Abstract
In chronic inflammatory diseases such as rheumatoid arthritis, joint macrophages/monocytes are the major source of pro- and anti-inflammatory cytokines. Little is understood regarding the signalling pathways which determine the production of the pro-inflammatory cytokine, tumour necrosis factor-α (TNF-α) and the anti-inflammatory cytokine, interleukin-10 (IL-10). Two pathways integral to macrophage function are the protein kinase C (PKC)- and the cAMP-dependent pathways. In this report, we have investigated the involvement of PKC and cAMP in the production of TNF-α and IL-10 by peripheral blood monocyte-derived macrophages. The utilization of the PKC inhibitors Go6983, Go6976 and RO-32-0432 demonstrated a role for conventional PKCs (α and β) in the production of TNF-α in response to stimulation by lipopolysaccharide and phorbol 12-myristate 13-acetate (PMA)/ionomycin. PKC stimulation resulted in the downstream activation of the p42/44 mitogen-activated protein kinase (MAPK) pathway which differentially regulates TNF-α and IL-10. The addition of cAMP however, suppressed activation of this MAPK and TNF-α production. Cyclic-AMP augmented IL-10 production and cAMP response element binding protein activation upon stimulation by PMA/ionomycin. In addition, cAMP activated PKCζ; inhibition of which, by a dominant negative adenovirus construct, selectively suppressed IL-10 production. These observations suggest that pro-inflammatory and anti-inflammatory cytokines are differentially regulated by PKC isoforms; TNF-α being dependent on conventional PKCs (α and β) whereas IL-10 is regulated by the cAMP-regulated atypical PKCζ.
Keywords: cytokines, inflammation, macrophages, protein kinase C (PKC), signalling
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by the dysregulated expression of many pro-inflammatory cytokines including tumour necrosis factor-α (TNF-α) with increased yet insufficient production of anti-inflammatory cytokines including interleukin-10 (IL-10).1 The identification of TNF-α as a therapeutic target has encouraged the investigation of signalling pathways regulating the production of TNF-α by cells relevant to the pathophysiology of such a chronic inflammatory disease.
Previously, we have observed that there is a differential utilization of pathways regulating pro-inflammatory and anti-inflammatory cytokines by monocytes/macrophages. We described that TNF-α production was dependent on p42/44 mitogen-activated protein kinase (MAPK) activity whereas IL-10 was independent;2 in contrast IL-10 production was dependent on phosphatidylinositol 3-kinase (PI3K) and TNF-α was negatively regulated by this signal pathway.3,4 In addition, these two cytokines display a differential utilization of the cAMP-dependent protein kinase A (PKA) pathway where IL-10 is dependent5,6 and TNF-α is potently suppressed upon elevation of cAMP.7–9 Both the p42/44 extracellular signal-related kinase (ERK) MAPKs and PI3K signal pathways have been described to regulate or be regulated by the activation of protein kinase C (PKC) although this would appear to be cell type- and isoenzyme-specific. Thus, we wished to investigate the role of PKC in the production of macrophage IL-10 and TNF-α and how PKC activity relates to other discriminatory signal cascades such as p42/44 MAPK and cAMP-dependent pathways.
PKC is a family of multifunctional protein serine/threonine kinases that express a pseudosubstrate site and a membrane interaction phosphatidylserine binding site and differ in structure, function and cofactor requirements.10,11 The PKC family comprises of four classes of 12 isoenzymes: conventional (cPKCs; α, βΙ, βII, γ) that require Ca2+ and diacylglycerol (DAG), novel (nPKCs; δ, ε, η, θ and µ) that require DAG only, atypical (aPKCs; ζ, ι, λ the mouse homologue of ι) that require neither DAG nor Ca2+, and PKD (may be PKCµ– see 12). Eight of these isoenzymes (α, βI, βII, δ, ε, η, ζ, µ) are expressed in monocytes and macrophages, yet their respective roles in macrophage function is relatively poorly understood.13
PKC regulation of monocyte/macrophage andRA-smooth muscle cell function is cell type- and isoenzyme-specific. Recently, PKCα has been shown to modulate macrophage cyclo-oxygenase-2 and prostaglandin E2 (PGE2) expression14,15 as well as FcγR-mediated phagocytosis.16 The novel isoenzyme, PKCε appears to be required for murine macrophage activation (NO, TNF-α, IL-1β, inhibitor of nuclear factor κB (IκB) kinase) and defence against bacterial infection.17 PKCs have also been described to regulate cytokine production where monocyte chemotactic protein-1 (MCP-1) release by murine macrophages is mediated by PKCβ and PKCδ; isozyme utilization being dependent on the stimulus encountered.18 Both PKCβII and PKCδ are also involved in monocyte IL-10 induction by HIV-1 Tat protein.19 In RA, aPKC regulate the synergistic effect of IL-1α and TNF-α to stimulate PGE2-dependent production of IL-11 in synovial fibroblasts.20 Thus it is possible that the PKC group determines whether the monocyte/macrophage response is pro- or anti-inflammatory: cPKCs and nPKCs being pro-inflammatory and aPKCs being anti-inflammatory.
One pathway known to down-regulate pro-inflammatory TNF-α production and consequently, up-regulation of anti-inflammatory IL-10 is that elicited by the second messenger, cAMP.21,22 This pathway may represent a good therapeutic target itself because of the opposing effects on TNF-α and IL-10. Previously, this group has demonstrated that rolipram, a phosphodiesterase (PDE) IV inhibitor, hence elevator of cAMP, reduced clinical and histological severity of collagen-induced arthritis (CIA).23 These studies demonstrate a role for the cAMP/PKA pathway in mediating autoimmune diseases such as RA. Interestingly, cAMP-dependent pathway antagonises the pro-inflammatory IL-1 regulation of stromelysin by RA synovial fibroblasts (RA-SFs).24 Recent data has suggested cross-talk between the cAMP/PKA and PKC pathways.25 Indeed, PKC and cAMP pathways not only co-operate26 but can also negatively regulate each other. In addition, PKC isoforms can cross-talk where cPKCα activation inhibits aPKCζ function.27 Other discriminatory pathways regulate PKC activation: atypical PKCζ can be activated in a PI3K/PDK1-dependent manner.28–31 Regulation is further complicated by the multifactorial regulation of transcription factors. cAMP response element binding protein (CREB), a factor which binds CREs, is primarily activated by phosphorylation of Ser 119/133 originally described as the PKA site. However, recent studies show CREB to be regulated by PKB, pp90RSK, p70S6K, MAPKAPK2, ERK and PKC, to name a few. The relative contributions of each signal determine phosphorylation and hence cellular distribution of CREB, in addition to complex cross-talk upstream of such transcription factors.
