cell surface protein expression by flow cytometry. When splenocytes from vaccinated mice were antibody-stained for CD4, CD8, CD3 and DX5 expression, the percentage of total splenocytes expressing CD4 decreased slightly, while the percentage of CD8+ cells increased in a progressive manner in animals treated with no vaccine, AGN2a-CD80, AGN2a-CD80/CD86, AGN2a-CD80/CD137L, and viable AGN2a-CD80/CD137L (non-mitomycin-C treated) vaccines (Table 1). When the percentage of total CD3 cells expressing DX5 was compared to expression on CD8 cells, an even greater percent increase was seen in the CD8 population in mice immunized with CD137L-containing vaccine preparations. Thus, inclusion of CD137L in a neuroblastoma tumour vaccine expanded CD8+ cells, and a substantial proportion of them coexpressed the DX5 marker.
Table 1.
Phenotypic analysis of splenocytes from tumour vaccine-immunized mice
| Tumour vaccine | % CD4* | % CD8† | CD3/ DX5‡ | CD8/ DX5§ |
|---|---|---|---|---|
| Naïve | 41·8 | 19·9 | 3·8 | 4·4 |
| AGN2a-CD80 | 33·3 | 15·1 | 2·8 | 5·0 |
| AGN2a-CD80/86 | 40·6 | 19·2 | 5·7 | 4·7 |
| AGN2a-CD80/137L | 41·4 | 21·7 | 7·5 | 9·6 |
| AGN2a-CD80/137L, viable | 36·2 | 21·2 | 7·9 | 14·8 |
A/J mice were given two weekly vaccine treatments with mitomycin-C inactivated AGN2a cells transfected with CD80 (AGN2a-CD80), with CD80 and CD86 (AGN2a-CD80/86), or with CD80 and CD137L (AGN2a-CD80/137L). An additional group consisted of unvaccinated mice (naïve), and mice that were vaccinated with viable AGN2a-CD80/137L cells, which were rejected. Five days after the second vaccination, splenocytes were isolated and analysed by flow cytometry for the percentage of cells expressing CD4
A/J mice were given two weekly vaccine treatments with mitomycin-C inactivated AGN2a cells transfected with CD80 (AGN2a-CD80), with CD80 and CD86 (AGN2a-CD80/86), or with CD80 and CD137L (AGN2a-CD80/137L). An additional group consisted of unvaccinated mice (naïve), and mice that were vaccinated with viable AGN2a-CD80/137L cells, which were rejected. Five days after the second vaccination, splenocytes were isolated and analysed by flow cytometry for the percentage of cells expressing CD8.
Splenocytes were also analysed for CD3 and DX5 coexpression. Also shown are the percentages of
Splenocytes were also analysed for CD8 cells that coexpressed DX5. Data is representative of three independent immunization series.
Analysis of DX5 expression
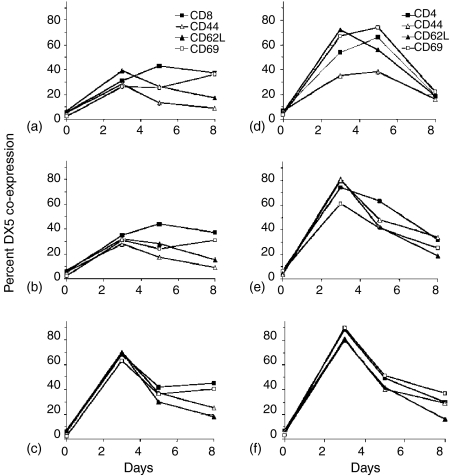
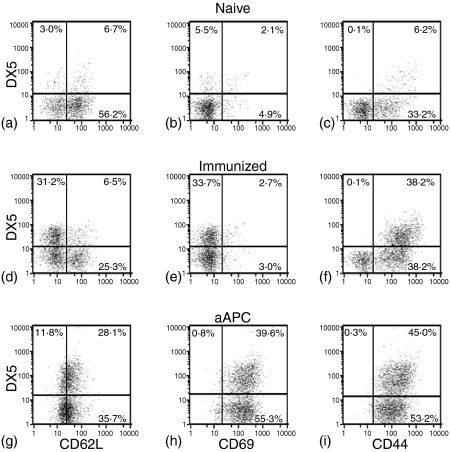
To determine the significance of the expansion of DX5-positive cells, we first sought to determine if DX5 expression was increased in a general manner upon lymphocyte activation. Naïve A/J splenocytes were separated into CD4 or CD8 populations by immunomagnetic bead sorting and subsequently coincubated with K562-based aAPC prepared to crosslink one, two or three receptor families. Figure 4 summarizes a large amount of flow cytometric demonstrating the activation of splenocytes and the subsequent patterns of DX5 coexpression on the CD8 or CD4 lymphocyte populations concomitantly stained with antibody specific