Abstract
Wegener's granulomatosis (WG) is a rare disease characterized by granulomatous lesions, small vessel vasculitis and the presence of anti-neutrophil cytoplasmic autoantibodies (C-ANCAs) in the sera of affected patients. Their main target antigen is proteinase 3 (PR3), a neutrophil and monocyte-derived neutral serine protease. Since the standard treatment of this severe autoimmune disease, with cyclophosphamide and corticosteroids, is associated with potential side-effects, the development of a more specific immunotherapeutic agent is warranted. The key role of ANCA in the pathogenesis of vasculitis and the effectiveness of anti-CD20 antibodies in patients with refractory WG points towards the importance of B cells in WG. We thus evaluated a new approach to selectively eliminate PR3-specific autoreactive B cells by targeting the B-cell receptor. For this purpose we used a bifunctional recombinant fusion protein consisting of the antigen PR3 and a toxin. The cytotoxic component of this novel fusion protein was the ribonuclease angiogenin, a human toxin with low immunogenicity. The toxin was stabilized by exchanging the catalytically relevant histidine in position 44 with glutamine to eliminate the autoproteolytic activity. PR3H44Q was fused either to the N terminus or to the C terminus of angiogenin. The recombinant proteins were expressed in 293T cells. Binding assays demonstrated the appropriate size and recognition by anti-PR3 antibodies. Using TUNEL technology, we demonstrated that these autoantigen toxins kill proteinase 3-specific B-cell hybridomas selectively by inducing apoptosis. The data indicate that autoantigen-toxins are promising tools in the treatment or co-treatment of autoimmune diseases in which the antigen is known.
Keywords: angiogenin, autoantibody, autoimmunity: Wegener's granulomatosis, fusion protein, targeted therapy
Introduction
Autoimmune diseases affect approximately 5% of the population in Western countries.1 Although the prevalence of most autoimmune diseases is low, their individual incidence has increased over the past few years. The understanding of the pathogenesis of autoimmune diseases has improved during the last years, but targeted therapeutic reagents are still lacking. Current treatment modalities are generally based on non-specific systemic immunosuppression.
Wegener's granulomatosis (WG) is a primary systemic vasculitis characterized by granulomatous inflammation in the upper and lower respiratory tract, necrotizing vasculitis and glomerulonephritis.2,3 Before the introduction of treatments with cyclophosphamide and glucocorticoids, the outcome of WG was usually fatal. However, prolonged use of cyclosphosphamide to sustain remission cannot be considered as standard because of a negative benefit to risk ratio. Lethal adverse effects, such as the occurrence of opportunistic infections and the development of malignancies, are common.4,5
Associated with WG are anti-neutrophil cytoplasmic autoantibodies (ANCA), which are thought to be involved in its pathogenesis.6,7 The main autoantigen of C-ANCA is proteinase 3 (PR3)8–10 a multifunctional serine protease localized in the azurophilic granules as well as in secretory vesicles of polymorphonuclear leucocytes (PMNs)11 and monocytes. In addition, PR3 translocates to the cell surface after activation of PMNs.12
Several observations suggest that PR3-ANCA play an important role in the pathophysiology of WG. In vitro ANCA have been shown to activate PMNs, thus increasing the subsequent release of lytic enzymes, toxic oxygen radicals and lipid metabolites from PMNs.13,14In vitro studies using endothelial monolayers demonstrated that in the presence of ANCA, neutrophils adhere to and lyse endothelial cells.15 Furthermore, ANCA can activate monocytes for the production and release of reactive oxygen species16 as well as interleukin-8,17 a potent attractant for PMNs. Also supporting a pathophysiological role of ANCA is the observation that high titres of ANCA precede clinical disease activity in a substantial portion of cases,18 indicating that ANCA are a major risk factor for subsequent relapse.
The current concept of whether ANCA directly or indirectly contribute to vascular damage (the ANCA-Cytokine-Sequence-Theory) was mainly developed from in vitro studies and is supported by data from clinical investigations as well as animal models.19 Recently, a direct causal link between ANCA and the development of glomerulonephritis and vasculitis has been demonstrated.20 Xiao et al. made use of a model in which myeloperoxidase (MPO) knockout mice are immunized with murine MPO.20 As reported, these mice developed a brisk anti-MPO-antibody response. When splenocytes from these animals are transferred to RAG2–/– mice, small-vessel vasculitis and a necrotizing crescentic glomerulonephritis develop. When mice are immunized with control antigens such as bovine serum albumin and splenocytes are transferred into them, no lesion occurs. These data indicate that the anti-MPO antibody is capable of creating a necrotizing glomerulonephritis and vasculitis.20 This model is the first convincing animal model for MPO-ANCA-vasculitis and provides the first evidence that ANCA are sufficient to cause systemic pauci-immune vasculitis and glomerulonephritis in vivo. Today, these findings are of major interest with regard to new treatment modalities. Furthermore, they suggest that elimination of ANCA is a promising approach for the treatment of, or as a suitable cotreatment of, WG. The recent observation from a controlled trial showed that the removal of antibodies by plasmapheresis is superior to conventional immunosuppressive treatment with methylprednisolone. This further supports the importance of ANCA in the development of vasculitis.21 In addition, the effectiveness of anti-CD20 antibodies in patients with refractory WG points towards a role of B cells in WG which warrants further investigation.22
A possible way to reduce autoantibodies is to deplete the autoreactive B lymphocytes by a recombinant fusion toxin consisting of the antigen and a toxin. Effective chimeric cytotoxins have already been constructed by fusion of cDNA encoding a variety of antibodies, cytokines and growth factors with Pseudomonas Exotoxin or other plant and bacterial toxins.23,24 Particularly with regard to prolonged application, the immunogenicity of the toxin moiety is still a major problem.25,26 Several attempts have been made to create human or humanized immunotoxins using human ribonucleases, such as angiogenin, as effector domain.27–29 We thus constructed a chimeric protein, fusing cDNA encoding PR3 to sequences coding for angiogenin.
