Abstract
Insemination elicits inflammatory changes in female reproductive tissues, but whether this results in immunological priming to paternal antigens or influences pregnancy outcome is not clear. We have evaluated indices of lymphocyte activation in lymph nodes draining the uterus following allogeneic mating in mice and have investigated the significance of sperm and plasma constituents of semen in the response. At 4 days after mating, there was a 1.7-fold increase in the cellularity of the para-aortic lymph node (PALN) compared with virgin controls. PALN lymphocytes were principally T and B lymphocytes, with smaller populations of CD3+ B220lo, NK1.1+ CD3– (NK) and NK1.1+ CD3+ (NKT) cells. CD69 expression indicative of activation was increased after mating and was most evident in CD3+ and NK1.1+ cells. Synthesis of cytokines including interleukin-2, interleukin-4 and interferon-γ was elevated in CD3+ PALN cells after exposure to semen, as assessed by intracellular cytokine fluorescence-activated cell sorting, immunohistochemistry and quantitative reverse transcriptase polymerase chain reaction. Matings with vasectomized males indicated that the lymphocyte activation occurs independently of sperm. However, in contrast, males from which seminal vesicle glands were surgically removed failed to stimulate PALN cell proliferation or cytokine synthesis. Adoptive transfer experiments using radiolabelled lymphocytes from mated mice showed that lymphocytes activated at insemination home to embryo implantation sites in the uterus as well as other mucosal tissues and lymph nodes. These findings indicate that activation and expansion of female lymphocyte populations occurs after mating, and is triggered by constituents of seminal plasma derived from the seminal vesicle glands. Moreover, lymphocytes activated at insemination may help mediate maternal tolerance of the conceptus in the implantation site.
Keywords: cytokines: interleukins, mucosal immunity, reproductive immunology, T cells
Introduction
The female reproductive tract is immunologically versatile as evidenced by its ability to generate protective immunity to infectious agents while tolerating semen and the semi-allogeneic conceptus in pregnancy. Semen contains several male gamete-specific antigens and paternal major histocompatibility complex (MHC) antigens1,2 and comprises a regular and substantial challenge to the cervical and uterine mucosa. However, insemination generally occurs without eliciting immunity to male transplantation or other antigens which would be incompatible with tolerance to future mating events and subsequent reproductive success.
It has been assumed that the explanation for this rests in the failure of semen to activate the afferent phase of the immune response by virtue of potent immunosuppressive moieties in seminal plasma.2,3 In the absence of seminal plasma, washed spermatozoa effectively elicit transplantation immunity against paternal antigens.4 However, immune suppression is not consistent with the dynamic changes in reproductive tract leucocytes elicited by semen, or observations of ensuing lymph node hypertrophy.4 In mice, exposure to semen at mating activates an inflammatory response in the uterine mucosa5 and in women intercourse causes similar inflammatory events in the cervix.6 These events have been well-characterized in mice, where specific factors in seminal plasma, including transforming growth factor-β (TGF-β) derived from the seminal vesicle gland,7 trigger synthesis of several pro-inflammatory cytokines and chemokines in uterine epithelial cells and leucocytes.8–10 Macrophages, dendritic cells and granulocytes recruited into the endometrial mucosa exhibit activated phenotypes5,10,11 and appear to trap seminal antigen and traffic to draining lymph nodes.12 The inflammation is transient and leucocyte cell numbers diminish by the time of embryo implantation.5,11
These observations suggest an alternative hypothesis, that insemination might activate a sequence of events leading to induction of active immune tolerance to paternal antigens, analogous to mechanisms operating in other mucosal tissues to mediate tolerance through activation of suppressive or regulatory T-lymphocyte populations or induction of type 2 immunity. Such responses are driven by potent immune-deviating cytokines, particularly TGF-β.13 This raises the possibility that the abundant TGF-β in semen acts to skew the immune response to male antigens as well as to regulate inflammatory cell recruitment.14 Consistent with this, unusual populations of regulatory lymphocytes with suppressive functions are present in the uterus15,16 and observations in rodents suggest that exposure to semen can cause hypo-responsiveness in type 1 immunity to paternal MHC antigens.17,18
This hypothesis also allows the possibility that semen has a role in initiating the immune responses required to accommodate pregnancy, since many of the same antigens are shared by semen and the conceptus. Exposure to semen is now recognized as beneficial in in vitro fertilization pregnancies19 and protective in pre-eclampsia and other pathologies of pregnancy.20 One potential mechanism explaining the benefits of semen in pregnancy is that insemination leads to activation and expansion of lymphocyte populations that are causally linked with those that later facilitate embryo implantation.21
Thus, the antigenic and cytokine composition of semen, the kinetics of antigen-presenting cell recruitment and activation in the endometrium, and observations of lymph node hypertrophy all implicate an active immune response to semen. However, changes in local lymphocyte populations indicative of activation have not been demonstrated. In rodents, organized lymphoid tissue is absent from the virgin uterus, implying that any primary immune response would be elicited in draining lymph nodes rather than in the uterine mucosa itself. The purpose of the present study is to explore evidence in mice for induction of lymphocyte activation following mating in the lumbar, or para-aortic lymph nodes (PALN) draining the uterus. We have analysed the effect of insemination on the abundance and activation status of different lymphocyte phenotypes present in the PALN, and have investigated phenotype skewing through measuring cytokine expression by fluorescence-activated cell sorting (FACS), immunohistochemistry and quantitative reverse transcriptase polymerase chain reaction (RT-PCR) analysis. The relative significance of the sperm and seminal plasma constituents of semen in eliciting the response has been assessed using vasectomized males and males from which the seminal vesicles were surgically excised. Finally the ability of PALN lymphocytes to home to early implantation sites in the pregnant uterus was evaluated using [125I]iodo-deoxyuridine (125IdUR)-labelled lymphocyte trafficking assays.
Materials and methods
Mice
C57BL/6 (H-2k) female mice (B6; 6–10 weeks old) and BALB/c (H-2d) male mice were obtained from the University of Adelaide Central Animal House, and maintained in pathogen-free facilities on a 12 hr/12 hr light/dark cycle, with food and water ad libitum. All experiments were approved by the University of Adelaide Animal Ethics Committee.
Vasectomized and seminal-vesicle-deficient (SV–) male mice were prepared surgically as previously described.5 They were allowed 2 weeks to recover, then proven capable of mating by caging with females and then detecting vaginal plugs and sperm deposition, respectively, prior to experimental use. For experimental matings, naturally cycling B6 female mice were caged 2 : 1 with intact, vasectomized or SV– BALB/c males and checked daily for vaginal plugs. For female mice caged with SV– males, vaginal smears were taken each morning and examined for the presence of sperm. The day on which a vaginal plug or sperm-positive smear was detected was designated day 1 of pregnancy.
