Abstract
Exogenous antigens endocytosed in large amounts by antigen-presenting cells (APC) are presented on major histocompatibility complex (MHC) class I molecules as well as on class II molecules, a process called cross-presentation. Among APC, dendritic cells (DC) play a key role in cross-presentation by transporting internalized antigen to the cytosol. The present study shows that ovalbumin (OVA) introduced with negative charges by succinylation (Suc-OVA), maleylation (Mal-OVA) or cis-aconitylation (Aco-OVA) was efficiently taken up by DC via scavenger receptors (SR). Mal-OVA and Aco-OVA were efficiently cross-presented by DC, while cross-presentation of Suc-OVA was hardly observed. MHC class I presentation of acylated OVA introduced directly into the cytosol was inefficient and presentation of exogenous native OVA but not of Aco-OVA was markedly augmented by chloroquine, an inhibitor of endosomal acidification, suggesting that deacylation in endosomes or lysosomes is necessary for cross-presentation of acylated OVA. MHC class I presentation of exogenous native OVA and Aco-OVA by DC was blocked by lactacystin and brefeldin A, demonstrating that exogenous antigens taken up by DC are cross-presented through the conventional cytosolic pathway. Therefore, SR-mediated delivery of antigen to DC leads to efficient cross-presentation, although the pathway of chemical modification should be considered.
Keywords: dendritic cells: cross-presentation, MHC class I presentation scavenger receptors; soluble antigens: negatively charged, ovalbumin
Inroduction
Successful immunotherapy against cancer or infectious diseases requires induction of cytotoxic T lymphocyte (CTL)-mediated immune responses. Antigen-specific CTL are generated when antigen-presenting cells (APC) present peptides from antigen on major histocompatibility complex (MHC) class I molecules to naïve CD8+ T cells. In general, however, proteins taken up by APC are degraded in endosomal/lysosomal compartments and presented on MHC class II molecules, while MHC class I molecules associate exclusively with peptides derived from endogenously synthesized proteins, such as virus-encoded proteins or tumour antigen.1 Therefore, it is not expected that exogenous antigen gain access to the MHC class I antigen presentation pathway and initiate CTL responses.
However, APC are able to present exogenous antigens on MHC class I molecules under certain conditions, a process called cross-presentation.2,3 Although macrophages and B cells are capable of cross-presentation in vitro,4–6 dendritic cells (DC) are most likely to be crucial in this process because of their distinctive ability to prime naïve T cells and generate CTL responses in vivo.7 In addition to phagocytosis4,8 and macropinocytosis,9,10 antigen endocytosed via Fcγ receptors11 and scavenger receptors (SR)5,12–14 are efficiently cross-presented to CD8+ T cells by APC.
SR, which recognize diverse polyanionic ligands,15 are expressed on macrophages, B cells and DC, and negatively charged proteins obtained by succinylation or maleylation are known to be SR ligands.16,17 Thus, using such chemical modifications, antigens are expected to be efficiently taken up by APC through SR-mediated endocytosis.
In this study, in order to investigate the possibility that efficient cross-presentation of soluble antigens by DC can be achieved through SR-mediated delivery and to study its mechanism, ovalbumin (OVA), which was selected as a model antigen, was introduced with negatively charges obtained via three forms of acylation, namely, succinylation (Suc-OVA), maleylation (Mal-OVA) and cis-aconitylation (Aco-OVA). Cellular uptake and subsequent MHC class I presentation of these chemically modified OVA were studied using a well-characterized DC line, DC2.4.18,19
Materials and methods
Chemicals
OVA, fluorescein isothiocyanate (FITC), polyinosinic acid (poly[I]), polycytidylic acid (poly[C]), bovine serum albumin (BSA), lactacystin, brefeldin A and chloroquine were purchased from Sigma (St. Louis, MO). Succinic anhydride was purchased from Nacalai Tesque (Kyoto, Japan). Maleic anhydride and cis-aconitic anhydride were purchased from Wako Pure Chemical Industries (Osaka, Japan). Diethylenetriaminepentaacetic acid (DTPA) anhydride was purchased from Dojindo Laboratory (Kumamoto, Japan). The OVA257−264 peptide, SIINFEKL was purchased from Bachem (Bubendorf, Switzerland). 111InCl3 was supplied by Nihon Medi-Physics (Takarazuka, Japan). All other chemicals were reagent-grade products obtained commercially.
