Abstract
The ezrin/radixin/moesin (ERM) family of actin-binding proteins act both as linkers between the actin cytoskeleton and plasma membrane proteins and as signal transducers in responses involving cytoskeletal remodelling. The Rho family of GTPases also regulate cytoskeletal organisation, and several molecular pathways linking ERM proteins and Rho GTPases have been described. This review discusses recent findings on ERM protein function in leucocytes and how these may be integrated with Rho GTPase signalling.
Keywords: adhesion molecules, signalling: signal transduction, cell polarity, phosphorylation
Introduction
Circulating leucocytes are some of the most dynamic cells in the adult body. As frontline defenders, leucocytes must undergo rapid changes in adhesion and morphology in order to respond to immunological and inflammatory stimuli. Such responses include polarization and migration towards a chemotactic source,1 exit from the bloodstream into the surrounding tissue,2 formation of a functional immune synapse with antigen-presenting cells (APCs)3 and apoptosis.4 Both Rho GTPases and ezrin/radixin/moesin (ERM) proteins have been implicated in these responses. Remodelling of the actin cytoskeleton mediates cell shape change and is regulated by the Rho family of GTPases.5,6 The ERM proteins were initially identified as being enriched in membrane structures such as microvilli, membrane ruffles, cell–cell junctions and cleavage furrows of dividing cells.7,8 They are now known to link integral membrane proteins to the cortical actin cytoskeleton that lies beneath the plasma membrane.9
A novel feature of the ERM proteins is their ability to act both upstream and downstream of Rho GTPases, which suggests the existence of a positive feedback loop between the two types of proteins. This review will focus on what is currently known about ERM proteins in leucocytes and how they may be linked to Rho GTPase signalling.
Genetics and expression of erm proteins
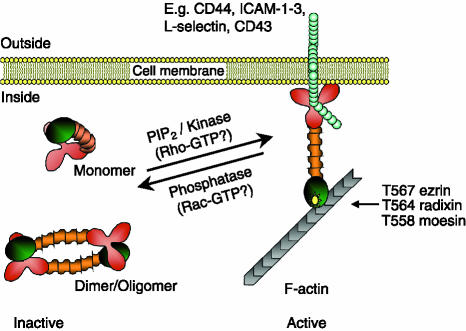
The three ERM proteins (≈75 000 molecular weight) share a high level of amino acid identity (70–85%), and it is likely that they have diverged from a common ancestral gene, as lower eukaryotes (e.g. fruit flies and nematodes) only have a single ERM gene.10 ERM proteins are not found in yeast, suggesting that they are required specifically in multicellular organisms. The ERM proteins can be subdivided into three domains (Fig. 1): a highly conserved N-terminal FERM (band Four point one Ezrin Radixin Moesin) domain; a central α-helical domain that is predicted to form coiled coils; and an actin-binding C-terminal domain. Many other proteins contain FERM domains (including the erythrocyte protein band 4·1), which appear to be generally involved in linking proteins to the plasma membrane.11 ERM proteins share a high level of homology with merlin (moesin/ezrin/radixin-like; also known as NF2), a tumour suppressor gene that is mutated in neurofibromatosis. To date, ERM proteins have no proven role in cancer progression, although a recent review explores this possibility.12
Figure 1.
Schematic representation of ezrin/radixin/moesin (ERM) protein activation in leucocytes. In their inactive conformation, the N-terminal FERM (band Four point one Ezrin Radixin Moesin) domain of ERM proteins (red) binds to the C-terminal actin-binding domain (green) via either an intramolecular or an intermolecular interaction. The α-helical domain is coloured in orange. Phosphatidylinositol 4,5 bis-phosphate (PIP2) binding to the FERM domain and phosphorylation of the critical C-terminal threonine (amino acid position indicated for each ERM protein) induce and stabilize the unfolded active conformation of ERM proteins, allowing the FERM domain to bind to transmembrane receptors and the actin-binding domain to interact with actin filaments (F-actin). Activation of ERM proteins can be stimulated by Rho. Conversely, dephosphorylation of the C-terminal threonine may be regulated by Rac. Yellow star indicates phosphorylation of a critical C-terminal residue. ICAM-1–3, intercellular adhesion molecule types 1–3.