Intracellular cytokine staining and flow cytometry
PALN were located anterior to the bifurcation of the abdominal aorta and excised from virgin mice or from mated mice killed at 08.00–09.00 hr on day 4 of pregnancy (80–81 hr after insemination). Single cell suspensions were prepared from PALN using a manually operated glass homogenizer and washed twice in RPMI-1640 (JRH Biosciences, Lenexa, KS) prior to counting using a haemocytometer.
To measure cytokine synthesis by flow cytometry, PALN lymphocytes were stimulated in vitro with polyclonal activators. Cell suspensions (2 × 106 cells/ml) were incubated for 6 hr at 37° in 5% CO2 in RPMI-FCS (RPMI-1640 supplemented with 20 mm HEPES, 10% fetal calf serum, 5 × 10−7β-mercaptoethanol and penicillin/streptomycin) with the following additions: phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO; 50 ng/ml) and calcium ionophore (Sigma; 1 µg/ml). Monensin (Calbiochem, La Jolla, CA; 2 μm) was added to all cultures to inhibit cytokine translocation to the cell membrane. Cells were washed in RPMI-1640 and resuspended in 0·1% FCS/phosphate-buffered saline (PBS; FACS buffer) to a concentration of 107 cells/ml.
For flow cytometry, 100 μl aliquots of 106 cells were treated with anti-Fc-γIIR antibody (Pharmingen, San Diego, CA) to block non-specific binding (5 min at 4°). Thereafter, fluorescein isothiocyanate- (FITC) and/or phycoerythrin-labelled monoclonal antibodies (mAbs; all Pharmingen) were added to the cells (30 min at 4°). The mAbs were reactive with the following surface markers; B220 (clone RA3-6B2); CD3 (clone 17A2); CD4 (clone RM4-5); CD8 (clone 53-5.8); NK1.1 (clone PK136) and CD69 (clone H1.2F3). When only surface markers were analysed the cells were subsequently washed with FACS buffer twice and finally resuspended in 300 μl FACS buffer and analysed in a FACScan (Becton Dickinson, San Jose, CA). For intracellular cytokine detection the cells were washed once in FACS buffer and then resuspended in 5% paraformaldehyde and stored for up to 1 week at 4° until staining with cytokine-reactive antibodies.
Intracellular cytokine staining was carried out according to Pharmingen recommendations and as previously described.22,23 FACS buffer containing 0·1% saponin (Sigma) was added to permeabilize the cells (1 ml for 15 min at 4°). The cells were washed and incubated with FITC and/or phycoerythrin labelled mAbs (Pharmingen) or unlabelled rat anti-mouse antibodies in the presence of 0·1% saponin (30 min at 4°). Antibodies were reactive with interleukin-4 (IL-4) (clone 11B11); interferon-γ (IFN-γ) (clones AN18 and R4-6A2) and IL-5 (clone TRFK-5). The cells were washed and when unlabelled mAb was used they were subsequently incubated with FITC-labelled secondary antibody (Silenus, Melbourne, Australia) (30 min at 4°). The cells were washed again and resuspended in 300 μl FACS buffer. Lymphocytes were analysed by using forward and side scatter to exclude other cells and dead cells. Data analysis was conducted using cellquest software (Becton Dickinson).
Cryosections and immunohistochemistry
Whole PALN collected at 10.00–12.00 hr on day 4 of pregnancy or from virgin mice were placed in OCT Compound (TissueTek, Sakura Finetek, Torrance, CA), snap frozen in liquid nitrogen and subsequently stained for intracellular cytokines as previously described.24 Briefly, 6-μm thick cryostat sections were mounted and fixed in 5% paraformaldehyde–PBS (15 min at 4°). Thereafter endogenous peroxidase was blocked by incubation in 1% hydrogen peroxide in PBS containing 0·1% saponin (60 min at room temperature). To block non-specific antibody binding, the slides were first incubated with normal rat serum (15 min at room temperature). Endogenous biotin was then blocked using an avidin–biotin blocking kit (Vector, Burlingame, CA), according to the manufacturer's instructions. Sections were incubated with primary antibodies reactive with IFN-γ and IL-5 (as above) diluted in 0·1% saponin/PBS (16 hr at room temperature). To detect bound primary antibodies slides were incubated with biotin-conjugated rabbit anti-rat immunoglobulin G (IgG; Vector) followed by peroxidase-conjugated avidin (ABComplex; DAKO, Glostrup, Denmark). Intracellular cytokine deposits were visualized by addition of diaminobenzidine (Sigma; 5 mg/ml in 0·05 m Tris–HCl pH 7·2) plus 0·02% hydrogen peroxide (10 min at room temperature). The tissue was then washed and counter-stained with haematoxylin (Sigma), washed and dried in ethanol and Safsolvent (APS Ajax Finechem, Auburn, Australia) and mounted in DPX (d.b.h. Laboratory Supplies, Poole, UK). The specificity of labelling was confirmed using an isotype-matched irrelevant control antibody.
Sections were evaluated and photographed using an Olympus BH-2 microscope and an Olympus C-35AD-2 camera (Olympus, Japan). The number of labelled cells per lymph node section was determined by manual counting in one to three sections of lymph nodes from 10 virgin and eight mated mice.
Quantitative real-time RT-PCR
Whole PALN collected at 10.00–12.00 hr on day 4 of pregnancy or from virgin mice were snap frozen in liquid nitrogen and stored at −70° prior to RNA extraction. Total cellular RNA was extracted using RNAzol B solution (Tel.Test, Friendswood, TX) and following treatment with RNase-free DNase I (500 IU/ml; 60 min at 37°) (Boehringer Mannheim, Mannheim, Germany), first-strand cDNA was reverse transcribed from 1 μg RNA employing a Superscript II RNase H Reverse Transcriptase kit (90 min at 43°) (Invitrogen, Carlsbad, CA). The cDNA solution was diluted to 100 μl and stored at − 20°. Primer pairs specific for published cytokine cDNA sequences were designed using primer designer software (Scientific and Educational Software, State Line, PA) or primer express software (Applied Biosystems, Foster City, CA). The PCR amplification employed reagents supplied in a 2× SYBR Green PCR Master Mix (Applied Biosystems), and each reaction volume (20 μl total) consisted of 0·5–1 μm 5′- and 3′ primers and 3 μl cDNA. The negative control included in each reaction consisted of H2O substituted for cDNA. PCR amplification was performed in an ABI Prism 5700 Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions to allow amplicon quantification. In preliminary experiments, serial dilutions of cDNA were analysed to confirm a linear relationship between cDNA content and quantity of product across the amplification range. PCR primers and optimized PCR reaction conditions for each primer pair are listed in Table 1. Reaction products were analysed by dissociation curve profile and by electrophoresis in 2% agarose gel containing 0·5 μg/ml ethidium bromide and visualized over an ultra-violet light box. Data were normalized for β-actin mRNA expression and expressed as a percent of the mean of virgin tissue samples.
Table 1.