Monocyte/macrophage isoenzyme expression and the range of functions regulated by PKCs would suggest that PKC modulates human monocyte-derived macrophage cytokine production and that the differential utilization of specific isoenzymes determines the macrophage response to be pro-inflammatory or anti-inflammatory. In this report we have found evidence of differential PKC regulation, where conventional PKC regulate macrophage TNF-α production but not IL-10 through a p42/44 MAPK-mediated pathway and that a cAMP/PKCζ/CREB-dependent pathway may antagonise this effect suppressing TNF-α and augmenting IL-10 production.
Materials and methods
Reagents
Capture and detection antibodies for human TNF-α and IL-10 enzyme-linked immunosorbent assays (ELISAs) were purchased from Pharmingen International (Oxford, UK). Macrophage colony-stimulating factor (M-CSF) was obtained from Genetics Institute (Boston, MA). PDE-resistant dibutyryl cAMP, PKA inhibitor H89, PDEIV inhibitor Rolipram, adenylate cyclase activator Forskolin and PKC inhibitors (Go6983, Go6976 and RO-32-0432) were purchased from Sigma (Poole, UK). Go6983 inhibits α, β, γ, δ; Go6976 inhibits α, βI and µ and RO-32-0432 inhibits α, βI and ε. Antibodies to PKC, PKCζ, p42/44 MAPK and CREB were all purchased from New England Biolabs (Hitchin, UK). All reagents used in these tissue culture experiments were tested for the presence of LPS/endotoxin contamination and were found to be below the lower level of detection of the limulus amaebocyte assay (BioWhittaker, Wokingham, UK). In addition, dibutyryl cAMP, H89 and PKC inhibitors were tested for cytotoxicity and displayed no toxicity at the concentrations being used in this study as determined by methyl thiazolyl tetrazolium (MTT) assay and trypan blue exclusion.
Purification of monocytes
Human peripheral blood mononuclear cells (PBMCs) were obtained from density centrifugation of human venous blood buffy coats purchased from the North London Blood Transfusion Service (Colindale, UK) through ficoll/hypaque (specific density 1·077 g/ml, Nycomed Pharma A.S. Oslo, Norway). The resulting PBMCs were centrifugally elutriated in 1% fetal calf serum (FCS) RPMI-1640 medium in a Beckman JE6 elutriator. Monocyte purity was assessed by flow cytometric analysis of binding of fluorochrome-conjugated anti-CD3, anti-CD19, anti-CD14 and anti-CD45 antibodies (Becton Dickinson, Oxford, U.K). Monocytes obtained were routinely >90% purity.
Differentiation of monocytes to macrophages
Peripheral blood monocytes obtained by centrifugal elutriation were seeded at a density of 1 × 106/ml in assay medium in T-75 medium tissue culture flasks. M-CSF was added to a final concentration of 100 ng/ml. Cells were cultured for 7 days at 37°/5% CO2. Adherent cells were then washed twice in FCS-free RPMI-1640 and removed from plastic by cell dissociation medium (Sigma). The resulting adherent cells were washed twice more and resuspended in RPMI-1640/10% FCS ready for use.
Cytokine determination by ELISA
Sandwich ELISAs were used to measure human IL-10 and TNF-α and were carried out in accordance with the manufacturer's specifications (PharMingen International, Oxford, UK). Briefly, in the IL-10 assay, the anti-IL-10 monoclonal antibody (mAb), 9D7 was used as the capture antibody and biotinylated 12G8 was used as the detection antibody. The ELISA was performed as was previously described with a standard curve of rhuIL-10 from 10 000 to 13 pg/ml32 TNF-α ELISA was carried out as described using 61E71 as the coating antibody and a rabbit polyclonal anti-TNF-α antibody as the detection antibody. This polyclonal anti-TNF-α antibody was in turn detected by a horseradish-peroxidase (HRP) conjugated goat anti-rabbit IgG(H + l) (Jackson ImmunoResearch Laboratories, West Grove, PA). The standard curve of rhuTNF-α covered the range of 20 000–8 pg/ml33 Both ELISAs were quantified by tetramethyl benzidine (TMB) activity in response to the HRP conjugate and read on a Labsystems Multiscan Bichromatic plate reader at 450 nm and analysed by Deltasoft II program (BioMetallics,Inc., Princeton, NJ). The minimal sensitivity of the ELISAs were 8 pg/ml for the TNF-α ELISA and 13–40 pg/ml for the IL-10 ELISA. All results are expressed as the mean concentration of cytokine ± SD obtained per condition.
Western blot analysis of phospho-CREB, PKC and p42/44 MAPK
Macrophages were seeded at a density of 5 × 106 cells/ml in 12-well plates in RPMI-1640/10% FCS. Macrophages were pretreated for 1 hr with inhibitors prior to stimulation for 20 min with LPS or PMA/ionomycin, after which cell lysates were harvested. The stimulation time was previously defined as optimal for activation of CREB, PKC and p42/44 MAPK. Following stimulation cells were lysed on ice for 15 min in lysis buffer (1% NP-40, 200 mm NaCl, 0·1 mm ethylenediaminetetra-acetic acid, 1 mm dithiothreitol, 1 mm Na3VO4, 1 mm NaF, 1 mm phenylmethylsulphonyl fluoride, 10 µg/ml leupeptin, 10 µg/ml pepstatin and 10 µg/ml aprotinin). Lysed samples (10 µg) were separated on a 10% sodium dodecyl sulphate–polyacrylamide gel and Western blotted onto a nitrocellulose membrane. Phosphorylated proteins were detected using antibodies raised against phospho-CREB followed by anti-rabbit HRP conjugate and enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech UK Ltd, Little Chalfont, UK). Total proteins were also detected for purpose of loading controls and are presented in the figures below the corresponding phospho-Western. Protein bands were visualised by autoradiography using Hyperfilm (Amersham Pharmacia Biotech UK Ltd).