ERM protein expression can be cell type- and organ-specific, and several reports have compiled a comprehensive list of ERM protein expression in different cell types.7,8,13,14 Moesin is the predominant isoform expressed in leucocytes, which generally express much lower levels of ezrin. Some leucocytes, such as T cells, do not express detectable levels of radixin, whereas other subtypes [e.g. natural killer (NK) cells and neutrophils] express trace amounts of radixin.15,16 Given the level of moesin expression in leucocytes, it is probable that moesin-null mice will have defects in leucocyte responses, but, to date, no detailed immunological studies have been reported in either moesin- or radixin-null mice. Moesin-null mice are viable and display no obvious phenotype,17 whereas radixin-null mice display defects in bile production that subsequently manifests in mild liver damage, suggesting that ezrin and moesin are either absent or unable to compensate for the loss of radixin expression/function in bile canalicular membranes.18
Regulation of erm protein activity
As with many proteins involved in cytoskeletal dynamics, ERM proteins exist in an auto-inhibited conformation that, when relieved, becomes activated.19 The discovery that the N and C termini of ERM proteins can interact with each other led to the hypothesis that ERM proteins exist as folded monomers or complexes of antiparallel dimers and/or hetero- or homo-oligomers (Fig. 1).19–22 Interaction between the N and C termini masks binding sites that that would otherwise bind to the cytoplasmic tails of cell-adhesion molecules and F-actin, respectively. Inactive ERM proteins reside in the cytosolic fraction of cells, whereas activated ERMs are membrane-associated and bind with integral membrane proteins via their N-terminal FERM domain and with F-actin via their C termini (Fig. 1), contributing to the formation of various membrane structures and to signal transduction pathways, as described below.
Regulation by phosphorylation
Phosphorylation of a threonine residue (T558 in moesin, T567 in ezrin, T564 in radixin) within the C-terminal actin-binding domain of ERM proteins is considered a hallmark of ERM activation (Fig. 1). Phosphorylation of moesin on T558 was first demonstrated in thrombin-activated platelets.23 Phosphorylation is believed to stabilize the active open conformation of ERM proteins by preventing interaction between the N-terminal FERM domain and the C-terminal F-actin-binding domain. Mutation of T567 in ezrin, or T558 in moesin, has been used to study the role of phosphorylation in ERM protein function. Mutation to aspartate (mimicking phosphorylation) has been used to generate ‘constitutively active’ ezrin/moesin. Conversely, mutation to alanine permits the study of ERM proteins that are unable to be phosphorylated.22,24
Several kinases have been implicated in regulating ERM protein function through phosphorylation of the C-terminal threonine residue (Fig. 2). Protein kinase C (PKC) α has been shown to interact with ezrin, both in vitro and in vivo, and can phosphorylate ezrin at T567 in vitro.25 Similarly, PKCθ can phosphorylate moesin at T558 in vitro,26 but whether these PKCs directly phosphorylate ERM proteins in vivo is still unclear. The Rho-activated kinase, ROCK, can also phosphorylate ERM proteins in vitro, but the effect of ROCK inhibitors on ERM phosphorylation in vivo varies27 and it is unclear whether ROCK acts directly or indirectly to modify ERM phosphorylation (see below). A relative of ROCK, MRCK, has also been reported to phosphorylate ERM proteins (Fig. 2).28
Figure 2.
Reciprocal regulation of Rho GTPases and ezrin/radixin/moesin (ERM) proteins. ERM proteins are able to act both upstream (red arrows) and downstream (green arrows) of Rho GTPases. Blue arrows represent inhibitory pathways. Kinases indicated in grey are not directly linked to Rho GTPases. Note that only ezrin is phosphorylated by a tyrosine kinase (Tyr-K) at position Y353, and that it specifically interacts with the regulatory subunit of phosphatidylinositide 3-kinase (PI3-K). PI3-K is coloured red because it has the potential to activate Rac, but this is not indicated in the diagram. Calpain specifically cleaves ezrin and not moesin in lymphocytes. The calpain cleavage site is currently unknown. Phosphorylation of residues Y145, T235 (both in the FERM domain), Y353 (in the α-helical domain) and T558 (moesin numbering – in the C-terminal actin-binding domain) is indicated with a blue ‘P’. Dashed lines indicate pathways that are known to exist, but not known to regulate ERM proteins. Cdk5, cyclin-dependent kinase 5; FERM, band Four point one Ezrin Radixin Moesin; MBS, myosin-binding subunit; PKC, protein kinase C, Myp, myosin phosphatase; PPase, phosphatase.