PCR primer sequences, product size and GenBank accession numbers for cytokine RT-PCR
| Cytokine | PCR conditions | Primer sequence | 5′/3′ bp position | GenBank accession no. | Product size (bp) |
|---|---|---|---|---|---|
| IL-2 | 15 s at 95°/1 min at 60·5° | 5′-acc tct gcg gca tgt tct g | 341–359/499–479 | X01772 | 159 |
| 3′-tcc acc aca gtt gct gac tca | |||||
| IL-4 | 15 s at 95°/1 min at 60·5° | 5′-tcg gca ttt tga acg agg tc | 188–207/311–292 | M25892 | 124 |
| 3′-agc acc ttg gaa gcc cta c. | |||||
| IL-5 | 15 s at 95°/1 min at 60° | 5′-tgg aga ttc cca tga gca c. | 105–124/194–174 | X06270 | 90 |
| 3′-gcc tca tcg tct cat tgc ttg | |||||
| IFN-γ | 20 s at 95°/20 s at 58°/1 min at 72° | 5′-gc cac ggc aca gtc at | 134–149/395–379 | K00083 | 262 |
| 3′-ttc gcc ttg ctg ttg ct | |||||
| β-actin | 20 s at 95°/20 s at58°/1 min at72° | 5′-cgtgggccgccctaggcacca | 24–44/209–193 | M12481 | 186 |
| 3′-acacgcagctcattgta |
125IdUR-labelling and trafficking studies
PALN were excised from B6 female mice mated with BALB/c males and killed at 10.00 hr on day 3 of pregnancy. Single cell suspensions were prepared from PALNs by pushing the tissues through a 70-μm nylon mesh (Becton Dickinson) and washing twice in RPMI-FCS. Cells were incubated for 24 hr at 37° in 5% CO2 in RPMI-FCS containing 1 μCi/ml 125IdUR (Amersham Biosciences, Little Chalfont, UK), 10−6 m fluorodeoxyuridine (FudR; Aldrich Chemical Company, St Louis, MO) and 50% supernatant from MLA-144 T cells25 as a source of IL-2. Labelled cells were washed four times in RPMI-FCS and adoptively transferred to day 6 pregnant B6 mice mated with BALB/c males, by injection of 1 × 107−2 × 107 donor cells [approximately 50 000 counts per minute (c.p.m.)] in 200 μl PBS via the lateral tail vein. Individual recipients received cells pooled from two to three donors. Twenty-four hours later, recipient mice were weighed, bled by cardiac puncture under avertin anaesthesia, killed by cervical dislocation and dissected for collection of the following organs: liver, kidney, heart, brain, lung, thymus, spleen and brachial, inguinal, mesenteric and para-aortic lymph nodes. Samples of ∼200 mg skin (after plucking to remove hair), and large and small intestine were also taken. The female reproductive tract was dissected and segments of intact implantation sites visible as decidualized swellings, or of uterus from between implantation sites, as well as ovaries were collected. Radioactivity in each sample was measured in an Auto-Gamma counter (10 min/sample; Packard Instrument Co., Downer's Grove, IL) with automatic subtraction of background. Data was expressed as ‘relative tissue content’ (RTC; c.p.m. per 100 mg tissue/c.p.m. per 100 mg total recovered tissue), as a measure of the density of donor cells accumulating in a given tissue relative to the average across all tissues. For example, if y = c.p.m./100 mg of a given tissue and z = c.p.m./100 mg total recovered tissues, then RTC = y/z.
Statistical analysis
All statistical analysis was carried out using spss 9·0 software (Chicago, IL). Data were analysed by non-parametric Kruskal–Wallis followed by Mann–Whitney rank sum test, or by parametric one-way analysis of variance followed by Student's t-test as indicated.
Results
Effect of insemination on para-aortic lymph node cellularity
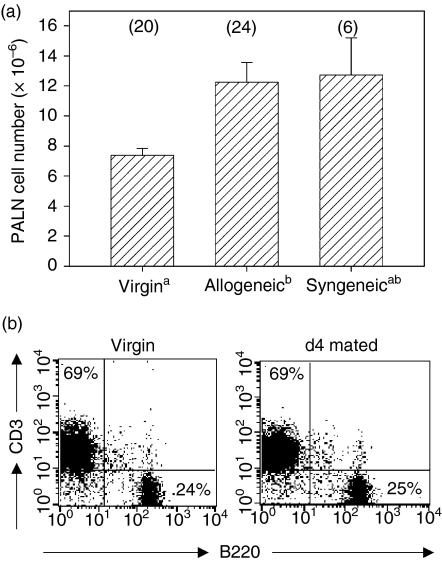
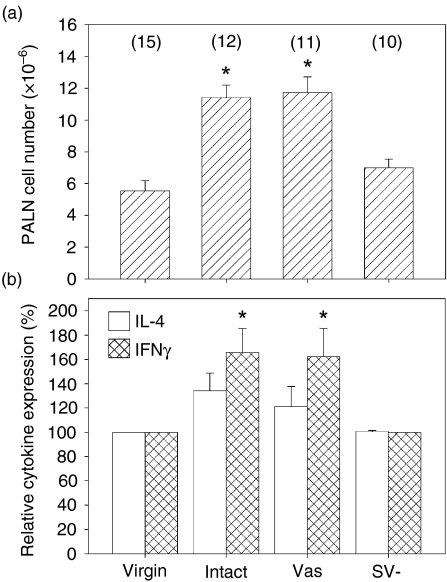
The PALN have been identified previously as the major lymph nodes draining the uterus in mice4 and we confirmed this in experiments evaluating the sites of accumulation of colloidal carbon injected into the uterus (data not shown). To determine the effect of insemination on the number and phenotype of lymphocytes in the PALN, tissues were excised from virgin and inseminated B6 mice on day 4 following allogeneic or syngeneic mating. There was a 1·7-fold increase in the mean number of cells after allogeneic mating, and syngeneic mating elicited a similar response (Fig. 1a). PALN preparations were evaluated by flow cytometry to determine whether the increase in cell numbers after mating was attributable to expansion of specific subpopulations. In virgin mice, the majority of PALN lymphocytes were identified as CD3+ T cells expressing CD4+ or CD8+, or B220+ B cells (Fig. 1b, Table 2). Smaller populations of NK cells (CD3– NK1.1+ approximately 3% of total lymphocytes) and unusual populations of CD3+ B220lo (approximately 5% of CD3+ cells), and NKT cells (CD3+ NK1.1+ approximately 2·5% of CD3+ cells) were also identified. No difference in the distribution of subpopulations was evident in PALN from mice after allogeneic mating compared with virgin controls, other than an approximately two-fold reduction in the proportion of NKT cells.
Figure 1.
Insemination increases cell numbers in PALN. (a) Female B6 mice were killed before mating or 4 days after mating with BALB/c (allogeneic) or B6 (syngeneic) males and the numbers of cells retrieved from PALN were determined. Data are mean (± SEM) number of PALN cells. The number of mice in each group is given in parentheses. Data were compared by independent samples t-test. a,bDifferent superscripts represent statistical significance between groups. (b) The proportion of CD3+ and B220+ lymphocytes in PALN of virgin and mated B6 mice characterized by flow cytometry. Quantitative data are given in Table 2. The data shown are representative of 10 independent experiments.
