Abstract
Initiation of an antitumour immune response is characterized by a complex process of chemokine-mediated cell migration and cell–cell interactions. Overexpression of chemokine CCL19 in tumour cells has been shown to result in accelerated tumour rejection under certain experimental conditions, suggesting a novel approach in the therapy of neoplastic malignancies. To investigate CCL19-mediated modulations of cellular immune responses in vivo, we generated a chimeric CCL19-immunoglobulin G2b (IgG2b) Fc fusion protein, which binds to the chemokine receptor CCR7 comparable to native CCL19. CCL19-IgG2b possesses a long-lasting potent chemotactic activity as a result of the extended half-life of Fc fusion proteins. Stable overexpression of CCL19-IgG2b in BALB/c-derived J558L tumour cells fails to support tumour cell rejection following transplantation in syngeneic mice. Moreover, overexpression of CCL19-IgG2b hinders tumour rejection in allogeneic C57BL/6 mice. This phenomenon was accompanied by a six-fold increase of dendritic cells (DCs) isolated from CCL19-IgG2b-secreting tumours when compared to the number of DCs isolated from control parental J558L tumours. While mice bearing the allogeneic parental tumour showed an intense hypercellularity in the draining lymph nodes, no such response could be observed in the draining lymph nodes of mice carrying the CCL19-IgG2b-secreting tumour. We could demonstrate that overexpression of CCL19-IgG2b in tumour cells retains antigen-presenting cells in the tumour mass and prevents DCs from migrating into draining lymph nodes to present antigens and to activate T cells, resulting in an impaired immune response against the tumour.
Keywords: chemokines, lymph nodes, migration: traffic
Introduction
Chemokines represent a family of structurally related chemotactic proteins that selectively bind to seven transmembrane-spanning G protein-coupled receptors.1,2 They may attract leucocytes to sites of inflammation.3 Certain chemokines support immune homeostasis and control the microarchitecture of secondary lymphoid organs in particular.4 A key role in lymphocyte trafficking to secondary lymphoid organs is played by CCL19 and CCL21, which are expressed constitutively in high endothelial venules of lymph nodes and Peyer's patches as well as in the T-cell zones of spleen and lymph nodes.5 Both, CCL19 and CCL21 recognize the CC chemokine receptor CCR7 and exhibit chemotactic activity for T cells, B cells and mature dendritic cells (DCs) but not for monocytes and neutrophils.6,7 These chemokines induce specific homing of naive T cells and DCs into the T-cell zone of secondary lymphoid organs, the place where naive T cells are primed and become activated by DCs. This represents the initial step during an activation process of an antigen-specific immune response. A mutant mouse strain, paucity of lymph node T cells (plt), lacks detectable CCL19 and CCL21 expression in lymphoid organs and consequently exhibits impaired T-cell homing to secondary lymphoid organs.8,9 Comparably, mice lacking CCR7 show a defect in lymph node architecture as a result of impaired migration of immune cells to and within lymphoid organs.10 Both defective expression of the chemokines and defective expression of the receptor resulted in a reduced immune response to peripherally encountered antigens.8–10
Recently, it has been shown that genetically modified tumour cells or DCs expressing CCL19 or CCL21, as well as injection of these chemokines into an established tumour, generate a potent antitumour response in vivo, suggesting therapeutic utilization of CCL19 or CCL21 in an antitumour gene therapy programme.11–14 However, overexpression of the same cytokine may induce both tumour rejection under certain circumstances and tumour progression in another model.15,16 Since the effects of chemokines depend on several parameters, including the chemokine gradient and the microenvironment, as well as the amount and duration of chemokine expression, the outcome in therapeutic applications may be difficult to predict. Therefore, we studied the antitumour immune modulatory effect of CCL19 in more detail. In contrast to previous reports, we show that overexpression of a CCL19-immunoglobulin G2b (IgG2b) fusion protein in J558L plasmocytoma cells leads to immunosuppression and prolonged tumour survival after transplantation in major histocompatibility complex (MHC) mismatched recipients. The fusion protein possesses properties that favour recruitment and localization of host-derived DCs within the tumour site. This was accompanied by absence of an immune reaction within the draining lymph nodes. Thus, overexpression of CCL19-IgG2b within the tumour may prevent antigen-presenting DCs from initiation of an antitumour immune response in secondary lymphoid organs.
Materials and methods
Generation of recombinant CCL19-IgG2b fusion protein
Mouse CCL19 cDNA17 was amplified using reverse-transcribed total spleen cells RNA.18
The forward primer (5′-CTGCCTAAGCTTTCTGCCATGGCCCCCCGT-3′) contained a HindIII restriction site followed by the Kozak sequence upstream of the translational start codon.
The reverse primer (5′-CACATCTCGGATCCCAAGACACAGGGCTCCTTCTG-3′) contained a BamHI restriction site in place of the CCL19 translational stop codon. The polymerase chain reaction product was digested with the restriction enzymes HindIII and BamHI, and cloned into the pcDNA3·1(+) vector (Invitrogen, Groningen, the Netherlands).
This construct was then digested with BamHI/NotI and fused to the BamHI/NotI-digested mouse IgG2b Fc domain, prepared from the interleukin-2 (IL-2) -IgG2b-CDM8 plasmid, described previously.19 This plasmid was used for transfection of COS and J558L cells.