Macrophage infection by dominant negative (DN) PKCζ adenovirus
A kinase-defective dominant negative mutant PKCζ was engineered into a tetracycline-regulated adenoviral system. DN PKCζ was under the control of the tetracycline-response element and expression of the transgene is dependent upon the presence of a second virus expressing the Tet-OFF transcriptional regulator under constitutive cytomegalovirus control. In the presence of tetracycline, the Tet-OFF transactivator can no longer bind and induce transcription of the DN PKCζ transgene.34,35 The transactivator and PKCζ adenovirus vectors were kindly provided by Dr Andrew Newby, Bristol, UK.36 Human macrophages were plated out at a density of 1 × 105 cells/well in 96-well plates and exposed to virus at a titration of multiplicity of infection (m.o.i. of 100 : 1 up to 400 : 1 for transactivator virus and PKCζ virus added together at a ratio of 1 : 1 and Ad0 control virus used at the top moi of 400 : 1) for 2 hr in serum-free medium, followed by washing and reculturing in growth medium (RPMI-1640/5% FCS) for 24 hr. Infected cells were then stimulated with 1 ng/ml LPS or PMA/ionomycin in the presence or absence of dibutyryl cAMP for 24 hr.
Statistical analysis
Comparison of data was assessed using GraphPad Prism version 3·0 (GraphPad Software, Inc., San Diego, CA). Statistical differences were determined by Student's t-test. Differences were regarded as significant when *P < 0·05, **P < 0·01 and ***P < 0·001.
Results
PKC selectively regulates macrophage TNF-α production without affecting IL-10
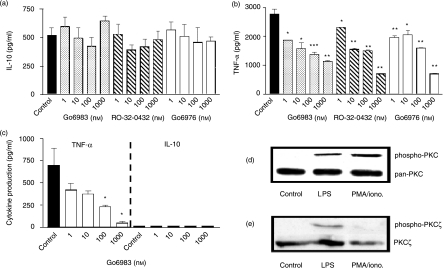
LPS is a commonly used stimulus for monocyte/macrophage cytokine production in vitro, inducing cytokines, which include IL-10, TNF-α, IL-1, IL-6 and IL-8. Several signal pathways, including MAPKs, have been described downstream of LPS binding to the cell surface and, in addition, other pathways such as PKC have been reported to be essential for macrophage activation and functions. We have investigated PKC involvement in M-CSF-primed peripheral blood monocytes (Mφs) with regard to IL-10 and TNF-α production; hypothesizing that PKC activation/isoform utilization discriminates between a pro-inflammatory and an anti-inflammatory response. PKC inhibitors dose-dependently suppress LPS-induced TNF-α production by 59%, 74·6% and 74% for 10−6 m Go6983, RO-32-0432 and Go6976, respectively, with IC50 values of 12·6 nm, 11·5 nm and 15·9 nm (Fig. 1b). In contrast, the anti-inflammatory cytokine, IL-10 is not significantly regulated upon PKC inhibition; control 522 ± 63 pg/ml compared to 648 ± 43 pg/ml, 482 ± 76 pg/ml and 471 ± 36 pg/ml at 10−6 m Go6983, RO-32-0432 and Go6976, respectively (Fig. 1a). In addition, macrophage c + nPKC were activated directly using PMA/ionomycin as a stimulus which resulted in TNF-α production of 700 ± 191 pg/ml whereas IL-10 failed to be produced. PKC inhibition by Go6983 potently suppressed TNF-α production by 67% at 100 nm, IC50 = 8·3 nm (Fig. 1c). These M-CSF-primed monocyte-derived macrophages displayed a differential PKC activation which was stimulus dependent. LPS and PMA/ionomycin activated macrophages showed activation of PKC (α,β isoforms; Fig. 1d). On the other hand, PKCζ was activated by LPS but not PMA/ionomycin (Fig. 1e).
Figure 1.
PKC selectively regulates macrophage TNF-α production without affecting IL-10. Human monocyte-derived macrophages were plated out at 1 × 105 cells per well in a flat-bottomed 96-well plate and pretreated with PKC inhibitors Go6983 (specificity for α, β, γ, δ, ζ), Go6976 (specificity for α and β1) or RO-32-0432 (specificity for α,β1 and ε) (a, b, c) for 1 hr prior to stimulation with 1 ng/ml LPS (a, b) or 50 ng/ml PMA/0·5 µg/ml ionomycin (c) and incubated for 24 hr at 37°/5% CO2, after which time supernatants were harvested and assayed for TNF-α and IL-10 by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD, showing a representative of n = 4 replicate experiments. Western blot analysis of activated phospho-PKC (d) shows PKC activation by LPS (lane 2) and PMA/ionomycin (lane 3). In addition, phospho-Western blot analysis of PKCζ (e) demonstrates LPS activation (lane 2), whereas PMA/ionomycin fails to activate this PKC isoform (lane 3). Loading controls are presented as total PKC and PKCζ blots below the corresponding phospho-Westerns. Data are representative of three replicate experiments. *P≤0·05, **P≤0·01, ***P≤0·001.
PMA/ionomycin activates macrophage p42/44 MAPK, which can be modulated by cAMP-dependent pathways
Previously, we have shown that monocytes display a differential requirement for p42/44 MAPK activity for the production of IL-10 and TNF-α: TNF-α is dependent whereas IL-10 is independent. PKC has been reported to activate p42/44 MAPK.37 In addition, PKC is antagonized by PKA; we and others have demonstrated PKA to differentially regulate IL-10 and TNF-α. We investigated p42/44 MAPK modulation by PKC and cAMP-dependent PKA activity. PMA/ionomycin activates/phosphorylates p42/44 MAPK in monocyte-derived Mφs (MDMs) obtained by 7-day M-CSF treatment of peripheral blood-derived monocytes. This cell type being more representative of Mφs present in the rheumatoid joint. PMA/ionomycin activated p44 MAPK but predisposed to stronger activation of p42 MAPK (Fig. 2a, lane 2). This activation was almost completely abrogated by the treatment with a PKC inhibitor, Go6983 (Fig. 2a, lane 3). These data suggest that PKC activation is upstream of p42/44 MAPK activation. In contrast, inhibition of the cAMP-dependent PKA by H89 had no effect on the activation of p42/44 MAPK (Fig. 2a, lane 4), suggesting that either PKA is not activated by PMA/ionomycin or that PKA activation is independent of the p42/44 MAPK pathway. Activation of cAMP-dependent PKA by utilizing the PDE-resistant dibutyryl cAMP down-regulated p42/44 MAPK activation; abrogating PMA/Ionomycin stimulation of p44 and partially suppressing p42 MAPK (Fig. 2b, lane 3). The activation of PKA alone by addition of dibutyryl cAMP in the absence of PMA/Ionomycin stimulation, failed to activate p42/44 MAPK (Fig. 2b, lane 4). This would suggest that the PKC and cAMP pathways have differential/antagonistic effects.
Figure 2.