for CD44, CD62L, and CD69. Splenocytes were activated with the following K562-based aAPC: aAPC loaded with anti-CD3 (Fig. 4a, d), with anti-CD3 and anti-CD28 (4b, 4e), or with CD137L-expressing aAPC loaded with anti-CD3 and anti-CD28 (4c, 4f). In general, the more complex the aAPC, the greater the degree of lymphocyte activation, as judged by coexpression of DX5 and CD69. While CD4 cells did not seem to increase their coexpression of activation antigens and DX5 with the inclusion of CD137L on the aAPC after 3 days of culture (Fig. 4f versus e), a clear difference was seen for CD8 cells (note the peak on day 3 in Fig. 4(c) as opposed to Fig. 4(a, b). The wide expression of DX5 on in vitro expanded CD4 and CD8 populations makes it unlikely that this antigen alone can serve as an NKT marker. Furthermore, the DX5 marker increased and decreased in tandem with CD69, CD62L, and CD44, thus allowing the graphical display of double-positive cells only to be an accurate reflection of the distribution of DX5+ cells in these populations. In contrast, when CD8-positive splenocytes from AGN2a-CD80/CD137L immunized mice were stained for CD62L, CD69, or CD44, approximately 30% of the total CD8 cells were demonstrated to be DX5+ CD62L–, DX5+ CD69–, and DX5+ CD44+, Fig. 5(d, e, f), respectively. These populations were not seen in naïve mice, Fig. 5(a, b, c). This unique population of CD8+ DX5+ CD62L– cells also was not seen in aAPC-expanded CD8 cells (Fig. 5g–i), nor were DX5+ CD69+ cells seen as would be expected for in vitro activated lymphocytes (compare Fig. 5d,e). The DX5+ CD69+ phenotype is likely to represent a previously described cell population shown to expand in vivo under lymphopenic conditions, as judged by the expression CD44.20
Figure 4.
Expression of the DX5 marker on naïve CD4 and CD8 splenocytes stimulated in vitro. Splenocytes were isolated from A/J mice, CD8 (a, b, c) and CD4 (d, e, f) populations isolated by immunomagentic bead selection, and then cultured with irradiated K562-based aAPC loaded with anti-CD3 (a, d), anti-CD3 and anti-CD28 (b, e) or irradiated K562/CD137L aAPC coated with anti-CD3 and anti-CD28 (c, f). Cells were stained for CD4 or CD8 (according to the population isolated, closed squares), CD44 (open triangles), CD62L (closed triangles), CD69 (open squares), and DX5 on days 3, 5 and 8. The percentage of cells coexpressing DX5 and CD4 (d, e, f) or DX5 and CD8 (a, b, c) and one of the other three markers as detected by three-colour flow cytometric analysis is plotted against days in culture.
Figure 5.
Analysis of memory cell markers on naïve, immunized, and aAPC expanded CD8+-splenocytes. Expression of CD62L (a, d, g), CD44 (c, f, i), and CD69 (b, e, h) was analysed by three-colour flow cytometric analysis of CyChrome-CD8-gated splenocytes from naïve mice (a, b, c), naïve splenocytes cultured for 8 days with K562/CD137L aAPC loaded with anti-CD3 and anti-CD28 (g, h, i), or splenocytes isolated directly from AGN2a-CD80/CD137L immunized A/J mice. Two-colour flow cytometric profiles for DX5 and CD62L (a, d, g), CD69 (b, e, h), or CD44 (c, f, i) expression are shown.
