Abstract
The objective of this study was to determine whether a DNA vaccine encoding bovine CD154 linked to a truncated version of bovine herpesvirus-1 (BHV-1) glycoprotein D (tgD-CD154) induces enhanced tgD-specific immune responses in cattle. In vitro characterization demonstrated that tgD and tgD-CD154 both bind to cultured bovine B cells, whereas only tgD-CD154 induces interleukin-4-dependent proliferation, suggesting that tgD-CD154 specifically binds the CD40 receptor and induces receptor signalling. Calves were immunized with plasmid encoding either tgD or tgD-CD154 by intradermal injection with a needle-free device. After two immunizations, tgD-specific immune responses were observed in both vaccinated groups and after challenge with BHV-1 these responses further increased. Animals immunized with plasmid encoding tgD-CD154 had significantly higher tgD-specific serum titres of immunoglobulins G and A but significantly lower numbers of tgD-specific interferon-γ-secreting cells than animals immunized with plasmid encoding tgD after BHV-1 challenge. This suggests that the expression of an antigen as a chimeric protein with CD154 can qualitatively alter immune responses in cattle. Since we previously showed that plasmid encoding tgD-CD154 induces significantly enhanced secondary tgD-specific antibody responses in sheep, there appear to be interspecies differences in the immune responses induced by tgD-CD154, which suggests that both proteins in the chimeric molecule may influence protein targeting and the induction of an immune response.
Keywords: antigen presentation, BHV-1, bovine CD154, DNA vaccines, glycoprotein
Introduction
Bovine herpes virus-1 (BHV-1) causes respiratory and genital infections in cattle and predisposes the animals to fatal secondary bacterial infections. Currently available vaccines against BHV-1 include live viral vaccines and subunit vaccines. DNA vaccines may be a suitable alternative over both types of vaccines as they are non-infectious, relatively inexpensive, easy to construct, stable during transport and storage, and pose no risk of establishing latency.
One of our long-term goals is to develop strategies for effective DNA immunization of large animal species. As an approach to achieve this, we have focused on creating a DNA vaccine that protects cattle against BHV-1 infection. DNA vaccines have been extremely successful in inducing strong humoral and cellular immune responses in mice. However, similar vaccine efficacy is not observed in large animals. This does not appear to be because of the plasmid itself, but rather to inefficient delivery methods resulting in poor transfection of the plasmid in vivo. Several strategies have been used to improve the immunogenicity of DNA vaccines.1–3 These include (i) utilization of plasmid-encoded cytokines,4 (ii) targeting of antigen to antigen-presenting cells (APCs) with molecules such as cytotoxic T-lymphocyte antigen-4, l-selectin,5 or CD154,6 (iii) the addition of immunostimulatory CpG sequences, and (iv) facilitating effective presentation through major histocompatibility complex class I7 and class II8 pathways. We have focused on the interaction between the CD40 receptor and its ligand, CD154, to enhance immune responses against one of the glycoproteins of BHV-1, glycoprotein D (gD).
Glycoprotein D, one of the antigens present in the viral envelope, is involved in virus penetration9 and induces apoptosis in activated lymphocytes.10 In comparison to other BHV-1 glycoproteins, gD is most effective at providing protection against BHV-1 infection.11 Advances in plasmid design and optimization of immunization parameters have resulted in the induction of strong gD-specific cellular immune responses by gD-expressing plasmids in cattle.12–15 However, significant improvements in the kinetics and magnitude of the antibody responses have yet to be accomplished. Since effective antibody and cellular immune responses are both required for viral clearance, gD-based DNA vaccines have not been completely effective in preventing clinical disease and virus shedding in BHV-1-challenged cattle.14,15
CD40–CD154 interactions play a crucial role in the development and regulation of immune responses.16 CD40 has been identified on a wide variety of cells including B cells, macrophages, dendritic cells (DCs), fibroblasts and epithelial cells, while CD154 is transiently expressed on activated T cells, basophils, mast cells and platelets.16 Biological consequences of CD40-CD154 signalling that can influence induction of an immune response include induction of DC migration to lymph nodes,17 enhancement of DC survival18 and increased efficiency of T-cell priming.19 Other biological responses to CD40 signalling include increased ability of vascular endothelial cells to bind leucocytes20 and enhanced cytokine production by skin fibroblasts21 and monocytes.22,23 CD40 signalling has a significant impact on B-cell selection, because cross-linking of the B-cell receptor (BCR) and CD40 induces B cells to express a phenotype similar to that of germinal centre B cells.24 The use of CD154 as an adjuvant for DNA vaccines has been described previously. Co-injection of plasmids that express murine CD154 and either β-gal25,26 or HSV-2 gD27 enhanced antigen-specific antibody and cellular immune responses in mice. Bovine CD154 has been cloned and sequenced28 and the kinetics and expression of CD154 on bovine T cells29 and its role in bovine B-cell development and differentiation30,31 have been determined.
In a previous study, we utilized the targeting approach to direct effectively a plasmid-encoded antigen to APCs. A plasmid encoding a truncated, secreted version of BHV-1 gD (tgD), linked to bovine CD154, was constructed. Sheep immunized with plasmid encoding tgD-CD154 showed a significant increase in tgD-specific antibody responses in comparison to animals immunized with plasmid encoding tgD.6 We hypothesized that CD154 linked to tgD might have enhanced antigen binding to CD40-expressing APCs, resulting in efficient antigen uptake, presentation and more rapid induction of an immune response. CD154 may also have functioned as an immunological adjuvant through increased DC activation.
The objective of this study was to investigate whether enhanced tgD-specific immune responses can be induced in cattle upon immunization with plasmid encoding tgD-CD154 and if such responses can protect the animals against a BHV-1 challenge.
Materials and methods
Generation of DNA constructs
All restriction enzymes were purchased from Amersham Pharmacia (Baie d'sUrfe, QC). The construction of pSLIAtgD [6372 base pairs (bp)]32 and pSLIAtgD-CD154 (7104 bp)6 has been described previously. To create pSLKIAtgD-CD154 for immunization of cattle, the coding sequence of ampicillin was replaced with the BspHI fragment containing the coding sequence of the kanamycin gene. Plasmids were grown in Escherichia coli DH5α and purified by anion exchange resin (Qiagen, Mississauga, ON), followed by Triton-X-114 (Sigma, Oakville, ON) extraction33 to reduce endotoxin levels to < 10 endotoxin units (< 10 pg/ml of DNA). The DNA concentration was assessed spectrophotometrically, and constructs were confirmed by restriction enzyme digestion and agarose gel electrophoresis. The plasmids used for immunization of cattle were tested for expression of the respective proteins by transient transfection and radioimmunoprecipitation.
Transfection and production of recombinant proteins
All cell culture incubations were performed at 37° in a humidified, 5% CO2 atmosphere unless otherwise specified. To obtain tgD and tgD-CD154 for in vitro assays, Cos-7 cells were cultured at a concentration of 2·5 × 105 cells/well in six-well plates (Corning, Corning, NY) in Dulbecco's modified Eagle's medium (Canadian Life Technologies, Burlington, ON) with 10% fetal bovine serum (FBS) (Canadian Life Technologies). Subsequently, the cells were transfected with pSLIAtgD or pSLIAtgD-CD154 using Lipofectamine Plus and Lipofectamine Reagents (Canadian Life Technologies) according to the manufacturer's instructions and cultured in Optimem (Canadian Life Technologies) for 36 hr. Culture supernatants were then harvested, concentrated 10-fold using Biomax Filters (Millipore, Bedford, MA) and analysed by Western blotting to confirm protein expression by both plasmid constructs.