Table 2.
The distribution of lymphocyte subsets in the draining lymph nodes
| Virgin | Mated day 4 | |
|---|---|---|
| CD3+ | 60±10 | 56±20 |
| B220+ | 37±8 | 34±7 |
| CD3+ B220lo | 3±3 | 3±2 |
| CD4+ | 36±5 | 34±5 |
| CD8+ | 25±3 | 28±4 |
| NK1.1+ | 3±0·8 | 2±0·4 |
| NK1.1+ CD3+ | 1·5±0 | 0·7±0·3 |
Data are mean per cent positive cells ± SD of n = 8 virgin mice and n = 13 day 4 mated mice, respectively.
CD3+ lymphocytes are activated following mating
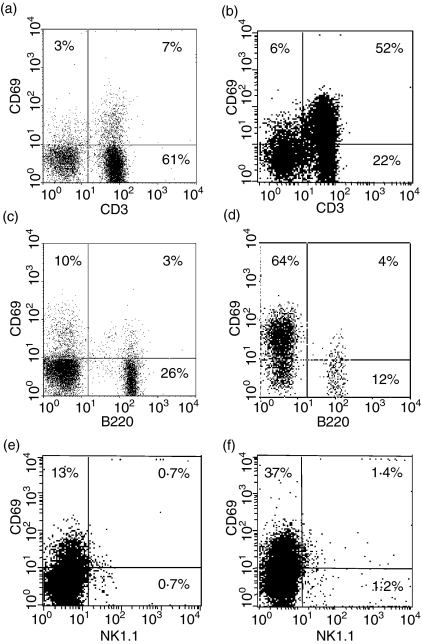
To assess the effect of insemination on activation status of PALN lymphocytes, expression of the early activation marker CD69 was analysed in PALN cells recovered from pregnant B6 mice mated with BALB/c males. Approximately 66% of lymphocytes recovered from PALNs of mated mice expressed high levels of CD69 with the majority of these cells being CD3+ T lymphocytes or NKT cells (Fig. 2b). Only a small proportion of B lymphocytes expressed high levels of CD69 (Fig. 2d), and approximately 50% of NK1.1+ cells expressed CD69 (Fig. 2f).
Figure 2.
PALN T lymphocytes are activated following insemination. CD69 expression was assessed by flow cytometry in (a,b) CD3+ lymphocytes, (c,d) B220+ lymphocytes and (e,f) NK1.1+ cells from PALN of virgin mice (left hand panel; a,c,e) and day 4 mated mice (right hand panel; b,d,f). The data shown are representative of at least 10 independent experiments.
Insemination stimulates cytokine synthesis in PALN lymphocytes
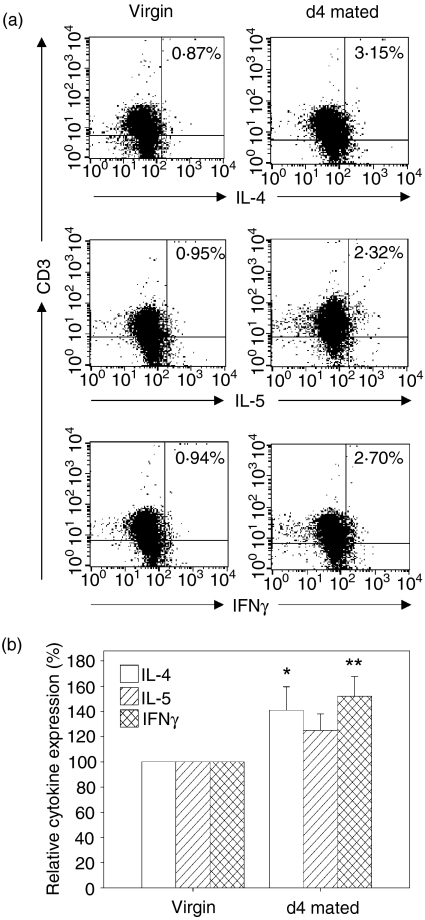
To investigate further the effect of insemination on the activation status of lymphocytes in PALN, production of cytokines was analysed by flow cytometry, immunohistochemistry and quantitative RT-PCR in cells recovered from virgin and pregnant B6 mice mated with BALB/c males. For flow cytometry, PALN lymphocytes were stimulated with polyclonal mitogens for 6 hr in vitro in the presence of a protein transport inhibitor to allow cytoplasmic accumulation of cytokines. Cytokine synthesis was evident in less than 1% of stimulated CD3+ lymphocytes from virgin mice and this population was used to set the baseline level of cytokine production. Insemination induced significant increases in the production of both IL-4 (1·4-fold increase, P = 0·037) and IFN-γ (1·5-fold increase, P = 0·003) (Figs 3a,b), with 46% and 55% of the mated mice yielding cells with IL-4 and IFN-γ synthesis above the threshold value in virgin mice. IL-5 synthesis was elevated in 27% of the PALN preparations at 4 days postmating (1·3-fold increase, P = 0·07) (Figs 3a,b).
Figure 3.
Cytokine synthesis assessed by intracellular FACS is increased in PALN T lymphocytes after insemination. (a) PALN cells from virgin mice (left hand panel) and day 4 mated mice (right hand panel) were stimulated for 6 hr with PMA and calcium ionophore in the presence of monensin, then stained for intracellular IL-4, IL-5, or IFN-γ. The data shown are representative of at least 10 independent experiments. (b) The percentage of cytokine-producing cells in CD3+ lymphocytes from PALN of day 4 mated mice was determined using the level of production in stimulated cells from virgin mice to set the baseline level. Data are mean ± SEM number of IL-4-, IL-5- and IFN-γ-positive cells. PALN from n = 25 virgin and n = 24 day 4 mated mice were assessed. Data were compared by independent samples t-test. *P = 0·04; **P = 0·003.
Immunohistochemistry was employed to identify cytokine-producing cells in PALN tissue sections. IFN-γ-producing cells and, to a lesser extent, IL-5-producing cells were detected in both virgin and mated PALN tissues. The mean number of IFN-γ-producing cells was increased 3·8-fold (P = 0·02) in lymph nodes retrieved from mated mice compared with virgin controls (Figs 4a,b,g). The mean number of IL-5-producing cells was increased 4·3-fold in mated mice, but the difference failed to reach statistical significance, with considerable between-animal variation in the data (P = 0·11) (Figs 4c,d,g). Labelled cells were not found in sections from virgin or mated mice (Figs 4e,f) stained either with detection reagents alone or with an isotype-matched control antibody.
Figure 4.