Cell lines
The murine DC line DC2.4 (haplotype H-2b) was a generous gift from Dr K. L. Rock (University of Massachusetts Medical Center, Worcester, MA).18 DC2.4 cells display dendritic morphology, express a series of DC-specific markers, MHC class I and II molecules, costimulatory molecules, and have phagocytic property and antigen-presenting capacity.18 CD8OVA1.3 T hybridoma cells, which secrete IL-2 upon stimulation with SIINFEKL-Kb complexes,20 were kindly provided by Dr C. V. Harding (Case Western Reserve University, Cleveland, OH).
DC2.4 cells were cultured in RPMI-1640 medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum (Equitech-Bio, Kerrville, TX), 50 µm 2-mercaptoethanol, 2 mm l-glutamine, and antibiotics (all from Invitrogen, Carlsbad, CA). CD8OVA1.3 cells were grown in Dulbecco's modified Eagle medium (Nissui) supplemented as described for RPMI-1640 medium.
Chemical modification of OVA
OVA was modified with succinic, maleic or cis-aconitic anhydride (Aco-OVA) at alkaline pH.16,21,22 In brief, OVA was dissolved in 0·2 m Tris buffer (pH 8·65), and an appropriate amount of each anhydride was added to the solution. The mixture was stirred until all the anhydride dissolved and kept at pH > 8 during the reaction. After the reaction was complete, free anhydride was removed by gel filtration with a Sephadex G-25 column (Amersham Biosciences, Piscataway, NJ). The protein fractions were then concentrated by ultrafiltration, and finally lyophilized. The purity of these products was confirmed by isoelectric focusing and unmodified OVA was not detected in all the derivatives. Thus, the products were used in following experiments without further purifications. The degree of modification was assessed by estimating the loss of free amino groups as measured by trinitrobenzenesulphonic acid (TNBS).23
Evaluation of deacylation of acylated OVA by hydrolysis
Each acylated OVA was dissolved in phosphate-buffered saline at pH 5·0 or 7·4, and incubated at 37°. At appropriate intervals, aliquots were collected and free amino groups were determined using TNBS. Deacylation at each time point was evaluated as the ratio of the remaining acylated amino groups.
Confocal microscopy
OVA, Suc-OVA and Aco-OVA were labelled with FITC by the method of Monsigny et al.24 DC2.4 cells cultured on glass coverslips were incubated with FITC-labelled proteins at 37°. After 6 hr, cells were washed and fixed with 4% paraformaldehyde. Confocal images were observed with a laser scanning confocal microscope (MRC-1024, Bio-Rad, Hercules, CA).
Cellular association experiments
For cellular association experiments, proteins were radiolabelled with 111In using the bifunctional chelating agent DTPA anhydride according to the method of Hnatowich et al.25 This radiolabeling method is suitable for the study of cellular association because any radioactive metabolites produced after cellular uptake are retained within the cells.26 DC2.4 cells cultured on 24-well plates were added to Hanks' balanced salt solution (HBSS) containing indicated concentrations of 111In-labelled proteins and incubated at 37°. After indicated times, the protein solution was removed and cells were washed with ice-cold HBSS. Cells were then solubilized with 0·3 n NaOH with 0·1% Triton-X-100 and the radioactivity in the cell lysate was measured using a well-type NaI-scintillation counter (ARC-500, Aloka, Tokyo, Japan). The amount of cellular protein in each cell lysate was estimated using a protein quantification kit (Dojindo Laboratory).
In competition experiments, 111In-labelled Suc-OVA was added to DC2.4 cells concomitantly with a variety of unlabelled macromolecules.
Antigen-presentation assays
Various concentrations of antigen were added to DC2.4 cells (5 × 104/well) cultured on 96-well plates and incubated with CD8OVA1.3 T hybridoma cells (105/well) at 37°. After 24 hr, the cell culture supernatants were collected and freeze–thawed. Then, the response of CD8OVA1.3 T cells was determined by measuring interleukin-2 (IL-2) levels in the supernatants with enzyme-linked immunosorbent assay (ELISA; AN'ALYZA mouse IL-2, Genzyme-Techne, Minneapolis, MN). The antigen presentation experiments were carried out in the presence of serum to maintain normal cellular functions of DC2.4 and CD8OVA1.3 T cells during the experiments. Although serum might contain SR ligands including lipoproteins that may affect the antigen uptake, the effect of these putative ligands would be negligible since the antigen concentration was high (up to 1–5 mg/ml).