In vitro and in vivo antitumour activity of CD137L-bearing tumour vaccines
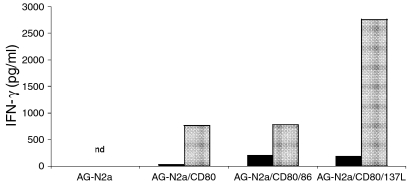
Functional cellular immunity induced by CD137L-expressing tumour vaccines was assessed by isolating CD8+ splenocytes from AGN2-CD80/137 L vaccinated animals and determining lytic activity and cytokine expression upon coculture with AGN2a targets. To measure secretion of IFN-γ, CD8+ cells from vaccinated mice were cocultured with the tumour cell type corresponding to the type use for vaccination. For example, mice immunized with AGN2a-CD80 were cocultured with inactivated AGN2a-CD80 tumour cells. Supernatants from tumour-splenocyte cocultures were collected on day 3 and IFN-γ concentration measured by cytometric bead array. Data presented is representative of three independent sets of immunization experiments and clearly demonstrates that CD137L-expressing tumour increased the level of IFN-γ production by more than threefold when used in concert with CD80, as compared to CD80 expression alone or coexpression of CD80 and CD86, Fig. 6. Robust IFN-γ secretion directly correlated with the presence of active cytolytic T-cell effectors. When CD8 splenocytes were harvested directly from immunized mice, without any in vitro culture or re-stimulation, strong antitumour lytic activity was displayed in mice that had been vaccinated with AGN2a-CD80/CD137L tumour vaccine, Fig. 7. This was true with both mitomycin-C inactivated and live tumour cell vaccine. Importantly, the level of cytolytic activity seen with freshly isolated lymphocytes from mice immunized with CD137L-bearing vaccines was far in excess of that seen with the other tumour cell vaccine preparations. Figure 7 shows lytic activity at 12 hr. Although lysis with CD137L-containing vaccine was seen at 4 hr, no lysis at all was seen for the other vaccine preparations, thus we presented data at 12 hr in order to facilitate comparison with our previous work in the AGN2a-CD80/86 vaccine system.
Figure 6.
IFN-γ production by lymphocytes from tumour vaccinated mice. Splenocytes, 2 × 105, from either naive mice (filled) or vaccinated mice (grey) were coincubated with their respective vaccine preparations. That is, splenocytes from AGN2a (wild-type), AGN2a-CD80, AGN2a-CD80/86 or AGN2a-CD80/137 L vaccinated mice were incubated in triplicate in 96-well plates with 2 × 104 mitomycin C-inactivated tumour cells of the same origin as those used for vaccine. Supernatants from tumour-splenocyte cocultures were collected on day 3 and IFN-γ concentrations measured by cytometric bead array. The data is representative of three independent experiments. nd, below the level of detection of our assay (approximately 20 pg/ml).
Figure 7.
Induction of CTL populations in the spleens of immunized mice. CD8+ T cells, purified from splenocytes by immunomagnetic beads, are the same as those used in Fig. 4. 51Cr-labelled tumour cell transfectants of the same origin as those used for vaccination in each group (a) and wild-type non-transfected tumour cell targets (b) were coincubated with effector cell populations at the indicated effector to target (E : T) ratios for 12 hr. At the indicated times, 50 µl of supernatant was collected from individual wells and added to Lumaplate-96 microplates, and radioactivity measured on a Packard TopCount. The data is representative of three independent experiments.