We herein report the construction, expression, purification and cytotoxic function of the PR3-angiogenin fusion protein. We demonstrate that this chimera is an effective and selective agent for targeting and specifically killing anti-PR3 B-cell hybridomas.
Materials and methods
Bacterial strains, oligonucleotides and plasmids
Escherichia coli XL1-Blue (Stratagene, Amsterdam, the Netherlands) was used as host for cloning and sequencing. Synthetic oligonucleotides were synthesized by MWG (Martinsried, Germany). Plasmids were prepared by the alkaline lysis method and purified using plasmid kits from Qiagen (Hilden, Germany). Restriction fragments and polymerase chain reaction (PCR) products were separated by horizontal agarose gel electrophoresis and extracted with Qiaex Gel extraction Kit500 (Qiagen). Ligation was performed using the Rapid DNA Ligation Kit (Roche, Mannheim, Germany).
Amplification of PR3 cDNA and plasmid construction
For the amplification of PR3 cDNA (GenBank Accession No. NM_002777), whole-cell RNA was isolated from THP1 cells using the RNeasy RNA Extraction Kit (Qiagen) according to the manufacturer's suggestions. After first-strand cDNA synthesis (SMART PCR cDNA synthesis kit, Clontech, CA) different PR3-specific primers were used for the amplification of PR3 variants. In a first step, the N-terminal and C-terminal portions of PR3 variants were generated separately using the outer primer 5′-act-ggt-gac-gcg-gcc-cag-ccg-gcc-ATC-GTG-GGC-GGG-CAC-GAG-3′ (underlined letters indicate the SfiI restriction site) forward combined with 5′-gg-tat-gtc-ccg-cag-gca-TTG-cgc-ggc-cgt-cag-cac-3′ (PR3H44Q), 5′-ctg-gat-gag-gag-aac-GGC-gtt-cag-ttt-gtt-ctc-3′ (PR3D91A) or 5′-cag-ggg-gcc-acc-TGC-gtc-tcc-gaa-gca-gat-gcc-3′ (PR3S176A) as inner reverse primers. The C-terminal fragments of the PR3 variants were synthesized using the reverse primer 5′-ctg-tct-aga-ctc-gag-tgc-ggc-cgc-GGG-GCG-GCC-CTT-GGC-CTC-CAC-ACG-3′ (bold letters indicate the NotI restriction site) combined with 5′-gtg-ctg-acg-gcc-gcg-CAA-tgc-ctg-cgg-gac-ata-cc-3′ (PR3H44Q), 5′-gag-aac-aaa-ctg-aac-GCC-gtt-ctc-ctc-atc-cag-3′ (PR3D91A) or 5′-ggc-atc-tgc-ttc-gga-gac-GCA-ggt-ggc-ccc-ctg-3′ (PR3S176A) as inner forward primers. The inner primers inserted a specific point mutation to substitute the amino acids histidine, aspartic acid, or serine, which form the catalytic triad. In a second step, matching fragments were joined together by PCR using the outer primer. PCR was performed in 50-μl reaction mixtures applying buffer and dNTP mix from Promega (Mannheim, Germany). Dimethyl sulphoxide was added to a final concentration of 2%. General cycling conditions were : 95° for 2 min; 30 cycles of (95° for 30 seconds; 58° for 1 min; and 72° for 1 min); followed by 72° for 6 min in a RoboCycler Gradient 96 (Stratagene). Products from this PCR were restricted with SfiI and NotI and cloned in the multiple cloning site of pMS III/G, pMS NAng III/G and pMS AngII/G30 by direct insertion using the SfiI and NotI restriction sites. In these plasmids the multiple cloning site is followed by a tandem Myc- and His-Tag epitope at their 3′ ends for detection and purification. The eukaryotic pMS-expression plasmids are derived from the pSecTag2-plasmids (Invitrogen, Karlsruhe, Germany) which contain the IVS/IRES-EGFP sequence of the pIRES-EGFP-plasmid from Clontech (Palo Alto, CA). This allows transcription of a bi-cistronic mRNA for the parallel expression of enhanced green fluorescent protein (EGFP) and recombinant protein.
To verify cloning, all constructs were sequenced with the BigDye Terminator Cycle Sequencing Ready Reaction Kit on an ABI PRISM. Besides plasmid located primers, the specific primers used for amplification of the PR3 variants have been used for the sequence reaction.
Cell culture
All cell lines including the hybridoma cell lines WGM2 and WGM3, the embryonic kidney-derived cell line 293T and the Hodgkin cell line L428, which is derived from B cells, were cultivated in complex medium (RPMI-1640, PAA, Pasching, Germany) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Invitrogen, Karlsruhe, Germany), 50 μg/ml penicillin, 50 μg/ml streptomycin and 2 mm l-glutamine. Cells were cultured at 37° in a 5% CO2 in air atmosphere. PR3-ANCA specific cell lines (designated WGH1, WGM2 and WGM3) were generated by hybridoma technology as earlier described.31 For the selection of the transfected cells, Zeocin (Invitrogen) was added to a final concentration of 200 μg/ml. For higher productivity transfected 293T cells were cultivated in CD293 medium (Invitrogen) instead of RPMI-1640 with supplements remaining unchanged.