Expression and purification of fusion protein
To obtain larger amounts of CCL19-IgG2b fusion proteins, the baculovirus expression system was used. The CCL19-IgG2b fusion gene was prepared from the CCL19-IgG2b-pcDNA3·1(+) plasmid by NheI/XbaI digest, isolated, and ligated into the NheI/XbaI-digested baculovirus transfer vector pBlueBac4·5 (Invitrogen). The construct was expressed and purified as previously described.20 The fusion protein was purified using Protein A Sepharose as previously described19 and stored at − 20° until use.
Quantification of fusion proteins by enzyme-linked immunosorbent assay
The concentration of the fusion protein was determined by an enzyme-linked immunosorbent assay (ELISA) specific for the murine IgG-Fc domains. Briefly, 96-well microtitre plates (Nunc, Life Technologies, Wiesbaden, Germany) were coated with rabbit α-mouse Fcγ-specific antibody (2·5 μg/ml; dianova, Hamburg, Germany) overnight at 4° and blocked with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). A soluion containing the fusion protein was added in duplicates following incubation with alkaline phosphatase-conjugated rabbit α-mouse Fcγ-specific antibody (0·15 μg/ml; dianova). Each plate also included titrated chrompure mouse IgG (dianova) to obtain a standard curve for quantification. The absorbance was determined after the addition of substrate for the alkaline phosphatase (1 mg p-nitrophenylphosphate/ml diethanolamine buffer; Bio-Rad, Munich, Germany) by an automated ELISA reader at 405 nm. The plates were washed three times between each incubation step.
Cell culture and transfections
J558L is a heavy-chain-loss variant of the plasmacytoma cell line J558 of BALB/c origin. J558L cells express MHC class I and the intercellular adhesion molecule 1 but not detectable MHC class II molecules.16 The human T-cell line HUT78 (American Type Culture Collection, Manassas, VA) expresses endogenously the chemokine receptor CCR7 at high levels.21 Both cell lines were grown in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, streptomycin (0·1 mg/ml) and penicillin (200 U/ml). Monkey kidney COS-7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, and antibiotics. All cultures were maintained at 37° in 5% CO2.
The plasmid CCL19-IgG2b in pcDNA3·1(+) was prepared for transfection using Qiagen MaxiPrep kits (Qiagen, Hilden, Germany). Transient transfection of COS-7 cells using PolyFect reagent (Qiagen) was performed according to the manufacturer's instructions by using 4 μg DNA per 100-mm dish. The recombinant fusion protein (CCL19-IgG2b) was released into the supernatant, which was collected 72 h postinfection.
The plasmid was also stably transfected by electroporation (GenePulser II, Bio-Rad) into the plasmacytoma cell line J558L, according to standard methods.22 After an additional 48 h, the medium was replaced with medium containing 1 mg/ml G418 (AppliChem, Darmstadt, Germany).
Flow cytometry
HUT78 were stained with the recombinant CCL19-IgG2b fusion protein for 30 min at 4° followed by a fluorescein isothiocyanate (FITC)-labelled rabbit α-mouse IgG, Fcγ-specific antibody (dianova).
For the flow cytometric analysis of T cells, B cells and DCs, tumours and lymph nodes were harvested at the indicated time-point, mechanically disrupted and prepared as single cell suspensions. All antibodies used for these experiments were purchased from PharMingen (BD Bioscienes, Heidelberg, Germany). Samples were stained with Cy-Chrome-conjugated antibodies to CD3, peridinin chlorophyll protein (PerCP)-conjugated antibodies to B220, FITC-conjugated antibodies to CD11b and R-phycoerythrin (R-PE)-conjugated antibodies to CD11c.
Flow cytometry was performed on a Coulter Epics XL flow cytometer (Beckman Coulter, Krefeld, Germany).
Migration assay
To test the chemotactic activity of the recombinant CCL19-IgG2b fusion proteins, chemotaxis assays were performed in 5-μm pore Transwells (Costar, Bodenheim, Germany) for 1 h at 37° in 5% CO2. Briefly, 600 μl RPMI-1640 (w/o FCS) supplemented with 1% BSA in the presence of 0–3000 nm CCL19-IgG2b fusion protein (based on the CCL19 part of the whole molecule) were placed in the lower chamber, and 1 × 106 HUT78 cells/100 μl (suspended in RPMI-1640 and 1% BSA) were placed in the upper chamber. The cells that had migrated to the bottom surface were collected after 4 h for each well and counted under a light microscope. All experiments were conducted in triplicate and repeated at least three times.
Determination of mitogen-activated protein kinase (MAPK) activation
Single cell suspensions were generated from spleens. Mouse T-cell enrichment columns (R & D Systems, Wiesbaden, Germany) were used to prepare purified T-cell populations. Cells (107 per dish) were treated for 5 min at 37° with different concentrations (10−7 or 10−6 m) of recombinant CCL19 or CCL19-IgG2b, respectively. Cell lysis was performed as previously described.20 The protein content was normalized and equal amounts of proteins (10 μg/lane) were separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Proteins were transferred to nitrocellulose membrane and analysed by immunoblotting. MAPK activation was visualized using phospho-p44/42-specific MAPK antibody (New England BioLabs, Frankfurt, Germany) and an ELCTM detection system (Amersham, Braunschweig, Germany).