Flow cytometry
Monoclonal antibodies (mAbs) specific for bovine CD3 [Clone MMAI; immunoglobulin G1 (IgG1)] on T cells, IgM (Clone PIg45A; IgG2b) on B cells, or CD172a on monocytes (Clone DH59B; IgG1) were purchased from VMRD Inc. (Pullman, WA). Fluorescein isothiocyanate (FITC) -conjugated goat IgG specific for murine IgG1, and phycoerythrin (PE) -conjugated goat IgG specific for murine IgG2a and IgG2b were purchased from Southern Biotechnology (Birmingham, AL). Glycoprotein d-specific mAb (Clone 1G6; IgG2a) was obtained as described earlier.34 Single labelling of bovine peripheral blood mononuclear cells (PBMCs) and bovine B cells and dual labelling of bovine PBMCs was performed by incubating the cells with medium or Cos-7 cell supernatants containing tgD or tgD-CD154 for 30 min at 4°. The cells were washed in phosphate-buffered saline (PBS) before incubation for 30 min at 4° with 1G6 for single labelling, or 1G6 and antibodies to various cell surface molecules for dual labelling. After three washes, appropriate isotype-specific, PE- and FITC- conjugated goat anti-mouse IgG (Southern Biotechnology) were added, and the cells were further incubated for 30 min at 4°. After a final wash, the cells were fixed with 2% paraformaldehyde until analysed. All samples were analysed with a FACScan (Becton Dickinson, Mountain View, CA) flow cytometer and the cell quest program (Becton Dickinson) was used for data acquisition and analysis. Non-specific mAb binding was quantified with isotype-matched, irrelevant mAbs (Sigma) and 5000 events were analysed for each sample.
Interleukin-4 bioassay
The interleukin-4 (IL-4) bioassay was performed with B cells isolated from bovine PBMCs using the same protocol described for ovine B cells.35 Briefly, bovine PBMCs were grown for 2–3 weeks in the presence of recombinant human IL-4 (Peprotech EC, London, UK) and murine cells expressing membrane-bound CD154.35 The resulting cell population was > 95% sIgM+ B cells as determined by flow cytometry. Fifty microlitres containing 8 × 105 bovine B cells per ml and Cos-7 cell supernatants containing tgD, tgD-CD154, or medium were incubated for 72 hr with 100 μl of medium or recombinant human IL-4 at 10 ng/ml in a U-bottom microtitre plate (Nalgene International, Rochester, NY). Methyl-[3H]thymidine (Amersham Pharmacia) was added at 0·4 μCi/well during the last 16 hr of culture prior to harvesting. Incorporation of [3H]thymidine was measured using a liquid scintillation counter (Top Count; Packard Instruments, Downer's Grove, IL).
DNA immunization and challenge of cattle
Twenty-one 8–9-month-old BHV-1 seronegative Hereford and Angus cross-bred calves were randomly allocated into three experimental groups (n = 7 in each group) and immunized with saline (group I); pSLKIAtgD (group II), or pSLKIAtgD-CD154 (group III). The vaccines were administered intradermally (i.d.) in the hip with a needle-free delivery device, a biojector (Biojector Medical Technologies, Portland, OR). Vaccinated animals received 500 μg plasmid per immunization. A secondary immunization was performed in the same manner 5 weeks later. At 3 weeks post-secondary immunization, the calves were challenged with BHV-1. All calves were moved to an isolation pen, weighed and examined clinically, and then individually exposed for 4 min to an aerosol of 1 × 107 plaque-forming units/ml of BHV-1 strain 108, generated by a Devilbis Nebulizer, model 099 HC (Devilbis, Barrie, ON). Shedding of infectious virus in nasal fluids was monitored throughout the course of infection. The attending veterinarian, who was unaware of the identity of the treatment groups, clinically evaluated the calves for 10 days post-infection. Evaluation consisted of daily body weight measurements, assessment of rectal temperatures and a visual examination for nasal lesions. The experiment was conducted in accordance with the guidelines provided by the Canadian Council on Animal Care.
Enzyme-linked immunosorbent assay (ELISA)
To test tgD-specific antibody production, polystyrene microtitre plates (Immulon IV, Dynatech Laboratories, Inc., Alexandria, VA) were coated overnight at 4° with 0·05 μg/well of tgD. Plates were washed four times in PBST (PBS with 0·05% Tween-20; Sigma) prior to the addition of four-fold dilutions of bovine sera prepared in PBST. Affinity-purified alkaline phosphatase (AP)-conjugated goat anti-bovine IgG (Kirkegaard and Perry Laboratories) diluted 1 : 5000 in PBST was used to detect IgG. To detect IgA, rabbit anti-bovine IgA Veterinary and Medical Research and Development (VMRD) was biotinylated and added at a dilution of 1 : 1000, followed by streptavidin-AP (Canadian Life Technologies) diluted 1 : 2000. Murine antibodies recognizing bovine IgG1 (provided by Dr K. Nielson, Canada Food Inspection Agency, Ottawa, ON) or IgG2 (Clone K1924F10; IgG1; Cedarlane Laboratories Ltd, Hornby, ON) diluted 1 : 40 000 and 1 : 400, respectively, followed by affinity-purified AP-conjugated goat anti-mouse IgG (Kirkegaard and Perry Laboratories) diluted 1 : 5000 were used to detect IgG1 and IgG2. After 1 hr at room temperature, plates were washed and reactions were visualized with 0·01 mp-nitrophenyl phosphate (Sigma-Aldrich). Absorbances were read on a model 3550 Microplate Reader (Bio-Rad Laboratories) at 405 nm with a reference wavelength of 490 nm. The OD reading (+ 2SD) corresponding to a known reciprocal dilution of standard positive control serum was used as the cut-off value to calculate the ELISA titres.
ELISPOT assay
Blood was collected into EDTA-treated vacutainers (Becton Dickinson), and PBMCs were isolated using Ficoll-Paque PLUS (Pharmacia) and resuspended in minimum essential medium (Canadian Life Technologies) containing 10% FBS (Canadian Life Technologies), 2 mm l-glutamine (Canadian Life Technologies), 50 μg/ml gentamycin and 5 × 10−5 mm 2-mercaptoethanol. Nitrocellulose plates (Millipore) were coated overnight at 4° with 100 μl of bovine interferon-γ (IFN-γ) -specific mAb (clone 2-2-136) diluted 1 : 1500 in sterile coating buffer. The plates were washed in PBST to remove unbound antibody and 1 × 106 PBMCs were cultured in triplicate wells for 24 hr in the presence of 1 μg/ml gD. After an overnight incubation at 37°, the plates were washed and incubated for 2 hr at room temperature with bovine IFN-γ-specific rabbit serum36 diluted 1 : 1500 in PBST with 5% gelatin (Sigma; PBST-g). Subsequently, the plates were incubated for 2 hr at room temperature with biotinylated rat anti-rabbit IgG (Zymed, San Francisco, CA) diluted 1 : 1500 in PBST-g, followed by 2 hr at room temperature with streptavidin-AP (Canadian Life Technologies) diluted 1 : 1000 in PBST-g. The spots were visualized with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Sigma) substrate. After 30–60 min at room temperature, the plates were washed in double-distilled water and air-dried before counting the number of stained spots in the wells. The number of antigen-specific IFN-γ-secreting cells was expressed as the difference between the number of spots per 106 cells in gD-stimulated wells and the number of spots per 106 cells in non-stimulated wells.