Eukaryotic expression and purification of recombinant PR3 (rPR3)
Transfection of 293T-cells was performed with Lipofectamine™ 2000 (Invitrogen). According to the manufacturer's instructions 0·8–1·0 μg DNA and 2–3 μl Lipofectamine™ 2000 were used to transfect 0·5 × 105−2 × 105 cells in a 24-well cell-culture plate. Two days after transfection, cell culture supernatants were analysed for secreted recombinant protein. Transfected cells were transferred into medium-size cell culture flasks (Nunc 85 cm2; Nunc, Roskilde, Denmark) and grown in RPMI-1640 supplemented with 200 μg/ml Zeocin. As a result of the bi-cistronic mRNA the recombinant proteins were simultaneously expressed together with EGFP. Thus, successfully transfected cells exhibit green fluorescence which allowed direct monitoring of transfected cells. After 1–2 weeks, single transfected clones could be detected by fluorescence microscopy. Transfected cell populations were established by subcultivation of these clones. Purification of His-tagged proteins was accomplished by the Ni-NTA metal-affinity method as previously described.30
SDS–PAGE and Western blotting
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) was performed using 4–15% gels. For immunodetection, proteins were transferred to a nitrocellulose membrane (Hybond-C, Amersham Pharmacia Biotech Inc., Freiburg, Germany) using the transfer apparatus supplied by Bio-Rad Labaratories (Munich, Germany). Immunoblots were blocked with Roti-Block (Roth, Karlsruhe, Germany) for 1 hr at room temperature and then incubated with a mouse anti-penta-His monoclonal antibody (mAb; Qiagen) at a dilution of 1/4000 in Tris-buffered saline with 0·2% bovine serum albumin and 0·2% Tween-20 for 1 hr. Bound antibody was detected by a horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin G (IgG) mAb (Dianova, Hamburg, Germany) or horseradish peroxidase-conjugated goat anti-mouse IgG (Dianova) diluted 1/10 000. The proteins were visualized using the ECL Western blotting detection reagents (Amersham Pharmacia) according to manufacturer's instructions. The membranes were then exposed to appropriate X-ray film (Roche) for various times.
Binding capacity
The binding activity of rPR3 and PR3 fusion toxin was determined by direct enzyme-linked immunosorbent assay (ELISA). Microtitre plates (Nunc) were coated with 1 μg/ml rPR3 or PR3 fusion toxin overnight at 4°. As a positive control, 1 μg/ml native PR3 was coated in the same manner. Plates were washed and blocked with phosphate-buffered saline supplemented with 2% bovine serum albumin for 1–2 hr. After washing, plates were incubated with 100 μl anti-PR3 mAb supernatant for 1–2 hr. Finally, the plates were incubated with horseradish peroxidase-conjugated donkey anti-human IgG + IgM (1/10 000, Dianova) (WGH1) or goat anti-mouse IgG + IgM (1/10 000, Dianova) (WGM2, WGM3). The substrate used in all assays was O-phenylenediamine dihydrochloride (Sigma-Aldrich, Munich, Germany). Absorbance values were measured at 450 nm with an ELISA reader (BioTek, Bad Friedrichshall, Germany).
Cytotoxic function of PR3 fusion toxin
CD293 cell culture supernatant of transfected 293T cells, containing either PR3H44Q, PR3H44Q-Ang, or NAng-PR3H44Q, was concentrated with Vivaflow 50 using Regenerated Cellulose membranes with a molecular weight cut-off of 10 000 or 30 000 (Vivascience, Hannover, Germany). Either 0·5 × 105−1 × 105 target cells (WGM2, WGM3) or control cells (L428) were incubated with 250 μl concentrated toxin-containing CD293 cell culture supernatant (approximately 50 μg/ml) for 6 hr at 37° in 5% CO2.
Detection of DNA fragmentation as a result of apoptosis
The toxin-treated cells were applied to slides by cytocentrifugation and dried overnight at room temperature. Then the cytospin preparations were incubated for 30 seconds in ice-cold acetone for fixation of the cells. Afterwards a TUNEL assay was performed using the In Situ Cell Death Detection Kit, TMR red (Roche) according to the manufacturer's instructions. Finally, cells were mounted with VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories, Burlingame, CA). Cytospin preparations were analysed by fluorescence microscopy.
Annexin V binding assay for detection of apoptotic cells
Following treatment with PR3H44Q, PR3H44Q-Ang or NAng-PR3H44Q, the cells were used for determining the translocation of phosphatidylserine to the outer leaflet of the plasma membrane during apoptosis. This was done by flow cytometry using annexin V conjugated with fluorescein (Annexin V-FITC apoptosis detection kit I, BD Biosciences, USA) as recommended.