The identity of the phosphatase(s) that dephosphorylates C-terminally phosphorylated ERM proteins remains elusive. One candidate is myosin phosphatase, as the myosin-binding subunit (MBS) of myosin phosphatase has been reported to interact with moesin.29 This interaction is thought to bring the phosphatase in close proximity to moesin, allowing dephosphorylation at T558. Interestingly, ROCK can phosphorylate myosin phosphatase MyP and, in doing so, inactivates the phosphatase activity, and thus ROCK could act both to phosphorylate ERMs and simultaneously prevent their dephosphorylation.30 Another possible ERM phosphatase is protein phosphatase 2C, which was biochemically purified as a protein phosphatase activity from platelet cell extracts that could dephosphorylate highly purified phospho-T558 moesin.31 Protein phosphatase 2C is insensitive to the phosphatase inhibitor, calyculin A. However, there is also an as-yet-unidentified calyculin A-sensitive phosphatase that dephosphorylates ERM proteins very rapidly and is present in cell extracts from lymphocytes stimulated with the chemokine stromal-cell-derived factor 1α (SDF-1α).24
In addition to C-terminal phosphorylation, ezrin has been reported to be phosphorylated at threonine 235 by cyclin-dependent kinase 5 (Cdk5).32 This threonine is conserved in all ERM proteins. Mutation of T235 to aspartic acid enhanced the membrane localization of ezrin,32 and thus phosphorylation of this site may increase ERM activity. Interestingly, Cdk5 and its associated cyclin, p35, are known effectors of Rac in neuronal cells33 and thus it would be interesting to determine whether Rac regulates T235 phosphorylation. It is currently not known whether ERM proteins are phosphorylated on this site in leucocytes, but Cdk5 is expressed in leucocytes and is involved in monocytic differentiation.34
Regulation by phosphoinositides
There is good evidence that phosphoinositides regulate ERM protein activity. Phosphatidylinositol 4,5 bis-phosphate (PIP2) is normally present in the inner leaflet of the plasma membrane and is thought to act in recruiting ERM proteins to the membrane, either by stabilizing the activated, C-terminally phosphorylated, form, or by unfolding inactive monomers (Fig. 1). Depletion of PIP2 using neomycin (which binds to and sequesters PIP2) led to microvillar collapse in L-cells, suggesting that PIP2 alone is sufficient for the activation of ERM proteins and subsequent formation of microvilli.35 Indeed, it appears that phosphoinositide binding to ezrin is required for the subsequent phosphorylation at T567.36
The FERM domain of ERM proteins contains at least three consensus phosphoinositide-binding sites: KK(X)(n)K/RK. Mutagenesis of some of these lysine residues (at positions 63, 64 and 253, 254, or 63, 64 and 262, 263) to asparagines within ezrin was sufficient to abrogate binding to PIP2 and targeting to the plasma membrane.37 However, X-ray crystallography revealed that radixin binding to inositol tris-phosphate (IP3) involved residues K60, K63 and K278, suggesting that the other lysine residues within the FERM domain may only indirectly affect binding to phosphoinositides.38 The crystal stucture of radixin binding to IP3 reveals how binding of phosphoinositides could alter the local conformation of the FERM domain, leading to dissociation from the C-terminal domain and unfolding.39
Regulation through proteolytic cleavage
Ezrin is cleaved by calpain following the stimulation of leucocytes with phorbol 12-myristate 13-acetate (PMA).40 Interestingly, moesin is not cleaved, implying that individual ERM proteins can be differentially regulated in the same cell. The functional significance of this proteolytic cleavage is currently unclear, although insights can be drawn from the study of a related FERM domain superfamily member, talin. Calpain cleaves the head domain of talin (which contains the FERM domain) from its C-terminal tail. It appears that the calpain-cleaved talin head domain has an increased affinity for the cytoplasmic tail of β3 integrin, facilitating clustering and activation of integrins.41 It is not yet known where ezrin is cleaved but if, as with talin, it is cleaved to release the FERM domain, this would lead to dissociation of ezrin's binding partners [e.g. intercellular adhesion molecules (ICAMs) 1–3, CD44 or L-selectin] from the actin cytoskeleton and might thereby alter their adhesive capacity. Clearly, more work is required to understand the significance of ezrin cleavage by calpain.
RECIPROCAL REGULATION BETWEEN ERM PROTEINS and RHO GTPases
Our understanding of the regulation and function of ERM proteins was broadened when signalling pathways linking ERM proteins and RhoA were discovered.42–44 This section describes the network of regulatory interactions between Rho GTPases and ERM proteins.