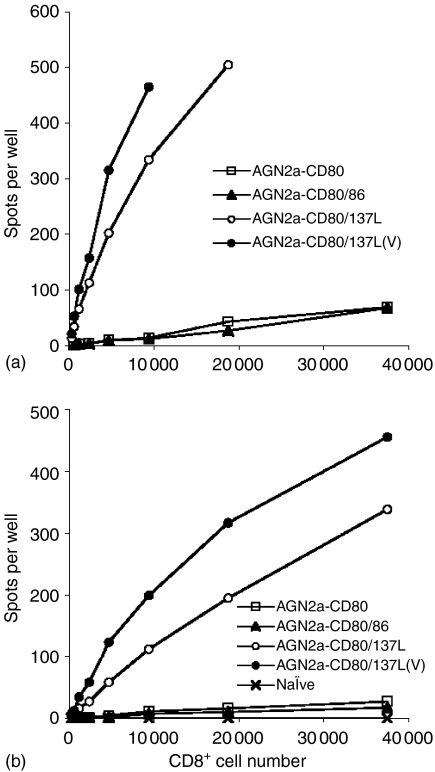
The strength and superiority of tumour cell vaccines incorporating CD137L was further demonstrated by the number of IFN-γ producing cells present in freshly isolated splenic CD8+ cells as determined by ELISPOT analysis (Fig. 8). These assays were performed by coincubating freshly isolated CD8 splenocytes with mitomycin-C treated tumour cell targets. Stimulation by the same tumour transfectants used for vaccination were able to induce better IFN-γ production than stimulation with wild-type (nontransfected) tumour cells (Fig. 8a versus b). Using ELISPOT analysis to examine the immune response to both tumour cell targets (wild-type and transfected) allowed a quantitative comparison of the contribution of the transfected cell surface molecules, as well as of viable tumour vaccine preparations versus inactivated, to the production in vivo and subsequent detection in vitro of tumour-specific IFN-γ-producing lymphocytes. The remarkable presence of both tumour-lytic cells and IFN-γ secreting cells in freshly isolated CD8+ T cells (i.e. not stimulated or cultured in vitro) could be in part due to amplification of T-cell activity by low levels NK or NKT cell activity. However, background levels of lysis were very low using YAC-1 targets (less than 10% lysis, below all experimental tumour results). When the C1498 cell line was used as an HLA-mismatched tumour control, no specific lysis was seen at all (not shown). Thus, NK effector cells are not likely to be the final mediators of antitumour immunity using CD137L/CD80-N2a vaccines.
Figure 8.
ELISPOT analysis of CD8 cells in immunized mice. A/J mice were vaccinated s.c with 2 × 106 inactivated AGN2a-CD80, AGN2a-CD80/86, AGN2a-CD80/137L or viable AGN2a-CD80/137L cells twice weekly. At day 5 following the second immunization, CD8+ splenocytes were purified with anti-CD8 immunomagnetic beads from each group, including CD8 splenocytes from naive mice as controls. Decreasing numbers of freshly isolated CD8 T cells (i.e. no in vitro culture) were incubated with 2 × 104 mitomycin C-inactivated cell transfectants of the same origin as those used for vaccination (a) or inactivated wild-type AGN2a cells (b) IFN-γ spots were developed 30–35 hr following incubation, based on the Pharmingen protocol.
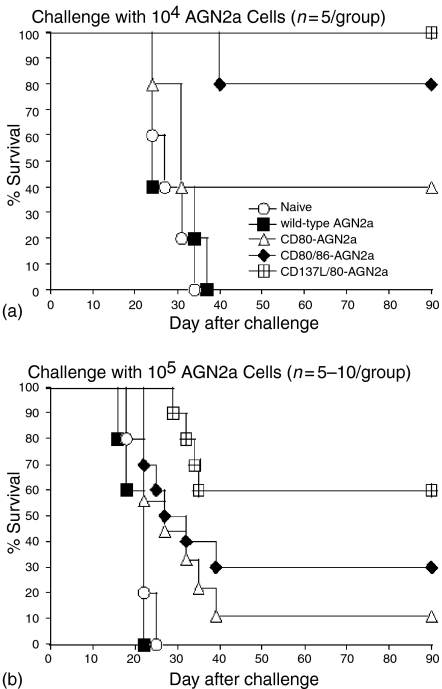
A final investigation into the immunity conferred by CD137L-expressing vaccines was carried out in tumour challenge studies. Tumour vaccine experiments compared the following tumour transfectants: AGN2a-CD80, AGN2a-CD80/CD86, and AGN2a-CD80/CD137L. Control groups consisted of naïve, non-immunized mice, and mice ‘immunized’ with unmodified AGN2a (wild-type). The AGN2a-CD80/CD137L tumour vaccine induced a better tumour-protective effect than either the AGN2a-CD80 or the AGN2a-CD80/CD86 vaccines (Fig. 9). The protection afforded by the AGN2a-CD80/CD137L was superior in terms of both the number of mice that finally succumbed to challenge with viable tumour and in the length of time it took for animals that did fail immunoprotection to evidence palpable tumour. Protection was especially evident when immunized mice were challenged with higher doses of viable wild-type tumour (Fig. 9b).
Figure 9.
