Abstract
Neutrophil chemotaxis has been shown to be regulated by two different signalling pathways that allow strong chemoattractants, such as bacterial-derived formylated peptides, to dominate over endogenous attractants, such as interleukin-8 (IL-8). Here we show that triggering of the formyl peptide receptor (FPR) with f-Met-Leu-Phe (fMLF) substantially reduced the neutrophil superoxide production induced by activation of the CXC receptors with IL-8. When the order of agonists was reversed, the cells were primed in their response to fMLF, suggesting that the signalling hierarchy between strong, so-called end-type (i.e. fMLF) and weak or intermediate-type (i.e. IL-8) chemoattractants, is also operating during activation of the NADPH-oxidase. The same result was obtained when fMLF was replaced with the hexapeptide, WKYMVM, specific for the formyl peptide-like receptor 1 (FPRL1). There were additional differences between the agonist receptor pairs fMLF/FPR, WKYMVM/FPRL1 and IL-8/CXCR. In contrast to FPR and FPRL1, no reserve pool of CXCR was present in subcellular granules and it was impossible to prime the oxidative response transduced through CXCR by the addition of priming agents such as tumour necrosis factor-α and platelet-activating factor. Moreover, the cytoskeleton-disrupting substance, cytochalasin B, had no effect either on IL-8-triggered oxidase activation or on CXCR reactivation. A pertussis toxin-sensitive G-protein is involved in signalling mediated through both FPR and CXCR, and the signalling cascades include a transient intracellular calcium increase, as well as downstream p38 MAPK and phosphoinositide 3-kinase activation. The data presented in this study provide support for two different signalling pathways to the neutrophil NADPH-oxidase, used by ligand binding to FPR/FPRL1 or CXCR, respectively.
Keywords: cytoskeleton, GPCR, priming
Introduction
Neutrophils play a key role in the innate immune response to infection. These phagocytic cells act at inflammatory sites, which they reach after extravasation from the bloodstream.1 The extravasation process is induced and directed by different chemoattractants that work either in parallel or in sequence to recruit the inflammatory cells to the infected site. The neutrophil chemoattractants comprise bacterial products, processed complement components and lipid metabolites, as well as a large number of chemokines.2 The chemoattractants are recognized by specific cell-surface receptors, which, upon occupation, prime or activate different downstream functional molecules. The cells are then desensitized for subsequent stimulation with the same stimulus.3 The magnitude of the cellular response thus depends not only on the nature of the agonist, but also on the level of expression of the specific receptors involved (the signalling capacity) as well as on the status (ready to go/primed/down-regulated), of these receptors.3–6
Despite large structural differences between chemoattractants, they all bind to (and activate) specific receptors that belong to the same family of pertussis toxin-sensitive G-protein linked receptors (GPCR). These receptors possess a high degree of structural similarities in the signalling domains, which has made it reasonable to assume that the receptors transduce the same downstream signals, even though they are activated by different agonists.7 Accordingly, binding of a chemoattractant to its specific cell-surface receptor induces a dissociation of the G-protein βγ subunit from its α subunit. The dissociated βγ subunit (and possibly also the α subunit) directly activates downstream signalling molecules, including phosphoinositide 3-kinase (PI3K), G-protein-coupled receptor kinases (GPKs), small GTP-binding proteins, and mitogen-activated protein kinase (MAPK). The dissociated subunits also activate phosphoinositide-specific phospholipase C (PLC) that, upon cleavage of membrane phospholipids, produce the second messengers responsible for activation of protein kinase C and an elevation of intracellular free calcium.2,3,8
Chemoattractants differ regarding their functional repertoire, illustrated by the fact that although equally potent as chemotactic agents, they might differ in their abilities to function as effective secretagogues.3 These differences suggest that the signalling events induced by chemoattractants are, in some respects, receptor specific. The fact that activation and desensitization of one receptor may also affect a non-ligated receptor of another specificity (heterologous desensitization) suggests that there is a hierarchical cross-talk between the different receptors. The distinctive character of chemoattractant receptors was recently highlighted when Bryan and co-workers disclosed the existence of an intracellular signalling hierarchy between what was termed intermediate- and end-type attractants.9 An end-type chemoattractant was defined as one that is of importance during the latter phase of the tracking process, bacterial derived formylated peptides (fMLF) and activated complement component (C5a) being prominent examples. An intermediate-type attractant was defined as one of importance in the early stage of neutrophil recruitment, e.g. interleukin-8 (IL-8) and LTB4.9 The hierarchy between the different receptor–ligand pairs is evident in a model system containing competing gradients. Neutrophils migrate towards strong end-target chemoattractants, even when the cells at the same time experience a steep concentration gradient, in the opposite direction, of an intermediate-type chemoattractant. The basis for this hierarchy has been suggested to be a dominance on the receptor signalling level, involving the P38 MAPK or PI3K pathways activated by end-type or intermediate-type chemoattractants, respectively.9
The formylated peptide, fMLF (an end-type attractant), and IL-8 (an intermediate-type attractant), tightly regulate many cellular activities, including activation of the NADPH-oxidase,3 and it is reasonable to assume that the receptor hierarchy and the difference in signalling between the receptors involved has implications also for activation of the oxidase. The aim of this study was to further characterize the neutrophil NADPH-oxidase activity triggered through the formyl peptide receptors (FPR and FPRL1) and chemokine IL-8 receptors (CXCR1 and CXCR2). We show that the NADPH-oxidase activity triggered by FPR/FPRL1, on the one hand, and CXCR1/CXCR2, on the other, differed with respect to the time required for onset and termination. Desensitization experiments revealed a receptor hierarchy where FPR/FPRL1 dominated over CXCR. Furthermore, only FPR/FPRL1 could be reactivated by dissociation of the receptor from the cytoskeleton and augmented by classical priming agents. There was no reserve pool of CXCR present in the neutrophil granules, but the basic signalling pathways activated were the same for the agonists, including calcium influx, and dependency for PI3K and p38 MAPK.