Results
Mutated PR3 is enzymatically inactive and recognized by anti-PR3 mAb
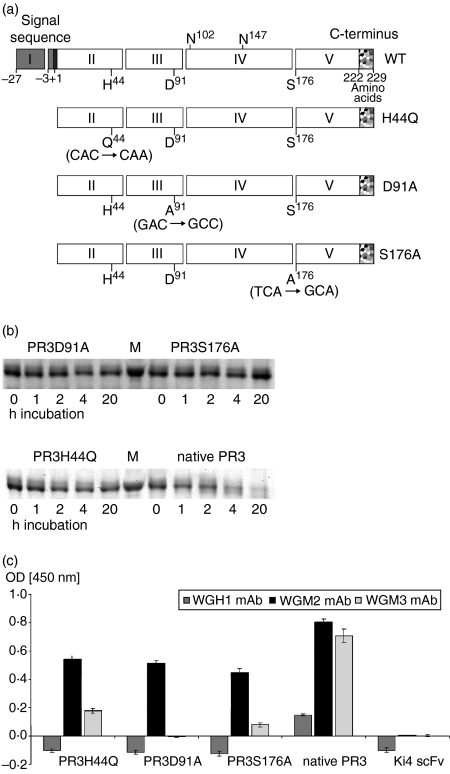
PR3 is a potent serine protease. The majority of PR3 antibodies recognize conformational epitopes on PR3 in the catalytic centre. To prevent autoproteolytic decomposition we generated an inactive PR3. With respect to their recognition by anti-PR3 mAbs, three differently mutated PR3 were synthesized. This was achieved by inserting a point mutation in the coding sequence, substituting one of the amino acids of the catalytic triad, histidine (PR3H44Q), aspartic acid (PR3D91A) or serine (PR3S176A), respectively (Fig. 1a). PR3 was expressed in 293T cells and purified from cell culture supernatant by Ni2+-NTA metal affinity chromatography.
Figure 1.
Design, expression and characterization of mutated PR3. (a) Design of mutated PR3. Schematic representation of the PR3 variants. The signal sequence, prosequences, and C-terminal sequence are shown as grey, black and speckled boxes, respectively. Exons are indicated with the exon number in the box. The amino acids H44, D91 and S176 forming the catalytic triad and the two glycosylation sites on N102 and N147 are marked at the wild-type PR3. PR3 variants are listed below, the introduced point mutations are written in parenthesis. (b) Enzymatic activity of rPR3; 500 ng PR3 variants were incubated with 500 ng PR3H44Q-Ang fusion protein as substrate for 1, 2, 4 and 20 hr at room temperature. Then, protein decomposition was measured by SDS–PAGE analysis. Proteins were visualized by Coomassie staining. (c) Affinity of rPR3 variants to anti-PR3 mAb. The rPR3 constructs were tested by direct ELISA using WGH1, WGM2, or WGM3 mAb. Bound antibody was detected by horseradish peroxidase-conjugated goat anti-mouse antibody. Therefore rPR3 was coated with a final concentration of 100 ng. The anti-CD30 scFv fragment was used as a non-specific control. Error bars represent SD values of triplicates.
To select the most feasible construct we first tested the proteolytic activity. As shown in Fig. 1(b) all constructs were unable to degrade PR3H44Q-Ang, which was used as substrate. In contrast, native PR3 elicited strong proteolytic activity towards the PR3-based construct.
The antibody-binding capacity of the PR3 variants was verified by ELISA with WGH1, WGM2 and WGM3 mAb, respectively (Fig. 1c). Comparing the different forms of PR3, we found that the affinities of WGM2 to PR3H44Q, PR3D91A and PR3S176A were nearly the same, whereas WGM3 bound best to PR3H44Q. WGH1 mAb did not bind to any of the PR3 variants and native PR3 was recognized somewhat better by all anti-PR3 mAb. The screening revealed that all tested PR3 mutants served equally well in respect of the lack of enzymatic activity. Sufficient binding of WGM2 and WGM3 mAb could be detected for PR3H44Q and to a lesser extent for PR3S176A.
293T cells produce functional PR3-angiogenin fusion protein
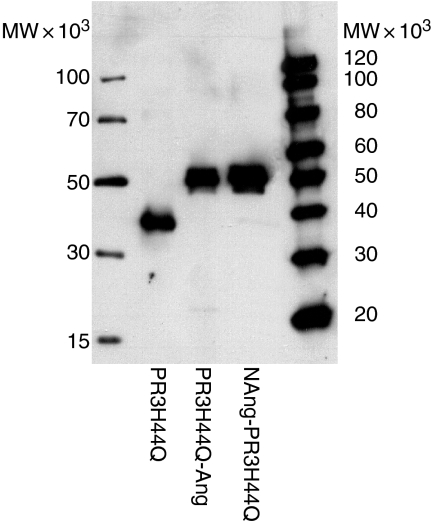
Based on the findings that the WGM2 and WGM3 mAb affinity is highest towards PR3H44Q, this PR3 variant was used as the basis to generate the immunotoxic agent. PR3H44Q was fused either to the N terminus or the C terminus of angiogenin (PR3H44Q-Ang and NAng-PR3H44Q). Production and appropriate size of the purified PR3 constructs were surveyed by Western blot analysis (Fig. 2). PR3H44Q had an apparent molecular weight of 38 000, PR3H44Q-Ang and NAng-PR3H44Q resulted in a band at approximately 50 000.
Figure 2.
Expression and purification of PR3 fusion proteins. Western blot analysis of PR3H44Q, PR3H44Q-Ang and NAng- PR3H44Q. The proteins were expressed in 293T cells and purified by Ni2+-NTA metal affinity chromatography. Proteins were analysed by SDS–PAGE under non-reducing conditions and visualized by immunostaining of the Western blot using mouse anti-penta-His mAb and a horseradish peroxidase-conjugated secondary donkey anti-mouse antibody, followed by ECL mediated chemiluminescence reaction.