Virus neutralization assay
Virus neutralization assays were performed as described previously.11 Madin Darby bovine kidney (MDBK) cells were cultured overnight in 96-well tissue culture plates (Becton Dickinson) to obtain a confluent monolayer. Bovine sera were serially diluted three-fold and known BHV-1 high-positive, low-positive and negative sera were used as controls. The Cooper strain of BHV-1 at 103 plaque-forming units/ml was diluted 1 : 1 with 100 μl of each serum sample and allowed to incubate at 37° for 1 hr. The serum–virus mixture was then added to duplicate MDBK cell cultures and incubated for 48 hr. The plates were stained with crystal violet to visualize viral plaques before counting and virus neutralization titres were expressed as the reciprocal of the highest dilution of serum that caused a 50% reduction in plaques relative to the virus control.
Virus isolation
Nasal secretions were collected on days 0, 2, 4, 6, 8 and 10 post-challenge using tampons. Virus was recovered from the nasal fluids and quantified by plaque titration in microtitre plates with an antibody overlay as described.37 Briefly, MDBK cells were cultured overnight in 96-well tissue culture plates (Becton Dickinson) to obtain a confluent monolayer. Nasal fluids from cattle were serially diluted 10-fold in culture medium and allowed to incubate at 37°. After 1 hr, the medium was removed and an overlay consisting of minimum essential medium, 2% FBS and 1 : 250 diluted standard BHV-1 hyperimmune serum, was added to each well and further incubated at 37° for 48 hr. The plates were then stained with crystal violet to visualize the plaques before counting. The virus titre was expressed as the number of plaque-forming units per ml of nasal fluid.
Statistical analyses
All data were analysed with the aid of a statistical software program (systat 10·0; SPSS Inc., Chicago, IL). One-way analysis of variance with Bonferroni's post-test was used to measure the differences between selected groups. Prior to performance of analyses, data were transformed by log transformation to obtain normally distributed data.
Results
In vitro expressed tgD-CD154 binds to bovine PBMCs and induces a tgD-specific proliferative response
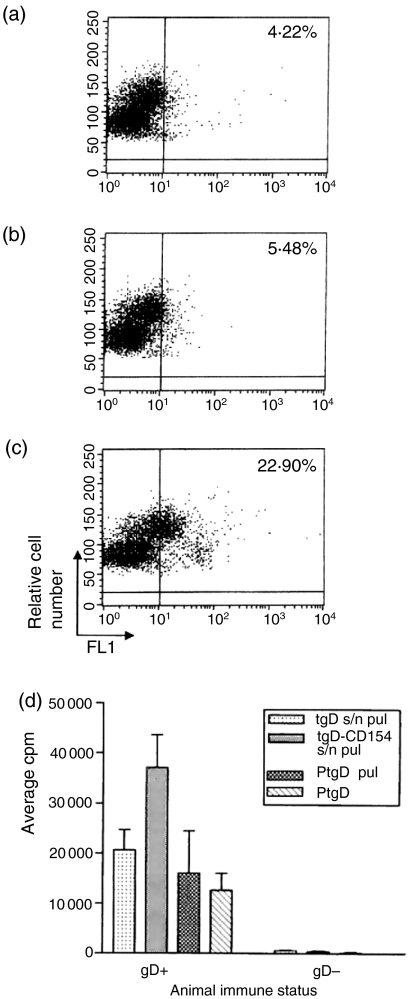
We previously showed that in vitro expressed tgD and tgD-CD154 both bind to bovine epithelial cells.6 To evaluate binding of tgD and tgD-CD154 to leucocytes, PBMCs were isolated and incubated with mock-, pSLIAtgD-, or pSLIAtgD-CD154-transfected Cos-7 cell culture supernatants and analysed by flow cytometry. There was some background binding of mock-transfected supernatant (4·22%; Fig. 1a) and slightly higher binding of supernatant containing tgD (5·48%; Fig. 1b) to the bovine leucocytes. Furthermore, there was substantially increased binding of supernatant containing tgD-CD154 (∼22·90%; Fig. 1c). To determine if the binding of tgD-CD154 results in the processing and presentation of tgD to T cells, we subsequently compared the proliferative responses of PBMCs isolated from naïve (tgD–) or tgD-immunized (tgD+) cattle after pulsing or incubation with tgD or tgD-CD154. PBMCs from tgD+ animals but not tgD– animals proliferated when incubated with pure tgD, pulsed with pure tgD, or pulsed with cell culture supernatant containing either tgD or tgD-CD154 (Fig. 1d). The proliferative responses of the cells pulsed with transfected cell supernatants containing tgD-CD154 were two-fold higher than the responses of the cells cultured with pure tgD, or pulsed with pure tgD or tgD in cell culture supernatants. Therefore, these observations support the conclusion that, while tgD can bind to PBMCs and augment gD-specific proliferation, these responses appeared to be enhanced when tgD is linked to CD154.
Figure 1.
Antigen-specific lymphocyte proliferation induced by binding of tgD or tgD-CD154 to bovine PBMCs. Supernatants from (a) mock-transfected, (b) pSLIAtgD-transfected, or (c) pSLIAtgD-CD154-transfected Cos-7 cells were incubated with bovine PBMCs. Bound protein was detected with a gD-specific mAb cocktail, followed by FITC-conjugated goat anti-mouse immunoglobulin. (d) PBMCs were isolated from naïve (tgD–) and tgD immunized (tgD+) cattle (n=3) and incubated for 30 min at 4° with either pSLIAtgD (tgD s/n pul) or pSLIAtgD-CD154 (tgD-CD154 s/n pul) transfected Cos-7 cell supernatants or with 3 μg/well of purified tgD (PtgD pul). Subsequently, PBMCs were washed twice and 2 × 105 cells/well were cultured for 72 hr. As a positive control, 2 × 105 PBMCs/well were also cultured for 72 hr with 3 μg of purified tgD (PtgD). Cell proliferation was detected by adding methyl-[3H]thymidine during the last 8 hr of culture. Data are shown as average c.p.m.+SEM for triplicate cultures.
We then identified which PBMC subpopulation could bind tgD and tgD-CD154. Only monocytes bound tgD, but both monocytes and B cells bound tgD-CD154 (Table 1). These results indicate that CD154 enhanced tgD targeting to monocytes (∼10-fold increase) and greatly enhanced tgD targeting to B cells.
Table 1.
Binding of tgD and tgD-CD154 to blood leucocyte population*
| % protein binding† | ||
|---|---|---|
| tgD | tgD-CD154 | |
| T cells | 0·5 | 0·7 |
| B cells | 0·0 | 46·5 |
| Monocytes | 1·2 | 11·6 |
| Total | 1·7 | 58·8 |
Dual labelling was performed by first incubating PBMCs with medium or transfected culture supernatants containing tgD or tgD-CD154, and then with a mixture of gD-specific mAb 1G6 and antibodies to various cell surface molecules. Isotype-specific goat anti-mouse immunoglobulin conjugated to either FITC or PE was used to detect anti-gD and anti-leucocyte mAbs.
Calculated based on the formula cells positive for tgD + cell marker/cells positive for cell marker.
Values obtained after subtracting autofluorescence following incubation with medium alone.