Materials and methods
Chemicals and reagents
Isoluminol, fMLF, platelet-activating factor (PAF), tumour necrosis factor-α (TNF-α), cytochalasin B and fluorescein isothiocyanate (FITC)-conjugated phalloidin were obtained from Sigma Chemical Co. (St Louis, MO). The hexapeptide Trp-Tyr-Met-Val-Met-NH2 (WKYMVM) was synthesized and purified, using HPLC, by Alta Bioscience (University of Birmingham, Birmingham, UK). Horseradish peroxidase (HRP) was purchased from Boehringer-Mannheim (Mannheim, Germany). Recombinant IL-8, from R & D Systems (Minneapolis, MN), was diluted in KRG [Krebs-Ringer phosphate buffer containing glucose (10 mm), Ca2+ (1 mm), and Mg2+ (1·5 mm), pH 7·3] containing 0·5% bovine serum albumin (BSA) and stored at −70°. Wortmannin and SB 203580 were from Calbiochem (La Jolla, CA). Dextran and Ficoll–Paque were from Pharmacia (Uppsala, Sweden). Fura2/AM was from Molecular Probes Inc. (Eugene, OR). Polyclonal antibodies against CXCR1 and CXCR2 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Isolation of human neutrophils
Neutrophil granulocytes were isolated from buffy coats obtained from healthy adults. After dextran sedimentation at 1 g, hypotonic lysis of the remaining erythrocytes and centrifugation in a Ficoll–Paque gradient, the neutrophils were washed twice and resuspended (1 × 107/ml) in KRG, pH 7·3. The cells were stored on ice until use. This isolation process permits cells to be purified with minimal granule mobilization.10
Neutrophil NADPH-oxidase activity
Neutrophil superoxide anion production and release was determined using isoluminol-enhanced chemiluminescence (CL).11 The CL activity was measured in a 6-channel Biolumat LB 9505 (Berthold Co., Wildbad, Germany) using disposable 4-ml polypropylene tubes with a 0·5-ml reaction mixture. The measuring tubes containing cells (106/ml), isoluminol (2 × 10−5m), and HRP (2 U) were equilibrated at 37° for a minimum of 5 min before 50 µl of stimulus was added. The light emission was recorded continuously.
Neutrophil priming, desensitization and resensitization
To prime the neutrophils, cells (106/ml) were incubated at 37° with TNF-α (25 ng/ml; 20 min), cytochalasin B (2 µg/ml; 5 min) or PAF (100 nm; 10 min), giving an optimal priming effect, according to the literature.12 Control cells were incubated at 37° for the corresponding time without priming agent. After priming, the cells were stimulated with fMLF (10 nm) or IL-8 (12·5 nm), and the release of superoxide was recorded. To induce desensitization without accompanying oxidase activation, neutrophils were incubated with an agonist for 10 min at 15°. Desensitized cells were then transferred to 37° and equilibrated for 10 min, followed by stimulation (resensitization) with the cytoskeleton-disrupting agent, cytochalasin B (2 µg/ml).13
Determination of changes in F-actin content
Neutrophils (107 cells/ml) were allowed to equilibrate at 37° for 10 min before stimulation with fMLF (10 nm) or IL-8 (12·5 nm) for 30 seconds. The neutrophils were then fixed with ice-cold paraformaldehyde (4%) for 30 min, washed with phosphate-buffered saline (PBS) and incubated with FITC-phalloidin (100 µg/ml) in PBS supplemented with 1 mg/ml lysophosphatidylcholine. Phalloidin labelling was performed at room temperature for 40 min, and the fluorescence, corresponding to F-actin content, was analysed by fluorescence-activated cell sorter (FACScan; Becton Dickinson, Mountain View, CA).
Subcellular fractionation and marker analysis
Subcellular fractionation was performed as described by Borregaard et al.14 Briefly, neutrophils were treated with the serine protease inhibitor, diisopropyl fluorophosphate (DFP, 8 µm), disintegrated by nitrogen cavitation (Parr Instruments Co., Moline, IL), and the postnuclear supernatant was fractionated on Percoll gradients. Fractions of 1·5 ml were collected by aspiration from the bottom of the centrifuge tube. The localization of subcellular granules was determined by granule marker analysis. Alkaline phosphatase (marker for secretory vesicles/plasma membrane) was measured by hydrolysis of p-nitrophenyl phosphate, at pH 10·5, in a sodium barbital buffer. The concentration of vitamin B12-binding protein (a marker for specific granules) was determined using the cyanocobalamin technique15 and myeloperoxidase (a marker for azurophil granule) was analysed by enzyme-linked immunosorbent assay (ELISA).
Neutrophil granule mobilization and surface receptor expression
The exposure/mobilization of the IL-8 receptors CXCR1 and CXCR2 was determined by flow cytometry. Neutrophils (final concentration, 106 cells/ml) were either stored at 4° or 37°, or primed with PAF (100 nm) or TNF-α (25 ng/ml) at 37° for 20 min, and then fixed with ice-cold paraformaldehyde (4%) before staining. Cells were labelled with anti-CXCR1 and FITC-conjugated secondary immunoglobulin, or phycoerythrin-conjugated anti-CXCR2 immunoglobulin. The amount of fMLF-receptors expressed on the cell surface was determined by incubating the cells with radiolabelled fMLF in the presence or absence of unlabelled fMLF, as described previously.16 CR3 mobilization, determined through binding of a phycoerythrin-conjugated anti-CD11b immunoglobulin, was used to monitor the degree of mobilization of neutrophil granules.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting
Percoll gradient fractions, prepared as described above, were diluted in non-reducing sample buffer, boiled for 5 min, and applied to 10% SDS–polyacrylamide gels. The separated proteins were transferred to nitrocellulose membranes, followed by blocking the membrane at room temperature for 1 hr in 1% BSA. After blocking, the blots were incubated overnight at 4° with primary antibodies against CXCR1 or CXCR2. Non-bound antibodies were removed by washing with PBS-Tween. An HRP-labelled secondary antibody and a peroxidase substrate (VIP kit; Vector Laboratories, Burlingame, CA) was used for visualization of the receptors.