Analysis of tumour growth in vivo
Tumour measurements were performed as described previously.23 Briefly, parental or transfected J558L cells (5 × 106 cells in 0·2 ml PBS) were injected subcutaneously (s.c.) into the abdominal skin of 6–8-week-old female BALB/c (H-2d) and C57BL/6 (H-2b) mice. Tumour size was measured using a dial gauge calliper (Oditest OD 1020T10; Kroeplin, Schlüchtern, Germany) and was recorded as the mean of the largest diameter and the diameter perpendicular to that.
Immunization and measurement of immune response in vivo
To test the delayed type hypersensitivity (DTH) reaction during constitutive CCL19-IgG2b expression, parental or CCL19-IgG2b-transfected J558L cells (5 × 106 cells in 0·2 ml PBS) were injected s.c. into the abdominal skin of 6–8-week-old female C57BL/6 mice. Before establishing the maximal tumour on day 7 these mice were sensitized on day 3 by intravenous injection of 2 × 105 sheep red blood cells (SRBC) in 100 μl PBS as described previously.19 Briefly, mice were challenged 4 days after immunization by injection of 2 × 108 SRBC in 50 μl of PBS intracutaneously into the left hind footpad (specific swelling). Non-immunized mice were challenged with the same dose of SRBC to determine non-specific swelling. Swelling of the footpad was measured 24 and 48 h after challenge with a dial gauge calliper. Results were calculated by subtracting the nonspecific swelling from the specific increment.
Fluorescent labelling of splenocytes
Spleens from syngeneic donor mice (C57BL/6) were harvested and single cell suspensions were prepared and labelled with CFDA-SE (Calbiochem-Novabiochem, Schwalbach, Germany), as previously described.24 Seven days after injection with parental or CCL19-IgG2b-transfected J558L cells, 1 × 107 labelled cells were injected retro-orbitally into anaesthetized recipient animals. The recipients were killed 4 h post injection. Inguinal lymph nodes from these individuals were harvested and incubated simultaneously with PerCP-conjugated rat α-mouse CD45R/B220 antibody (BD Biosciences, Heidelberg, Germany) and Cy5-conjugated rat α-mouse CD3. Migration of labelled cells into the ipsi- and contralateral lymph nodes was quantified by flow cytometry.
Results
Expression and in vitro potency of recombinant CCL19-IgG2b
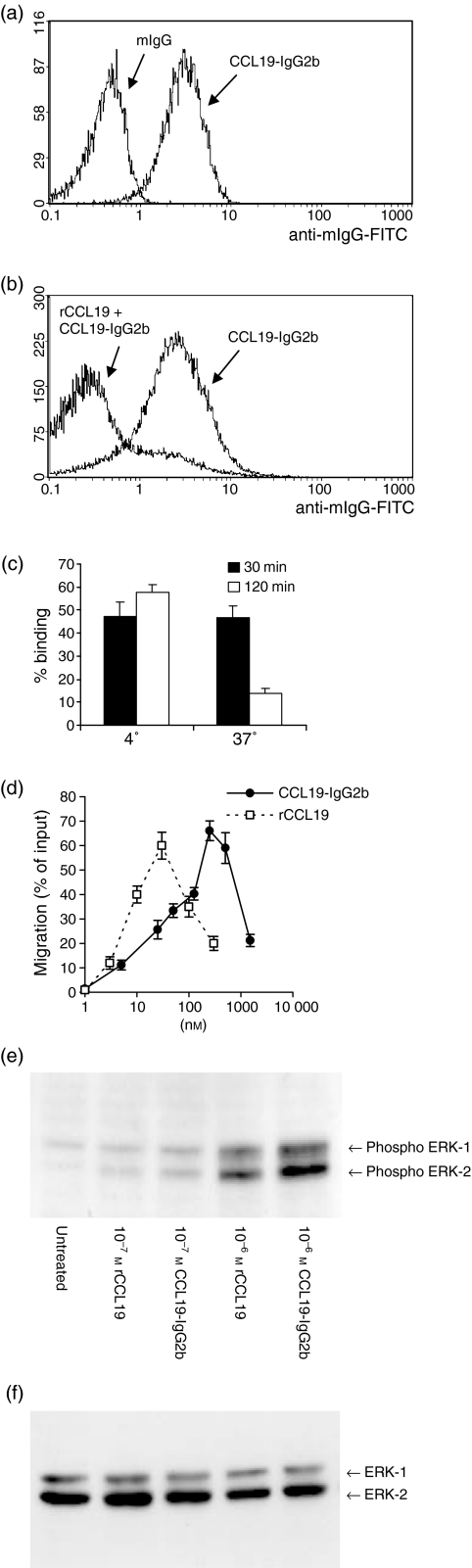
To investigate the immunomodulatory potency of high amounts of CCL19 during immune responses and tumour development, a CCL19-IgG2b chimeric protein was produced by fusing CCL19 to Fc part of mouse IgG2b.19 CCL19-IgG2b-transfected COS or J558L cells as well as infected insect cells produced identical proteins with a relative mass of a 39 000 in the monomeric form and a 78 000 molecular weight biologically active dimer, respectively, as estimated by SDS–PAGE and Coomassie staining (data not shown). The binding of CCL19-IgG2b to CCR7 was examined by flow cytometry using the human T-cell line HUT78.21 Figure 1(a) shows that CCL19-IgG2b binds specifically to CCR7 via the CCL19 domain with no apparent binding of the Fc part of IgG2b. The binding of CCL19-IgG2b to CCR7 was significantly inhibited by pretreatment of HUT78 cells with 100 nm recombinant CCL19 for 30 min, followed by another 30-min incubation with 100 nm CCL19-IgG2b at 4° (Fig. 1b). CCL19-IgG2b did not stain J558L cells (data not shown). As previously demonstrated for native CCL1925 binding of CCL19-IgG2b induced a strong down-regulation of surface CCR7 after incubating cells at 37° (Fig. 1c). Next, the chemotactic responses of CCR7-expressing cells to CCL19-IgG2b were examined in comparison with rCCL19. As shown in Fig. 1(d), HUT78 cells responded to rCCL19 by cell migration in a dose-dependent manner. The chemotaxis induced by recombinant CCL19-IgG2b showed a similar dose–response with an approximately 10-fold lower activity (maximum chemotactic effect at 250 nm CCL19-IgG2b versus 30 nm rCCL19). These results indicated that recombinant CCL19-IgG2b is a specific high-affinity ligand for CCR7.