PMA/ionomycin activates macrophage p42/44 MAPK which can be modulated by cAMP-dependent pathways. Human monocyte-derived macrophages were plated out at 5 × 106 cells per well in a flat-bottomed 12-well plate and pretreated with PKC inhibitor Go6983, H89 PKA inhibitor or the phosphodiesterase resistant dibutyryl cAMP for 1 hr prior to stimulation with 50 ng/ml PMA/0·5 µg/ml ionomycin and incubated for 20 min at 37°/5% CO2, after which time cell lysates were harvested. Western blot analysis of activated phospho-p42/44 MAPK shows PKC-dependence and PKA-independence of p42/44 MAPK (a). Lane 1, Mφ control; 2, Mφ + PMA/iono; 3, Mφ + PMA/iono. +Go6983; 4, Mφ + PMA/iono. + H89. Dibutyryl cAMP suppresses p42/44 MAPK activation (b). Lane 1, Mφ control; 2,Mφ + PMA/iono; 3, Mφ + PMA/iono. + cAMP; 4, Mφ + cAMP. Loading controls are presented as total p42/44 MAPK blots below the corresponding phospho-Westerns. Data are representative of three replicate experiments.
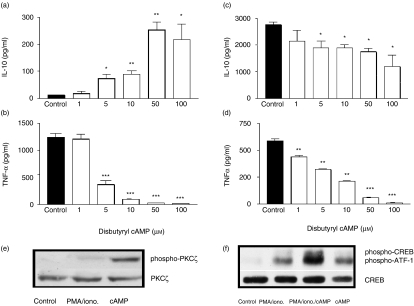
CAMP modulates PMA/ionomycin-stimulated macrophage cytokine profile
It is well established that IL-10 and TNF-α are differentially regulated by cAMP. We wished to determine the relationship between PKC and cAMP-dependent PKA in the context of macrophage cytokine production. Elevation of macrophage cAMP augments the human anti-inflammatory IL-10 response however, potently inhibits the TNFα response. In this study, macrophages were stimulated by PMA/ionomycin activating PKC. Elevation of i[cAMP], by the addition of dibutyryl cAMP augmented IL-10 production with a corresponding decrease in TNF-α production. Dibutyryl cAMP augmented IL-10 production from control levels of 13 ± 1 pg/ml to 253 ± 29 pg/ml at a concentration of 50 µm (ED50 = 6·4 µm, Fig. 3a). TNF-α production was suppressed by 97% at the same concentration (IC50 = 6 µm, Fig. 3b). This trend of augmentation of IL-10 and suppression of TNF-α by PMA/ionomycin-stimulated Mφs upon addition of dibutyryl cAMP was further confirmed by the utilization of the PDE IV inhibitor, rolipram and the adenylate cyclase activator, forskolin (see Table 1). In contrast, cAMP failed to augment macrophage IL-10 production upon LPS stimulation and even partially suppressed IL-10 yet exhibited suppression of TNF-α resulting in an IC50 = 2·5 µm (Fig. 3c,d). This partial suppression of LPS-induced IL-10 production was likely to be as a consequence of potent suppression of TNF; endogenous TNF regulates IL-10 production2,38. Addition of forskolin or rolipram to LPS-stimulated Mφs again had similar results as cAMP, where TNFα production was suppressed (rolipram IC50 = 50 nm; forskolin IC50 = 0·6 µm) with little effect on IL-10 (see Table 2). Furthermore, cAMP on its own activates PKCζ (Fig. 3e) and when added in combination with the PMA/ionomycin costimulated the phosphorylation/activation of the downstream transcription factor to the cAMP-dependent pathway, CREB (Fig. 3f, lane 3). Neither stimulus, on their own, was able to activate CREB. Of particular interest however, is the observation that ATF-1 is activated by PMA/ionomycin-stimulation (ATF-1 is also recognized by the CREB antibody used) (lane 2), the addition of PDE-resistant cAMP costimulates CREB, which is detected along with ATF-1 (lane 3). The addition to macrophages of cAMP alone failed to activate CREB. Although cAMP plays an important regulatory role in Mφ cytokine production which is stimulus-specific; this is independent of PKA, a major signalling component activated by cAMP. PMA/ionomycin and LPS-stimulated Mφ cytokine production is PKA-independent; PKA inhibitor, H89, had little effect on Mφ production of IL-10 and TNF-α (see Table 3). It must be noted however, that variations in baseline control cytokine expression for PMA/ionomycin-stimulated Mφs between Table 1 and Table 3 (IL-10 production at 203 pg/ml against 47 pg/ml; TNF-α production at 18680 pg/ml against 3303 pg/ml) and LPS-stimulated Mφs in Table 2 (IL-10 production at 1786 pg/ml against 819 pg/ml; TNFα production at 183 pg/ml against 3624 pg/ml) is representative of experimental/donor variation. The overall response, i.e. percentage change of control is highly reproducible from donor to donor and experiment to experiment.
Figure 3.
cAMP modulates LPS- and PMA/ionomycin-stimulated macrophage cytokine profile. Human monocyte-derived macrophages were plated out at 1 × 105 cells per well in a flat-bottomed 96-well plate and pretreated with dibutyryl cAMP for 1 hr prior to stimulation with 50 ng/ml PMA/0·5 µg/ml ionomycin (a, b) or 1 ng/ml LPS (c, d) and incubated for 24 hr at 37°/5% CO2, after which time supernatants were harvested and assayed for IL-10 (a, c) and TNF-α (b, d) by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD, showing a representative of four replicate experiments. Western blot analysis demonstrates (e) cAMP modulation of activated phospho-PKCζ: Lane 1, macrophage control; 2, macrophage + PMA/ionomycin; 3, macrophage + cAMP; and (f) costimulation required for CREB activation: Lane 1, macrophage control; 2, macrophage + PMA/ionomycin; 3, macrophage + PMA/ionomycin + cAMP; 4, macrophage + cAMP. Loading controls are presented as total PKCζ and CREB blots below the corresponding phospho-Westerns. Data are representative of three replicate experiments. *P≤0·05, **P≤0·01, ***P≤0·001.
Table 1.