Inclusion of CD137L increases tumour vaccine efficacy. A/J mice were either not vaccinated (Naïve, open circles) or vaccinated s.c with 2 × 106 mitomycin C-treated wild-type AGN2a (filled squares), AGN2a-CD80 (open triangles), AGN2a-CD80/86 (filled diamonds) or AGN2a-CD80/137 L (open squares) cells twice at weekly intervals. One week after the second immunization, 1 × 104 (a) or 1 × 105 (b) live wild-type AGN2a cells were injected s.c. into each animal. Data in (a) is from a single experiment (n = 5 per group) and the data in (b) represents pooled results from two trials, n = 5 for control groups (naïve and wild-type ‘vaccinated’ AgN2a), and n = 9–10 for the experimental groups.
Discussion
The long-term expansion of murine CD8 lymphocytes has been problematic, and thus has limited the modelling of adoptive immunotherapy in mouse models. To address this challenge we produced artificial antigen presenting cells (aAPC) from both mouse and human cell lines (YAC-1 and K562) to facilitate long-term expansion of mouse T lymphocytes. Interestingly, long-term expansion of either murine CD4 or CD8 cells required the presence of CD137L. This differs from previous studies of human CD4 lymphocytes, where anti-CD3 and anti-CD28 coated beads were sufficient for long-term expansion.21 Recent reports have highlighted that CD137L can expand both CD4 and CD8 cells, as we have demonstrated here.22 We also found that the cellular substrate for aAPC construction was critical for the expansion of murine CD4 cells with aAPC. The K562 and YAC-1 cell lines are commonly used as indicators of NK activity in human and murine cytolytic assays, respectively, and have little to no MHC antigen expression on their surface thus reducing the possibility of allo-reactivity.23,24 While K562-based aAPC were required for maximal expansion of CD4 cells, either K562- or YAC-1-based aAPC could expand CD8 cells, highlighting a CD4 lymphocyte specific effect. This difference in expansion is not likely to be caused by a cell-specific difference in the ability of antibody to saturate the FcR activity of transfected CD32. Differences in the concentration of antibody required to saturate aAPCs were determined in titration experiments designed to insure meaningful comparison between the aAPC formats. We do not have a mechanistic reason for the different concentration of antibody required to saturate CD32-mediated FcR activity in K562- and YAC-1-based aAPC, but report them here in order to highlight the need to validate transduced FcR function in each cell-based system as the concentrations of antibody required to saturate identical FcR differed according to cellular context.
CD137L, when coimmobilized on plastic with anti-CD3, can induce IL-2 production from both CD28+ and CD28– T cells through a TNF receptor associated factor (TRAF)-2-dependent pathway, indicating that this receptor can mediate costimulatory activity independent of other cell surface proteins.25 Secondary signalling through aggregated CD137 on the cell surface activates TRAF-1 as well, having an additive effect on the inhibition of caspase activity and the stimulation of nuclear factor (NF)-κB.26 NF-κB-mediated stimulation through CD137 has been shown to induce the anti-apoptotic proteins Bcl-xl and Bfl-1 in CD8 cells.27 Interestingly, Saoulli et al. reported that CD137 was inferior to CD28 for the induction of IL-2 secretion.25 This activity, however, is not the sole function of CD137, as Maus et al. demonstrated that CD137L on K562-based aAPC in conjunction with B-7 molecules was required for large scale expansion of human cytotoxic T lymphocytes (CTL).18 CD137L-mediated stimulation of CD4 and CD8 cells has also been shown to result in different cytokines being produced, with IL-4 secretion induced in CD4 cells and IFN-γ secretion induced in CD8 cells in a non-CD28-dependent manner.26 In addition to the well-described induction and growth of CD4 and CD8 T lymphocytes by signalling through CD137, NK1.1+ cells also express CD137 and functionally respond to CD137 binding.28 NK cells have also been shown to secrete IFN-γ upon CD137-mediated activation without the induction of lytic activity, demonstrating that the CD137 on NK cells plays a primarily immunoregulatory role.16,28 This immunoregulatory role is essential in vivo for the amplification of antitumour immunity by NKT (NK1.1+ CD3+) cells. Although these cells may not play a direct role in tumour cell lysis, in some model systems their ability to amplify antitumour immune responses mediated by CTL is essential for tumour cell clearance.28
The finding that an aggressive subclone of Neuro-2a, AGN2a, requires more than a single costimulatory molecule for induction of antitumour immunity is similar to the results reported by Melero et al. where the less aggressive P815 mastocytoma was immunogenic upon introduction of CD137L, whereas the AG104 sarcoma required expression of CD137L and CD80.17 Another report found that each CD80, CD86, and CD137L each contributed to the generation of anti-A20 B-cell lymphoma immunity.29 Yang et al. demonstrated that blocking of both CD80 and CD86 significantly inhibited NKT cell activation, while blocking a single costimulatory antigen, CD80, CD86, or CD40, had little effect on NKT activity.30 Recent work with the K1735 melanoma cell line engineered to express single-chain Fv fragments specific for CD137, showed the induction of a strong Th1 response in which CD4 cells were found to be the primary mediators of antitumour immunity in conjunction with NK cells, without significant CTL production.31 Taraban et al. recently compared the relative importance of CD134 (OX40)- and CD137-mediated lymphocyte activation and concluded that CD134 better potentiated CD4 activation while CD137 was better able to activate CD8 cells and corresponded to better antitumour immunity in model systems where CD8 responses are required for tumour clearance.32 Thus, individual tumours may require alternate mechanisms of T-cell costimulation depending on which set of cellular immune effectors are required to clear the tumour.