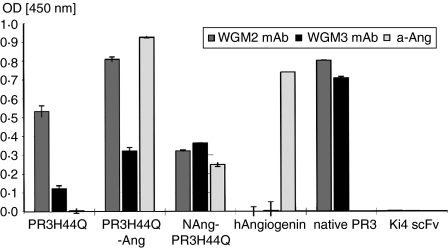
The identity of the PR3 fusion proteins was confirmed by ELISA using WGM2 mAb and WGM3 mAb for primary binding and horseradish peroxidase-conjugated anti-mouse IgG antibody for subsequent detection (Fig. 3). The anti-CD30 Ki4 scFv, which was used as nonspecific control, was not recognized by any anti-PR3 mAb. Figure 3 shows that the anti-PR3 mAb binds specific to PR3H44Q and angiogenin did not interfere.
Figure 3.
Binding of anti-PR3 mAb to PR3 fusion proteins by direct ELISA. Cell culture supernatant of WGM2 and WGM3 cells or mouse anti-angiogenin mAb (1 : 2000) was used for binding analysis; bound antibody was detected by horseradish peroxidase-conjugated goat anti-mouse antibody. Error bars represent SD values of triplicates.
Thus, eukaryotic expression of rPR3 variants results in the generation of feasible constructs: SDS–PAGE, Western blotting and ELISA data confirm that 293T cells produce PR3H44Q, PR3H44Q-Ang and NAng-PR3H44Q that had the correct size, tertiary structure and antigenic property.
TUNEL assay
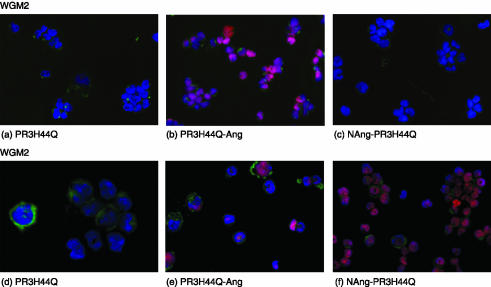
We tested the potency of the constructs against IgM- and IgG-type target cells. For this purpose we used the hybridoma cell lines WGM2 and WGM3 which were incubated for 6 hr at 37° with cell culture supernatant containing approximately 50 μg/ml rPR3 or PR3-fusion toxin (data not shown). To visualize apoptotic cells, the TUNEL assay was used, allowing analysis at the single-cell level by fluorescence microscopy. DNA fragmentation during apoptosis can be identified by labelling free 3′-OH termini with TMR red-labelled nucleotides in an enzymatic reaction, resulting in a red staining of the nuclei. To preclude the possibility of PR3-specific cytotoxicity, we incubated WGM2, WGM3 and L428 cells with PR3H44Q without effector domain. All cell lines remained unaffected, as the absence of red staining indicates (Fig. 4a and d). In contrast, incubation with PR3H44Q-Ang resulted in apoptosis of WGM2 and WGM3 cells whereas NAng-PR3H44Q proved to be toxic only to WGM3 cells but not to WGM2 (Figs 4c,f). As expected, none of the PR3 variants had any effect on the Hodgkin-derived control cell line L428 (data not shown).
Figure 4.
Detection of PR3-toxin-induced apoptosis by TUNEL technology, staining apoptotic cells red. To preserve fluorescence, cytospin preparations were coverslipped with mounting medium containing DAPI. WGM2 cells treated with PR3H44Q (a), PR3H44Q-Ang (b) and NAng-PR3H44Q (c); WGM3 cells treated with PR3H44Q (d), PR3H44Q-Ang (e) and NAng-PR3H44Q (f).
Annexin V binding assay.
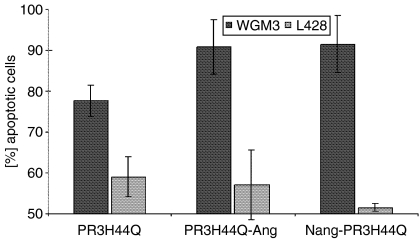
To verify the cytotoxicity of the autoantigen toxins in a quantitative assay, we performed an annexin V binding assay. One of the early features in the apoptotic process is the loss of plasma membrane asymmetry, leading to the exposure of phosphatidylserine on the outer leaflet of the plasma membrane. This process can be determined with annexin V, an anticoagulant protein that binds to negatively charged phospholipids. We used fluorescein-conjugated annexin V (Annexin V-FITC) to determine apoptotic cell death in cultures of WGM3 and L428 cells treated with PR3H44Q, PR3H44Q-Ang and NAng-PR3H44Q, respectively. Figure 5 shows a representative annexin V FACS analysis of WGM3 cells and L428 cells after incubation with cell culture supernatant containing different fusion toxins. The data clearly show, that the treatment with any of the concentrated cell culture supernatants did not lead to autoantigen-toxin-induced apoptosis in Hodgkin-derived L428 cells, whereas WGM3 exhibited PR3 fusion-toxin-specific cell death. The high background apoptosis of about 60% results from the incubation of the cells on ice, which is a necessary step in the method used here.
Figure 5.
Flow cytometric analysis of PR3-toxin-induced apoptosis by annexin V binding assay. Cells were incubated with CD293 cell culture supernatant of 293T cells containing PR3H44Q, PR3H44Q-Ang or NAng-PR3H44Q (approx. 50 μg/ml), respectively. Cells were incubated for 6 hr at 37°, 5% CO2. Apoptotic cell death was measured by flow cytometry using fluorescein-conjugated annexin V as described in the Materials and methods section. The error bars represent the standard deviation of three independent experiments.