In vitro expressed tgD and tgD-CD154 bind cultured bovine B cells but only tgD-CD154 induces IL-4-dependent proliferation
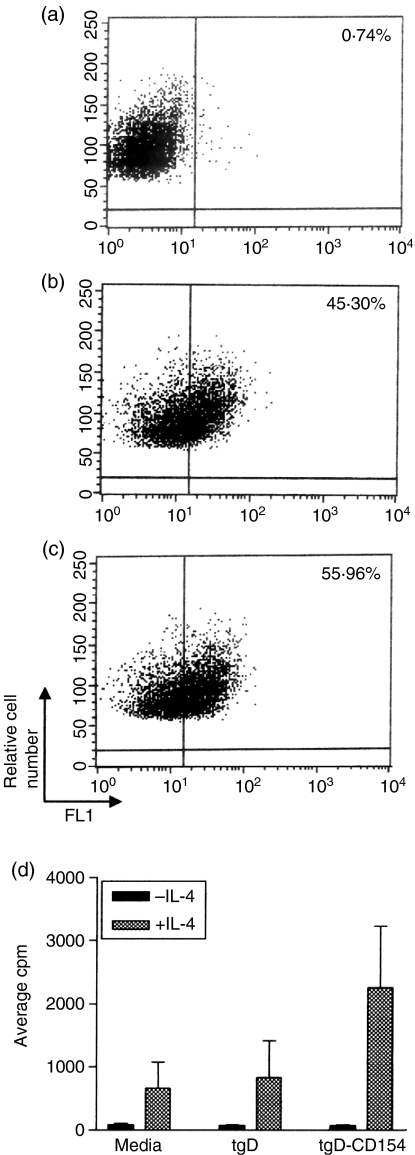
CD40 signalling can induce IL-4-dependent proliferation of cultured B cells.35 This signalling may be mediated by various forms of CD154 including the membrane-associated, trimeric soluble and dimeric forms of CD154. We have previously shown that plasmid encoded tgD-CD154 exists as monomers and dimers.6 Flow cytometric analysis (Table 1) indicated that tgD-CD154 primarily binds to bovine B cells in a population of PBMCs. Therefore, we compared the binding of tgD and tgD-CD154 by cultured bovine B cells and further investigated whether binding induced CD40 signalling. A culture of bovine B cells was established (∼95% IgM+) and incubated with mock-transfected cell supernatans or transfected cell supernatants containing tgD or tgD-CD154. Flow cytometric analysis revealed similar levels of B-cell binding by tgD (Fig. 2b) and tgD-CD154 (Fig. 2c). Increased binding of tgD by isolated cultures of B cells in comparison to B cells in the PBMC population may be the result of the activated state of the B cells, which has been previously associated with an increased expression of gD-specific receptors on B cells.10 However, only the pSLIAtgD-CD154-transfected Cos-7 cell supernatants induced increased B-cell proliferative responses in the presence of recombinant human IL-4 (Fig. 2d). These results support the conclusion that tgD-CD154 specifically binds the CD40 receptor and induces receptor signalling.
Figure 2.
Induction of IL-4 responsiveness following tgD-CD154 binding to bovine B cells. Supernatants from (a) mock-transfected, (b) pSLIAtgD-transfected, or (c) pSLIAtgD-CD154-transfected Cos-7 cells were incubated with bovine B cells. Bound protein was detected with a gD-specific mAb cocktail, followed by FITC-conjugated goat anti-mouse immunoglobulin. (d) Triplicate cultures of bovine B cells (4 × 104 cells/well) were incubated with medium or with pSLIAtgD- or pSLIAtgD-CD154 transfected Cos-7 cell supernatants. Cultures were incubated for 72 hr with either 100 μl/well of 10 ng/ml recombinant human IL-4 (+IL-4) or medium (–IL-4). Cell proliferation was detected by adding methyl-[3H]thymidine during the last 18 hr of culture. Data are shown as average c.p.m. + SEM for triplicate cultures.
Immunization of calves with pSLKIAtgD-CD154 induces reduced tgD-specific cellular immune responses
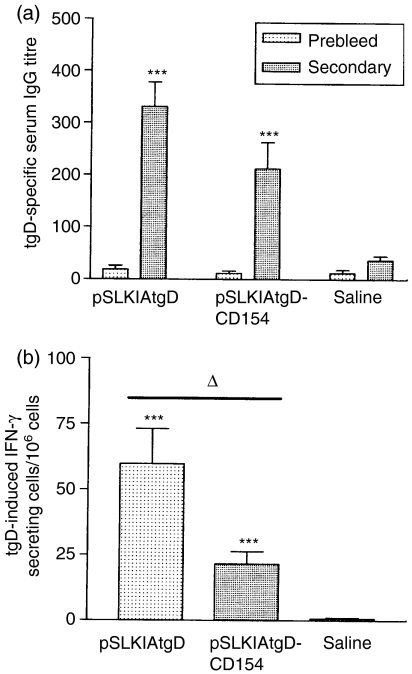
Since immunization of sheep with plasmid encoding tgD-CD154 resulted in significantly enhanced tgD-specific humoral immune responses compared to vaccination with pSLKIAtgD, we investigated if a similar augmentation of immune responses was induced in cattle. After two immunizations, pSLKIAtgD- and pSLKIAtgD-CD154-vaccinated calves displayed significantly higher tgD-specific serum IgG titres than animals injected with saline (P<0·001; Fig. 3a). Although the tgD-specific serum IgG titres tended to be lower in calves immunized with pSLKIAtgD-CD154 than in animals immunized with pSLKIAtgD, this difference was not statistically significant. Both vaccinated groups also developed significantly higher numbers of tgD-specific IFN-γ-secreting cells in the blood when compared to animals injected with saline (P<0·001: Fig. 3b). However, calves immunized with pSLKIAtgD-CD154 tended to develop weaker T-cell proliferation (data not shown) and had significantly lower numbers of tgD-specific IFN-γ-secreting cells in comparison to calves immunized with pSLKIAtgD (P<0·05; Fig. 3b). This suggests that the T-cell responses are lower in the pSLKIAtgD-CD154-immunized calves. To confirm that these responses were induced by the vaccine and not by prior exposure to BHV-1, antibody levels specific for glycoprotein C (gC) were measured in all calves. None of the calves had gC-specific antibody titres prior to challenge (data not shown).
Figure 3.
Immune responses induced in calves by immunization with plasmids encoding tgD or tgD-CD154. Three groups (n=7) of calves were injected intradermally with saline, pSLKIAtgD or pSLKIAtgD-CD154. Responses were measured prior to primary immunization (prebleed) and 3 weeks post-secondary immunization (secondary). (a) tgD-specific mean IgG titres+SEM in sera. The OD reading (+2SD) corresponding to a known reciprocal dilution of standard positive control serum was used as the cut-off value to determine the ELISA titres. (b) Mean number of tgD-induced IFN-γ-secreting cells+SEM in blood. Values were calculated based on the difference between the number of spots per 106 cells in gD-stimulated wells and the number of spots per 106 cells in non-stimulated wells. Asterisks (***P<0·001) indicate significance of differences from the saline injected group. Open triangle (▵, P<0·05) indicates significance of difference between pSLKIAtgD- and pSLKIAtgD-CD154-immunized groups.