Rolipram and forskolin augment PMA/ionomycin stimulated Mφ IL-10 and suppress TNF-α production
| IL-10 pg/ml (% control) | TNF-α pg/ml (% control) | |
|---|---|---|
| PMA/ionomycin | 202·9 ± 9·233 (100) | 18 680 ± 2477 (100) |
| + rolipram 10 µm | 869·6 ± 230·6 (429) | 12460 ± 591·1 (67) |
| 100 µm | 1582 ± 444·6 (780) | 9319 ± 966·0 (50) |
| + forskolin 1 µm | 523·3 ± 119·2 (258) | 15280 ± 4907 (82) |
| 10 µm | 1556 ± 33·31 (767) | 8314 ± 469·1 (45) |
| 20 µm | 1594 ± 44·36 (786) | 6661 ± 384·2 (36) |
Monocytes primed with M-CSF for 7 days (Mφs) were plated at a density of 1 × 105 cells per well in 96-well flat bottomed plates and were preincubated for 1 hr with the PDE IV inhibitor, rolipram or the adenylate cyclase activator, forskolin or the appropriate vehicle control, afterwhich cells were stimulated with PMA/ionomycin (50 ng/ml and 0·5 µg/ml, respectively) and incubated for a further 24 hr. Supernatants were harvested and assayed for IL-10 and TNF-α levels by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD and their percentage change over control levels (100%), showing a representative of two replicate experiments.
Table 2.
Rolipram and forskolin suppress LPS-stimulated Mφ TNF-α production
| IL-10 pg/ml (% control) | TNF-α pg/ml (% control) | |
|---|---|---|
| LPS control | 1786 ± 90·44 (100) | 182·6 ± 21·78 (100) |
| + rolipram 0·1 µm | 1365 ± 42·66 (76) | 65·31 ± 16·78 (36) |
| 1 µm | 1305 ± 156·7 (73) | 34·86 ± 9·402 (19) |
| 10 µm | 1061 ± 58·97 (60) | 17·14 ± 2·308 (9·4) |
| 100 µm | 862·6 ± 135·1 (48) | 13 ± 0 (7) |
| LPS control | 819·0 ± 91·08 (100) | 3624 ± 383·5 (100) |
| + forskolin 0·1 µm | 928·3 ± 155·6 (113) | 2936 ± 561·4 (81) |
| 1 µm | 866·5 ± 215·7 (106) | 1956 ± 211·7 (54) |
| 10 µm | 846·3 ± 119·0 (103) | 1178 ± 143·6 (33) |
| 20 µm | 762·9 ± 71·33 (93) | 1033 ± 55·21 (29) |
Monocytes primed with M-CSF for 7 days (Mφs) were plated at a density of 1 × 105 cells per well in 96-well flat bottomed plates and were preincubated for 1 h with the PDEIV inhibitor, rolipram or the adenylate cyclase activator, forskolin or the appropriate vehicle control, afterwhich cells were stimulated with 1 ng/ml LPS and incubated for a further 24 hr. Supernatants were harvested and assayed for IL-10 and TNF-αlevels by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD and their percentage change over control levels (100%), showing a representative of seven replicate experiments.
Table 3.
PMA/ionomycin and LPS-stimulated Mφ IL-10 and TNF-α are PKA independent
| IL-10 pg/ml (% control) | TNF-α pg/ml (% control) | |
|---|---|---|
| PMA/ionomycin | 46·77 ± 1·285 (100) | 3303 ± 207·4 (100) |
| + H89 10 µm | 44·73 ± 2·236 (96) | 3079 ± 25·32 (93) |
| 100 µm | 45·36 ± 2·445 (97) | 3312 ± 40·22 (100) |
| 1000 µm | 41·57 ± 2·950 (89) | 2690 ± 207·4 (81) |
| LPS | 1946 ± 57·81 (100) | 1296 ± 39·06 (100) |
| + H89 10 µm | 2085 ± 81·75 (107) | 1265 ± 126·4 (98) |
| 100 µm | 1912 ± 223·6 (98) | 1357 ± 25·90 (105) |
| 1000 µm | 1681 ± 318·0 (86) | 1288 ± 344·4 (99) |
Monocytes primed with M-CSF for 7 days (Mφs) were plated at a density of 1 × 105 cells per well in 96-well flat bottomed plates and were preincubated for 1 hr with the PKA inhibitor, H89 or the appropriate vehicle control, afterwhich cells were stimulated with PMA/ionomycin (50 ng/ml and 0·5 µg/ml, respectively) or LPS (1 ng/ml) and incubated for a further 24 hr. Supernatants were harvested and assayed for IL-10 and TNF-αlevels by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD and their percentage change over control levels (100%), showing a representative of two replicate experiments.
PKCζ selectively regulates macrophage IL-10 production without affecting TNF-α
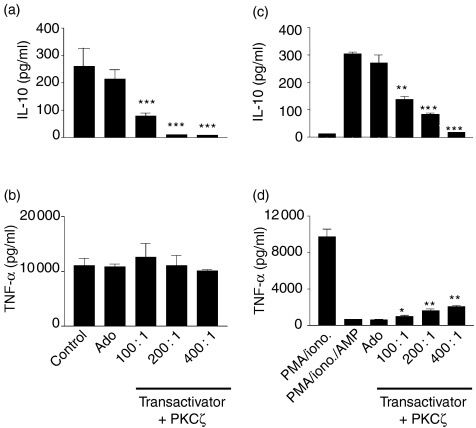
PKCζ is activated by PDK/phosphatidylinositides and by elevations of intracellular cAMP. Both of these mechanisms selectively regulate IL-10 production with a corresponding negative regulation of TNF-α. We wished to investigate if PKCζ represented an anti-inflammatory pathway whereas earlier data demonstrated c+nPKCs to be pro-inflammatory regulators. Selective inhibition of PKCζ using the dominant negative PKCζ system described (transactivator virus and PKCζ virus added together at a ratio of 1 : 1) showed IL-10 production to be suppressed. LPS induction of macrophage IL-10 was suppressed by 69·2% (P = 0·005) at m.o.i. of 100 : 1 (Fig. 4a). This inhibition was increased further by the addition of the transactivator virus to a ratio of 2 : 1 transactivator:PKCζ: at a PKCζ virus m.o.i. of 100 : 1, IL-10 production of 80 ± 10 pg/ml was reduced to 58 ± 14 (data not shown). No such suppression of LPS-induced TNF-α production was observed where control levels of 11 020 ± 1373 pg/ml were not significantly altered at m.o.i. of 400 : 1 (10 070 ± 267 pg/ml, 8·6% suppression, not significant; Fig. 4b). In addition we investigated the influence of PKCζ on the cAMP-driven augmentation of IL-10 production upon stimulation of n + cPKCs by PMA/ionomycin. As in Fig. 3(a), PMA/ionomycin failed to induce IL-10, which however, was augmented upon costimulation with PDE-resistant cAMP. This augmentation of IL-10 production by cAMP was inhibited by DN-PKCζ where control levels were suppressed by 54·7% (P = 0·0041) and 72·5% (P = 0·0002) at m.o.i. of 100 : 1 and 200 : 1, respectively (Fig. 4c). Conversely, the cAMP suppression of PMA/ionomycin-stimulated macrophage TNF-α (9777 ± 780 pg/ml TNF-α, suppressed by cAMP to 657 ± 33 pg/ml) was partially rescued upon PKCζ inhibition. This suppression by cAMP was partially rescued by DN-PKCζ resulting in 11% (P = 0·0080) and 15·2% (P = 0·0031) rescue of PMA/ionomycin induced TNF-α production at m.o.i. of 200 : 1 and 400 : 1, respectively (Fig. 4d).