In some assays, antigen was introduced directly into the cytosol by osmotic lysis of pinosomes.27 Briefly, each antigen was dissolved in hypertonic medium (RPMI-1640 medium containing 0·5 m sucrose, 10% polyethylene glycol 1000 and 10 mm HEPES) at 5 mg/ml. DC2.4 cells were pelleted and resuspended at 2 × 106/ml in antigen-containing hypertonic medium for 10 min at 37°. The suspension was diluted to 30-fold with hypotonic medium (60% RPMI-1640 and 40% water) and left for 2–3 min at 37°. Cells were then pelleted, resuspended in culture medium and plated at different cell numbers. After a 3-hr incubation at 37°, cells were fixed with 1% paraformaldehyde and incubated with CD8OVA1.3 T hybridoma cells (105/well) for 20 hr. IL-2 levels in the culture supernatants were determined by ELISA.
To study the pathway for antigen presentation, DC2.4 cells (105/well) were incubated with 100 µm chloroquine, 10 µm lactacystin, or 5 µg/ml brefeldin A for 30 min prior to the addition of antigen. Cells were further incubated for 6 hr at 37° in the continued presence of inhibitor, washed and fixed. Then CD8OVA1.3 T hybridoma cells (105/well) were added and, after 20 hr, the IL-2 levels in the supernatants were measured.
Results
Chemical modification of OVA
As shown in Table 1, the 18·3, 19·1, and 14·8 amino groups of OVA were modified with succinic (Suc-OVA), maleic (Mal-OVA) and cis-aconitic anhydride (Aco-OVA), respectively. These three derivatives seemed to possess enough negative charges to have an affinity for SR.28
Table 1.
Characteristics of acylated OVA*
| Compound | Number of modified amino groups | Number of free amino groups | Estimated molecular weight |
|---|---|---|---|
| OVA | 0 | 21 | 46 000 |
| Suc-OVA | 18·3 | 2·7 | 47 800 |
| Mal-OVA | 19·1 | 1·9 | 47 900 |
| Aco-OVA | 14·8 | 6·2 | 48 300 |
The numbers and molecular weights were estimated using TNBS.
Deacylation of acylated OVA by hydrolysis
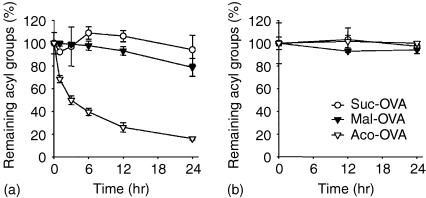
In the recognition of antigenic peptide presented on MHC molecules, T cells strictly discriminate the structure of the peptide using T-cell receptors (TCR). Accordingly, the acyl group on the antigenic peptide may affect the TCR recognition. It has been demonstrated that maleyl and cis-aconityl groups introduced into proteins are removed by hydrolysis at an acidic pH, unlike succinyl groups.29 Thus, deacylation of each acylated OVA by hydrolysis was investigated at pH 7·4 and 5·0, assuming physiological and endosomal/lysosomal conditions, respectively. At pH 5·0, the acyl groups of Aco-OVA were removed by hydrolysis in a time-dependent manner (t1/2 = 3·97 hours) (Fig. 1a). Mal-OVA also underwent hydrolysis, although the rate was slower than Aco-OVA (t1/2 = 78·8 hr). On the other hand, deacylation of Suc-OVA was hardly observed under these conditions. All three derivatives were stable at pH 7·4 (Fig. 1b). These results suggest that after being endocytosed, Aco-OVA and Mal-OVA are deacylated in acidic compartments such as endosomes and lysosomes and returned to native OVA, whereas they are stable in the extracellular fluid.
Figure 1.
Deacylation of acylated OVA under acidic or neutral conditions. Acylated OVA in phosphate buffered saline pH 5·0 (a) or 7·4 (b) was incubated for the indicated times at 37°. The ratio of remaining acyl groups on each protein was determined by estimating the number of acylated amino groups at each time point using TNBS. Results are expressed as the mean ± SD (n = 3).