The Rho family of GTPases
Rho family GTPases are small (≈ 21 000 molecular weight) GTP-binding proteins and, in mammals, consist of approximately 20 family members. The most extensively investigated are RhoA, Rac and Cdc42. Rho proteins are involved in regulating cytoskeletal dynamics in response to extracellular stimuli.6 With few exceptions, Rho family members have an intrinsic GTPase activity that catalyses the conversion of bound GTP to GDP. When bound to GTP, Rho proteins are active and interact with their downstream targets. Their conformational changes, when bound to GDP, are such that they have a much reduced affinity for targets, and are inactive. Cycling of the Rho GTPases between active and inactive forms is influenced by the action of guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and GDP dissociation inhibitors (GDIs) (Fig. 3). GEFs catalyse exchange of GDP for GTP, thereby promoting activation of Rho GTPases,45 whereas GAPs stimulate the intrinsic GTPase activity, leading to inactivation.46 As with ERM proteins, the Rho family of GTPases usually become associated with membranes when they are activated, allowing them to exert their effects locally. Rho GTPases are post-translationally modified by farnesylation or geranylgeranylation, and these isoprenyl groups are required for targeting to membranes (Fig. 3). The RhoGDIs sequester Rho GTPases in the cytosol by binding to the isoprenyl groups, thereby inhibiting their activity.47 Phosphorylation of RhoGDI by PKCα leads to dissociation from Rho, allowing Rho to become activated (Fig. 3).48
Figure 3.
Regulation of Rho GTPases. In unstimulated cells, Rho GTPases are maintained in an inactive state in the cytoplasm by binding to RhoGDI (purple) via their isoprenylated C-termini (shown in red). Dissociation of RhoGDI from Rho may occur through phosphorylation or interaction with activated and unfolded ezrin/radixin/moesin (ERM) proteins, allowing the isoprenyl group to incorporate into membranes. Membrane recruitment and interaction with RhoGEFs (e.g. Dbl) induces the exchange of GDP for GTP on Rho GTPases. When bound to GTP, Rho GTPases interact with and activate downstream effectors (e.g. ROCK). RhoGAPs (green) inactivate Rho GTPases by increasing their intrinsic GTPase activity, resulting in accelerated hydrolysis of GTP to GDP. RhoGDIs are able to extract GDP-bound Rho GTPases from membranes. PKCα, protein kinase C α.
To date, a multitude of genes with potential GAP and GEF activities have been identified.5 In contrast, only three GDIs have been identified in mammals and little is known about how their inhibitory actions are regulated. Mice with targeted disruption of GDI-D4 (a haematopoietic-specific GDI), show a mild reduction in macrophage superoxide production, but otherwise appear normal.49
RhoGDI displacement by ERM proteins: upstream regulators of Rho GTPases?
In 1997, Hirao et al. were the first to report that RhoGDI associates with CD44 and moesin.42 It was later shown that RhoGDI and ERM proteins could interact directly with one another.50 Direct association between ERM proteins and RhoGDI is thought to displace RhoGDI from Rho GTPases, allowing them to become loaded with GTP and subsequently activated. A crystal of the radixin FERM domain and RhoGDI has been produced, but the structure has yet to be resolved.51
A novel role for GDIs in localizing Rho GTPases has recently been proposed.52 As well as binding Rac-GDP, RhoGDI can interact with constitutively activated (GTP-loaded) Rac, preventing interactions with GAPs and downstream effectors.52,53 The Rac-GTP–RhoGDI complex appears to be targeted to sites of local integrin activation, where RhoGDI is displaced from Rac, allowing Rac to interact with its effectors. Further research will determine whether ERM proteins are involved in displacing RhoGDI from Rac-GTP.
Dbl exchange factor and ERM proteins
In vitro interaction between the RhoGEF Dbl and ERM proteins has been reported, suggesting a pathway whereby ERM proteins can act upstream of Rho GTPases (Fig. 2).54 Further in vivo work is required to assess whether this interaction occurs physiologically and the nature of its impact on Rho GTPase activity.
Regulation of ERM proteins by Rho GTPases
RhoA
Direct inhibition of RhoA function, using the Clostridium botulinum toxin C3 transferase, can have a dramatic effect on ERM protein activity. Treatment of most cell types with C3 transferase results in microvillar collapse and concomitant inactivation of ERM proteins.35 Lysophosphatidic acid (LPA), a constituent of serum,55 can signal to Rho through the LPA receptor and activate ERM proteins.56 This effect may be mediated, in part, by ROCK, which, as described above, can phosphorylate ERM proteins. The LPA receptor is a G-protein-coupled receptor that signals, via Gα13, to activate p115RhoGEF and subsequently RhoA.45 Interestingly, Gα13 is known to interact with and conformationally activate radixin,57 which in turn could activate RhoA by displacing RhoGDI. LPA receptors and related receptors are found on the surface of leucocytes,58 but links between Gα12/13 subunits, ERM proteins and RhoA have not, to date, been described in leucocytes.