Figure 4.
PKCζ selectively regulates macrophage IL-10 production without affecting TNF-α. Human monocyte-derived macrophages were plated out at 1 × 105 cells per well in a flat-bottomed 96-well plate and coinfected with DN-PKCζ and transactivator adenovirus vectors for 24 hr prior to stimulation with 1 ng/ml LPS (a, b) or 50 ng/ml PMA/0·5 µg/ml ionomycin in the presence or absence of cAMP (c, d) and incubated for 24 hr at 37°/5% CO2, after which time, supernatants were harvested and assayed for IL-10 (a, c) and TNF-α (b, d) by ELISA. Data are mean cytokine levels in pg/ml of triplicate culture supernatants ± SD, showing a representative of three replicate experiments. *P≤0·05, **P≤0·01, ***P≤0·001.
Discussion
PKC differentially controls IL-10 and TNF-α production in monocyte-derived macrophages. LPS induction of TNF-α is dependent on PKC α/β activation whereas IL-10 production is independent of n and cPKCs. The use of selective PKC inhibitors suggests that LPS induced TNF-α is dependent on cPKC isoforms α and β. This result is backed up by direct stimulation of cPKC (require DAG and calcium) using PMA/ionomycin where TNF-α is stimulated and IL-10 is not produced which is consistent with that observed in human alveolar macrophages.39 LPS-induced IL-10 expression however, is down-regulated by PMA suggesting complex control by PKC likely to be stimulus- and isoform-specific. PMA/ionomycin- or PMA-stimulation (PMA being an analogue of DAG) of macrophages activates the conventional PKCs (α, βI and βII) and novel PKCs (δ, ε, η), respectively. The TNFα production being sensitive to the PKC inhibitors, Go6983, RO-32-0432 and Go6976, all of which share a common inhibition of α and β isoforms. Stimulation of nPKCs by PMA alone however, induced TNF-α production but at much smaller levels than for PMA/ionomycin. PKCε isoform is a major signal component in murine macrophages17 activated by PMA (DAG analogue) alone; it is thus possible that PKCε may regulate TNF-α production however, PKCα/β isoforms predominate. Although cPKCα/β were activated by PMA/ionomycin they were less so upon LPS stimulation; however, LPS-induced TNF-α was suppressed by all three PKC inhibitors whose specificity overlaps for the α and β PKC isoforms.
LPS has been demonstrated to activate PKCζ,37 an atypical PKC that does not require DAG or calcium, and is activated by phosphatidylinositides and, as such, PMA/ionomycin does not activate PKCζ. The macrophages used in these studies also demonstrate activation of PKCζ upon LPS stimulation. These results alone suggest that TNF-α production selectively requires cPKC activation over that of aPKC or PKCζ. PKCζ is activated by PIP2 and PIP3; products of PI3K activation. We have previously demonstrated that IL-10 production selectively utilises PI3K in macrophages3,4 and it is possible that PKCζ lies downstream of PI3K activation in stimulated macrophages.
PMA/ionomycin (cPKCs)-stimulated macrophages activate the downstream effector kinase p42/44 MAPK, which is sensitive to PKC inhibition but insensitive to PKA inhibition. The cAMP/PKA pathway has long since been established as an important pathway in IL-10 expression, inducing IL-10 mRNA but not protein secreted into the supernatant.5 Upon stimulation by the PDE-resistant dibutyryl cAMP, PMA/ionomycin activation of p42/44 MAPK was suppressed which suggests a regulatory role for a cAMP-dependent pathway on the cPKC pathway with ragard to p42/44 MAPK activation. Previous data has already described that TNF-α, unlike IL-10, requires p42/44 MAPK activation for production by monocytes.2 Thus, it is suggested that TNF-α production is dependent on the cPKC/p42/44 MAPK pathway which in turn is negatively regulated by a cAMP-dependent pathway. Interestingly, ERK2 and PKA activation can regulate intracellular cAMP levels through interaction with PDE4, modulating enzymic breakdown of cAMP.40,41 Conversely, ERKs can be modulated by cAMP-dependent PKA which modifies phosphatase binding to ERKs (reviewed in 42). Indeed, upon the addition of cAMP to macrophages stimulated by PMA/Ionomycin (high TNF-α/no IL-10): IL-10 is now produced and secreted into the supernatant. Cyclic-AMP augments IL-10 production and at the same time suppresses TNF-α production; this regulation of TNF-α may be direct or indirect as a result of induction of IL-10. Thus, in the case of TNF-α production, a cAMP-dependent pathway antagonizes cPKC pathway, whereas in the case of IL-10 production, cAMP activation co-operates with cPKC. This, however, is not the case upon LPS stimulation. Cyclic-AMP suppresses TNF-α production but does not augment IL-10 production, in fact is slightly suppressive. This suppression of IL-10 was thought to be an indirect effect caused by the potent inhibition of TNF-α, which partially regulates LPS-induced IL-10 expression. PKCζ was activated by cAMP, this suggests that IL-10 production requires activation of both cPKC and PKCζ.