Figure 1.
Binding of CCL19-IgG2b to CCR7+ HUT78 cells and chemotactic activity. (a) Cells were incubated either with murine IgG (control) or CCL19-IgG2b (100 nm) followed by antimIgG-FITC-antibody. (b) Cells were preincubated with 100 nm recombinant CCL19 before staining with CCL19-IgG2b and anti-mIgG-FITC. (c) CCR7 is down-regulated by binding of CCL19-IgG2b in a temperature-dependent manner. FACS analysis of HUT78 cells after incubation with the CCL19-IgG2b fusion protein for the indicated times. Binding of fusion protein was detected by staining with α-murine IgG-FITC antibody. (d) Chemotactic responses of CCR7-expressing cells to rCCL19 and CCL19-IgG2b. HUT78 cells are stimulated with indicated concentrations of rCCL19, respectively, CCL19-IgG2b by using a 24-well Transwell chemotaxis chamber. The assay was done in triplicate. Shown is the percentage of migrated cells ± SD. (e) Effects of CCL19 on MAPK activation. Freshly isolated murine T cells were treated with rCCL19 or CCL19-IgG2b (10−7 and 10−6 m) at 37° for 5 min. The activity of MAPKs (ERK-2 and ERK-1 are indicated) was measured by specific phosphorylation. (f) As control for sample variations the blot was stripped according to the manufacturer's instructions (Amersham) and reprobed with p44/42 MAPK antibody, which detects total MAPK status independent of its phosphorylation, showing equal protein levels of ERK-2 and ERK-1.
To confirm that the CCL19-IgG2b fusion protein and recombinant CCL19 share the same activation properties the activation of the MAPK pathway was analysed as a downstream signalling event in response to chemokine stimulation of murine CCR7+ lymphocytes. Freshly isolated T cells were stimulated for 5 min with indicated concentrations of recombinant CCL19 or CCL19-IgG2b (10−7 and 10−6 m) lysed and subjected to Western blot analysis for activated ERK-1 and ERK-2, two MAPK-family members which are phosphorylated in response to biologically active CCL19.26 The phosphorylation of ERKs shown in Fig. 1(e) demonstrates that there is no substantial difference in the activation properties of recombinant CCL19 and CCL19-IgG2b.
CCL19-IgG2b does not induce tumour regression in syngeneic BALB/C mice
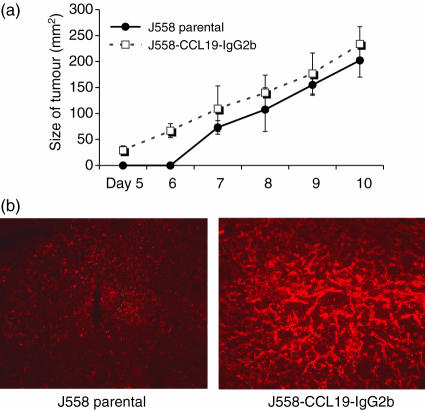
To assess the ability of CCL19 to modulate the antitumour immune response in a syngeneic mouse model the murine plasmacytoma cell line J558L was stably transfected with the CCL19-IgG2b construct. After a 48-h culture period for 106 transfected J558L cells/ml, 50–100 ng/ml of the fusion protein was secreted as determined by ELISA. Non-transfected parental tumour cells did not secrete measurable amounts of mIgG2b. No difference in doubling time was detected between the transfected and parental J558 cells in vitro (data not shown). To assess the tumorigenicity, 106 parental or 106 CCL19-IgG2b-secreting J558L cells were injected subcutaneously into the abdomen of BALB/c mice, and tumour growth was monitored for up to 10 days. As shown in Fig. 2(a), CCL19-IgG2b failed to induce a significant delay of tumour growth or a reduction of final tumour size (CCL19-IgG2b: 233·8 ± 33·2 mm2 versus parental: 202·3 ± 32·3 mm2). As these results are in contrast to the previously described antitumour effects for CCR7 ligands12,27,28 we assessed the possibility that transfected J558L cells lost their ability to secrete CCL19-IgG2b in vivo. Mice were sectioned on day 7, the tumour mass was homogenized, the cells were cultured and the supernatant was analysed by ELISA after 48 h. The secreted amount of fusion protein in these supernatants was in a range identical to that in non-injected cells (data not shown). Furthermore, stable expression of CCL19-IgG2b within J558-CCL19-IgG2b tumours was confirmed by immunohistology (Fig. 2b).