Rac
Rac and ERM proteins are both required for membrane ruffling and lamellipodium extension and are important for cell migration, as well as for the production of reactive oxygen species in phagocytes (see below).5,11 Although molecular links between Rac and ERM proteins have not been studied extensively, different studies suggest that Rac might either positively or negatively regulate ERM proteins. On the one hand, Rac can regulate PIP2 production (Fig. 2)59 and could thereby increase ERM activity. ERM proteins can be activated by PIP2 independently of Rho35 and it is possible that Rac is involved here. On the other hand, Rac has recently been proposed to down-regulate ERM activity by stimulating dephosphorylation (see below).60
Cdc42
Cdc42 induces the formation of filopodia, actin-rich protrusions involved in sensing the extracellular environment.5 In fibroblasts, Cdc42-induced filopodial extensions are rich in C-terminally phosphorylated ERM proteins.28 Myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), a relative of ROCK, is a downstream target of Cdc42 and is able to phosphorylate moesin at position 558 in vitro. It would be interesting to determine whether phosphorylation of ERM proteins is regulated by Cdc42/MRCK in leucocytes.
Binding partners of erm proteins
Since their discovery, a variety of proteins have been shown to interact with ERM proteins. In this section we will focus predominantly on binding partners of ERM proteins that are expressed in leucocytes.
Integral membrane proteins
ERM proteins can associate with integral membrane proteins in one of two ways: either through direct interaction or via an adaptor molecule known as EBP50 (ERM-binding phospho-protein of 50 000 molecular weight).61 As a general rule, ERM proteins interact with seven-pass transmembrane proteins (e.g. cystic fibrosis transmembrane conductance regulator62 or β2-adrenergic receptor63) via EBP50. However, the sodium–hydrogen exchanger, NHE1, interacts with ERM proteins both directly and via EBP50.64,65 Interestingly, EBP50 has recently been reported to link lipid rafts in T cells to the actin cytoskeleton via ERM proteins, and to play a negative role in immune synapse formation.66
The best characterized integral membrane ERM-binding partners belong to the single-pass transmembrane proteins. Table 1 lists known interactions between ERM proteins and single-pass transmembrane proteins that are expressed in leucocytes. There appear to be two different modes of interaction between ERM proteins and transmembrane receptors. For some receptors, ERM proteins bind to juxta-membrane clusters of basic amino acids (Table 2).67 For other receptors, including syndecan 2, ICAM-2 and ICAM-3, non-basic amino acid residues are necessary for interaction with ERM proteins (Table 2).68,69 Indeed, the crystal structure of the ICAM-2 cytoplasmic tail complexed with the radixin FERM domain does not include the basic amino acid clusters.38 Radixin's affinity for different cytoplasmic tails of cell-adhesion molecules varies dramatically when measured in vitro: it is highest for ICAM-2 and lowest for ICAM-3, although both ICAM-2 and ICAM-3 can co-immunoprecipitate with ERM proteins in cell lysates.70,71 It is possible that other proteins, or protein modifications such as phosphorylation, contribute to the association between ERM proteins and transmembrane receptors in cells.
Table 1.
Ezrin/radixin/moesin (ERM)-binding partners in leucocytes
| Binding partner | Cell type | ERM? | Possible role of interaction | Reference |
|---|---|---|---|---|
| Integral membrane proteins | ||||
| ″ICAM-1 | T cells | M, E | Uropod localization | 115 |
| ″ICAM-2 | Thymoma | E | Susceptibility to cytotoxic NK cell killing | 38,67,105 |
| ″ICAM-3 | T cells | M | Polarization | 68–71 |
| ″CD43 | T cells | M, E | Immune synapse formation | 67,100,102,103 |
| ″CD44 | T cells | M, E | Uropod localization | 67,70,71 |
| ″CD95 (Fas) | T cells | E | Uropod localization Apoptosis | 72,73 |
| ″PSGL-1 | K562 (myeloid) | M | Rolling Uropod localization | 84,85 |
| ″L-selectin | Lymphocytes | M, E | Microvillar positioning PMA-induced shedding | 85,86, and personal communication |
| ″P-glycoprotein | CEM-VBL100 | E, R, M | Uropod localization Multi-drug resistance | 116 |
| Cytosolic proteins | ||||
| ″EBP-50 | T cells | M, E | Negative regulation of IS formation | 66 |
| ″Calpain | T cells | M | Specific cleavage of moesin | 40 |
| ″P40phox and P47phox | U937 cells | M | Phagosome formation? | 114 |
| ″P56Lck | Jurkat T cells | E | Tyrosine phosphorylates ezrin on Y145 | 104 |
| ″Syk | K562 cells | M | PSGL-1 signalling | 75 |
| ″PI3-kinase | LLC and lymphocytes | E | Apoptotic survival | 107,108 |
In the table above, the known binding partners for ERM proteins in leucocytes are listed, together with the cell type in which the interaction was identified, the ERM proteins with which they are known to interact (E = ezrin, R = radixin M = moesin), and the role of the interaction in leucocyte responses.EBP-50, ERM-binding phospho-protein of 50 000 molecular weight; ICAM-1, intercellular adhesion molecule type 1; ICAM-2, intercellular adhesion molecule type 2; IS, immunological synapse; NK, natural killer; PI3-kinase, phosphatidylinositide 3-kinase; PMA, phorbol 12-myristate 13-acetate; PSGL, P-selectin glycoprotein-ligand-1.