Calves immunized with pSLKIAtgD-CD154 develop higher tgD-specific serum antibody responses after challenge
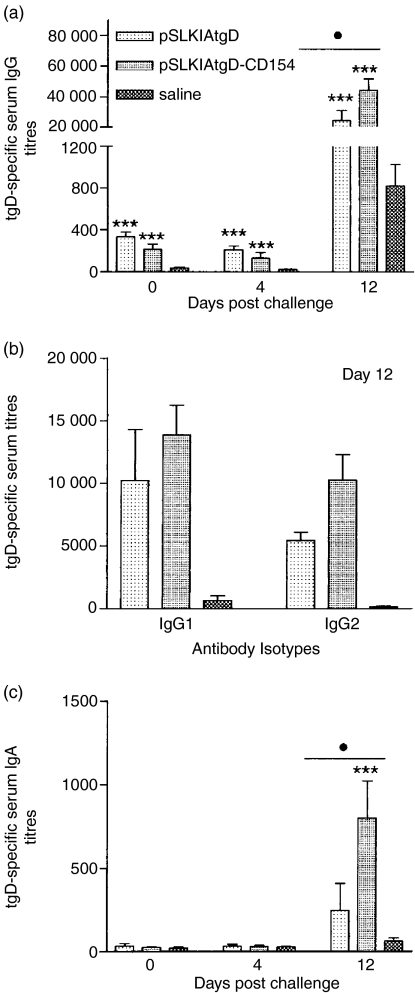
Although the immunogenicity has generally been poor in outbred species, DNA vaccines can effectively prime antigen-specific immune responses.38,39 Therefore, we next investigated the immune responses following challenge with BHV-1. Truncated gD-specific serum IgG titres were significantly higher in both vaccinated groups in comparison to the saline-injected group on days 4 and 12 post-challenge (P<0·001; Fig. 4a). In addition, the tgD-specific IgG titres of the pSLKIAtgD-CD154-immunized calves were significantly higher than the titres of the pSLKIAtgD-immunized animals (P<0·05) on day 12 post-challenge. Consistent with this observation, the tgD-specific IgG1 and IgG2 titres were also higher in the pSLKIAtgD-immunized animals than in the pSLKIAtgD-vaccinated calves, but the differences were not significant (Fig. 4b). No significant serum IgA titres were detected in any of the groups prior to challenge or post-challenge on day 4, but pSLKIAtgD-CD154 immunized animals had significantly higher titres than both pSLKIAtgD-immunized (P < 0·05) and saline-injected (P < 0·001) animals on day 12 post-challenge (Fig. 4c).
Figure 4.
Antigen-specific IgG and IgA titres in sera of calves after BHV-1 challenge. Three groups (n = 7) of calves were injected intradermally with saline, pSLKIAtgD or pSLKIAtgD-CD154. Truncated gD-specific (a) mean IgG titres + SEM, (b) mean IgG1 and IgG2 titres+SEM and (c) mean IgA titres + SEM. The OD reading (+2SD) corresponding to a known reciprocal dilution of standard positive control serum was used as the cut-off value to determine the ELISA titres. Asterisks (***P < 0·001) indicate significance of differences from the saline injected group. Closed circle (•, P < 0·05) indicates significance of differences between the pSLKIAtgD- and pSLKIAtgD-immunized groups. Two animals injected with saline died on days 11 and 12 post-challenge.
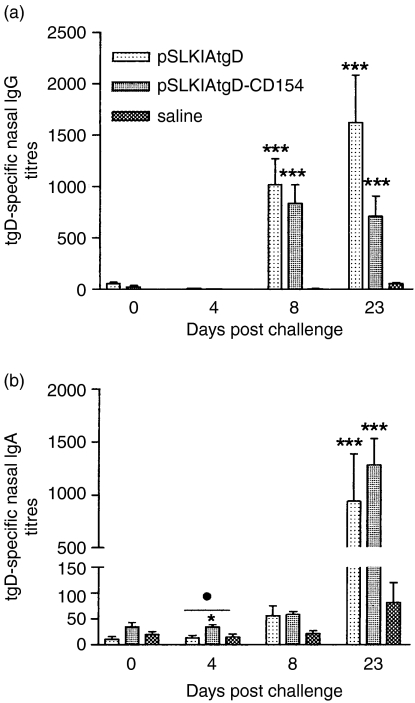
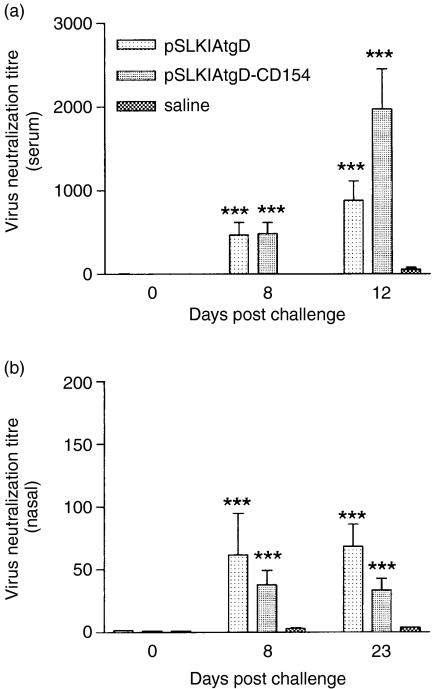
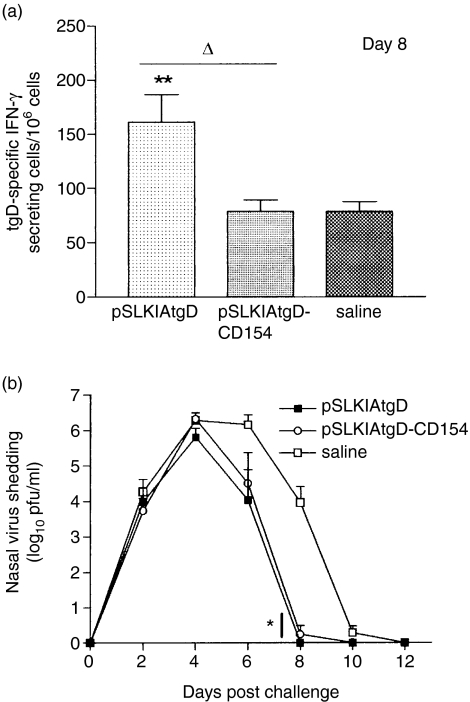
Before challenge there was no tgD specific nasal IgG or IgA in either the pSLKIAtgD or the pSLKIAtgD-CD154 vaccinated group (Figs 5a,b). In contrast, both vaccinated groups had tgD-specific IgG titres in nasal secretions on days 8 and 23 post-challenge, which were significantly higher than the titres of the saline-injected group (P < 0·001; Fig. 5a). Animals immunized with pSLKIAtgD-CD154 also had significantly higher tgD-specific nasal IgA titres than both pSLKIAtgD- and saline-immunized animals on day 4 post-challenge (P < 0·05; Fig. 5b). However, no biological advantage of the enhanced IgA titres in the pSLKIAtgD-CD154 immunized animals was observed. Both vaccinated groups had significantly higher IgA titres than the saline group on day 23 post-challenge (P < 0·001; Fig. 5b). Virus neutralization by serum and nasal secretions was similar for both vaccinated groups and significantly stronger when compared to the saline group on days 8 and 23 post-challenge (P < 0·001; Fig. 6). With regards to the cellular immune responses, animals immunized with pSLKIAtgD had significantly higher numbers of tgD-specific IFN-γ-secreting cells than both pSLKIAtgD-CD154-immunized and saline-injected animals on day 8 post-challenge (P < 0·01; Fig. 7a).
Figure 5.