The impact of including CD137L in a tumour vaccine preparation is complex and is likely to include not only activation but also differentiation of T lymphocyte effectors. Using human cord blood-derived CD8+ CTL, CD137 ligation was shown to up-regulate CD28 expression in naïve T cells induced to down-regulate CD28 in response to IL-12 and IL-15. CD137 ligation also induced the expression of the memory-type markers CCR6 and CD45RO on T cells, suggesting differentiation into a cytotoxic memory phenotype.33 Further along the differentiation pathway of T cells, Cannons et al. reported that while CD28-mediated signals play a primary role in initial T-cell expansion, it is CD137 that mediates signals that sustain T-cell responses.26 It therefore appears that alternate and non-overlapping roles are played by CD137 and CD28 that when appropriately triggered lead to Th1-like differentiation and expansion of CD8 cells. The ability of CTL precursors, effector cells, and memory cells to transit from a CD28–/CD137+, to a CD28+/CD137– stage, and then to re-express CD137 upon stimulation, once more highlights the fact that how costimulation signals are introduced, and in what cellular and cytokine milieu they are encountered, greatly influences the phenotypic and functional properties of the resultant cell population. Our report highlights the ability of CD137 to expand murine CD8 lymphocytes in the context of aAPC and that this expansion correlated to in vivo antitumour immunity that is likely to feature both antigen-specific CTL activity and the potential amplification of Th1-like signals by NK or NKT cells. It should also be noted that the presence of CD137L has triggering effects in the in vitro assays used to measure antitumour immunity. Thus in Figs 7 and 8, it is clear that tumour targets containing CD137 ligand are more readily lysed than wild type tumour and induce a greater number of IFN-γ-producing cells as measured by ELISPOT. Nevertheless the large increase in native tumour lysis and ELISPOT activity when CD137L is combined with CD80, as opposed to CD86, documents the importance of including of CD137L in experimental tumour vaccines. Finally, the magnitude of the cellular immune responses initiated by CD137L-expressing cell-based tumour vaccines far exceed those we have previously studied. While CD80 and CD86 do induce protective immunity in vivo (Fig. 9), in vitro demonstration of this activity required restimulation of splenocytes with tumour in vitro.15 With the CD137L/CD80-based vaccine, strong in vitro activity was observed in fresh splenic lymphocytes (Fig. 7). The high level of in vitro activity is correlated with a relatively high percentage of DX5+ cells present in the spleens of CD137/CD80-N2a vaccinated mice (Fig. 5).