Table 2.
Cytoplasmic tails of leucocyte adhesion molecules known to interact with ezrin/radixin/moesin (ERM) proteins
| Adhesion molecule | Cytoplasmic tail |
|---|---|
| L-selectin | 356-RRLKKGKKSKRSMNDPY-372 |
| CD44 | 289-VNSRRRCGQKKKLVINSGNGAVEDRKPSGLNG-320 |
| PSGL-1 | 334-RLSRKGHMYPVRNYSPTEMVCISSLLPDGGEG-365 |
| CD43 | 278-RRRQKRRTGALVLSRGGKRNGVVDAWAGPAQ-308 |
| Syndecan-2 | 170-RMRKKDEGSYDLGERKPSSAAYQKAPTKEFYA-201 |
| ICAM-2 | 249-QHLRQQRMGTYGVRAAWRRLPQAFRP-275 |
| ICAM-3 | 482-REHQRSGSYHVREESTYLPLTSMQPTEAMGEEPSRAE-518 |
Underlined amino acids are residues that have been shown to be important for interaction with ERM proteins. Amino acid residues within the cytoplasmic tails of intercellular adhesion molecule type 1 (ICAM-1), P-glycoprotein, CD95 (Fas) and CD31 involved in ERM protein interaction have not been identified and these tails are therefore not included.
PSGL, P-selectin glycoprotein-ligand-1.
In vitro experiments suggest that interactions between ERM proteins and the intracellular domains of CD43, CD44 and ICAM-2 are stabilized by PIP2,42 possibly by maintaining ERM proteins in an active conformation.
Although most receptors appear to bind all three ERM proteins, the death receptor Fas/CD95 binds to ezrin, but not moesin, in T lymphocytes.72 The F2 region of the ezrin FERM domain (residues 149–168) is involved in interacting with Fas and is only 60–65% identical with moesin and radixin.73 In contrast, the radixin FERM domain interacts with the ICAM-2 cytoplasmic tail via its F3 region (predominantly residues 245–252). The crystal structure of the EBP50 and moesin FERM domain shows that EBP50 interacts with a different region of F3 to ICAM-2.74 These results suggest that ERM proteins use different regions within the FERM domains to interact with different binding partners, and could therefore potentially interact simultaneously with EBP50 and/or more than one integral membrane protein. It will be interesting to know whether the FERM domain can bind to transmembrane receptors and RhoGDI at the same time.
Cytosolic proteins
ERM proteins have been shown to interact with many cytosolic proteins. The lower part of Table 1 lists the cytosolic proteins that may be involved in leucocyte responses. Two of particular relevance to leucocyte signalling are phosphatidylinositide 3-kinase (PI3-K) (Fig. 2) and the leucocyte-specific tyrosine kinase, Syk. Binding of PI3-K to ezrin might protect leucocytes from apoptosis (see below). ERM proteins have recently been reported to interact directly with Syk following the antibody-mediated crosslinking of P-selectin glycoprotein-ligand-1 (PSGL-1).75 All three ERM proteins contain a cryptic immunoreceptor tyrosine-based activation motif (ITAM). Tyrosine residues within ITAMs are normally phosphorylated prior to the recruitment of signalling molecules such as Syk. Although it has been demonstrated that two tyrosine residues (Y191 and Y205) in the FERM domain are important for the recruitment of Syk, it remains unclear whether these tyrosines need to be phosphorylated to bind Syk.