Downstream of cAMP-dependent PKA, the transcription factor, CREB is also activated by cAMP. In the presence of PMA/ionomycin, cyclic-AMP costimulates CREB activation which heterodimerises with ATF-1. CREB and other CRE-binding proteins share a wide array of activating kinases which include PKA, PKB, PKC, pp90RSK (a substrate of ERK), MAPKAPK2 (a substrate of p38 MAPK), p70S6K, calmodulin kinases II and IV, casein kinases I and II and glycogen synthase kinase III.42,43 Thus, specificity of response may not be solely dictated by upstream signalling cascades but also by the dimerization partner, hence the binding characteristics to the CRE in the promotor region. ATF-1 and CREB respond differentially to cAMP where ATF-1 has a lower activity than CREB in supporting cAMP inducibility whereas these two transcription factors are almost identical in conferring Ca2+ inducibility.44 In our hands, further investigation into the cAMP-dependent mechanism lead to an interesting observation where ATF-1 was activated upon PMA/ionomycin stimulation (c+nPKCs) and CREB was activated by cAMP and c + nPKC stimulation. This might suggest a dichotomy in mechanisms regulating pro- and anti-inflammatory cytokines: PMA/ionomycin stimulating a c+nPKC/ATF-1 pro-inflammatory pathway whereas PMA/ionomycin+cAMP stimulates a PKCζ/CREB anti-inflammatory pathway. The utilization of these pathways however, being cell- and stimulus-specific.
This study suggests that there is differential regulation of macrophage cytokine production by PKC where TNF-α is regulated by a cPKC/p42/44 MAPK pathway and that cAMP/PKCζ/CREB controls IL-10 production. Additionally, these two pathways exhibit a level of cross-regulation. The utilization of an adenoviral system expressing a dominant negative PKCζ demonstrated that inhibition of PKCζ suppresses LPS- and PMA/ionomycin/cAMP-induced IL-10. On the other hand, PMA/ionomycin induced TNF-α production was suppressed by cAMP; inhibition of PKCζ partially reversed this suppression of TNF-α production. In combination with phospho-Western results, PKCζ is activated by cAMP which is capable of inhibiting phosphorylation of p42/44 MAPK. It would appear that cross regulation between these two pathways exists at this point; whether PKCζ is capable of regulating the activity of cPKC and vice versa is not known and will be the subject of research in the future.
We propose that mechanistically, Mφ induction of TNF-α production is driven through a conventional- or novel-PKC, activating p42/44 MAPK which in turn activates transcription factors such as ATF-1 leading to gene transcription. The activation of p42/44 MAPK is antagonized by cAMP which in turn activates the atypicalPKCζ and the transcription factor CREB resulting in IL-10 expression. In this study, cAMP is at a pivotal point regulating Mφ responses as either pro- or anti-inflammatory.
In conclusion, we have provided evidence to suggest that there is a differential PKC regulation of macrophage production of IL-10 and TNF-α. Conventional PKCs regulate the pro-inflammatory cytokine, TNF-α, in a p42/44 MAPK-dependent manner whereas, anti-inflammatory IL-10 is regulated by a cAMP/PKCζ/CREB-dependent pathway. In addition, cross-regulation between these pathways exists where cAMP suppresses TNF-α production possibly at the level of p42/44 MAPK activation.
Acknowledgments
This work was funded by a Wellcome Trust project grant and the Kennedy Institute of Rheumatology is supported by a core grant from the Arthritis and Rheumatism Council of Great Britain.
Abbreviations
- IL-10
interleukin-10
- TNF-α
tumour necrosis factor-α
- Mφ
macrophage
- PKA
protein kinase A
- cAMP
cyclic adenosine monophosphate
- M-CSF
macrophage colony-stimulating factor
- LPS
lipopolysaccharide
- RA
rheumatoid arthritis
- CREB
cAMP response element binding protein
- p42/44 MAPK
p42/44 mitogen-activated protein kinase
References
- 1.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BMJ, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNFα: Role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–8. [PubMed] [Google Scholar]
- 3.Foey AD, Feldmann M, Brennan FM. CD40 ligation induces macrophage IL-10 and TNFα production: differential use of the PI3K and p42/44 MAPK pathways. Cytokine. 2001;16:131–42. doi: 10.1006/cyto.2001.0954. [DOI] [PubMed] [Google Scholar]
- 4.Foey AD, Green P, Foxwell BMJ, Feldmann M, Brennan FM. Cytokine-stimulated T cells induce macrophage IL-10 production dependent on phosphatidylinositol 3-kinase and p70S6K: implications for rheumatoid arthritis. Arthr Res. 2002;4:64–70. doi: 10.1186/ar385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platzer C, Meisel C, Vogt K, Platzer M, Volk H-D. Up-regulation of monocytic IL-10 by tumour necrosis factor-a and camp elevating drugs. Int Immunol. 1995;7:517–23. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 6.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk H-D, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–104. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Taffet SM, Singhel KJ, Overholtzer JF, Shurtleff SA. Regulation of tumour necrosis factor expression in a macrophage like cell line by lipopolysaccharide and cyclic AMP. Cell Immunol. 1989;120:291–300. doi: 10.1016/0008-8749(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 8.Endres S, Fulle H-J, Sinha B, Stoll D, Dinarello CA, Gerzer R, Weber PC. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-α and interleukin-1β by human mononuclear cells. Immunology. 1991;72:56–60. [PMC free article] [PubMed] [Google Scholar]
- 9.Semmler J, Wachtel W, Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumour necrosis factor-α production by human mononuclear cells. Int J Immunopharmacol. 1993;15:409–13. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- 10.Parker PJ. Protein kinase C. a structurally related family of enzymes. In: Epand RM, Lester DS, editors. Protein Kinase C. Current Concepts and Future Perspectives. Chichester: Ellis Horwood; 1992. pp. 3–24. [Google Scholar]
- 11.Nishizuka Y. Protein kinase C and lipid signalling for sustained cellular responses. FASEB J. 1995;9:484–95. [PubMed] [Google Scholar]
- 12.Ron D, Kazanietz MD. New insight into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–76. [PubMed] [Google Scholar]
- 13.Webb BLJ, Hirst SJ, Giembycz MA. Protein kinase C isoenzymes. a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br J Pharmacol. 2000;130:1433–52. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Denis A, Chano F, Tremblay P, St. Pierre Y, Descoteaux A. Protein kinase C-alpha modulates lipopolysaccharide-induced functions in a murine macrophage cell line. J Biol Chem. 1998;273:32787–92. doi: 10.1074/jbc.273.49.32787. [DOI] [PubMed] [Google Scholar]