Figure 2.
(a) Constitutive secretion of CCL19-IgG2b does not suppress J558 tumour growth in syngeneic BALB/c mice. 5 × 106 cells/200 μl PBS were injected s.c. in female 6–8-week-old BALB/c mice. The size of tumour was measured using a dial-gauge calliper (largest diameter × diameter at right angle). The groups differed not significantly regarding tumour size. Representative of three independent experiments, mean ± SD. (b) Immunohistochemically detection of CCL19 within the tumour of J558-CCL19-IgG2b-transfected cells documented intratumoral CCL19 expression in vitro. Indirect immunofluorescent staining of CCL19 with 10 μg/ml goat anti-mouse MIP-3β (R & D Systems, Wiesbaden, Germany) using peroxidase-conjugated mouse-anti-goat IgG (dianova) as secondary antibody and detection with tyramide signal amplification direct red (NEN Life Science Products, Boston, MA, USA), according to the manufacturer's instructions. Micrographs are representative of organs from three different recipient mice.
Constitutively secreted CCL19-IgG2b inhibits tumour rejection in allogeneic C57BL\6 mice
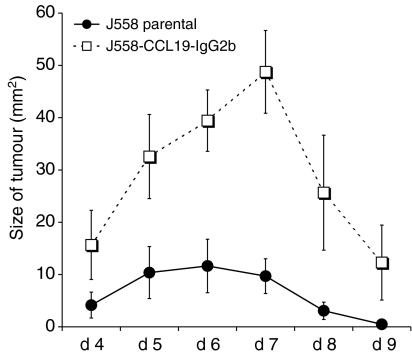
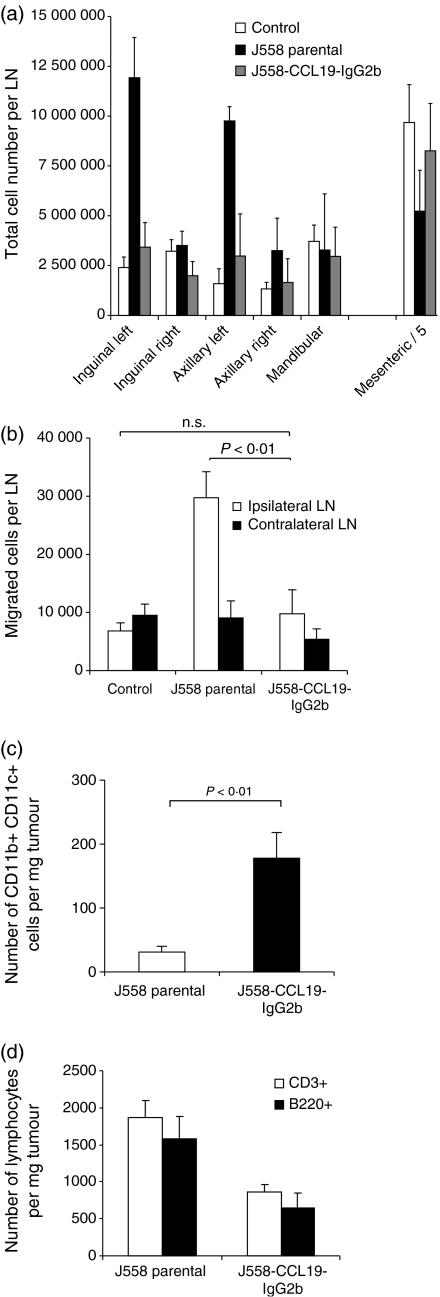
To analyse further the potential immunoregulatory effects of CCL19-IgG2b, either J558L-CCL19-IgG2b cells or J558L cells (5 × 106/200 μl PBS) were injected into allogeneic C57BL/6 mice. As shown in Fig. 3, mice injected with transfected tumour cells showed a maximal tumour growth (49 ± 7 mm2) after 6–7 days. In contrast, in C57BL/6 mice receiving untransfected J558L cells a significantly lower maximum tumour size (10 ± 3 mm2; P = 0·003) was observed. Neither group showed a difference in the occurrence of tumours (day 3–4). Parental as well as transfected tumours were rejected after 10 days on average. Since the immunomodulatory effect of this chemokine fusion protein may affect lymphocyte homing and/or DC migration, the cell content of skin-draining lymph nodes was analysed from tumour-bearing C57BL/6 mice. The ipsilateral, but not the contralateral, inguinal and axillary lymph nodes of mice receiving parental J558L cells s.c. into the left abdomen, were heavily enlarged showing a significantly increased cell content at day 7 (Fig. 4a). The enlargement of the regional lymph nodes as well as increased cell number could not be detected in mice receiving CCL19-IgG2b-secreting tumour cells nor in sham-treated control mice.