Internalization of acylated OVA into endocytic compartments
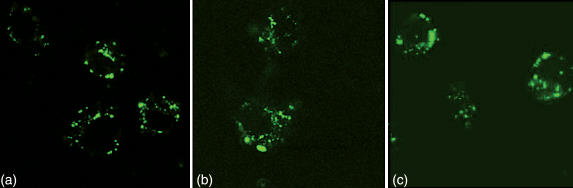
Figure 2 shows confocal microscopic images of uptake of FITC-labelled native and acylated OVA by DC2.4 cells. Punctate vesicular staining by FITC-labelled proteins was observed within DC2.4 cells, suggesting that after binding to the DC, acylated OVA was internalized into endocytic compartments.
Figure 2.
Intracellular localization of FITC-labelled native OVA (a), Suc-OVA (b), and Aco-OVA (c) in DC2.4 cells. DC2.4 cells were incubated with 1 mg/ml FITC-labelled proteins for 6 hr at 37°.
Cellular association experiments
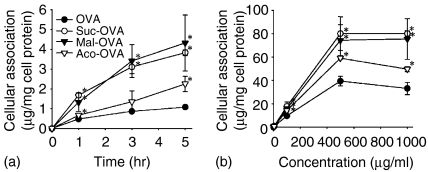
All the 111In-labelled proteins were taken up by DC2.4 cells in a time- and concentration-dependent manner, and acylated OVA were more efficiently taken up by DC2.4 cells than native OVA (Fig. 3). Because the association of each protein was saturable and its amount was higher in acylated OVA than in native OVA which is taken up through mannose receptors,30 it is likely that other receptors, probably SR also participate in the uptake of acylated OVA.
Figure 3.
Time-course and concentration–dependence of cellular association of 111In-labelled native or acylated OVA in DC2.4 cells. DC2.4 cells were incubated with 111In-labelled proteins at 5 µg/ml for indicated times (a) or at various concentrations for 3 hr (b) at 37°. Results are expressed as the mean ± SD (n = 3). *P < 0·05 versus OVA by Student's t-test.
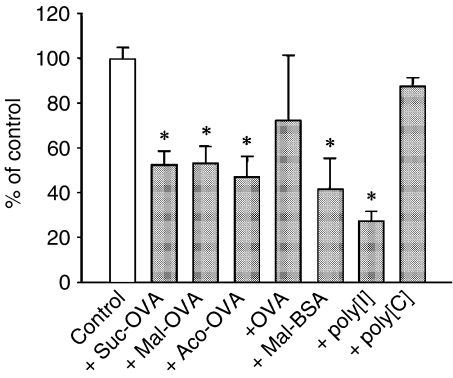
To study the uptake mechanism of acylated OVA, competition experiments were performed using 111In-labelled Suc-OVA and excess amounts of unlabelled native or acylated OVA, maleylated BSA (Mal-BSA), poly[I], or poly[C]. The amount of cellular association of 111In-Suc-OVA with DC2.4 cells was significantly reduced by all kinds of unlabelled acylated OVA (Fig. 4). Furthermore, poly[I] and Mal-BSA, but not poly[C], inhibited the cellular association of 111In-Suc-OVA. These results demonstrate that each acylated OVA is endocytosed by DC via the same receptors, that is, SR.
Figure 4.
Inhibition of cellular association of 111In-labelled Suc-OVA with DC2.4 cells. 111In-Suc-OVA (10 µg/ml) was added to DC2.4 cells concomitantly with unlabelled protein (500 µg/ml), poly[I] or poly[C] (100 µg/ml) and incubated for 3 hr at 37°. Results are expressed as the mean ± SD (n = 3). *P < 0·01 versus control by Student's t-test.
Cross-presentation of native and acylated OVA
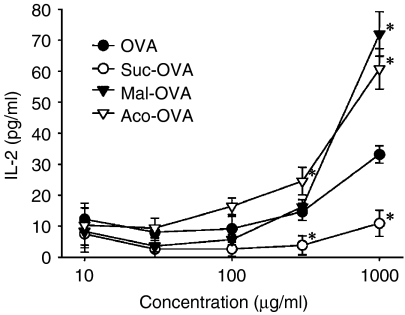
Figure 5 shows MHC class I presentation of exogenous native and acylated OVA by DC2.4 cells. Aco-OVA and Mal-OVA, which showed enhanced uptake by DC2.4 cells and deacylation under acidic conditions, were efficiently cross-presented to CD8OVA1.3 T hybridoma cells. In contrast, despite the higher association with DC2.4 cells, Suc-OVA induced a lower response of CD8OVA1.3 cells than native OVA.