Antigen-specific IgG and IgA titres in nasal secretions of calves following BHV-1 challenge. Three groups (n + 7) of calves were injected intradermally with saline, pSLKIAtgD or pSLKIAtgD-CD154. Truncated gD-specific (a) mean IgG titres + SEM, (b) mean IgA titres + SEM. The OD reading (+ 2SD) corresponding to a known reciprocal dilution of standard positive control serum was used as the cut-off value to determine the ELISA titres. Asterisks (***P < 0·01; *P < 0·05) indicate significance of differences from the saline injected group. Closed circle (•, P < 0·05) indicates significance of difference from the pSLKIAtgD-immunized group. Two animals injected with saline died on days 11 and 12 post-challenge.
Figure 6.
Virus neutralization titres in sera and nasal secretions of cattle after BHV-1 challenge. Virus neutralization titres of (a) sera and (b) nasal secretions. Mean titres + SEM are expressed as the reciprocal of the highest dilution of serum that caused a 50% reduction in viral plaques relative to the virus control. Asterisks (***P<0·001) indicate significance of differences from the saline group. Two animals injected with saline died on days 11 and 12 post-challenge.
Figure 7.
Cellular immune responses and virus shedding in cattle after BHV-1 challenge. Three groups (n = 7) of calves were injected intradermally with saline, pSLKIAtgD or pSLKIAtgD-CD154. (a) tgD-specific mean number of IFN-γ-secreting cells + SEM, 8 days post-challenge. Values were calculated based on the difference between the number of spots per 106 cells in gD-stimulated wells and the number of spots per 106 cells in non-stimulated wells. (b) Mean virus shedding + SEM in nasal secretions of calves challenged with BHV-1. Asterisks (**P < 0·01, *P < 0·05) indicate significance of differences with saline-injected group. Open triangle (▵, P < 0·05) indicates significance of differences between pSLKIAtgD- and pSLKIAtgD-CD154-immunized groups. Two animals injected with saline died on days 11 and 12 post-challenge.
These results indicate that both vaccine constructs primed for effective tgD-specific immune responses following BHV-1 infection. However, significant differences in antibody and cellular immune responses between the vaccinated groups suggest possible differences in the priming of the immune responses between pSLKIAtgD-CD154- and pSLKIAtgD-immunized calves.
pSLKIAtgD- and pSLKIAtgD-CD154-immunized animals develop similar clinical signs of disease after BHV-1 challenge
Infected calves were monitored daily for clinical signs of disease by measuring rectal temperatures and body weights, and by examining the nasal passages for lesions. The cumulative temperature gains and weight loss after challenge were similar and no significant differences were observed among the groups (data not shown). The pattern of virus shedding was also similar in all groups, with the peak of virus shedding on day 4 post-challenge. All vaccinated animals showed a decrease in virus shedding on day 6 and significantly lower virus shedding on day 8 post-challenge when compared to animals injected with saline (P < 0·05; Fig. 7b). There were no differences in virus shedding between the vaccinated groups at any time post-infection. These results suggest that the anamnestic responses induced in pSLKIAtgD- and pSLKIAtgD-CD154-immunized animals resulted in more rapid clearance of virus when compared to saline-injected animals.
Discussion
DNA vaccines offer potential advantages over conventional vaccines in terms of economy and simplicity of vaccine production. However, improving the immunogenicity of DNA vaccines still remains a major challenge. Antigen targeting to APCs represents one of the various approaches developed to enhance immune responses to plasmid-encoded antigens.
In a previous study, we utilized bovine CD154 to target a soluble version of BHV-1 tgD to CD40-expressing APCs. Our preliminary experiment conducted in sheep revealed that plasmid expressing tgD linked to bovine CD154 induced significantly higher tgD-specific antibody titres when compared to plasmid expressing tgD.6 This was an encouraging observation, as it is a challenge to induce satisfactory antibody responses in large animals by DNA immunization. In the present study, we investigated whether we could elicit similar responses in cattle, because enhanced tgD-specific antibody responses may greatly aid the protection of cattle upon a BHV-1 challenge. Unfortunately, biojector-mediated immunization of cattle with plasmid expressing tgD-CD154 did not result in significantly higher tgD-specific antibody titres in comparison to immunization with plasmid expressing tgD. Reasons for the discrepancy between the results in sheep and cattle may be two-fold. First, antibody responses have generally been shown to be dependent on the plasmid dose and it is possible that in this study, inadequate amounts of plasmids were administered or inefficient transfection occurred using the biojector, resulting in limited antigen expression. This would indicate that further optimization is required for delivery of DNA vaccines using the biojector in cattle. Secondly, tgD bound to receptors on a number of bovine cells in vitro, including epithelial cells and activated B cells, in contrast to activated ovine B cells, which did not bind tgD present in transfected Cos-7 cell supernatants.6 This suggests that BHV-1 gD may recognize fewer receptors in sheep than in cattle or perhaps recognize the ovine cellular receptors at very low affinity. Indeed, differences in utilizing species-specific receptors have previously been reported for BHV-1, where human and rat forms of CD155 (PvR, Tage4), but not the mouse homologue, have facilitated entry of BHV-1 (reviewed in ref. 40). The competitiveness between tgD and CD154 in binding to their respective receptors apparently was not strong enough to cause an effect in the sheep, but may have reduced the targeting efficiency in the calves. These conflicting results between the model and target systems, even though very closely related, stress the importance of performing the experiments in the natural host of the pathogen.
DNA vaccines encoding tgD administered by intradermal needle injection have been shown to induce significant tgD-specific antibody titres and high numbers of IFN-γ-secreting cells.15 In our study, although the tgD-specific IgG titres were comparable between the groups immunized with plasmids encoding tgD or tgD-CD154, the numbers of tgD-specific IFN-γ-secreting cells were significantly lower in the pSLKIAtgD-CD154-vaccinated group in comparison to the group immunized with pSLKIAtgD, prior to challenge. The proliferative responses of the calves immunized with plasmid encoding tgD-CD154 also tended to be lower than the responses of the animals immunized with plasmid encoding tgD (data not shown). This observation parallels our earlier observations in sheep, where the proliferative responses induced by plasmid encoding tgD also tended to be lower than those elicited by plasmid encoding tgD-CD154. These results would argue that there may be qualitative differences in the immune responses induced by tgD and tgD-CD154.
The anticipated increase in tgD-specific antibody titres in the pSLKIAtgD-CD154 group, however, appeared after challenge of the animals with BHV-1. One of the interesting differences between the groups immunized with plasmids encoding tgD and tgD-CD154 was that the latter group showed significantly higher levels of not only tgD-specific serum IgG, but also IgA, post-challenge, while the numbers of tgD-specific IFN-γ-secreting cells still remained significantly lower than in the group immunized with plasmid encoding tgD. It may be argued that the herpesvirus infection in itself can cause the production of a wide variety of cytokines that may have influenced the enhanced production of the tgD-specific antibodies in animals immunized with plasmid encoding tgD-CD154. However, if such an effect exists, similar increases in IgA and IgG titres should have been observed in pSLKIAtgD immunized animals. Furthermore, as there was no significant difference in virus shedding between the vaccinated groups, the difference in tgD-specific IgG and IgA titres between the groups is not likely to be the result of enhanced virus replication, but is possibly the result of differences in the ability of tgD and tgD-CD154 to prime the immune responses. However, because we vaccinated systemically, the nasal antibodies are probably a transudate from the serum. IgG2 appeared to be the antibody isotype that is most enhanced, so this might not have translated in enhanced transudate and therefore not affected nasal IgG levels or virus shedding.