Figure 3.
Increased tumour growth of CCL19-IgG2b-secreting J558 cells in allogeneic C57BL/6 mice; 5 × 106 cells/200 μl PBS were injected s.c. in 6–8-week-old mice and tumour size was measured every day. Results are expressed as mean size ± SEM (representative of three independent experiments).
Figure 4.
(a) CCL19-IgG2b-secreting J558 tumours suppress regional lymph node enlargement. Single cell suspensions of the presented lymph nodes were prepared and total cell numbers were counted. Data represent mean total cell numbers per lymph node ± SD (n = 5 mice per group). Numbers of mesenteric cells have been divided by 5. Experiment was performed in duplicate. (b) CCL19-IgG2b-secreting tumours decrease homing of lymphocytes into draining lymph nodes. Splenocytes from syngeneic donor animals were labelled with CFDA SE and injected intravenously into CCL19-IgG2b-secreting J558 tumour or parental J558 tumour-bearing mice on day 7, respectively. Representative results of independent experiments with five recipient animals each are shown. (c) increase of CD11b+CD11c+ DCs within the tumour mass of mice receiving CCL19-IgG2b-transfected tumour cells. Tumours of each group (untransfected J558 cells vs. J558-CCL19-IgG2b-secreting tumours) were harvested 7 days after tumour injection and analysed by FACS for the presence of infiltrated cells. (d) Distribution of infiltrated lymphocytes (CD3+ and B220+ cells) per mass of tumour. Comparison of parental J558 tumour cells vs. J558-CCL19-IgG2b-secreting tumour cells. Tumours of each group were harvested 7 days after tumour injection and analysed by FACS for the presence of infiltrated lymphocytes.
To test whether CCL19-IgG2b-secreting tumours might interfere with lymphocyte homing to draining lymph nodes, splenocytes from untreated syngeneic donor mice (C57BL/6) were labelled with carboxy fluorescein succinimidyl ester (CFSE) and the cells were injected intravenously into C57BL/6 mice that had received parental J558L or CCL19-IgG2b-secreting J558L cells 7 days before. The distribution of transferred cells was evaluated by flow cytometry on single cell suspensions of isolated ipsi- and contralateral lymph nodes. Based on the green fluorescence of CFSE labelling, the absolute cell numbers of transferred splenocytes migrating into lymphoid tissues was determined 4 h after transfer. As shown in Fig. 4(b) an increased migration of labelled donor cells into the ipsilateral lymph nodes was recorded in mice carrying the untransfected parental J558L tumour cells, reflecting the increase in size of this lymph node. In contrast, mice receiving CCL19-IgG2b-secreting tumour cells as well as control (untreated) mice displayed an equal distribution of transferred cells to all lymph nodes examined.
The reduced immune response was accompanied by an altered pattern of cell composition observed within the tumours. Compared to tumours established by untransfected J558L cells, CCL19-IgG2b-secreting tumours displayed a six-fold increase of CD11b+ CD11c+ DCs per tumour mass (Fig. 4c). In addition, CCL19-IgG2b-secreting tumours exhibited a reduced infiltration of CD3+ and B220+ lymphocytes compared to the parental tumours (Fig. 4d). Immunohistological analyses did not reveal any differences with regard to the localization of tumour-infiltrating T cells, B cells, or DCs caused by CCL19-IgG2b expression (data not shown).
Local secretion of CCL19-IgG2b does not influence the systemic immune response
We aimed to analyse whether CCL19-IgG2b-secreting tumours might extend their immunosuppressive effects systemically by inducing a delayed-type hypersensitivity (DTH) response in tumour-bearing mice. Therefore, parental or CCL19-IgG2b-secreting J558L cells were injected s.c. into C57BL/6 mice. Three days later both groups were sensitized by an intravenous injection of SRBC and challenged on day 7. The recombinant CCL19 fusion protein secreted by J558L cells failed to suppress a DTH reaction in vivo, further demonstrating a locally restricted action of the tumour-expressed chemokine (data not shown).
Discussion
Antigen-engaged mature DCs move to the T-cell zone of secondary lymphoid organs along a chemotactic gradient of CCL19 and CCL21. Recirculating naive T cells are also instructed by CCL19 and CCL21 to enter secondary lymphoid organs, and consequently encounter antigen-presenting DCs. Thus, the constitutive expression of CCL19 and CCL21 in a discrete microenvironment provides a mechanism for the colocalization of mature antigen-presenting DCs and naive T cells since both share the chemokine receptor CCR7. Colocalization of DCs and naive T cells is required to initiate an adaptive immune response by T-cell receptor engagement. This initial step has been targeted for antitumour gene therapy by local expression of CCL19 within the tumour. Since local expression or local injection of CCL19 into the tumour has been shown effectively to induce an antitumour immune response in certain models14,27,28 modulation of this initial step of the immune response has been suggested to be of therapeutic value. However, the expression level of cytokines after gene transfer in tumours is poorly controlled. High levels of cytokine expression may not necessarily induce a stronger antitumour immune response, the opposite might occur.