Discussion
In this study, we investigated the potential of a newly constructed PR3-human angiogenin fusion toxin against anti-PR3-specific B cells. The major findings to emerge from our studies are as follows. (i) Mutated rPR3 is enzymatically inactive and recognized by anti-PR3 mAb. (ii) Embryonic kidney-derived 293T-cells produce functional PR3-fusion toxin. (iii) PR3-fusion toxin develops specific cytotoxicity against WGM2 and WGM3 hybridomas.
The standard treatment of autoimmune diseases consists of immunosuppressive drugs, corticosteroids, or plasmapheresis. The rationale is to reduce the level of autoantibodies, thus intervening at a rather late phase of pathogenesis. This unspecific immunosuppression results in a general immunodeficiency, often causing severe side-effects. Consequently, the development of a specific and selective immunotherapeutic agent is warranted, which ideally interferes at an early pathogenic stage.
We report here the construction of a bi-functional recombinant fusion protein, consisting of the antigen and a toxin, as an agent for specific depletion of autoantibody-producing B-cell hybridomas. We chose WG to exemplify this new therapeutic approach for autoimmune diseases, because the autoantigen of WG, PR3, is well characterized and several anti-PR3-specific B-cell hybridomas are available. This was of particular interest, as antigen-specific B cells are not suitable for cytotoxicity assays after Epstein–Barr virus transformation because of their blocked B-cell receptor signalling and antigen transport.32
To construct the chimeric protein we used inactivated mature PR3 as the targeting moiety, because the majority of PR3-ANCA recognize conformational epitopes on PR3.33 The proteolytic activity of PR3 was eliminated by exchanging one of the amino acids of the catalytic triad, respectively, thus modifying the antigen as little as possible. Analyses of the three generated PR3 variants showed that the substitution of H44Q resulted in the loss of autoproteolytic activity and had the least effect on the binding of anti-PR3 mAb (Fig. 1c). Several studies were conducted to define relevant epitopes for anti-PR3 mAbs and PR3-ANCA sera of WG patients,34,35 showing that different epitope areas are recognized, some of them being surface-accessible36,37 or even coinciding with the catalytic site.37 The reduced binding activity of WGM3 to PR3D91A and PR3S176A indicates that these mutations cause stronger interference with the epitope area. The total loss of binding capacity of WGH1 towards any of the PR3 variants suggests that the epitope coincides with the catalytic site of PR3.
One of the major limitations of clinically using recombinant or chemically linked chimeric toxins is the development of antibodies against exogenous moieties of the protein. This restricts the treatment in terms of duration and number of patients.38,39 To diminish immunogenicity of the immunotoxins (IT), we chose the human ribonuclease angiogenin as effector domain. Human angiogenin has proven specific cytotoxic activity when fused to a ligand, which is internalized on binding to a given target cell.29 In addition, angiogenin is expected to have a very low immunogenic potential because of its endogenous origin. PR3H44Q was recombinantly linked either to the N terminus or the C terminus of angiogenin considering possible impairment of the binding capacity or the cytotoxic activity, respectively. The binding capacity of the different fusion proteins was analysed by direct ELISA. Interestingly, PR3H44Q is not, as one would expect, recognized best by anti-PR3 mAb. In fact, the binding capacity of PR3H44Q-Ang seems to be elevated. As the ELISA data exclude unspecific binding of anti-PR3 mAb towards the toxin moiety, the apparent increase of WGM2 mAb binding can be ascribed to the method used. Direct ELISA bears the difficulty that the epitopes are eventually not accessible for the antibody after binding to the surface. An additional peptide sequence at the C terminus increases the probability that even after coating the antigen moiety remains free for antibody binding.
The ELISA data show reduced autoantigen-binding activity of WGM2 mAb if angiogenin is located at the N terminus of the fusion protein (Fig. 3). Thus, in this new construct the epitope area is not freely accessible probably as a result of steric hindrance. These findings correspond to the results of the cytotoxicity assays. While the treatment with PRH44Q-Ang resulted in effective apoptosis of both anti-PR3 specific B-cell hybridomas, NAng-PR3H44Q was only toxic to WGM3 cells (Fig. 4). The potency of the autoantigen-toxins was also verified by an annexin V binding assay, proving that both constructs exhibit specific toxicity towards WGM3 cells, whereas the Hodgkin-derived L428 cells show no PR3-fusion protein-induced apoptosis (Fig. 5).
The almost 95% specificity of C-ANCA for WG6,14 and the ample evidence for a pathogenic role of C-ANCA, suggest that depletion of anti-PR3-specific B cells may be a sensible way to intervene at an early stage of the pathogenesis. ANCA are capable of stimulating human neutrophils, provoking a respiratory burst and generating reactive oxygen species.13 These effects are markedly enhanced by primed neutrophils. When being primed with cytokines, as occurs in episodes of infection or inflammation, neutrophils express PR3 on their surface,12,40 which thus becomes accessible to autoantibody binding. PR3 expression was also observed on the plasma membrane of resting neutrophils11,31 and monocytes.41 Furthermore, increase in ANCA titre frequently precedes relapse42,44 and development of relapses can be successfully prevented by treatment based on changes in ANCA.45,46 The most convincing clinical evidence that ANCA cause vasculitis and glomerulonephritis is the observation that certain drugs, such as hydralazine, thiouracils and penicillamine, are capable of inducing ANCA formation that is associated with the development of small-vessel vasculitis and glomerulonephritis. This phenomenon is pathologically identical to idiopathic pauci-immune ANCA-vasculitis and glomerulonephritis and has been described most often with propylthiouracil treatment.47,49
Eventually, all these ANCA-associated effects may result in damage of endothelial cells and lead to vasculitis, indicating the reduction of antigen-specific antibody titre by depletion of anti-PR3-specific B cells to be a reasonable approach for the treatment of WG.