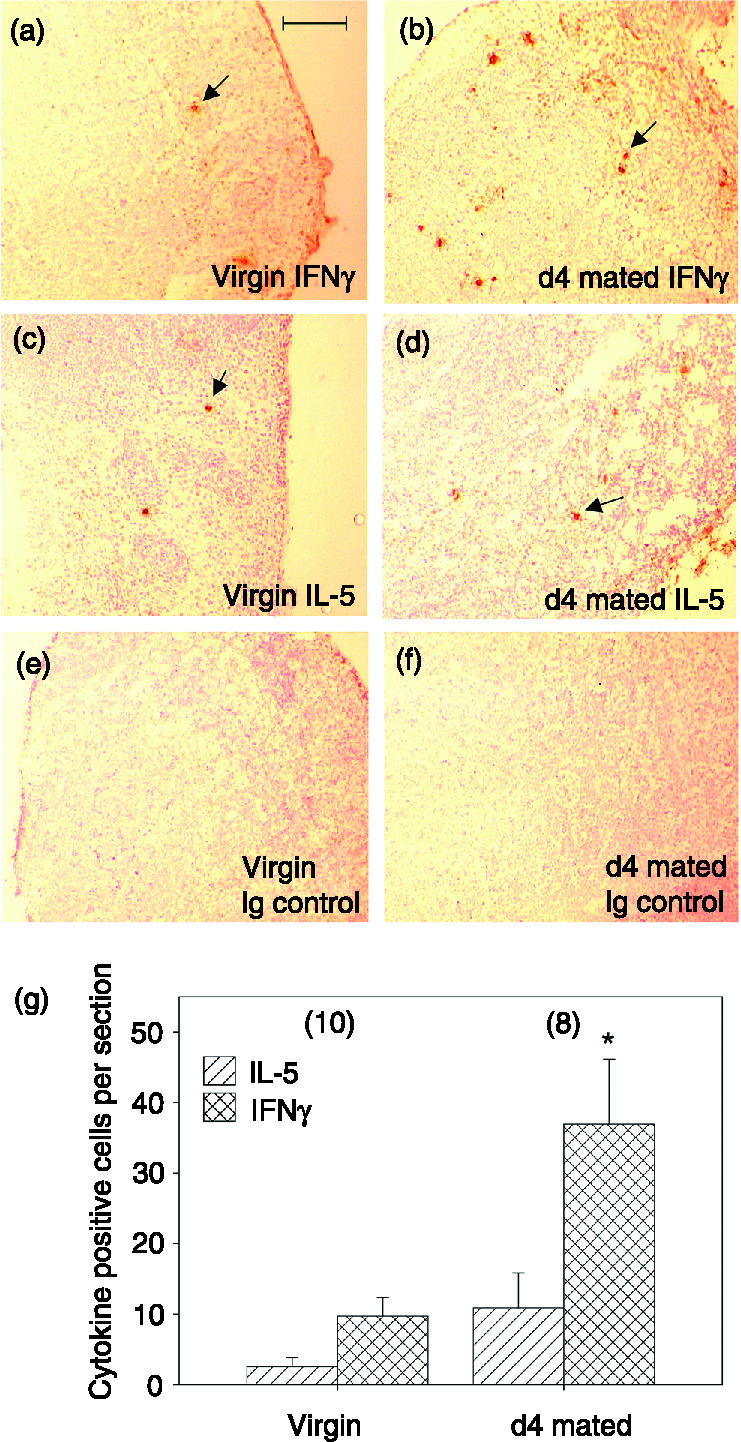
Cytokine synthesis assessed by immunohistochemistry is increased in PALN lymphocytes after insemination. Frozen sections of PALN tissue taken from virgin or day 4 mated mice were labelled with rat anti-IFN-γ or anti-IL-4 antibodies followed by biotin-conjugated rabbit anti-rat IgG. (a) IFN-γ-producing cells were sparsely scattered within the PALN of virgin mice, and (b) were considerably increased in abundance following mating. (c) IL-5-producing cells were rare in the PALN of virgin mice, and (d) were increased following mating. Staining was absent in tissues from both virgin (e) and day 4 mated mice (f) probed with an isotype-matched control antibody. Scale bar = 100 μm. (g) Stained cells were quantified by manual counting and are expressed as the mean ± SEM number of positive cells per section in virgin and day 4 mated PALN sections. One to three PALN sections from n = 10 virgin and n = 8 day 4 mated mice were examined. *P = 0·02.
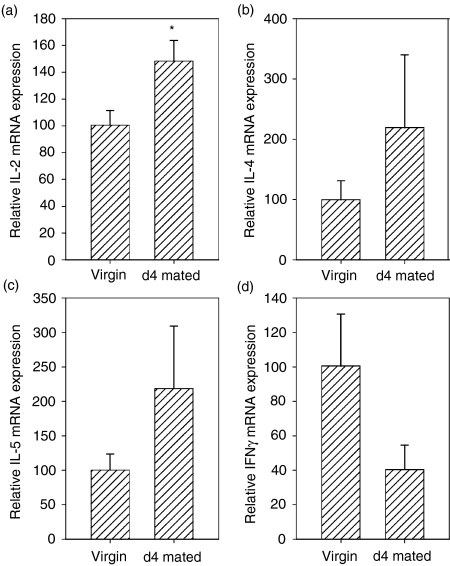
To investigate whether increases in cytokine protein were accompanied by up-regulated mRNA expression, PALN tissues were analysed by quantitative RT-PCR. In addition to IFN-γ, IL-4 and IL-5 mRNAs, IL-2 mRNA expression was also quantified. Insemination induced a 1·5-fold increase in mean IL-2 mRNA content (P = 0·03, Fig. 5a), and a 2·2-fold increase in both IL-4 and IL-5 mRNA expression although these latter increases failed to reach statistical significance (Fig. 5b,c). No increase in IFN-γ mRNA content was detected (Fig. 5d).
Figure 5.
Cytokine synthesis assessed by quantitative real-time RT-PCR is increased in PALN lymphocytes after insemination. cDNA was prepared from whole PALN from virgin or day 4 mated mice and mRNAs for (a) IL-2; (b) IL-4; (c) IL-5 and (d) IFN-γ were quantified using primers sets and reaction conditions detailed in Table 1. Data are mean ± SEM relative mRNA expression in arbitrary units, where data are normalized to β-actin mRNA expression and expressed as per cent of the mean of data from virgin PALN tissues. PALN tissue from n = 6 virgin and n = 6 day 4 mated mice was evaluated. *P = 0·03.
Seminal plasma is necessary for lymphocyte activation
To investigate the relative importance of the sperm and seminal plasma constituents of semen in inducing lymphocyte activation after insemination, female B6 mice were mated with intact, vasectomized or SV– BALB/c males. Mating with vasectomized males elicited significant increases in PALN cell numbers comparable to those seen after mating with intact males (mean values were increased 2·1-fold in both cases, P < 0·001). However, there was no significant difference in cell numbers after mating with SV– males (Fig. 6a).
Figure 6.