Given that Syk can tyrosine phosphorylate and activate the RacGEF Vav1,76 it is conceivable that Syk binding to PSGL-1/ERM initiates a negative feedback loop whereby Vav1-induced activation of Rac would activate an ERM-specific C-terminal threonine phosphatase that dephosphorylates and inactivates ERMs, leading to disassociation of ERMs from PSGL-1. It is not known whether PSGL-1 activates Rac, but cross-linking of other ERM-binding cell-adhesion molecules, such as ICAM-1 and L-selectin, leads to the activation of Rho family GTPases77–79 and thus it is possible that they act via Syk, or other ITAM-binding proteins, to regulate Rho/Rac activity.
ERM PROTEINS and RHO GTPases IN LEUCOCYTE RESPONSES
Leucocytes alter their shape and become polarized in response to stimuli during migration and cell-to-cell communication (e.g. during interaction with endothelial cells, antigen presentation or cell killing). Leucocyte polarization involves the partitioning of the cell into separate domains. For example, in migrating leucocytes, the actin polymerizing network, chemokine receptors, Rac, Cdc42 and integrins are found predominantly at the front, whereas Rho, the microtubule organizing centre (MTOC) and cell–cell adhesion molecules (such as ICAMs 1–3, CD43, PSGL-1 and CD44) are found at the rear. Several recent reports have described the involvement of ERM proteins and Rho family GTPases in establishing and maintaining leucocyte polarization.
Microvilli and leucocyte–endothelial interactions
Circulating leucocytes have abundant microvilli on their cell surface, which are thought to present adhesion molecules involved in the capture of leucocytes by endothelial cells. ERM proteins are required for the formation and maintenance of microvilli, and, in fact, ezrin was first isolated from chicken intestinal microvilli.80 ERM protein activation correlates with the formation or elongation of microvilli in both adherent and suspension cells. Expression of constitutively active RhoA can activate ERM proteins and lead to the formation of microvillus-like structures.81 Over-expression of an ERM-binding integral membrane protein can also enhance microvillar length and ERM protein recruitment to the plasma membrane.82 Conversely, treatment of murine thymoma cells with antisense oligonucleotides that prevent the expression of all three ERM proteins led to the complete loss of microvilli.83
The initial capture and subsequent rolling of leucocytes on endothelial cells is generally considered to require attachment of the adhesion molecules involved (e.g. L-selectin and PSGL-1) to the leucocyte actin cytoskeleton in microvilli.84,85 Indeed, treatment of leucocytes with toxins that inhibit actin polymerization, such as cytochalasin B or D, dramatically inhibits leucocyte rolling. ERM proteins bind to both L-selectin and PSGL-185,86 (Table 1) and thus could be good candidates for regulating leucocyte capture/rolling by mediating receptor–actin cytoskeleton interaction. In agreement with this, disruption of the moesin–actin interaction correlates with the inhibition of PSGL-1-mediated rolling.85 Interestingly, neutrophils isolated from Rac2 knockout mice are defective in L-selectin-dependent rolling,87 but whether this effect is linked to the ability of Rac to regulate ERM protein phosphorylation is not known.
Rolling leucocytes are stimulated by chemokines presented on the surface of endothelial cells, which activate leucocyte integrins and thereby induce stronger adhesion to the endothelium. When stimulated with the chemokine SDF-1α, peripheral blood lymphocytes (PBLs) lose surface microvilli concomitant with rapid C-terminal dephosphorylation of ERM proteins.24 In these cells, exogenous expression of the moesin FERM domain was sufficient to induce microvillar collapse, whereas the constitutive expression of active moesin (threonine mutated to aspartate to mimic phosphorylation) led to an increase in microvillar length. In addition, SDF-1α-induced polarization of PBLs was retarded in cells constitutively expressing active moesin, suggesting that dephosphorylation of ERM proteins is a necessary prerequisite for chemokine-induced polarization. SDF-1α-induced collapse of microvilli may be necessary to redistribute transmembrane receptors during cell polarization and to release actin monomers for new actin polymerization at the leading edge. More insight into the pathway that leads from chemokine receptor stimulation to ERM dephosphorylation will reveal whether microvillar collapse is caused by chemokine-induced changes in Rho GTPase activity.