Determination of changes in cytosolic calcium
Neutrophils (5 × 107 cells/ml) were incubated with acetoxymethylated fura-2 (2 µm), in Ca2+-free KRG supplemented with BSA (0·1%), at room temperature for 30 min, protected from light. The cells were then washed twice, resuspended in KRG and adjusted to 2 × 107/ml. For some experiments, the cells were desensitized at 15° with fMLF (10 nm) or IL-8 (12·5 nm) before use. To measure [Ca2+]i, the cells (2 × 106/ml) were equilibrated at 37° for 10 min in 4-ml cuvettes before adding the stimulus. The fura-2 fluorescence was measured using a luminescence spectrometer (LS50B; Perkin-Elmer Life Sciences, Wellesley, MA), and the changes in fluorescence were followed at an emission wavelength of 510 nm and a dual-excitation wavelength system at 340 nm and 380 nm, respectively. The change in intracellular Ca2+ was expressed as the ratio between the emission values of Fura-2 when excited at 340 nm and 380 nm.
Statistic analysis
Two-tailed, paired Student's t-tests were performed to determine statistical significance, and a P-value of < 0·05 was regarded as significant.
Results
The neutrophil NADPH-oxidase is activated by the end-type chemoattractant, fMLF, as well as by the intermediate-type chemoattractant, IL-8
Assembly and activation of the neutrophil NADPH-oxidase leads to the production and release of reactive oxygen species (ROS). Neutrophils can generate ROS both intracellularly and extracellularly, depending on the localization of oxidase assembly which, in turn, is dependent on the nature of the stimulus.17 When stimulating neutrophils with the end-type chemoattractant, fMLF, or the intermediate-type chemoattractant, IL-8, extracellular ROS release was triggered (Fig. 1a). No intracellular ROS production was detected with the chemoattractants as triggering agents (data not shown). Both fMLF and IL-8 induced dose-dependent extracellular ROS production in neutrophils (Fig. 1b), but, compared with fMLF, IL-8 was a fairly poor activator. fMLF induced maximal oxidase activity at 100 nm, with a calculated 50% effective concentration (EC50) value of ≈ 20 nm. For IL-8, the concentration interval used in the study did not reach a maximal cellular response, making it impossible to determine the EC50. We can, however, estimate the EC50 to be higher than 100 nm of the cytokine.
Figure 1.
Human neutrophil NADPH-oxidase activation induced by the chemoattractants f-Met-Leu-Phe (fMLF) and interleukin-8 (IL-8). Neutrophils (1 × 106/ml) were preincubated for 5 min at 37° and then stimulated with different concentrations of fMLF (the concentrations used are shown in the left panel of b) or IL-8 (the concentrations used are shown in the right panel of b). The release of superoxide was measured and the time course of the response determined. (a) Representative curves corresponding to the production induced by four different concentrations (see b) of fMLF and three different concentrations (see b) of IL-8. The higher concentration of ligand resulted in a larger magnitude of cellular response. (b) The peak values were determined from three independent dose–response experiments (see a) and the numerals represent the mean ± standard deviation (SD) values for the different concentrations of fMLF (left) and IL-8 (right). (c) Time to reach the peak value for superoxide release after fMLF- (left panels) and IL-8- (right panels) induced stimulation. Results are expressed as mean ± standard deviation (SD) (n = 4; P < 0·05). (d) Time to reach a background value (duration of response) for superoxide release after fMLF- (left) and IL-8- (right) induced stimulation. Results are expressed as mean ± SD (n = 4; P < 0·05).
To compensate for the different EC50 values, all subsequent experiments were performed with a concentration of fMLF (10 nm) that activates the oxidase to a degree similar to that induced by IL-8. The NADPH-oxidase activity induced by fMLF and IL-8 differed not only with respect to the magnitude of the responses, but also with respect to the kinetics. The fMLF-induced response was characterized by a rapid onset, peaking after ≈ 60 seconds (Fig. 1c), and the time course was similar, regardless of whether a high or a low concentration of fMLF was used to trigger the burst. The NADPH-oxidase activity induced by IL-8 had an even more rapid onset, with a peak value reached in ≈ 20 seconds (Fig. 1c). The fMLF-induced response returned to baseline after ≈ 5 min, whereas the IL-8-induced response returned to baseline after only 2 min (Fig. 1d). This suggests that the time during which the receptors are actively signalling differs between occupied FPR and CXC receptors.
When the neutrophils were challenged with the hexapeptide, WKYMVM, the basic characteristics of the NADPH-oxidase response were the same as those for fMLF (data not shown), suggesting that the signalling properties of the formyl peptide receptor (FPR; activated by fMLF) and the formyl peptide like receptor 1 (FPRL1; activated by WKYMVM) are similar.
Termination of the response and linkage to the cytoskeleton
The IL-8-induced NADPH-oxidase response was terminated more rapidly than those induced by fMLF or WKYMVM, suggesting differences in the termination/deactivation process. It is well known that the cytoskeleton plays an important role in this process, and that it is affected by the cytoskeleton-disrupting agent, cytochalasin B. The response induced by fMLF was augmented by up to threefold in the presence of cytochalasin B, both with regard to peak value (Fig. 2a) and duration (data not shown) of the response. This confirms that the cytoskeleton takes part in terminating the signalling from FPR. This was true also for the FPRL1-mediated response (data not shown). In contrast, no such cytochalasin B-dependent effect was seen on the IL-8-induced oxidative response, regarding neither the magnitude nor the kinetics (Fig. 2a). Despite the fact that IL-8 triggered ROS production independently of the cytoskeleton, the signals generated by the occupied receptor still included the second messengers responsible for the induction of actin polymerization, in a manner similar to the induction induced by fMLF, as measured by phalloidin staining (Fig. 2a).