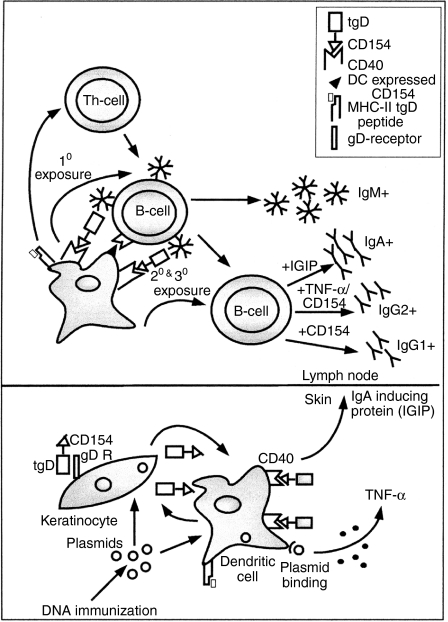
The role of T cells in the induction of an immune response in animals immunized with plasmid encoding tgD-CD154 cannot be ruled out because of the presence of higher numbers of IFN-γ-secreting T cells compared to the saline-injected animals prior to challenge. However, the enhanced tgD-specific IgG and IgA titres in animals immunized with tgD-CD154 and challenged with BHV-1, without increased T-cell proliferation or numbers of IFN-γ-secreting cells, suggest that tgD-CD154 may induce an immune response that is partially independent of T cells41,42(Fig. 8). This line of reasoning agrees with recent results published by Bajer et al.43 who have demonstrated a role of bovine peripheral blood DCs in eliciting a T helper type 2-biased response in the absence of a polarized T helper type 2 cell population.
Figure 8.
A model to illustrate priming/induction of immune responses by tgD-CD154 upon DNA immunization.
In our study, the expression of tgD-CD154 upon intradermal immunization can result in binding to CD40-expressing DCs present in the skin. This binding of tgD-CD154 on DCs may provide the necessary signals for up-regulation of various costimulatory molecules, including CD15444 and rapid migration of DCs to the secondary lymphoid organs, where the DCs may directly prime tgD-specific IgM+ B cells independent of T cells (Fig. 8). Upon subsequent immunizations, a similarly activated DC may also participate in switching the IgM+ B cells to other isotypes. It has been shown that DCs stimulated by CD154 can induce B cells to secrete IgG1.43 As well, DCs stimulated by CD154 in the presence of IFN-α can induce B cells to secrete IgG2.43,45 In our study, IFN-α may be produced by DCs stimulated by the plasmid backbone, because bacterial DNA has been shown to induce the production of type I IFNs by DCs.46 Furthermore, CD154 stimulation of DCs has also been shown to induce the production of IgA-inducing protein (IGIP), which has been responsible for promoting the isotype switching from IgM+ to IgA+ B cells.47 This mechanism of inducing immune responses may be different from that elicited by tgD, which most likely induces a T-cell-dependent immune response. The mechanism described above and shown in Fig. 8 may have not been very efficient after DNA vaccination as a result of the poor transfection efficiency of DNA vaccines resulting in low levels of antigen. However, the persistent and enhanced amounts of viral gD present after challenge may have effectively expanded the DC-primed, tgD-specific IgG1-, IgG2- and IgA-secreting B-cell population in animals immunized with plasmid encoding tgD-CD154, resulting in augmented IgG and IgA production.
In summary, the success of targeting proteins with CD154 or any other ligand to receptors on APCs may be influenced by the presence of receptors that can bind the vaccine antigen. The inability to observe enhanced immune responses in cattle following DNA immunization with plasmid encoding tgD-CD154 may be because of the general limitation of achieving adequate antigen expression in large animals with current delivery methods and/or of the competition of tgD and CD154 to bind to their respective receptors. Indeed, to our knowledge, there is no evidence yet for effective targeting of a protective antigen to APCs in vivo in the natural host. Nevertheless, the presence of CD154 did affect the immune responses induced. With improvements in the delivery of DNA vaccines to large animals, we believe that the use of CD154 to target antigens that do not bind multiple receptors may facilitate induction of immune responses that can enhance disease protection in animals.
Acknowledgments
We thank Brenda Karvonen, Laura Latimer, Tamela King and Reno Pontarollo for their expert technical assistance, Donna Dent for providing with biotinylated anti-bovine IgA and Terry Beskorwayne for performing FACS analyses. We also want to thank Barry Carroll, Tracy Bruneau, Jamie Mamer, Brock Evans, Don Wilson and Carolyn Olson for animal care. L.A.B. is holds a Canada research chair in vaccinology.
References
- 1.Saffery R, Choo KH. Strategies for engineering human chromosomes with therapeutic potential. J Gene Med. 2002;4:5–13. doi: 10.1002/jgm.236. [DOI] [PubMed] [Google Scholar]
- 2.Pachuk CJ, McCallus DE, Weiner DB, Satishchandran C. DNA vaccines – challenges in delivery. Curr Opin Mol Ther. 2000;2:188–98. [PubMed] [Google Scholar]
- 3.Babiuk LA, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21:649–58. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 4.Pasquini S, Xiang Z, Wang Y, He Z, Deng H, Blaszczyk-Thurin M, Ertl HC. Cytokines and costimulatory molecules as genetic adjuvants. Immunol Cell Biol. 1997;75:397–401. doi: 10.1038/icb.1997.62. [DOI] [PubMed] [Google Scholar]
- 5.Boyle JS, Brady JL, Lew AM. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408–11. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 6.Manoj S, Griebel PJ, Babiuk LA, Van Drunen Littel-Van Den Hurk S. Targeting with bovine CD154 enhances humoral immune responses induced by a DNA vaccine in sheep. J Immunol. 2003;170:989–96. doi: 10.4049/jimmunol.170.2.989. [DOI] [PubMed] [Google Scholar]
- 7.Leachman SA, Shylankevich M, Slade MD, et al. Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. J Virol. 2002;76:7616–24. doi: 10.1128/JVI.76.15.7616-7624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch N, van Driel IR, Gleeson PA. Hijacking a chaperone: manipulation of the MHC class II presentation pathway. Immunol Today. 2000;21:546–50. doi: 10.1016/s0167-5699(00)01717-5. [DOI] [PubMed] [Google Scholar]
- 9.Liang X, Pyne C, Li Y, Babiuk LA, Kowalski J. Delineation of the essential function of bovine herpesvirus 1 gD. an indication for the modulatory role of gD in virus entry. Virology. 1995;207:429–41. doi: 10.1006/viro.1995.1102. [DOI] [PubMed] [Google Scholar]
- 10.Hanon E, Keil G, van Drunen Littel-van den Hurk S, Griebel P, Vanderplasschen A, Rijsewijk FA, Babiuk L, Pastoret PP. Bovine herpesvirus 1-induced apoptotic cell death: role of glycoprotein D. Virology. 1999;257:191–7. doi: 10.1006/viro.1999.9620. [DOI] [PubMed] [Google Scholar]
- 11.van Drunen Littel-van den Hurk S, Parker MD, Massie B, van den Hurk JV, Harland R, Babiuk LA, Zamb TJ. Protection of cattle from BHV-1 infection by immunization with recombinant glycoprotein gIV. Vaccine. 1993;11:25–35. doi: 10.1016/0264-410x(93)90336-v. [DOI] [PubMed] [Google Scholar]
- 12.Loehr BI, Rankin R, Pontarollo R, King T, Willson P, Babiuk LA, van Drunen Littel-van den Hurk S. Suppository-mediated DNA immunization induces mucosal immunity against bovine herpesvirus-1 in cattle. Virology. 2001;289:327–33. doi: 10.1006/viro.2001.1143. [DOI] [PubMed] [Google Scholar]