Seminal fluid is necessary for lymphocyte activation in PALN following mating. The effect of sperm and seminal vesicle fluid on the immune response elicited in the PALN following mating was evaluated using surgically altered male mice. Females were mated with intact BALB/c males or with BALB/c males rendered sperm deficient by vasectomy (Vas) or seminal vesicle deficient (SV–) by surgical removal of the seminal vesicles. (a) The mean ± SEM number of cells retrieved from PALN. (b) The percentage of cytokine-producing cells in CD3+ lymphocytes from PALN of day 4 mated mice was determined by flow cytometry using the level of production in stimulated cells from virgin mice to set the baseline level. Data are mean ± SEM number of IL-4- and IFN-γ-positive cells. Data were compared by independent samples t-test. The number of mice per group are shown in parentheses. *P < 0·02.
Analysis of cytokine synthesis by flow cytometry also revealed an effect of male accessory gland status. The abundance of PALN cells expressing IFN-γ in females mated to vasectomized males was significantly increased compared to virgin controls (P = 0·02), and comparable to females mated with intact BALB/c males (Fig. 6b). However, IFN-γ synthesis was not detected in PALN cells of females mated to SV– males. Furthermore, IL-4-expressing PALN cells were detected more frequently in females mated with intact or vasectomized males than in females mated with SV– males, although statistical significance was not achieved (Fig. 6b).
PALN lymphocytes activated at insemination home to the uterus at implantation
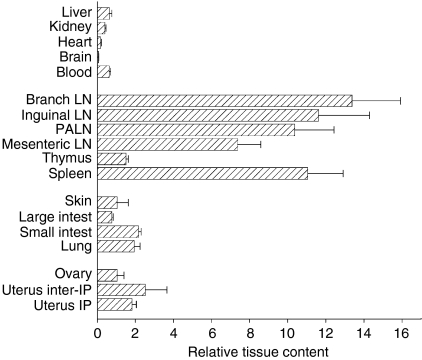
To investigate the homing patterns of PALN cells activated after insemination, and specifically to determine whether PALN cells can traffic into implantation sites in the uterus, 125IdUR-labelled PALN cells from day 3 pregnant donors were adoptively transferred into day 6 pregnant recipients. There was differential recruitment of PALN cells into different tissues examined in recipient mice, with mean RTC values of < 1 in liver, kidney, heart, brain and large intestine (Fig. 7). In contrast there was considerable accumulation of transferred cells into lymph nodes and spleen, with mean RTC values of 7–14 in these tissues. Selective accumulation of donor cells was also seen in mucosal tissues, particularly the small intestine, lung and, to a lesser extent, the skin, with mean ± SEM RTC values of 2·2 ± 0·1, 1·9 ± 0·3 and 1·0 ± 0·6, respectively. Most notably, PALN cells were also selectively recruited into uterine tissues, although there was no significant difference in the densities of cells found between implantation sites (mean ± SEM RTC = 1·8 ± 0·2) and interimplantation sites (RTC =2·5 ± 1·1). Fewer cells were seen to traffic into the ovaries (RTC = 1·1 ± 0·3).
Figure 7.
Activated PALN cells can home to uterine tissue. PALN lymphocytes from day 4 mated donors were labelled with 125IdUR and adoptively transferred to day 6 pregnant recipients. 125IdUR associated with each tissue was determined by gamma-counting. The relative density of donor cells recruited into each tissue is given as mean ± SEM relative tissue content (as defined in the Materials and methods) from n = 17 recipient mice. LN = lymph node; IP = implantation site.
Discussion
The present study demonstrates that a single, physiological exposure to semen by natural insemination triggers an immune response involving the lymph nodes draining the uterus. Both T and B lymphocytes contribute to an increase in the cellularity of the PALN 4 days after mating, when CD3+ lymphocyte activation, as assessed by CD69 expression and synthesis of IL-2, IFN-γ and IL-4, is clearly evident. Activated, cytokine-expressing cells appear to be predominantly conventional CD4+ or CD8+ T lymphocytes but also include NKT cells and potentially CD3+ B220lo cells and NK cells. The presence of seminal vesicle fluid is crucial to the increased cellularity and production of cytokines, showing that lymphocyte activation depends upon specific factors in the plasma fraction of the ejaculate. However, these indices of lymphocyte activation were not affected by the vasectomy of males, indicating that neither sperm in the ejaculate nor the presence of the conceptus is required to elicit immune responses early in pregnancy.
Factors in semen are known to induce an inflammatory cascade in the endometrial mucosa, but whether this results in lymphocyte activation has not previously been examined. It was reported decades ago that hypertrophy occurs in draining lymph nodes following mating in rodents and that the para-aortic nodes exhibit the greatest response.4,26 The present study confirms that hypertrophy is the consequence of expansion in the leucocyte pool associated with local lymphocyte proliferation. Antigen-presenting cells arriving from the uterus via afferent lymphatics probably also contribute, with phagocytes containing engulfed spermatozoa known to traffick to the lymph nodes following insemination.12,27 Extravasation of blood-borne lymphocytes via postcapillary venules probably provides a third source of new cells.
Our results indicate that the plasma fraction of seminal fluid is required to achieve activation and expansion of lymph node cell populations after mating. The seminal vesicle contributes the majority of the seminal fluid in mice, with lesser amounts derived from the prostate, coagulating and bulbourethral glands. In earlier studies we have reported that the endometrial inflammatory response elicited after mating fails to occur in the absence of seminal vesicle secretions5 and that TGF-β of seminal vesicle origin provides the major pro-inflammatory stimulus in semen.7 Macrophages and dendritic cells are not recruited into the uterus after mating with SV– males, and the up-regulation in granulocyte–macrophage colony-stimulating factor and tumour necrosis factor-α synthesis that is expected after intact mating does not occur.5 Lymph node changes presumably do not occur when mating fails to stimulate these initial antigen-processing and presentation events.
Sperm, on the other hand, appear not to be required either for cellular increase or for cytokine production in lymph nodes. Uterine inflammatory events after mating with a vasectomized male are indistinguishable from those elicited by intact males,5 implying that the trafficking and lymphocyte-activating behaviour of antigen-presenting cells occurs similarly despite the absence of sperm. The lymph node changes seen after mating with vasectomized males indicate that paternal antigens are probably derived from constituents of semen in addition to sperm, such as leucocytes and epithelial cell debris, or soluble MHC antigens in the seminal plasma.28 Moreover, since lymph node hypertrophy occurred regardless of MHC disparity between male and female, antigens in addition to MHC such as H-Y or other moieties in semen might be involved. Further studies are required to resolve the effects of antigenic composition on the phenotype of activated lymphocytes.