Our study aimed to analyse the influence of locally expressed CCL19-IgG2b fusion proteins on an antitumour immune response. This molecule possesses the biological functions of the CCL19 moiety and contains the prolonged circulating half-life of cytokine-immunoglobulin fusion proteins. In vitro, CCL19-IgG2b and native CCL19 were equally potent on a molar basis to activate MAPK, whereas CCL19 exhibited a 10-fold stronger chemotactic potency. However, in contrast to the very short half-life of chemokines in vivo, half-lives of cytokine fusion proteins in mice have been measured up to 24 h.19,29 To express CCL19-IgG2b constitutively within a tumour we selected a well-characterized murine tumour model using J558L cells. Gene targeting of J558L cells with various cytokines or IL-2-IgG2b fusion protein genes resulted in a complete tumour rejection in syngeneic BALB/c mice.23
Interestingly, a different behaviour of J558L tumour cells was observed when transfected with CCL19-IgG2b, which grew comparable to parental J558L cells without any sign of tumour rejection in the syngeneic animals. In contrast to the expected regression of tumour size a tendency towards increased growth of chemokine-secreting tumours was observed. We therefore postulated a possible CCL19-IgG2b-mediated immunosuppressive effect. To investigate this effect of locally expressed CCL19-IgG2b, we used an allogeneic mouse model in which parental J558L cells barely grow to measurable tumour sizes. J558L cells, that express H-2d MHC I but no MHC class II molecules on the cell surface are rapidly rejected in MHC I mismatched C57BL/6 mice mainly by cytotoxic CD8+ T cells.30 In contrast to parental J558L cells, we observed a gain of tumour size, a reduction in the size of regional lymph nodes, and a decreased homing of lymphocytes to regional lymph nodes in C57BL/6 mice bearing allogeneic J558L CCL19-IgG2b-producing tumours. Concomitantly, we observed a decreased lymphocytic infiltration of the CCL19-IgG2b-secreting tumours indicating that the immune response is already impaired prior to the effector phase. This assumption is also consistent with the observation that regional lymph nodes of CCL19-IgG2b-secreting tumours lack the enlargement that occurs after transplantation of parental J558L cells and act like lymph nodes from untreated mice. These observations imply an impaired priming in MHC-restricted immune response against CCL19-IgG2b-secreting tumours.
Innate immunity against tumour cells may also be modified by CCL19. Particularly, the function of subpopulations of natural killer cells, which express CCR7, may have been altered in response to CCL19-IgG2b-secreting tumours. The role of natural killer cells in this mouse model has been intensively examined previously. It could be demonstrated that natural killer cells do play a role in rejection of J558L tumours but their contribution to retard tumour progression is visible only after 3–4 weeks of unimpaired tumour growth as shown in scid (severe combined immunodeficiency) mice and NK cell depletion experiments.16
Several studies addressing the antitumour effects of CCR7-ligand chemokines report an increased infiltration into the tumour of lymphocytes and DCs.11,13,27,28 Indeed, sustained expression of CCL19-IgG2b within the tumour leads to a substantially increased number of DCs, accompanied by a reduced infiltration of T and B cells in the study presented here. Physiologically, DCs enter the tumour for antigen uptake and maturation. This process leads to up-regulation of CCR7 that guide DCs along the chemotactic gradient towards lymph nodes or secondary lymph organs to encounter antigen-searching T cells. The abundant local concentration of CCL19-IgG2b within the tumour might prevent the egress of DCs. Although CCL19 as well as CCL19-IgG induce down-regulation of CCR725 the high local concentration of CCL19-IgG2b may overcome the declining number of CCR7 receptors on DCs. In addition, the reversed chemotactic gradient as well as the CCL19-IgG2b induced down-regulation of CCR7 may also contribute to the insusceptibility of tumour DCs to move to secondary lymphoid organs in response to lymph-node-derived CCL19 or CCL21. These findings are paralleled by an impaired immune response in the draining lymph nodes, where normally the immune response should be initiated.
To address the question whether the Fc part of the CCL19-IgG2b fusion protein might contribute to the effects demonstrated, we would like to point out that the immune response against J558L cells stably transfected with murine CD8-IgG2b did not differ from untransfected parental cells in mice. Furthermore, secretion of IL-2-IgG2b conferred a more pronounced tumour-suppressive capacity compared to secretion of IL-2 alone23 demonstrating that the observed immunosuppressive effect of CCL19-IgG2b is specifically mediated by CCL19 and not by the IgG part of the fusion protein.
There were no indications for a systemic immunosuppressive effect of tumour-derived CCL19-IgG2b, since the DTH reaction was not impaired in mice carrying the CCL19-IgG2b tumour. We hypothesize that this is due to low serum concentrations of the fusion protein, which are not above the background level of endogenous CCL19 as determined by ELISA (data not shown).
It is concluded that high local concentrations and sustained expression of CCL19-IgG2b induce an antitumour (but not a systemic) immunosuppression by impaired DC trafficking, since DC migration from tumour to secondary lymphoid organ is mandatory for initiating a fast and effective antitumour immune response. The amount and duration of CCL19 expression as well as several other factors influencing an immune response has to be taken in account before consideration of CCL19 as a gene-targeted antitumour agent.