Figure 2.
Involvement of the cytoskeleton in f-Met-Leu-Phe (fMLF)- and interleukin-8 (IL-8)-triggered neutrophil activity. (a) The direct triggering effects of fMLF and IL-8 on neutrophil actin polymerization were determined, as were the indirect effects of cytochalasin B (CytB) on NADPH-oxidase activity induced by the two agonists. Neutrophils (1 × 106/ml) were incubated with or without CytB (2 µg/ml) for 5 min at 37° and then stimulated with fMLF (10 nm; upper panel) or IL-8 (12·5 nm; lower panel). The potentiating effect induced by CytB was calculated from the peak values and is expressed as the ratio (fold increase) between treated and untreated cells. Results are expressed as mean ± standard deviation (SD); n = 3. The amount of polymerized actin induced by fMLF (10 nm; upper panel) or IL-8 (12·5 nm; lower panel) was determined through phalloidin staining and is expressed as the ratio (fold increase) between stimulated (with fMLF or IL-8) and non-stimulated (mean ± SD; n = 3) cells. (b) A representative experiment showing reactivation by CytB. The neutrophils were desensitized (at 15°) to fMLF (10 nm; upper panel) or IL-8 (12·5 nm; lower panel). The arrow shows the addition of CytB. To illustrate the difference between the two stimuli, ordinary control experiments on non-desensitized cells activated by fMLF (upper panel; solid line) and IL-8 (lower panel; solid line) are also shown. Note that the duration of the experiments are different for fMLF and IL-8. CL, chemiluminescence; c.p.m., counts per minute.
The molecular basis for the cytoskeleton-dependent termination of FPR signalling is the direct cessation of the transmembrane signals when the ligated receptor segregates from the signalling G-protein through binding to the cytoskeleton,18 an event known to follow shortly after binding of fMLF to FPR.19 If binding of fMLF takes place at or below 15°, the chronological order in which the signalling/activation/deactivation steps normally occur is broken,20 and the receptor is directly associated with the cytoskeleton and desensitized without signalling to activate the oxidase. We have previously shown that if the association with the cytoskeleton is broken, the receptor (FPR) is resensitized and the NADPH-oxidase is activated (Fig. 2b).13 The same result is obtained with WKYMVM (data not shown). However, in contrast to FPR and FPRL1, the occupied CXCR could not be reactivated by disruption of the cytoskeleton (Fig. 2b).
TNF-α and PAF do not prime the neutrophil response mediated by CXCR
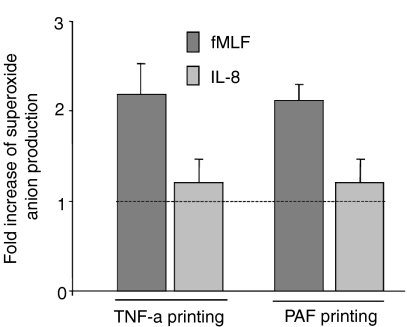
The level of NADPH-oxidase activity is dependent not only on the specific stimulus used, but also on the state of the cells that are triggered. With respect to the amount of superoxide released, neutrophils can be primed in their response (i.e. hyper-responsive) to fMLF or WKYMVM by prior exposure of the cells to priming agents such as TNF-α, granulocyte–macrophage colony-stimulating factor (GM-CSF) and low doses of chemoattractants.21,22 In order to determine the similarities/differences between FPR/FPRL1 agonists and IL-8 with respect to priming, neutrophils were pretreated with non-activating concentrations of TNF-α and PAF before stimulation with fMLF or IL-8, respectively. In agreement with previous findings, neutrophils exposed to TNF-α and PAF were primed with respect to the fMLF- (Fig. 3) and WKYMVM- (data not shown) induced responses. However, no such priming effect was seen for the IL-8-induced oxidative response (Fig. 3).
Figure 3.
Priming effect on the f-Met-Leu-Phe (fMLF)- or interleukin-8 (IL-8)-triggered neutrophil NADPH-oxidase response by platelet-activating factor (PAF) and tumour necrosis factor-α (TNF-α). Neutrophils were primed with PAF (100 nm, 10 min) or TNF-α (25 ng/ml, 20 min) at 37°. The cells were then activated with fMLF (10 nm) or IL-8 (12·5 nm) and the release of superoxide anions was followed. Oxidase activity was determined in parallel with control cells treated in the same way, but in the absence of any priming agent, and the results are presented as the ratio (fold increase of the peak values) between the responses in primed and non-primed cells. Results are expressed as mean ± standard deviation (SD); n = 3.
We have previously shown that one of the mechanisms involved in neutrophil priming relies on receptors in the neutrophil granules and secretory vesicles being recruited to the cell surface through granule mobilization.16,23,24 The inability of TNF-α and PAF to prime neutrophils in response to IL-8, suggests the lack of a reserve pool of receptors in the membranes of the granules.