The expansion of CD44hi CD62lo cells is a hallmark of antigen-experienced memory effector cells, although homeostatic proliferation under lymphopenic conditions can result in the antigen-independent up-regulation of CD44.20 Ma et al. have demonstrated that expansion of CD44hi CD62Lhi cells in a combined lymphodepletion-F10 melanoma tumour vaccine model was due to a generalized activation of the immune system seen with irradiation and immune reconstitution.34 However, in that model, expansion of CD44hi CD62lo cells in tumour vaccine draining lymph nodes was associated with a tumour protective immune response. Our data with CD137L-bearing aAPC and tumour vaccination supports these findings. Naïve CD8+ splenocytes expanded with CD3/28/137 l-aAPC in vitro remained CD62Lhi CD69hi (Fig. 4). The majority of these cells were DX5 positive when CD137L was included in the aAPC stimulation repertoire. A significant subset, approximately 30%, of these cells remained DX5+ out to day 8 poststimulation. CD8 cells from AGN2a-CD80/CD137L immunized mice were CD62lo D69lo, as would be expected for memory cells; however, approximately 30% of these cells also expressed the DX5 marker (Fig. 5). CD44 expression was similarly high on 38% of the DX5+ cells, negative on naïve cells, and high on aAPC-expanded cells, just as has been reported for lymphocytes undergoing homeostatic expansion.20 The DX5 marker remained up-regulated on approximately 30–40% of both tumour induced memory-effector cells, CD62Llo D69lo D44hi, as well as aAPC-induced lymphocytes expanded in vitro, CD62Lhi CD69hi CD44hi. The presence of CD137L in both the N2a-based vaccine and in aAPC correlates with the detection of DX5+ T cells. While the presence of DX5 on aAPC-expanded cells can be argued to simply be a result of CD137L further amplifying strong in vitro activation (thus making DX5 a general activation marker only), the presence of a DX5+ subset in memory/effector cells isolated from tumour immunized mice raises the possibility that DX5 on these cells identifies a specific functional subset present in antitumour immune responses. Our description of antigen-specific effector cells that display markers formerly thought to be restricted to NK cells (i.e. DX5) in the AGN2a tumour model are similar to recent findings in studies of the immune responses to viruses. Slifka et al. reported that LCMV-specific CD4 and CD8 cells coexpress DX5, and that approximately 30–40% of virus-specific (IFN-γ+) CD8+ cells were positive for DX5 and NK1.1 8 days postinfection, a percentage strikingly similar to the one we report in tumour vaccinated mice (Fig. 5e).35 Kambayashi et al. reported the induction of a similar DX5+ CD8+ subset displaying an activation/memory phenotype during influenza infection.36 Our report highlights the dual expression of DX5 during antigen-non-specific expansion, where it serves as a general marker of activation, as well as on a vaccine-induced population in mice immunized with a tumour vaccine expressing CD137L and CD80.
Acknowledgments
This work was supported by the Midwest Athletes Against Childhood Cancer (MACC Fund, Inc.), the Childrens's Hospital Foundation (Children's Hospital of Wisconsin), and the Medical College of Wisconsin Research Affairs Committee.
References
- 1.Schmidt ML, Lukens JN, Seeger RC, et al. Biologic factors determine prognosis in infants with stage IV neuroblastoma: a prospective Children's Cancer Group study. J Clin Oncol. 2000;18:1260–8. doi: 10.1200/JCO.2000.18.6.1260. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, Nakano T, Yondea A, et al. Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haplo-identical bone marrow transplantation. Bone Marrow Transpl. 2003;32:103–6. doi: 10.1038/sj.bmt.1704070. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Handretinger R, Leung W, Ihm K, et al. Tumor cell contamination of autologus stem cell grafts in high-risk neuroblastoma: the good news? Br J Cancer. 2003;88:1874–7. doi: 10.1038/sj.bjc.6601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truitt RL, Piaskowski V, Kirchner P, et al. Immunologic evaluation of pediatric cancer patients receiving recombinant interleukin-2 in a phase I trial. J Immunother. 1992;11:274–85. doi: 10.1097/00002371-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Marti F, Pardo N, Peiro M, Bertrna E, Amill B, Garcia J, Cubells J, Rueda F. Progression of natural immunity during one-year treatment of residual disease in neuroblastoma patients with high doses of interleukin-2 after autologous bone marrow transplantation. Exp Hematol. 1995;23:1445–52. [PubMed] [Google Scholar]