Figure 5.
MHC class I presentation of exogenous native or acylated OVA by DC2.4 cells. DC2.4 cells (5 × 104/well) were incubated with various concentrations of antigen and CD8OVA1.3 T hybridoma cells (105/well) at 37°. After 24 hr, IL-2 production from CD8OVA1.3 T hybridoma cells was measured by ELISA. Results are expressed as the mean ± SD (n = 3). *P < 0·05 versus OVA by Student's t-test.
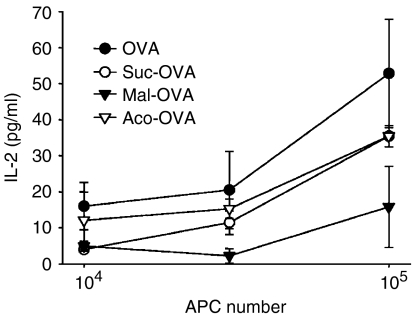
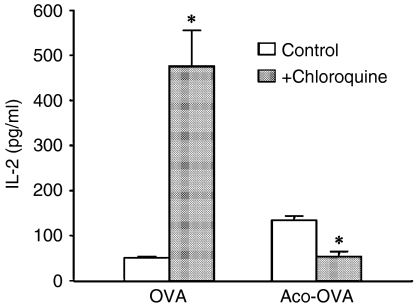
The most prominent difference between Suc-OVA and Aco-OVA or Mal-OVA seems to be the chemical stability under acidic conditions (Fig. 1). Therefore, to ascertain whether deacylation in endosomes or lysosomes is essential for MHC class I presentation, each antigen was delivered directly into the cytosol of DC2.4 cells, and the response of CD8OVA1.3 T cells to the antigen-introduced DC2.4 cells was examined. When cytosolically delivered, Aco-OVA and Mal-OVA failed to generate a higher response of CD8OVA1.3 cells than native OVA (Fig. 6). Furthermore, the effects of endosomal acidification on MHC class I presentation of exogenous native OVA and Aco-OVA was also studied using chloroquine, which raises the pH in the endosomal/lysosomal compartments. Treatment with chloroquine dramatically enhanced the MHC class I presentation of exogenous native OVA, while the presentation of Aco-OVA was reduced (Fig. 7). Taken together, these results indicate that acyl groups on lysine residues in OVA prevent processing for MHC class I presentation by DC and/or recognition of antigenic peptide SIINFEKL by T cells. Consequently, exogenous Suc-OVA failed to elicit the response of CD8OVA1.3 T cells, whereas Aco-OVA and Mal-OVA were efficiently presented because of their reversibility at acidic pH.
Figure 6.
MHC class I presentation of osmotically introduced native or acylated OVA in DC2.4 cells. Each antigen (5 mg/ml) was introduced directly into the cytosol of DC2.4 cells by osmotic lysis of pinosomes. After a 3-hr incubation at 37°, cells were fixed, further incubated with CD8OVA1.3 T hybridoma cells (105/well) for 20 hr, and the levels of IL-2 in the supernatants were determined by ELISA. Results are expressed as the mean ± SD (n = 3).
Figure 7.
Effects of chloroquine on MHC class I presentation of exogenous antigen by DC2.4 cells. DC2.4 cells (105/well) were incubated with or without chloroquine (100 µm) for 30 min at 37° and mixed with 3 mg/ml native OVA or Aco-OVA. After a 6-hr chase in the continued presence of chloroquine, cells were washed, fixed and incubated with CD8OVA1.3 T hybridoma cells (105/well) for 20 hr. IL-2 levels in the culture supernatants were determined by ELISA. Results are expressed as the mean ± SD (n = 3). *P < 0·005 versus control by Student's t-test.