Neutrophils lack an easily mobilizable reserve pool of IL-8 receptors
Subcellular localization studies on resting neutrophils have shown that complement receptor 3 (CR3), as well as receptors belonging to the FPR-family, are stored in the specific and gelatinase granules and, to a minor extent, also in the mobilizable secretory vesicles.23,25 To investigate the role of receptor up-regulation in TNF-α priming, we monitored the amount of CXCR1 and CXCR2 mobilized to the cell surface during the priming procedure by FACS analysis. In line with the lack of priming by TNF-α, in respect to IL-8-induced oxidase activation, there was no increase in the surface expression of either CXCR1 or CXCR2. In fact, a slight down-regulation of both receptors was observed (Fig. 4). In contrast, an increased amount of CR3 was exposed on the surface of TNF-α-primed cells (Fig. 4), suggesting that the integrin-storing organelles were mobilized. Accordingly, TNF-α priming was accompanied by an increased specific binding of radiolabelled fMLF [10·2 ± 3·3 fmol/106 cells bound to TNF-α-treated cells compared with 4·0 ± 1·6 fmol/106 cells for corresponding control cells; mean ± standard error of the mean (SEM), n = 6], reflecting an increased amount also of formyl peptide receptors (FPR) on the surface of the primed neutrophils. Hence, receptor mobilization may explain the increased oxidative response in the primed cells when challenged with fMLF. When TNF-α was replaced with PAF, a similar pattern of CXCR and CR3 expression was obtained (data not shown).
Figure 4.
The surface expression of CR3 (CD11b) and CXCR1/CXCR2 after priming with tumour necrosis factor-α (TNF-α). Neutrophils primed with TNF-α (25 ng/ml) (dotted lines) at 37° for 20 min, and the corresponding control cells (solid lines), were fixed in paraformaldehyde and labelled with antibodies directed against CXCR1, CXCR2 and CR3, respectively. The amount of receptor expression was determined by fluorescence-activated cell sorter (FACS) analysis, and, as the control curves for CXCR1 and CXCR2 were overlapping, only the curve corresponding to the exposure of CXCR1 is shown.
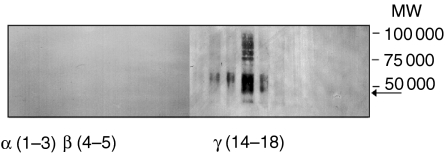
To find support for our hypothesis that the inability to prime the IL-8 response is the result of a lack of mobilizable receptors, we determined the subcellular localization of the IL-8 receptors in resting cells using a Percoll gradient fractionation technique. In accordance with our hypothesis, CXCR1, as well as CXCR2, were present exclusively in the neutrophil γ fraction (containing the plasma membrane and the easily mobilizable secretory vesicles, while no receptors were found in the granule-enriched α or β fractions (shown for CXCR2 in Fig. 5).
Figure 5.
Localization of CXCR2 in the subcellular fractions of neutrophils. Neutrophil subcellular organelles were isolated following fractionation of disintegrated cells on a Percoll gradient. Marker analysis revealed that the azurophil granules (α) were enriched in fractions 1–3, specific and gelatinase granules (β) in fractions 4–5, and the plasma membrane/secretory vesicles (γ) in fractions 14–18. Proteins from each fraction were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (10% gel), and the localization of CXCR2 was determined through blotting with antibodies directed against this receptor. The 40 000 molecular weight (MW) protein corresponding to CXCR2 was detected in the plasma membrane-enriched fractions, whereas no binding of the antibody was obtained to the proteins in the granule-enriched fractions. The MW of the monomeric form of the receptor, as determined from the amino acid sequence, is indicated by an arrow.
FPR and CXCR display a hierarchical pattern of receptor desensitization
The NADPH-oxidase activating signalling is rapidly initiated when cells are triggered by a chemoattractant, but the response does not persist for more than a couple of minutes. This transient response can partly be accounted for by receptor desensitization. Accordingly, we have shown that fMLF-desensitized neutrophils are unable to respond to a new dose of fMLF, while being fully responsive to an agonist that activate the cells through FPRL1 (e.g. WKYMVM). There is no dominance for either of these two receptors, as illustrated by the fact that the same result was obtained when the order of the two peptides was reversed.26,27 Here, similar experiments were carried out with fMLF and IL-8. When the cells were first challenged with fMLF, they were unable to respond to subsequent stimulation with IL-8 (Fig. 6). In contrast, the cells that received IL-8 first were still fully responsive (or actually even primed) to the second stimulation by fMLF (Fig. 6). The same desensitization hierarchy was found when fMLF was replaced with the hexapeptide WKYMVM (data not shown). These results strongly support the concept suggesting the existence of an intracellular hierarchical signal cross-talk between chemoattractant receptors.28
Figure 6.
Cross-desensitization between CXCR and the formyl peptide receptor (FPR). Neutrophils (1 × 106/ml), activated by (and subsequently desensitized to) f-Met-Leu-Phe (fMLF) (10 nm), were challenged with interleukin-8 (IL-8) (12·5 nm); the formyl peptide was added to the cells at time 0 and IL-8 was added 5 min later (dotted line). In parallel, neutrophils (1 × 106/ml) activated by (and subsequently desensitized to) IL-8 (12·5 nm), were challenged with fMLF (10 nm); IL-8 was added to the cells at time 0 and fMLF was added 5 min later (solid line). The release of superoxide anion was determined during a 10-min period following addition of the initial stimulus, and the curves are from a representative experiment. CL, chemiluminescence; c.p.m., counts per minute.
Common intracellular signalling molecules are shared by FPR and CXCRs
Treatment of neutrophils with pertussis toxin resulted in an inhibition of both FPR- and CXCR-induced oxidase activity (data not shown), in agreement with the fact that both receptors are coupled to pertussis toxin-sensitive G proteins. An immediate consequence of the association of a chemoattractant to its receptor, and the subsequent dissociation of the G-protein from the same receptor, is the activation of phospholipase C and the production of inositol 1,4,5-triphosphate, leading to a transient elevation of the intracellular calcium. Accordingly, a calcium response was detected within seconds after the cells were stimulated with either fMLF or IL-8 (Fig. 7a,b). Surprisingly, there was no direct link between the Ca2+ response and the oxidase activity, illustrated by the fact that a very low Ca2+ response was obtained in desensitized cells when they were reactivated with cytochalasin B, regardless of whether an oxidase activation was induced (Fig. 7c; positive with respect to activation of the oxidase) or not (Fig. 7d; negative with respect to activation of the oxidase).