Our data are consistent with T lymphocytes being predominant amongst the PALN lymphocytes responding to the mating stimulus. However, small populations of less conventional lymphocyte subsets were also evident in PALN. These included unusual CD3+ B220lo cells comparable to a prevalent uterine CD4– CD8– lymphocyte subset suggested to have a regulatory role in providing a suppressive uterine environment.29 Both NK and NKT cells, identified as important regulatory cells in the reproductive tract,30,31 were also present as minor but clearly distinguishable populations. We did not observe increases in the relative abundance of these cells after mating but small changes may not have been discernable. Increased expression of CD69 in NK1.1+ cells after mating is consistent with their activation, but whether this is accompanied by proliferation requires further investigation.
Up-regulated expression of IL-2, IL-4, IL-5 and IFN-γ was detected in lymph node cells after insemination using three experimental strategies to measure cytokine mRNA and protein. Increased IFN-γ mRNA expression was not detected by RT-PCR despite the more abundant IFN-γ-producing cells in flow cytometry and immunohistochemistry experiments, perhaps indicating post-transcriptional regulation or kinetics of IFN-γ mRNA induction not evident at the single time-point examined. Furthermore, considerable variation within groups of mated mice was seen and might reflect individual differences in the strength of activating signals stemming from variation in the quantity of semen accessing the uterine tissue or the intensity of the endometrial inflammatory reaction.
The phenotypes of lymphocytes primed at insemination might be expected to be instrumental in guarding against inappropriate immunity to future semen exposures. However, the current data do not provide evidence of phenotype skewing in lymph node cells. Since virgin mice were used in these experiments, the CD3+ T cells found in the PALN on day 4 of pregnancy are likely to be newly activated naïve Th0 cells, expected to produce both the type 1 cytokine IFN-γ and the type 2 cytokines IL-4 and IL-5. Day 4 might prove too early to detect an eventual skew in the immune response. Multiparous mice or mice exposed to semen previously could be expected to display greater evidence of immune deviation after affinity maturation and selective expansion of memory T-lymphocyte pools.
The physiological significance of the lymphocyte activation after mating is not clear, but it is tempting to speculate upon a role in preparation for pregnancy. Since many antigens are shared between semen and the conceptus, and almost every mating event in mice leads to a pregnancy, it seems reasonable to postulate that any immune response to insemination is causally linked to conceptus implantation 4 days later. It has been demonstrated that mating can induce changes in immune responsiveness to paternal MHC antigens; paternal alloreactivity is strongly suppressed in PALN cells during the pre- and peri-implantation periods of pregnancy in rats17 and exposure to semen mediates a transient state of functional immune tolerance to tumour cells bearing paternal MHC antigens.18 Although it might seem counter-intuitive to link an active immune response to semen with the immune suppression required for pregnancy, activation and expansion of lymphocytes can precede some forms of immune tolerance. Examples include anergic CD8+ cells induced after cross-tolerization by activated dendritic cells32 and regulatory T cells or Th3 cells, also acquired after active induction.33 The phenotypes of antigen-presenting cells, largely influenced by local microenvironmental signals in the site of antigen exposure, are instrumental in instructing the path of T-cell differentiation. In this context it is noteworthy that the uterus is richly populated with dendritic cells34 which in the presence of the very high concentration of TGF-β found in semen14 might be expected to favour the generation of Th3 cells and other regulatory T cells.13
The implantation site is richly populated with lymphocytes having similar phenotypes to those observed in the PALN, including α/β and γ/δ T cells,35,36 NKT cells31 and NK cells.30 The abundance of uterine NK cells and NKT cells, as well as a population of suppressor lymphocytes with a Th3-like phenotype, is linked with optimal placental development and successful pregnancy outcome.30,31,37 The possibility that lymphocytes activated and induced to proliferate at insemination might be selectively recruited into uterine implantation sites after recirculation via the blood was evaluated by passive transfer experiments in pregnant mice. We were able to show that PALN lymphocytes activated at insemination can indeed traffic into the uterus, with comparable affinity for implantation sites and interimplantation tissue. However, there was a similar propensity for PALN cells to home to other mucosal tissues particularly the small intestine and the lung. This is consistent with expression in uterine venule endothelial cells of mucosal addressin cell adhesion molecule-138 the ligand for integrin α4/β7 responsible for common lymphocyte homing properties across mucosal tissues.
Uterine NK cell precursor cells are known to originate in tissues other than the uterus, with the spleen identified as the richest source and a lesser contribution from peripheral lymph nodes39 so it is unlikely that PALN-derived cells add substantially to this lineage. It is possible that NKT cells activated in PALN at insemination home to the uterus and contribute to the dramatically (40-fold) expanded NKT population evident in the implantation site by day 6 of pregnancy.31 Uterine NKT cells are T-cell receptor Vα14+ but express a novel Vβ repertoire reactive with a class I/Ib molecule other than CD1 expressed by placental cells.40 It would be of great interest to examine whether semen can provide or induce in female tissues the yet to be identified class I/Ib molecule, and thus potentially contribute to NKT activation in early pregnancy. This possibility is supported by the presence of MHC class Ia and Ib molecules in semen1 and of α-galactosylceramide and other glycolipids in sperm.41
We conclude that exposure to semen at mating in mice elicits a sequence of events that result in an active immune response characterized by lymphocyte activation and proliferation in draining lymph nodes. The response is dependent on factors in seminal plasma, presumably including TGF-β which was previously identified as essential for stimulating the inflammatory response to semen.7 The kinetics of the response, the phenotypes of lymphocytes activated and the ability of activated cells to be recruited into uterine tissues supports the possibility that these cells contribute to the initial phase of the maternal immune response to the implanting conceptus, and would provide a mechanistic explanation for mounting evidence that exposure to semen facilitates successful pregnancy through mechanisms other than assisting fertilization.20,21 Confirmation of this will require investigation of the lymphocyte populations in pregnancies initiated in the absence of seminal plasma. A better understanding of the immune response to semen and its impact on early pregnancy might eventually lead to improved therapeutic strategies in miscarriage and other immune pathologies of pregnancy.
Acknowledgments
This study was supported by the NHMRC of Australia and the Swedish Royal Academy of Sciences, Sven and Dagmar Sahléns Foundation, Adlerbertska Research Foundation and Crafoordska Foundation. The skilful technical assistance of Katherine Pensa and Vicky Mau is greatly appreciated.