- 13.Pontarollo RA, Babiuk LA, Hecker R, Van Drunen Littel-Van Den Hurk S. Augmentation of cellular immune responses to bovine herpesvirus-1 glycoprotein D by vaccination with CpG-enhanced plasmid vectors. J General Virol. 2002;83:2973–81. doi: 10.1099/0022-1317-83-12-2973. [DOI] [PubMed] [Google Scholar]
- 14.Braun RP, Babiuk LA, Loehr BI, van Drunen Littel-van den Hurk S. Particle-mediated DNA immunization of cattle confers long-lasting immunity against bovine herpesvirus-1. Virology. 1999;265:46–56. doi: 10.1006/viro.1999.0032. [DOI] [PubMed] [Google Scholar]
- 15.van Drunen Littel-van den Hurk S, Braun RP, Lewis PJ, et al. Intradermal immunization with a bovine herpesvirus-1 DNA vaccine induces protective immunity in cattle. J General Virol. 1998;79:831–9. doi: 10.1099/0022-1317-79-4-831. [DOI] [PubMed] [Google Scholar]
- 16.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 17.Moodycliffe AM, Shreedhar V, Ullrich SE, Walterscheid J, Bucana C, Kripke ML, Flores-Romo L. CD40–CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J Exp Med. 2000;191:2011–20. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esche C, Gambotto A, Satoh Y, Gerein V, Robbins PD, Watkins SC, Lotze MT, Shurin MR. CD154 inhibits tumor-induced apoptosis in dendritic cells and tumor growth. Eur J Immunol. 1999;29:2148–55. doi: 10.1002/(SICI)1521-4141(199907)29:07<2148::AID-IMMU2148>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 20.Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissoan MC, Van Kooten C, Chomarat P, Galibert L, Durand I, Thivolet-Bejui F, Miossec P, Banchereau J. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol. 1995;25:1125–8. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 23.Wagner DH, Jr, Stout RD, Suttles J. Role of the CD40–CD40 ligand interaction in CD4+ T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur J Immunol. 1994;24:3148–54. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- 24.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza RB, Cantwell MJ, Kipps TJ. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J Immunol. 1997;159:5777–81. [PubMed] [Google Scholar]
- 26.Gurunathan S, Irvine KR, Wu CY, Cohen JI, Thomas E, Prussin C, Restifo NP, Seder RA. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161:4563–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Sin JI, Kim JJ, Zhang D, Weiner DB. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum Gene Ther. 2001;12:1091–102. doi: 10.1089/104303401750214302. [DOI] [PubMed] [Google Scholar]
- 28.Mertens B, Muriuki C, Gaidulis L. Cloning of two members of the TNF-superfamily in cattle: CD40 ligand and tumor necrosis factor alpha. Immunogenetics. 1995;42:430–1. doi: 10.1007/BF00179409. [DOI] [PubMed] [Google Scholar]
- 29.Hirano A, Brown WC, Trigona W, Tuo W, Estes DM. Kinetics of expression and subset distribution of the TNF superfamily members CD40 ligand and Fas ligand on T lymphocytes in cattle. Vet Immunol Immunopathol. 1998;61:251–63. doi: 10.1016/s0165-2427(97)00155-4. [DOI] [PubMed] [Google Scholar]
- 30.Estes DM, Brown WC, Hirano A. CD40 ligand-dependent signaling of bovine B lymphocyte development and differentiation. Vet Immunol Immunopathol. 1998;63:15–20. doi: 10.1016/s0165-2427(98)00077-4. [DOI] [PubMed] [Google Scholar]
- 31.Haas KM, Estes DM. Activation of bovine B cells via surface immunoglobulin M cross-linking or CD40 ligation results in different B-cell phenotypes. Immunology. 2000;99:272–8. doi: 10.1046/j.1365-2567.2000.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun R, Babiuk LA, van Drunen Littel-van den Hurk S. Compatibility of plasmids expressing different antigens in a single DNA vaccine formulation. J General Virol. 1998;79:2965–70. doi: 10.1099/0022-1317-79-12-2965. [DOI] [PubMed] [Google Scholar]
- 33.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–46. [PubMed] [Google Scholar]
- 34.Hughes G, Babiuk LA, van Drunen Littel-van den Hurk S. Functional and topographical analyses of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103:47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griebel P, Ferrari G. CD40 signalling in ileal Peyer's patch B cells: implications for T cell-dependent antigen selection. Int Immunol. 1995;7:369–79. doi: 10.1093/intimm/7.3.369. [DOI] [PubMed] [Google Scholar]
- 36.Raggo C, Habermehl M, Babiuk LA, Griebel P. The in vivo effects of recombinant bovine herpesvirus-1 expressing bovine interferon-gamma. J General Virol. 2000;81:2665–73. doi: 10.1099/0022-1317-81-11-2665. [DOI] [PubMed] [Google Scholar]
- 37.van Drunen Littel-van den Hurk S, Gifford GA, Babiuk LA. Epitope specificity of the protective immune response induced by individual bovine herpesvirus-1 glycoproteins. Vaccine. 1990;8:358–68. doi: 10.1016/0264-410x(90)90095-4. [DOI] [PubMed] [Google Scholar]
- 38.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–5. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 39.Loehr BI, Pontarollo R, Rankin R, Latimer L, Willson P, Babiuk LA, van Drunen Littel-van den Hurk S. Priming by DNA immunization augments T-cell responses induced by modified live bovine herpesvirus vaccine. J General Virol. 2001;82:3035–43. doi: 10.1099/0022-1317-82-12-3035. [DOI] [PubMed] [Google Scholar]
- 40.Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi T, Worgall S, Singh R, Moore MA, Crystal RG. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat Med. 2000;6:1154–9. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- 42.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–9. [PubMed] [Google Scholar]
- 43.Bajer AA, Garcia-Tapia D, Jordan KR, Haas KM, Werling D, Howard CJ, Estes DM. Peripheral blood-derived bovine dendritic cells promote IgG1-restricted B cell responses in vitro. J Leukoc Biol. 2003;73:100–6. doi: 10.1189/jlb.0302128. [DOI] [PubMed] [Google Scholar]
- 44.Pinchuk LM, Klaus SJ, Magaletti DM, Pinchuk GV, Norsen JP, Clark EA. Functional CD40 ligand expressed by human blood dendritic cells is up-regulated by CD40 ligation. J Immunol. 1996;157:4363–70. [PubMed] [Google Scholar]
- 45.Estes DM, Tuo W, Brown WC, Goin J. Effects of type I/type II interferons and transforming growth factor-beta on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology. 1998;95:604–11. doi: 10.1046/j.1365-2567.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tascon RE, Ragno S, Lowrie DB, Colston MJ. Immunostimulatory bacterial DNA sequences activate dendritic cells and promote priming and differentiation of CD8+ T cells. Immunology. 2000;99:1–7. doi: 10.1046/j.1365-2567.2000.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin AS, Haas KM, Naugler SM, Bajer AA, Garcia-Tapia D, Estes DM. Identification and characterization of a novel regulatory factor: IgA-inducing protein. J Immunol. 2003;171:1336–42. doi: 10.4049/jimmunol.171.3.1336. [DOI] [PubMed] [Google Scholar]