Figure 7.
Levels of cytosolic calcium in human neutrophils during the direct activation or cytochalasin B-induced reactivation of desensitized cells. (a, b) Direct activation of Fura-2 loaded control neutrophils with f-Met-Leu-Phe (fMLF) (10 nm; left) or interleukin-8 (IL-8) (12·5 nm; right). The arrows indicate addition of agonist. (c, d) Fura-2-loaded neutrophils desensitized with fMLF (10 nm; left) or IL-8 (12·5 nm; right), as described in the text, were reactivated through the addition of cytochalasin B (2 µg/ml). The arrows indicate the addition of cytochalasin B. The changes in cytosolic calcium levels were determined through measurement of the fluorescence, emitted at 510 nm, during excitation at 340 and 380 nm. The curves are from one representative experiment out of three. Abscissa, time of study in seconds; ordinate, the level of intracellular Ca2+ expressed as the 340/380 nm ratio.
To investigate whether the signalling pathways used by FPR and CXCR, and leading to activation of the oxidase, differed in a manner similar to the differences described for regulation of chemotaxis,9 PI3K and p38 MAPK inhibitors were used. Wortmannin (a PI3K-specific inhibitor) markedly inhibited both fMLF and IL-8-induced superoxide production with a similar concentration-dependency for both agonists (IC50 = 2·1 nm for fMLF and 3·8 nm for IL-8). There was no difference in inhibitory profile of the fMLF- and IL-8-induced response when Wortmannin was exchanged for the p38 MAPK-specific inhibitor, SB 203580 (IC50 = 38·2 µm for fMLF and 30·2 µm for IL-8). Hence, both PI3-kinase and p38 MAPK are essential components of the pathways leading to activation of neutrophil NADPH-oxidase, regardless of whether the activation is induced by the end-type chemoattractant fMLF or the intermediary type IL-8.
Discussion
Members of the CXC and FPR receptor families all induce multiple neutrophil functions, including cell migration, granule secretion and superoxide anion generation. As all belong to the pertussis toxin-sensitive GPCR superfamily, it has been generally accepted that they possess many signalling similarities. However, it was recently shown that the formylated peptide, fMLF (a ligand for the FPR) dominates over the chemotactic cytokine, IL-8, binding to the CXCRs when it comes to guide neutrophil migration.9 Here, we show that a signalling hierarchy, similar to that described in migration, exists between FPR/FPRL1 and CXCRs with regard to NADPH-oxidase activation, while there is an equal relationship (no domination) between FPR and FPRL1. In addition, we show that there are fundamental differences between FPR/FPRL1 and CXCR with respect to their utilization of different mechanisms leading to oxidase activation/deactivation.
TNF-α and PAF were both able to prime the neutrophils in response to fMLF and WKYMVM, while there was no priming of the IL-8 response. Based on previous studies,16,23,24,29,30 we know that receptor mobilization from intracellular storage granules is a major mechanism for neutrophil priming and we thus decided to investigate whether the lack of stored CXCRs could explain why the priming was missing in the IL-8 response. Accordingly, subcellular fractionation and receptor-mobilization studies revealed that the neutrophil CXCRs are present exclusively on the plasma membrane. These results are in agreement with previously published data showing that radiolabelled IL-8 binds exclusively to the neutrophil plasma membrane.31 The FPR/FPRL1 are, in contrast, to a large extent stored in granules. Minor fractions of these two receptors are distributed equally between the plasma membrane and the secretory vesicles.23,25 In agreement with their respective subcellular localization, no increased surface expression of CXCR was obtained, concomitant with FPR and CR3 mobilization induced by the priming agents. Rather, incubation of neutrophils in the presence of priming agents down-regulated the levels of surface-exposed CXCRs. This down-regulation of CXCRs, induced by TNF-α, has been suggested by others to be caused by receptor internalization and/or proteolytic cleavage of surface-exposed receptors.32,33 The difference in localization of FPRs and CXCRs probably reflects the different nature of their agonists as being end-point or intermediate chemoattractants, respectively. In the first stage of migration, neutrophils sense mainly intermediate chemoattractants, such as IL-8, and thus it is reasonable that the corresponding receptors are expressed on the cell surface. When end-type chemoattractant gradients take over later in the extravasation process, the neutrophils have already mobilized their granules and, by doing so, expose their receptors for end-point chemoattractants.
The neutrophil formyl peptide family of receptors comprise two members: the classical FPR, activated by formylated peptides; and FPRL1, also known as the lipoxin A4 receptor.34,35 FPRL1 is a promiscuous receptor that has been shown to recognize not only WKYMVM/m, but also two synthetic peptides derived from human immunodeficiency virus-1 (HIV-1) (a leucine zipper-like domain of the HIV-1 envelope glycoprotein 41 and a sequence from the V4-C4 region of glycoprotein 120), a cecropin-like peptide from Helicobacter pylori and the acute-phase reactant serum amyloid A (SAA).36–38 The two receptors not only possess a high degree of amino acid identity in the signalling cytoplasmic domains, but also have a high degree of functional identity.13,39 In line with this, we found that FPR and FPRL1 rank in the same category with regard to being superior to CXCR in activation of the NADPH-oxidase.
Chemotaxis has been shown to be regulated by two different signalling pathways, which allows for one receptor to dominate over another.9 The chemokine IL-8 receptor family comprises two members, CXCR1 and CXCR2, and although IL-8 has high affinity for both receptors, it has been suggested that IL-8-induced oxidase activation is mediated through CXCR1 exclusively.40 This suggestion was based on inhibition studies with monoclonal antibodies. It should, however, be noted that the CXCR1 antibody used blocked only ≈ 60% of the IL-8-induced oxidative response, indicating that CXCR2 may also be involved. This gains support from the fact that cytokines such as NAP-2 and Gro-α, also binding exclusively to CXCR2, can induce activation of the neutrophil NADPH-oxidase.41,42 It is, however, with respect to our data, of minor importance whether the activity is mediated through one or the other of these receptors, as they are both localized to the same subcellular compartment.