However, therapeutical interference with leucocyte recirculation has been recently proven to be a new tool for immunosuppression in human organ transplantation.31
Acknowledgments
We thank Stephanie Palffy, Andrea Hesse and Brigitte Schreiber for expert technical assistance, as well as Dirk Büscher (Salk Institute, La Jolla, CA, USA) for critically reading this manuscript.
References
- 1.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 4.Lipp M, Forster R, Schubel A, Burgstahler R, Muller G, Breitfeld D, Kremmer E, Wolf E. Functional organization of secondary lymphoid organs by homeostatic chemokines. Eur Cytokine Netw. 2000;11:504–5. [PubMed] [Google Scholar]
- 5.Yoshida R, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–9. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH, Pelus LM, Appelbaum E, Johanson K, Anzai N, Broxmeyer HE. CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKbeta-11/MIP-3beta/ELC, are chemoattractants for CD56(+) CD16(–) NK cells and late stage lymphoid progenitors. Cell Immunol. 1999;193:226–35. doi: 10.1006/cimm.1999.1483. [DOI] [PubMed] [Google Scholar]
- 7.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–102. [PubMed] [Google Scholar]
- 8.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 11.Braun SE, Chen K, Foster RG, Kim CH, Hromas R, Kaplan MH, Broxmeyer HE, Cornetta K. The CC chemokine CK beta-11/MIP-3 beta/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J Immunol. 2000;164:4025–31. doi: 10.4049/jimmunol.164.8.4025. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164:4558–63. doi: 10.4049/jimmunol.164.9.4558. [DOI] [PubMed] [Google Scholar]
- 13.Vicari AP, Ait-Yahia S, Chemin K, Mueller A, Zlotnik A, Caux C. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. J Immunol. 2000;165:1992–2000. doi: 10.4049/jimmunol.165.4.1992. [DOI] [PubMed] [Google Scholar]
- 14.Kirk CJ, Hartigan-O'Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–802. [PubMed] [Google Scholar]
- 15.Qin Z, Kruger-Krasagakes S, Kunzendorf U, Hock H, Diamantstein T, Blankenstein T. Expression of tumor necrosis factor by different tumor cell lines results either in tumor suppression or augmented metastasis. J Exp Med. 1993;178:355–60. doi: 10.1084/jem.178.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hock H, Dorsch M, Kunzendorf U, Qin Z, Diamantstein T, Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA. 1993;90:2774–8. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngo VN, Tang HL, Cyster JG. Epstein–Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Kunzendorf U, Pohl T, Bulfone-Paus S, Krause H, Notter M, Onu A, Walz G, Diamantstein T. Suppression of cell-mediated and humoral immune responses by an interleukin-2-immunoglobulin fusion protein in mice. J Clin Invest. 1996;97:1204–10. doi: 10.1172/JCI118534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krautwald S. IL-16 activates the SAPK signaling pathway in CD4+ macrophages. J Immunol. 1998;160:5874–9. [PubMed] [Google Scholar]
- 21.Schweickart VL, Raport CJ, Godiska R, Byers MG, Eddy RL, Jr, Shows TB, Gray PW. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21. 2 Genomics. 1994;23:643–50. doi: 10.1006/geno.1994.1553. [DOI] [PubMed] [Google Scholar]
- 22.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 2000. [Google Scholar]
- 23.Bulfone-Paus S, von Bernuth H, Ruckert R, et al. Vaccination with tumor cells engineered to secrete interleukin 2-immunoglobulin G fusion protein induces tumor rejection. Cancer Res. 1998;58:2707–10. [PubMed] [Google Scholar]
- 24.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 25.Bardi G, Lipp M, Baggiolini M, Loetscher P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol. 2001;31:3291–7. doi: 10.1002/1521-4141(200111)31:11<3291::aid-immu3291>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan SK, McGrath DA, Liao F, Boehme SA, Farber JM, Bacon KB. MIP-3alpha induces human eosinophil migration and activation of the mitogen-activated protein kinases (p42/p44 MAPK) J Leukoc Biol. 1999;66:674–82. doi: 10.1002/jlb.66.4.674. [DOI] [PubMed] [Google Scholar]
- 27.Arenberg DA, Zlotnick A, Strom SR, Burdick MD, Strieter RM. The murine CC chemokine, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol Immunother. 2001;49:587–92. doi: 10.1007/s002620000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Stolina M, Zhu L, Lin Y, Batra R, Huang M, Strieter R, Dubinett SM. Secondary lymphoid organ chemokine reduces pulmonary tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2001;61:6406–12. [PubMed] [Google Scholar]
- 29.Zheng XX, Steele AW, Nickerson PW, Steurer W, Steiger J, Strom TB. Administration of noncytolytic IL-10/Fc in murine models of lipopolysaccharide-induced septic shock and allogeneic islet transplantation. J Immunol. 1995;154:5590–600. [PubMed] [Google Scholar]
- 30.Cayeux S, Richter G, Becker C, Beck C, Aicher A, Pezzutto A, Dorken B, Blankenstein T. Lack of correlation between rejection of tumor cells co-expressing interleukin-2 and B7.1 and vaccine efficiency. Eur J Immunol. 1997;27:1657–62. doi: 10.1002/eji.1830270710. [DOI] [PubMed] [Google Scholar]
- 31.Budde K, Schmouder RL, Brunkhorst R, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13:1073–83. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]