- 7.Leimig T, Branner M, Ramsey J, et al. High-efficiency transduction of freshly isolated human tumor cells using adenoviral interleukin-2 vectors. Hum Gene Ther. 1996;7:1233–9. doi: 10.1089/hum.1996.7.10-1233. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–26. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 9.Hank JA, Surfus J, Gan J, et al. Treatment of neuroblastoma patients with antiganglioside GD2 antibody plus interleukin-2 induces antibody-dependent cellular cytotoxicity against neuroblastoma detected in vitro. J Immunother. 1994;15:29–37. doi: 10.1097/00002371-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ishizu H, Bove KE, Ziegler MM, Arya G. Immune-mediated regression of ‘metastatic’ neuroblastoma in the liver. J Pediatr Surg. 1994;29:155–60. doi: 10.1016/0022-3468(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 11.Hock RA, Reynolds BD, Tucker-McClung CL, Kwok WW. Human class II major histocompatibility complex gene transfer into murine neuroblastoma leads to loss of tumorigenicity, immunity against subsequent tumor challenge, and elimination of microscopic preestablished tumors. J Immunother. 1995;17:12–8. doi: 10.1097/00002371-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Bausero MA, Panoskaltsis-Mortari A, Blazar BR, Katsanis E. Effective immunization against neuroblastoma using double-transduced tumor cells secreting GM-CSF and interferon-γ. J Immunother. 1996;19:113–24. doi: 10.1097/00002371-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Katsanis E, Bausero MA, Xu H, et al. Transfection of the mouse ICAM-1 gene into murine neuroblastoma enhances susceptibility to lysis, reduces in vivo tumorigenicity and decreases ICAM-2-dependent killing. Cancer Immunol Immunother. 1994;38:135–41. doi: 10.1007/BF01526209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsanis E, Orchard PJ, Bausero MA, et al. Interleukin-2 gene transfer into murine neuroblastoma decreases tumorigenicity and enhances systemic immunity causing regression of preestablished retroperitoneal tumors. J Immunother. 1997;15:81–90. doi: 10.1097/00002371-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cell Immunol. 2003;222:15–26. doi: 10.1016/s0008-8749(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox RA, Tamada K, Strome S, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–6. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 17.Melero I, Bach N, Hellström KE, et al. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–21. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Maus MV, Thomas AK, Leonard DGB, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artifical APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20:143–8. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 19.Hellström KE, Hellström I. Therapeutic vaccination with tumor cells that engage CD137. J Mol Med. 2003;81:71–86. doi: 10.1007/s00109-002-0413-8. [DOI] [PubMed] [Google Scholar]
- 20.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading a memory T cells. J Immunol. 2000;165:1733–7. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 21.Levine BL, Cotte J, Small CC, et al. Large-scale prodiction of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation. J Hematother. 1998;7:437–48. doi: 10.1089/scd.1.1998.7.437. [DOI] [PubMed] [Google Scholar]
- 22.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol. 2002;168:4897–906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 23.Maziarz RT, Burakoff SJ, Faller DV. The regulation of exogenous and endogenous class I MHC genes in a human tumor cell line, K562. Mol Immunol. 1990;27:135–42. doi: 10.1016/0161-5890(90)90108-c. [DOI] [PubMed] [Google Scholar]
- 24.Carlow DA, Payne U, Hozumi N, et al. Class I (H-2Kb) gene transfection reduces susceptibility of YAC-1 lymphoma targets to natural killer cells. Eur J Immunol. 1990;20:841–6. doi: 10.1002/eji.1830200419. [DOI] [PubMed] [Google Scholar]
- 25.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–62. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–24. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 27.Lee H-W, Park S-J, Choi BK, Kim HH, Nam K-O, Kwon BS. 4-1BB Promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xl and Bfl-1. J Immunol. 2002;169:4882–8. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 28.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1 BB monoclonal antibody. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 29.Guinn B-A, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol. 1999;162:5003–10. [PubMed] [Google Scholar]
- 30.Yang Y, Ueno A, Bao M, et al. Control of NKT cell differentiation by tissue-specifc microenvironments. J Immunol. 2003;171:5913–20. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 31.Ye Z, Hellström I, Hayden-Ledbetter M, et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–8. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 32.Taraban VY, Rowley TF, O'Brien L, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–27. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y-J, Brutkiewicz RR, Broxmeyer HE. Role of 4-1BB (CD137) in the functional activation of cord blood CD28– CD8+ T cells. Blood. 2002;100:3253–60. doi: 10.1182/blood-2001-11-0136. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Urba WJ, Si L, et al. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–32. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 35.Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ cells. J Immunol. 2000;164:2009–15. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 36.Kambayashi T, Assarsson E, Chambers BJ, Ljunggren H-G. Expression of the DX5 antigen on CD8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. Eur J Immunol. 2001;31:1523–30. doi: 10.1002/1521-4141(200105)31:5<1523::AID-IMMU1523>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]