A similar approach as described in the present study has recently been described for multiple sclerosis. Zocher et al.50 designed a bispecific fusion protein composed of the extracellular immunoglobulin-like domain of human myelin oligodendrocyte glycoprotein and the CH2 and CH3 domains of the human IgG1 heavy chain (MOG-Fc). They were able to show selective elimination of autoantigen-reactive B lymphocytes in vitro as well as in vivo. Furthermore, using the autoantigen as binding domain to target autoreactive B cells not only bears the advantage of high selectivity but targets even plasma cells, which present the autoantibodies on the outer leaflet of the membrane.
Considering the development of the autoantigen-toxin PR3-Ang against autoantigen-reactive B lymphocytes in WG as a model, our findings indicate that this might be a new therapeutic strategy for autoimmune diseases in general.
Acknowledgments
This work was in part supported by the Ministerium für Wissenschaft und Forschung des Landes Nordrhein-Westfalen, Germany.
Abbreviations
- ANCA
anti-neutrophil cytoplasmic autoantibodies
- Ang
angiogenin
- IT
immunotoxin
- PR3
proteinase 3
- PR3D91A
rPR3 with the amino acid aspartic acid at position 91 substituted against alanine
- PR3H44Q
rPR3 with the amino acid histidine at position 44 substituted against glutamine
- PR3S176A
rPR3 with the amino acid serine at position 176 substituted against alanine
- rPR3
recombinant PR3
- WG
Wegener's granulomatosis
References
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS. Wegener granulomatosis. An analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 3.Langford CA, Hoffman GS. Rare diseases.3: Wegener's granulomatosis. Thorax. 1999;54:629–37. doi: 10.1136/thx.54.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stillwell TJ, Benson RC, Jr, DeRemee RA, McDonald TJ, Weiland LH. Cyclophosphamide-induced bladder toxicity in Wegener's granulomatosis. Arthritis Rheum. 1988;31:465–70. doi: 10.1002/art.1780310402. [DOI] [PubMed] [Google Scholar]
- 5.Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995;38:1120–7. doi: 10.1002/art.1780380815. [DOI] [PubMed] [Google Scholar]
- 6.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 7.Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 8.Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–93. [PubMed] [Google Scholar]
- 9.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, Hack CE, van den Ende ME, von Kallenberg CG, dem Borne AE. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludemann J, Utecht B, Gross WL. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990;171:357–62. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V, Cramer EM, Hieblot C, Guichard J, Nusbaum P, Lopez S, Lesavre P, Halbwachs-Mecarelli L. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94:2487–96. [PubMed] [Google Scholar]
- 12.Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–19. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross WL, Csernok E, Flesch BK. ‘Classic’ anti-neutrophil cytoplasmic autoantibodies (cANCA), ‘Wegener's autoantigen' and their immunopathogenic role in Wegener's granulomatosis. J Autoimmun. 1993;6:171–84. doi: 10.1006/jaut.1993.1015. [DOI] [PubMed] [Google Scholar]
- 15.Savage CO, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992;141:335–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Ewert BH, Jennette JC, Falk RJ. The pathogenic role of antineutrophil cytoplasmic autoantibodies. Am J Kidney Dis. 1991;18:188–95. doi: 10.1016/s0272-6386(12)80879-1. [DOI] [PubMed] [Google Scholar]
- 17.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997;100:1416–24. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boomsma MM, Damoiseaux JG, Stegeman CA, Kallenberg CG, Patnaik M, Peter JB, Cohen Tervaert JW. Image analysis. a novel approach for the quantification of antineutrophil cytoplasmic antibody levels in patients with Wegener's granulomatosis. J Immunol Meth. 2003;274:27–35. doi: 10.1016/s0022-1759(02)00273-9. [DOI] [PubMed] [Google Scholar]
- 19.Csernok E. Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun Rev. 2003;2:158–64. doi: 10.1016/s1568-9972(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 20.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luqmani RA, Jayne D, Gaskin G group E. Adjunctive plasma exchange is superior to methylprednisolone in acute renal failure due to ANCA-associated glomerulonephritis. Arthritis Rheum. 2002;46:S207/473. [Google Scholar]
- 22.Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener's granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum. 2001;44:2836–40. doi: 10.1002/1529-0131(200112)44:12<2836::aid-art471>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Brinkmann U, Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 24.Barth S, Huhn M, Matthey B, Tawadros S, Schnell R, Schinkothe T, Diehl V, Engert A. Ki-4 (scFv)-ETA′, a new recombinant anti-CD30 immunotoxin with highly specific cytotoxic activity against disseminated Hodgkin tumors in SCID mice. Blood. 2000;95:3909–14. [PubMed] [Google Scholar]