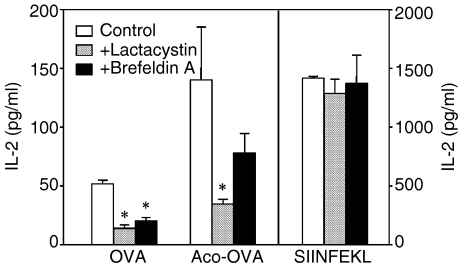
The results in Fig. 7 also suggest that after endocytosis of exogenous antigens, peptides to be presented on MHC class I molecules are generated outside the endosomal/lysosomal compartments in DC. Endogenous cytosolic proteins are primarily degraded into peptides by proteasomes. The peptides are then transported into the endoplasmic reticulum (ER) by transporters associated with antigen processing (TAP), where they bind to nascent MHC class I molecules. The newly assembled peptide-MHC complexes are transported to the cell surface and presented to CD8+ T cells.31 To examine whether exogenous native and acylated OVA is cross-presented by DC via this pathway, the effects of lactacystin, a proteasome inhibitor, and brefeldin A, an inhibitor of anterograde ER-Golgi transport were examined. Both lactacystin and brefeldin A blocked MHC class I presentation of exogenous native OVA and Aco-OVA but not of SIINFEKL, which does not require intracellular processing for presentation (Fig. 8). These results indicate that exogenous native and acylated OVA are transported to the cytosol from endosomes or lysosomes and processed via the conventional MHC class I presentation pathway.
Figure 8.
Effects of lactacystin and brefeldin A on MHC class I presentation of exogenous antigens by DC2.4 cells. DC2.4 cells (105/well) were incubated with or without lactacystin (10 µm) or brefeldin A (5 µg/ml) for 30 min at 37° and mixed with 3 mg/ml native OVA, Aco-OVA or 0·1 µg/ml SIINFEKL. After a 6-hr chase in the continued presence of inhibitors, cells were washed, fixed and incubated with CD8OVA1.3 T hybridoma cells (105/well) for 20 hr. IL-2 levels in the culture supernatants were determined by ELISA. Results are expressed as the mean ± SD (n = 3). *P < 0·05 versus control by Student's t-test.
Discussion
It is well-known that cross-presentation occurs when APC take up a large amount of exogenous antigens by phagocytosis,4,8 macropinocytosis,9,10 or receptor-mediated endocytosis.5,11–14
Among APC, DC are considered to be responsible for in vivo cross-presentation. It has been demonstrated that DC are the only type of APC that efficiently transport exogenous antigens to the cytosol.35 Therefore, specific receptor-mediated antigen delivery to DC can be a promising approach for the development of effective vaccines.
In the present study, OVA was introduced with negative charges for the purpose of efficient SR-mediated uptake and cross-presentation by DC. Three kinds of negatively charged OVA, Suc-OVA, Mal-OVA, and Aco-OVA were synthesized and evaluated with respect to cellular association and cross-presentation.
Acylated OVA was efficiently taken up by DC2.4 cells compared with native OVA (Fig. 3), and the uptake was mediated by SR (Fig. 4). SR family has been divided into six classes15 and most SR can bind a variety of anionic macromolecules, including modified low density lipoproteins (LDL), polynucleotides, and negatively charged proteins. It has been reported that SR are involved in the cross-presentation of antigen from apoptotic (CD36)12 and live (SR-A)14 cells by DC and that of maleylated antigen by macrophages and B cells.5 Moreover, SR-, especially lectin-like oxidized LDL receptor-1 (LOX-1)-mediated uptake and cross-presentation of heat-shock protein-antigen conjugates by DC has been recently reported,13 thereby supporting the usefulness of SR-mediated antigen delivery to DC. In this study, which SR are expressed on the surface of DC2.4 cells and involved in the uptake of acylated OVA was not examined in detail although it is of particular interest. The distinct, but partly overlapping, binding properties of the SR classes represent a complication in defining their respective activity in terms of ligand uptake. Therefore, the biological response after the recognition of acylated OVA might be unpredictable. However, this approach can also be efficacious in in vivo vaccination, since OVA-specific CTL were efficiently induced after subcutaneous injection of acylated OVA in mice (Yamasaki et al. manuscript in preparation). We speculated that LOX-1 might be involved in the uptake of acylated OVA because the inhibition profile shown (Fig. 4) was consistent with that of LOX-1.13 In fact, expression of LOX-1 on the surface of DC2.4 cells was confirmed by immunostaining (data not shown). However, the details remain to be elucidated.
After SR-mediated endocytosis by DC2.4 cells, enhanced presentation of antigenic peptides SIINFEKL on MHC class I molecules to CD8OVA1.3 T hybridoma cells was observed in the case of Mal-OVA and Aco-OVA, while presentation of Suc-OVA was inefficient (Fig. 5). Native and acylated OVA taken up by DC were accumulated in endocytic compartments (Fig. 2), and under acidic conditions, maleyl and cis-aconityl, but not succinyl groups on lysine residues in OVA were removed by hydrolysis (Fig. 1a). Thus, it appears that deacylation of Mal-OVA and Aco-OVA occurs in endosomes or lysosomes and that Suc-OVA remains unhydrolysed in such compartments. Reduced presentation of Suc-OVA is thought to be attributed to this chemical stability in the acidic compartments. Direct introduction of acylated OVA into the cytosol resulted in a lower response of CD8OVA1.3 T cells than that of native OVA (Fig. 6), supporting this speculation.