The differences between FPR and CXCR are not only reflected in the time-course of superoxide production induced by their respective ligand; more importantly, we found that the mechanism for termination of the FPR- and CXCR-mediated responses differed. The basic mechanism for termination of the FPR-induced response has been suggested to be a lateral segregation of the G-protein from the receptor–ligand complex in the plasma membrane, achieved through a direct binding of the ligand–receptor complex to the cytoskeleton.19,43,44 Accordingly, the presence of the cytoskeleton-disrupting agent, cytochalasin B, leads to augmentation and prolongation of the FPR-induced oxidative response. Furthermore, addition of this compound to fMLF-deactivated cells leads to a rapid reactivation of FPR. The fact that no such effects were seen with the IL-8-triggered response suggests that the termination mechanism for CXCR does not involve the cytoskeleton. Instead, phosphorylation and internalization of the agonist-occupied, cytoskeleton-uncoupled receptor might be alternative mechanisms for termination of the response.3
An immediate consequence of FPR/FPRL1 and CXCR activation is the production of inositol 1,4,5-triphosphate and a subsequent transient elevation of intracellular Ca2+. The lipid remodelling is mediated by phosphoinositide kinases, phospholipase (PL)D, PLA2 and a phosphoinositide-specific PLC. The transient elevation of Ca2+ has been claimed to be required, but not sufficient, for the generation of an NADPH-oxidase activating signal from FPR.45 However, previously published data,13 as well as those presented in this study, suggest that the neutrophil NADPH-oxidase can be activated without any significant increase of cytosolic Ca2+.
The ligation of FPR and CXCR activate multiple downstream signal cascades. Along the lines of hierarchical cross-talk used in neutrophil migration,9 we determined the inhibitory profiles of p38 MAPK and PI3K inhibitors on the neutrophil oxidative response. Distinct from the hierarchical signalling pattern used for cell migration, we found that fMLF, as well as IL-8, activated the neutrophil NADPH-oxidase using both p38 MAPK and PI3K. Hence, the molecular mechanism behind the chemotaxis hierarchy and the respiratory burst hierarchy is obviously not the same. A common and distinct intracellular signalling pathway in neutrophils has also been found by fMLF and the other intermediary chemoattractants, e.g. PAF. Stimulation with both PAF and fMLF result in equivalent phosphorylation and activation of p38 MAPK; however, fMLF, but not PAF, triggered a significant p42/44 (ERK) MAPK activation.46 Thus, a fine regulation of neutrophil function occurs through activation of a spectrum of signalling pathways. For each stimulus, a distinctive signal may exist, or, alternatively, various combinations of signalling pathways may be used for distinct cellular functions. The data presented provide support for two mechanisms operating during activation and termination of the neutrophil NADPH-oxidase by intermediate- and end-type chemoattractants. We have, however, not yet been able to directly identify the precise and unique signals generated by these two classes of receptors.
Acknowledgments
The work was supported by the Swedish Medical Research Council, the King Gustaf V 80-Year Foundation, the Royal Society of Arts and Sciences in Göteborg and the Swedish Medical Society.
References
- 1.Cramer EB. Cell biology of phagocyte migration from the bone marrow, out of the bloodstream, and across organ epithelia. In: Gallin JI, Goldstein IM, Snyderman R, editors. Infalmmation. Basic Principles and Clinical Correlates. New York: Raven Press; 1992. pp. 341–451. [Google Scholar]
- 2.Bokoch GM. Chemoattractant signalling and leukocyte activation. Blood. 1995;86:1649–60. [PubMed] [Google Scholar]
- 3.Uhing RJ, Snyderman R. Chemoattractant stimulus-response coupling. In: Gallin JI, Snyderman R, editors. Inflammation. Basic Principles and Clinical Correlates. Philadelphia, Lippincott: Williams & Wilkins; 1999. pp. 607–26. [Google Scholar]
- 4.Haribabu B, Richardson RM, Verghese MW, Barr AJ, Zhelev DV, Snyderman R. Function and regulation of chemoattractant receptors. Immunol Res. 2000;22:271–9. doi: 10.1385/IR:22:2-3:271. [DOI] [PubMed] [Google Scholar]
- 5.Christophe T, Rabiet MJ, Tardif M, Milcent MD, Boulay F. Human complement 5a (C5a), anaphylatoxin receptor (CD88), phosphorylation sites and their specific role in receptor phosphorylation and attenuation of G protein-mediated responses. Desensitization of C5a receptor controls superoxide production but not receptor sequestration in HL-60 cells. J Biol Chem. 2000;275:1656–64. doi: 10.1074/jbc.275.3.1656. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Levy BD, Clish CB, Gronert K, Chiang N. Lipoxins, aspirin-triggered 15-epi-lipoxin stable analogs and their receptors in anti-inflammation: a window for therapeutic opportunity. Ernst Schering Res Found Workshop. 2000;31:143–85. doi: 10.1007/978-3-662-04047-8_8. [DOI] [PubMed] [Google Scholar]
- 7.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 8.Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993;73:797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- 9.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signalling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson T, Dahlgren C, Lew PD, Stendahl O. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in the cytosolic free Ca2+ level and action of phorbol myristate acetate. J Clin Invest. 1987;79:1226–33. doi: 10.1172/JCI112941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic Biol Med. 1996;20:785–92. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 12.Gougerot-Podicalo MA, Elbim C, Chollet-Martin S. Modulation of the oxidative burst of human neutrophils by pro- and anti-inflammatory cytokines. Pathol Biol (Paris) 1996;44:36–41. [PubMed] [Google Scholar]