- 25.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol. 1999;11:570–8. doi: 10.1016/s0952-7915(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 26.Winkler U, Barth S, Schnell R, Diehl V, Engert A. The emerging role of immunotoxins in leukemia and lymphoma. Ann Oncol. 1997;8(Suppl. 1):139–46. [PubMed] [Google Scholar]
- 27.Rybak SM, Hoogenboom HR, Meade HM, Raus JC, Schwartz D, Youle RJ. Humanization of immunotoxins. Proc Natl Acad Sci USA. 1992;89:3165–9. doi: 10.1073/pnas.89.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zewe M, Rybak SM, Dubel S, Coy JF, Welschof M, Newton DL, Little M. Cloning and cytotoxicity of a human pancreatic RNase immunofusion. Immunotechnology. 1997;3:127–36. doi: 10.1016/s1380-2933(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 29.Huhn M, Sasse S, Tur MK, Matthey B, Schinkothe T, Rybak SM, Barth S, Engert A. Human angiogenin fused to human CD30 ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res. 2001;61:8737–42. [PubMed] [Google Scholar]
- 30.Stocker M, Tur MK, Sasse S, Krussmann A, Barth S, Engert A. Secretion of functional anti-CD30-angiogenin immunotoxins into the supernatant of transfected 293T-cells. Protein Expr Purif. 2003;28:211–19. doi: 10.1016/s1046-5928(02)00709-x. [DOI] [PubMed] [Google Scholar]
- 31.Csernok E, Ludemann J, Gross WL, Bainton DF. Ultrastructural localization of proteinase 3, the target antigen of anti-cytoplasmic antibodies circulating in Wegener's granulomatosis. Am J Pathol. 1990;137:1113–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Dykstra ML, Longnecker R, Pierce SK. Epstein–Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 33.Bini P, Gabay JE, Teitel A, Melchior M, Zhou JL, Elkon KB. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 34.Van Der Geld YM, Limburg PC, Kallenberg CG. Characterization of monoclonal antibodies to proteinase 3 (PR3) as candidate tools for epitope mapping of human anti-PR3 autoantibodies. Clin Exp Immunol. 1999;118:487–96. doi: 10.1046/j.1365-2249.1999.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Der Geld YM, Simpelaar A, Van Der Zee R, Tervaert JW, Stegeman CA, Limburg PC, Kallenberg CG. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 2001;59:147–59. doi: 10.1046/j.1523-1755.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 37.Griffith ME, Coulthart A, Pemberton S, George AJ, Pusey CD. Anti-neutrophil cytoplasmic antibodies (ANCA) from patients with systemic vasculitis recognize restricted epitopes of proteinase 3 involving the catalytic site. Clin Exp Immunol. 2001;123:170–7. doi: 10.1046/j.1365-2249.2001.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitetta ES, Thorpe PE, Uhr JW. Immunotoxins: magic bullets or misguided missiles? Immunol Today. 1993;14:252–9. doi: 10.1016/0167-5699(93)90041-I. [DOI] [PubMed] [Google Scholar]
- 39.LeMaistre C, Saleh M, Kuzel T, et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood. 1998;91:399–405. [PubMed] [Google Scholar]
- 40.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991;50:539–46. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 41.Muller Kobold AC, Kallenberg CG, Tervaert JW. Monocyte activation in patients with Wegener's granulomatosis. Ann Rheum Dis. 1999;58:237–45. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egner W, Chapel HM. Titration of antibodies against neutrophil cytoplasmic antigens is useful in monitoring disease activity in systemic vasculitides. Clin Exp Immunol. 1990;82:244–9. doi: 10.1111/j.1365-2249.1990.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Qjm. 1995;88:127–33. [PubMed] [Google Scholar]
- 44.Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, Limburg PC, Cohen Tervaert JW. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Cohen Tervaert JW, Huitema MG, Hene RJ, Sluiter WJ, The TH, van der Hem GK, Kallenberg CG. Prevention of relapses in WG by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990;336:709–11. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- 46.Boomsma MM, Stegeman CA, Kallenberg CGM, Cohen Tervaert JW. Prevention of relapsing disease in anti-neutrophil cytoplasmic antibody-related necrotizing small-vessel vasculitis: the role for autoantibody-guided and anti-bacterial treatment. In: Kallenberg CGM, Cohen Tervaert JW, editors. Disease-Modifying Therapy in Vasculitides. Basel: Birkhäuser-Verlag; 2001. pp. 181–99. [Google Scholar]
- 47.Dolman KM, Gans RO, Vervaat TJ, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet. 1993;342:651–2. doi: 10.1016/0140-6736(93)91761-a. [DOI] [PubMed] [Google Scholar]
- 48.Vogt BA, Kim Y, Jennette JC, Falk RJ, Burke BA, Sinaiko A. Antineutrophil cytoplasmic autoantibody-positive crescentic glomerulonephritis as a complication of treatment with propylthiouracil in children. J Pediatr. 1994;124:986–8. doi: 10.1016/s0022-3476(05)83199-3. [DOI] [PubMed] [Google Scholar]
- 49.D'Cruz D, Chesser AM, Lightowler C, Comer M, Hurst MJ, Baker LR, Raine AE. Antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis associated with anti-thyroid drug treatment. Br J Rheumatol. 1995;34:1090–1. doi: 10.1093/rheumatology/34.11.1090. [DOI] [PubMed] [Google Scholar]
- 50.Zocher M, Baeuerle PA, Dreier T, Iglesias A. Specific depletion of autoreactive B lymphocytes by a recombinant fusion protein in vitro and in vivo. Int Immunol. 2003;15:789–96. doi: 10.1093/intimm/dxg076. [DOI] [PubMed] [Google Scholar]