The speculation was further confirmed using chloroquine, an inhibitor of endosomal acidification. MHC class I presentation of exogenous native OVA by DC2.4 cells was markedly augmented by chloroquine treatment and the level of MHC class I presentation of Aco-OVA was reduced by this drug (Fig. 7), probably as a consequence of suppression of deacylation in the acidic compartments. It is conceivable that enhanced cross-presentation of native OVA was caused by the inhibitory effect of chloroquine on proteolysis by cathepsins, which require an acidic environment for activity. This finding also indicates that proteolysis of OVA in the endosomal/lysosomal compartments is not required for the cross-presentation by DC and that the processing takes place in the cytosol. Rather, endocytic degradation is likely to abrogate the cross-presentation. Chloroquine has been reported to slightly enhance MHC class I presentation of exogenous bead-conjugated OVA by DC2.4 cells,18 although the data on native OVA were not available. The difference in sensitivity to chloroquine between native and bead-conjugated OVA can be explained by the difference in size, because transport of internalized antigen to the cytosol within DC is size-selective.35
The cytosolic pathway for cross-presentation of native and acylated OVA within DC was examined using lactacystin, a proteasome inhibitor, and brefeldin A, an inhibitor of anterograde ER-Golgi transport. MHC class I presentation of exogenous native OVA and Aco-OVA by DC2.4 cells was inhibited by both drugs (Fig. 8), suggesting that internalized antigen in the endosomal/lysosomal compartments are delivered to the cytosol and presented on MHC class I molecules via the conventional pathway. It has been reported that in cross-presentation of Mal-OVA by macrophages, processing takes place in endosomes or lysosomes, not in the cytosol.5 These two results probably reflect the type of APC used in each study, because antigen transport to the cytosol is restricted to DC35 and the endosomal MHC class I presentation pathway is exclusively involved in the case of macrophages.8,20
In summary, exogenous Mal-OVA and Aco-OVA are efficiently taken up by DC via SR, deacylated in endosomes or lysosomes and transferred to the cytosol. The deacylated Mal-OVA and Aco-OVA in the cytosol are then processed via the conventional cytosolic pathway, which results in enhanced MHC class I presentation. Although DC also efficiently take up Suc-OVA by SR-mediated endocytosis, conceivably the succinyl groups on lysine residues are not removed since Suc-OVA is chemically stable under acidic conditions. As a result, even if transported to the cytosol, Suc-OVA induces a poor response of CD8OVA1.3 T-cell hybridomas. Possible reasons for this are that succinyl groups inhibit ubiquitin conjugation on lysine residues which is thought to be important in proteasomal degradation36 and that succinylated SIINFEKL cannot be recognized by CD8OVA1.3 cells through TCR, since lysine residue on SIINFEKL is important for TCR recognition.37,38
In conclusion, the present study reveals that efficient cross-presentation can be achieved by antigen delivery to DC by chemical modification. In applications involving various types of tumour antigen, cis-aconitylation might be superior to maleylation, because cis-aconitylation can introduce two minus charges per amino group and therefore it can give antigen an affinity for SR with less modification than maleylation.39 These findings should provide useful information for optimizing the design of in vivo cancer immunotherapy strategies based on the targeting of antigens in a soluble form to DC.