- 13.Bylund J, Bjorstad A, Granfeldt D, Karlsson A, Woschnagg C, Dahlgren C. Reactivation of formyl peptide receptors triggers the neutrophil NADPH-oxidase but not a transient rise in intracellular calcium. J Biol Chem. 2003;278:30578–86. doi: 10.1074/jbc.M209202200. [DOI] [PubMed] [Google Scholar]
- 14.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau KS, Gottlieb C, Wasserman LR, Herbert V. Measurement of serum vitamin B12 level using radioisotope dilution and coated charcoal. Blood. 1965;26:202–14. [PubMed] [Google Scholar]
- 16.Almkvist J, Faldt J, Dahlgren C, Leffler H, Karlsson A. Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl-Leu-Phe. Infect Immun. 2001;69:832–7. doi: 10.1128/IAI.69.2.832-837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 18.Klotz KN, Jesaitis AJ. Physical coupling of N-formyl peptide chemoattractant receptors to G protein is unaffected by desensitization. Biochem Pharmacol. 1994;48:1297–300. doi: 10.1016/0006-2952(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 19.Jesaitis AJ, Klotz KN. Cytoskeletal regulation of chemotactic receptors. molecular complexation of N-formyl peptide receptors with G proteins and actin. Eur J Haematol. 1993;51:288–93. doi: 10.1111/j.1600-0609.1993.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 20.Jesaitis AJ, Tolley JO, Allen RA. Receptor–cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J Biol Chem. 1986;261:13662–9. [PubMed] [Google Scholar]
- 21.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94:461–71. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 22.Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–8. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 23.Bylund J, Karlsson A, Boulay F, Dahlgren C. Lipopolysaccharide-induced granule mobilization and priming of the neutrophil response to Helicobacter pylori peptide Hp (2–20), which activates formyl peptide receptor-like 1. Infect Immun. 2002;70:2908–14. doi: 10.1128/IAI.70.6.2908-2914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faldt J, Dahlgren C, Ridell M, Karlsson A. Priming of human neutrophils by mycobacterial lipoarabinomannans: role of granule mobilisation. Microbes Infect. 2001;3:1101–9. doi: 10.1016/s1286-4579(01)01470-8. [DOI] [PubMed] [Google Scholar]
- 25.Sengelov H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J. 1994;299:473–9. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlgren C, Christophe T, Boulay F, Madianos PN, Rabiet MJ, Karlsson A. The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A (4) receptor. Blood. 2000;95:1810–8. [PubMed] [Google Scholar]
- 27.Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276:21585–93. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 28.Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. J Biol Chem. 1999;274:6027–30. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg M, Lofgren R, Zheng L, Stendahl O. Tumour necrosis factor-alpha potentiates CR3-induced respiratory burst by activating p38 MAP kinase in human neutrophils. Immunology. 2001;103:465–72. doi: 10.1046/j.1365-2567.2001.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem. 2000;275:36713–9. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 31.Barnett ML, Lamb KA, Costello KM, Pike MC. Characterization of interleukin-8 receptors in human neutrophil membranes: regulation by guanine nucleotides. Biochim Biophys Acta. 1993;1177:275–82. doi: 10.1016/0167-4889(93)90123-7. [DOI] [PubMed] [Google Scholar]
- 32.Khandaker MH, Mitchell G, Xu L, et al. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93:2173–85. [PubMed] [Google Scholar]
- 33.Tikhonov I, Doroshenko T, Chaly Y, Smolnikova V, Pauza CD, Voitenok N. Down-regulation of CXCR1 and CXCR2 expression on human neutrophils upon activation of whole blood by S. aureus is mediated by TNF-alpha. Clin Exp Immunol. 2001;125:414–22. doi: 10.1046/j.1365-2249.2001.01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–60. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells. inhibition of CD11/18 expression by lipoxin A4–lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–86. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 36.Deng X, Ueda H, Su SB, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999;94:1165–73. [PubMed] [Google Scholar]
- 37.Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su SB, Gao J, Gong W, Dunlop NM, Murphy PM, Oppenheim JJ, Wang JM. T21/DP107, a synthetic leucine zipper-like domain of the HIV-1 envelope gp41, attracts and activates human phagocytes by using G-protein-coupled formyl peptide receptors. J Immunol. 1999;162:5924–30. [PubMed] [Google Scholar]
- 39.Betten A, Dahlgren C, Hermodsson S, Hellstrand K. Histamine inhibits neutrophil NADPH oxidase activity triggered by the lipoxin A4 receptor-specific peptide agonist Trp-Lys-Tyr-Met-Val-Met. Scand J Immunol. 2003;58:321–6. doi: 10.1046/j.1365-3083.2003.01301.x. [DOI] [PubMed] [Google Scholar]
- 40.Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA. 1996;93:6682–6. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walz A, Dewald B, von Tscharner V, Baggiolini M. Effects of the neutrophil-activating peptide NAP-2, platelet basic protein, connective tissue-activating peptide III and platelet factor 4 on human neutrophils. J Exp Med. 1989;170:1745–50. doi: 10.1084/jem.170.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jesaitis AJ, Erickson RW, Klotz KN, Bommakanti RK, Siemsen DW. Functional molecular complexes of human N-formyl chemoattractant receptors and actin. J Immunol. 1993;151:5653–65. [PubMed] [Google Scholar]
- 44.Klotz KN, Krotec KL, Gripentrog J, Jesaitis AJ. Regulatory interaction of N-formyl peptide chemoattractant receptors with the membrane skeleton in human neutrophils. J Immunol. 1994;152:801–10. [PubMed] [Google Scholar]
- 45.Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin Biochem. 1990;23:159–66. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 46.Nick JA, Avdi NJ, Young SK, Knall C, Gerwins P, Johnson GL, Worthen GS. Common and distinct intracellular signalling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–86. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]