- 15.Giroux M, Descoteaux A. Cyclooxygenase-2 expression in macrophages: modulation by protein kinase C-α. J Immunol. 2000;165:3985–91. doi: 10.4049/jimmunol.165.7.3985. [DOI] [PubMed] [Google Scholar]
- 16.Breton A, Descoteaux A. Protein kinase C-alpha participates in FcgammaR-mediated phagocytosis in macrophages. Biochem Biophys Res Commun. 2000;276(2):472–6. doi: 10.1006/bbrc.2000.3511. [DOI] [PubMed] [Google Scholar]
- 17.Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. Protein kinase Cε is required for macrophage activation and defence against bacterial infection. J Exp Med. 2001;194:1231–42. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitti M, Domenicotti C, d'Abramo C, et al. Activation of PKC-beta isoforms mediates HNE-induced MCP-1 release by macrophages. Biochem Biophys Res Commun. 2002;294(3):547–52. doi: 10.1016/S0006-291X(02)00512-0. [DOI] [PubMed] [Google Scholar]
- 19.Bennasser Y, Bahraoui E. HIV-1 Tat protein induces interleukin-10 in human peripheral blood monocytes: involvement of protein kinase C-beta II and -delta. FASEB J. 2002;16(6):546–54. doi: 10.1096/fj.01-0775com. [DOI] [PubMed] [Google Scholar]
- 20.Mino T, Sugiyama E, Taki H, Kuroda A, Yamashita N, Maruyama M, Kobayashi M. Interleukin-1alpha and tumour necrosis factor alpha synergistically stimulate prostaglandin E2-dependent production of interleukin-11 in rheumatoid synovial fibroblasts. Arthr Rheum. 1998;41(11):2004–13. doi: 10.1002/1529-0131(199811)41:11<2004::AID-ART16>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Kambayashi T, Jacob CO, Zhou D, Mazurek N, Fong M, Strassmann G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNFα and IL-6 release by endotoxin-stimulated macrophages. J Immunol. 1995;155:4909–16. [PubMed] [Google Scholar]
- 22.Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk H-D. Differential regulation of monocytic tumour necrosis factor-α and interleukin-10 expression. Eur J Immunol. 1996;26:1580–6. doi: 10.1002/eji.1830260726. [DOI] [PubMed] [Google Scholar]
- 23.Ross SE, Williams RO, Mason LJ, Mauri C, Marinova-Mutafchieva L, Malfait A-M, Maini RN, Feldmann M. Suppression of TNFα expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–9. [PubMed] [Google Scholar]
- 24.Case JP, Lafyatis R, Kumkumian GK, Remmers EF, Wilder RL. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990;145(11):3755–61. [PubMed] [Google Scholar]
- 25.Miguel BG, Calcerrada MC, Mata F, Aller P, Clemente R, Catalan RE, Martinez AM. Differential dedistribution of protein kinase C isoforms by cyclic AMP in HL60 cells. Biochem Biophys Res Commun. 2000;274:596–602. doi: 10.1006/bbrc.2000.3194. [DOI] [PubMed] [Google Scholar]
- 26.Gubina E, Luo X, Kwon E, Sakamoto K, Shi YF, Mufson RA. βc Cytokine receptor-induced stimulation of cAMP response element binding protein phosphorylation requires protein kinase C in myeloid cells: a novel cytokine signal transduction cascade. J Immunol. 2001;167:4303–10. doi: 10.4049/jimmunol.167.8.4303. [DOI] [PubMed] [Google Scholar]
- 27.Condorelli G, Vigliotta G, Trencia A, et al. Protein kinase C (PKC)-α activation inhibits PKCζ and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes. 2001;50:1244–52. doi: 10.2337/diabetes.50.6.1244. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Velit P, Knutson KL, Reiner NE. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-ζ in bacterial lipopolysaccharide-treated human monocytes. J Biol Chem. 1997;272:16445–52. doi: 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- 29.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK-1. Science. 1998;281:2042–5. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 30.Chou MM, Hou W, Johnson J, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–77. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 31.Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR. A 3-phosphoinositide-dependent protein kinase-1 (PDK-1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta) and PKC-related kinase 2 by PDK-1. J Biol Chem. 2000;275:20806–13. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- 32.Abrams J, Roncorolo MG, Yssel H, Andersson U, Gleich GJ, Silver J. Strategies and practice of anti-cytokine monoclonal antibody development. immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 33.Engelberts I, Moller A, Schoen GJ, van der Linden CJ, Buurmann WA. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991;10:69–76. [PubMed] [Google Scholar]
- 34.Harding TC, Geddes BJ, Noel JD, Murphy D, Uney JB. Tetracycline-regulated transgene expression in hippocampal neurones following transfection with adenoviral vectors. J Neurochem. 1997;69(6):2620–3. doi: 10.1046/j.1471-4159.1997.69062620.x. [DOI] [PubMed] [Google Scholar]
- 35.Harding TC, Geddes BJ, Murphy D, Knight D, Uney JB. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nature Biotechnol. 1998;16(6):553–5. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 36.Hussain S, Assender JW, Bond M, Wong L-F, Murphy D, Newby A. Activation of PKCζ is essential for cytokine-induced metalloproteinases-1,-3 and -9 secretion from rabbit smooth muscle cells and inhibits proliferation. J Biol Chem. 2002;277(30):27345–52. doi: 10.1074/jbc.M111890200. [DOI] [PubMed] [Google Scholar]
- 37.Monick MA, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW. Protein kinase Cζ plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol. 2000;165:4632–9. doi: 10.4049/jimmunol.165.8.4632. [DOI] [PubMed] [Google Scholar]
- 38.Wanidworanun C, Strober W. Predominant role of tumour necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–61. [PubMed] [Google Scholar]
- 39.Boehringer N, Hagens G, Songeon F, Isler P, Nicod LP. Differential regulation of tumour necrosis factor-alpha (TNF-alpha) and interleukin-10 (IL-10) secretion by protein kinase and phosphatase inhibitors in human alveolar macrophages. Eur Cytok Netw. 1999;10:211–8. [PubMed] [Google Scholar]
- 40.Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase, HSPDE4D3 by phosphorylating it at Ser579. EMBO J. 1999;18:893–903. doi: 10.1093/emboj/18.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKenzie SJ, Baillie GS, McPhee I, Bolger GB, Houslay MD. ERK2 MAP kinase binding, phosphorylation and regulation of PDE4D cAMP specific phosphodiesterases: The involvement of C-terminal docking sites and N-terminal UCR regions. J Biol Chem. 2000;275:16609–17. doi: 10.1074/jbc.275.22.16609. [DOI] [PubMed] [Google Scholar]
- 42.Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signalling. Mol Pharmacol. 2000;58:659–68. [PubMed] [Google Scholar]
- 43.Habener JF. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990;4:1087–94. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Thompson MA, Wagner S, Greenberg ME, Green MR. Activating transcription factor-1 can mediate Ca2+- and cAMP-inducible transcriptional activation. J Biol Chem. 1993;268:6714–20. [PubMed] [Google Scholar]