Acknowledgments
We thank Dr Kenneth L. Rock (University of Massachusetts Medical Center, Worcester, MA) for providing DC2·4 cells and Dr Clifford V. Harding (Case Western Reserve University, Cleveland, OH) for providing CD8OVA1.3 T hybridoma cells.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- DC
dendritic cell
- SR
scavenger receptor
References
- 1.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–50. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo. implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 3.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal P, Mukherjee P, Basu SK, George A, Bal V, Rath S. MHC class I-restricted presentation of maleylated protein binding to scavenger receptors. J Immunol. 1999;162:4430–7. [PubMed] [Google Scholar]
- 6.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med. 1996;184:1179–84. doi: 10.1084/jem.184.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 9.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 10.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 11.Regnault A, Lankar D, Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–80. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 14.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–9. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 15.Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000;20:1860–72. doi: 10.1161/01.atv.20.8.1860. [DOI] [PubMed] [Google Scholar]
- 16.Takakura Y, Fujita T, Furitsu H, Nishikawa M, Sezaki H, Hashida M. Pharmacokinetics of succinylated proteins and dextran sulfate in mice: Implications for hepatic targeting of protein drugs by direct succinylation via scavenger receptors. Int J Pharm. 1994;105:19–29. [Google Scholar]
- 17.Haberland ME, Fogelman AM. Scavenger receptor-mediated recognition of maleyl bovine plasma albumin and the demaleylated protein in human monocyte macrophages. Proc Natl Acad Sci USA. 1985;82:2693–7. doi: 10.1073/pnas.82.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
- 19.Okada N, Saito T, Mori K, et al. Effects of lipofectin-antigen complexes on major histocompatibility complex class I-restricted antigen presentation pathway in murine dendritic cells and on dendritic cell maturation. Biochim Biophys Acta. 2001;1527:97–101. doi: 10.1016/s0304-4165(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 21.Butler PJG, Hartley BS. Maleylaion of amino groups. Methods Enzymol. 1972;25:191–9. doi: 10.1016/S0076-6879(72)25016-9. [DOI] [PubMed] [Google Scholar]
- 22.Schoen P, Corver J, Meijer DK, Wilschut J, Swart PJ. Inhibition of influenza virus fusion by polyanionic proteins. Biochem Pharmacol. 1997;53:995–1003. doi: 10.1016/s0006-2952(96)00876-3. [DOI] [PubMed] [Google Scholar]
- 23.Habeeb AF. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966;14:328–36. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 24.Monsigny M, Roche AC, Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51:187–96. doi: 10.1111/j.1768-322x.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 25.Hnatowich DJ, Layne WW, Childs RL. The preparation and labeling of DTPA-coupled albumin. Int J Appl Radiat Isot. 1982;33:327–32. doi: 10.1016/0020-708x(82)90144-2. [DOI] [PubMed] [Google Scholar]
- 26.Duncan JR, Welch MJ. Intracellular metabolism of indium-111-DTPA-labeled receptor targeted proteins. J Nucl Med. 1993;34:1728–38. [PubMed] [Google Scholar]
- 27.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki Y, Sumimoto K, Nishikawa M, Yamashita F, Yamaoka K, Hashida M, Takakura Y. Pharmacokinetic analysis of in vivo disposition of succinylated proteins targeted to liver nonparenchymal cells via scavenger receptors: importance of molecular size and negative charge density for in vivo recognition by receptors. J Pharmacol Exp Ther. 2002;301:467–77. doi: 10.1124/jpet.301.2.467. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K. The structure and function of ribonuclease T1. XXIII. Inactivation of ribonuclease T1 by reversible blocking of amino groups with cis-aconitic anhydride and related dicarboxylic acid anhydrides. J Biochem (Tokyo) 1977;81:641–6. doi: 10.1093/oxfordjournals.jbchem.a131499. [DOI] [PubMed] [Google Scholar]
- 30.Kindberg GM, Magnusson S, Berg T, Smedsrod B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990;270:197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–96. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 32.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanger NA, Wardwell K, Shen L, Tedder TF, Guyre PM. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol. 1996;157:541–8. [PubMed] [Google Scholar]
- 35.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 36.Grant EP, Michalek MT, Goldberg AL, Rock KL. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–8. [PubMed] [Google Scholar]
- 37.Jameson SC, Bevan MJ. Dissection of major histocompatibility complex (MHC) and T cell receptor contact residues in a Kb-restricted ovalbumin peptide and an assessment of the predictive power of MHC-binding motifs. Eur J Immunol. 1992;22:2663–7. doi: 10.1002/eji.1830221028. [DOI] [PubMed] [Google Scholar]
- 38.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–50. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamasaki Y, Hisazumi J, Yamaoka K, Takakura Y. Efficient scavenger receptor-mediated hepatic targeting of proteins by introduction of negative charges on the proteins by aconitylation: the influence of charge density and size of the proteins molecules. Eur J Pharm Sci. 2003;18:305–12. doi: 10.1016/s0928-0987(03)00021-6. [DOI] [PubMed] [Google Scholar]