Abbreviations
- FCS
fetal calf serum
- 125IdUR
[125I]iodo-deoxyuridine
- IFN-γ
interferon-γ
- IL
interleukin
- NK
natural killer
- PALN
para-aortic lymph node
- RTC
relative tissue content
- RT-PCR
reverse transcriptase polymerase chain reaction
- SV−
seminal vesicle deficient
- TGF-β
transforming growth factor-β
- Th
T-helper
References
- 1.Hutter H, Dohr G. HLA expression on immature and mature human germ cells. J Reprod Immunol. 1998;38:101–22. doi: 10.1016/s0165-0378(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 2.Alexander NJ, Anderson DJ. Immunology of semen. Fertil Steril. 1987;47:192–204. doi: 10.1016/s0015-0282(16)49990-5. [DOI] [PubMed] [Google Scholar]
- 3.Kelly RW, Critchley HO. Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod. 1997;12:2200–7. doi: 10.1093/oxfordjournals.humrep.a019559. [DOI] [PubMed] [Google Scholar]
- 4.Beer AE, Billingham RE. Host responses to intra-uterine tissue, cellular and fetal allografts. J Reprod Fert Suppl. 1974;21:59–88. [Google Scholar]
- 5.Robertson SA, Mau VJ, Tremellen KP, Seamark RF. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil. 1996;107:265–77. doi: 10.1530/jrf.0.1070265. [DOI] [PubMed] [Google Scholar]
- 6.Robertson SA, Sharkey DJ, Tremellen KP, Danielsson KG. Semen elicits immunological changes in the human cervix. J Soc Gynecol Invest. 2001;9:228A. [Google Scholar]
- 7.Tremellen KP, Seamark RF, Robertson SA. Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod. 1998;58:1217–25. doi: 10.1095/biolreprod58.5.1217. [DOI] [PubMed] [Google Scholar]
- 8.Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992;46:1069–79. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- 9.Sanford TRM, Wood GW. Expression of colony-stimulating factors and inflammatory cytokines in the uterus of CD1 mice during days 1–3 of pregnancy. J Reprod Fertil. 1992;94:213–20. doi: 10.1530/jrf.0.0940213. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SA, Allanson M, Mau VJ. Molecular regulation of uterine leukocyte recruitment during early pregnancy in the mouse. Trophoblast Res. 1998;11:101–20. [Google Scholar]
- 11.McMaster MT, Newton RC, Dey SK, Andrews GK. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol. 1992;148:1699–705. [PubMed] [Google Scholar]
- 12.Reid BL. The fate of isotope-labelled uterine spermatozoa in the mouse post coitum. Aust J Zool. 1965;13:525–31. [Google Scholar]
- 13.Weiner HL. Oral tolerance. Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–54. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 14.Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta–a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57:109. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 15.Robertson SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;5:164–74. doi: 10.1530/ror.0.0050164. [DOI] [PubMed] [Google Scholar]
- 16.Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16:43–9. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kapovic M, Rukavina D. Kinetics of lymphoproliferative responses of lymphocytes harvested from the uterine draining lymph nodes during pregnancy in rats. J Reprod Immunol. 1991;20:93–101. doi: 10.1016/0165-0378(91)90026-m. [DOI] [PubMed] [Google Scholar]
- 18.Robertson SA, Mau VJ, Hudson SA, Tremellen KP. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol. 1997;37:438–2. doi: 10.1111/j.1600-0897.1997.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 19.Tremellen KP, Valbuena D, Landeras J, et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod. 2000;15:2653–8. doi: 10.1093/humrep/15.12.2653. [DOI] [PubMed] [Google Scholar]
- 20.Dekker GA, Robillard PY, Hulsey TC. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. Obstet Gynecol Surv. 1998;53:377–82. doi: 10.1097/00006254-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13:243–54. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 22.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin precedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 23.Carter LL, Swain SL. Single cell analyses of cytokine production. Curr Opin Immunol. 1997;9:177–82. doi: 10.1016/s0952-7915(97)80132-x. [DOI] [PubMed] [Google Scholar]
- 24.Johansson M, Ward M, Lycke N. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunol. 1997;92:422–8. doi: 10.1046/j.1365-2567.1997.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabin H, Hopkins RF, 3rd, Ruscetti FW, Neubauer RH, Brown RL, Kawakami TG. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981;127:1852–6. [PubMed] [Google Scholar]
- 26.Hetherington CM, Humber DP. The effect of pregnancy on lymph node weight in the mouse. J Immunogenet. 1977;4:271–6. doi: 10.1111/j.1744-313x.1977.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 27.Watson JG, Carroll J, Chaykin S. Reproduction in mice: the fate of spermatozoa not involved in fertilization. Gamete Res. 1983;7:75–84. [Google Scholar]
- 28.Koelman CA, Coumans ABC, Nijman HW, Doxiadis IIN, Dekker GA, Claas FHJ. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46:155–66. doi: 10.1016/s0165-0378(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 29.Johansson M, Lycke N. A unique population of extrathymically derived alpha beta TCR+CD4–CD8– T cells with regulatory functions dominates the mouse female genital tract. J Immunol. 2003;170:1659–66. doi: 10.4049/jimmunol.170.4.1659. [DOI] [PubMed] [Google Scholar]
- 30.Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: key regulators of placental development (a review) J Reprod Immunol. 2002;57:151. doi: 10.1016/s0165-0378(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 31.Dang Y, Beckers J, Wang CR, Heyborne KD. Natural killer 1.1 (+) alpha beta t cells in the periimplantation uterus. Immunol. 2000;101:484–91. doi: 10.1046/j.1365-2567.2000.t01-1-00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 33.Cottrez F, Groux H. Specialization in tolerance. innate CD(4+) CD(25+) versus acquired TR1 and TH3 regulatory T cells. Transplantation. 2004;77:S12–15. doi: 10.1097/01.TP.0000106471.23410.32. [DOI] [PubMed] [Google Scholar]
- 34.Hudson Keenihan SN, Robertson SA. Diversity in phenotype and steroid hormone dependence in dendritic cells and macrophages in the mouse uterus. Biol Reprod. 2004 doi: 10.1095/biolreprod.103.024794. 10.1095/biolreprod.103.024794. [DOI] [PubMed] [Google Scholar]
- 35.Heyborne KD, Cranfill RL, Carding SR, Born WK, O'Brien RL. Characterization of gamma delta T lymphocytes at the maternal–fetal interface. J Immunol. 1992;149:2872–8. [PubMed] [Google Scholar]
- 36.Arck PC, Ferrick DA, Steele Norwood D, Croitoru K, Clark DA. Murine T cell determination of pregnancy outcome. I. Effects of strain, alpha/beta T cell receptor, gamma/delta T cell receptor, and gamma/delta T cell subsets. Am J Reprod Immunol. 1997;37:492–502. doi: 10.1111/j.1600-0897.1997.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 37.Lea RG, Flanders KC, Harley CB, Manuel J, Banwatt D, Clark DA. Release of a transforming growth factor(TGF)-beta 2-related suppressor factor from postimplantation murine decidual tissue can be correlated with the detection of a subpopulation of cells containing RNA for TGF-beta 2. J Immunol. 1992;148:778–87. [PubMed] [Google Scholar]
- 38.Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte–vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–26. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions fromself-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–8. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 40.Dang Y, Heyborne KD. Cutting edge: regulation of uterine NKT cells by a fetal class I molecule other than CD1. J Immunol. 2001;166:3641–4. doi: 10.4049/jimmunol.166.6.3641. [DOI] [PubMed] [Google Scholar]
- 41.Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E. New insights into the interaction between the gp120 and the HIV receptor in human sperm. J Reprod Immunol. 1998;41:213–31. doi: 10.1016/s0165-0378(98)00060-6. [DOI] [PubMed] [Google Scholar]