Leucocyte migration
Leucocytes migrate both on the endothelial surface (prior to transmigration) and through lymph nodes and peripheral tissues. In migrating leucocytes in vitro, Rac is required for actin polymerization and membrane protrusion at the front of the cell, whereas Rho is required to retract the rear.88 Mutual suppression of one GTPase by the other may allow Rac to predominate at the leading edge and Rho at the back.89 However, active Rac has also been observed at the rear of migrating neutrophils,90 so it may have a function there in addition to its role at the front. In neutrophils, polarization may be initiated by different G-protein-coupled receptors that differentially regulate Rac (via Gi) or Rho (via Gα12/13).91 In addition, RhoA could be asymmetrically distributed in polarized cells by targeted degradation of RhoA at the front, but not at the back of migrating fibroblasts.92
The rear of migrating leucocytes is either firmly attached to the substratum (for example monocytes on endothelial cells) or forms a uropod that stick upwards and outwards away from the substratum (for example lymphocytes), sometimes up to six times the height of the cell.4 What causes leucocytes to adopt these different migratory morphologies is currently not known. In leucocytes with a firmly adherent rear, tail retraction can be a rate-limiting step for migration, and is dependent on RhoA/ROCK, which may mediate both integrin deactivation and actomyosin-dependent contraction.93,94 In neutrophils, dephosphorylation of moesin at T558 was reported to precede tail retraction, and inhibition of moesin dephosphorylation by the serine/threonine phosphatase inhibitor, calyculin A, prevented tail retraction.16 As Rac can stimulate ERM dephosphorylation,60 it is possible that the active Rac observed in the tail of neutrophils is responsible for moesin dephosphorylation. This response could transiently allow release of the actin cytoskeleton from transmembrane receptors. It will be important to identify this calyculin A-sensitive phosphatase and determine whether it is regulated by Rac in the tail of migrating leucocytes.
In migrating lymphocytes with uropods, cell adhesion molecules, such as ICAMs, CD43 and CD44, relocate to the uropod.1 ERM proteins are important for anchoring these cell adhesion molecules to the uropod: for example, localization of ICAM-3 to the uropod is dependent on its ERM-binding site.69,71 Clustering of cell adhesion molecules at the uropod is proposed to act as a platform for the recruitment of additional leucocytes during leucocyte transendothelial migration. Indeed, leucocyte-to-leucocyte rolling has been shown to occur in vitro and in vivo.95,96
Rho GTPases have been implicated in regulating uropod formation. Constitutively active mutants of Rho, Rac and Cdc42 impair uropod formation and chemokine-induced lymphocyte polarization, whereas dominant negative mutants induce uropod formation.97 The effects of Cdc42 and Rac on uropod formation may be an indirect consequence of their roles in promoting membrane protrusion at the leading edge.89 Rho, however, is localized to the uropod and plays a major role in its formation.91 Understanding the spatial organization of ERM proteins and the kinases/phosphatases that regulate their activity will shed light on the inter-relationships between Rho GTPase activity, ERM phosphorylation, uropod formation and rear retraction.
T-cell interaction with antigen-presenting cells
T-cell recognition of cognate antigen is an essential prerequisite for T-cell proliferation and initiation of an immune response. T cells polarize towards antigen-presenting cells (APCs), such as dendritic cells or B cells, when they recognize antigen bound to major histocompatibility complex (MHC) molecules on the cell surface. The interaction zone that forms between a T cell and its APC is known as the immunological synapse (IS), and similar cell-to-cell interactions occur between NK cells and their target cells. Formation of the IS facilitates engagement of the T-cell receptor (TCR) with its cognate MHC–peptide complex on the APC, allowing subsequent TCR signalling, leading to proliferation. During IS formation, some cell adhesion and signalling proteins accumulate either in the centre (e.g. CD3, Lck and PKCθ) or the periphery (e.g. the integrin LFA-1) of the IS zone, whereas others (e.g. CD43) are excluded from the IS. Movement of cell adhesion molecules and cell-surface signalling receptors into the IS is not a passive event and requires remodelling of the actin cytoskeleton mediated by the RhoGEF Vav1, Rac and Cdc42 and the Cdc42 target, Wiscott–Aldrich Syndrome protein (WASP).98
Exclusion of CD43 (a known ERM-binding protein; Tables 1 and 2) from the IS occurs both in vitro and in lymph nodes in vivo.99 CD43 localizes in a cap-like structure, the distal pole complex (DPC), at the most distal site to the IS, where it co-localizes with ezrin and RhoGDI.100,101 Recent evidence indicates that ERM proteins coordinate the movement of molecules, such as CD43, away from the IS to the DPC.100,102,103 Upon IS formation, rapid dephosphorylation of the C-terminal threonine of ERM proteins was followed by rephosphorylation within a timescale of 3 min.100 Transient dephosphorylation of ERM proteins is believed to allow ERM-binding integral membrane proteins to detach from the cortical actin cytoskeleton and be ‘squeezed out’ of the central IS zone. Subsequent reactivation of ERM proteins would facilitate reattachment of CD43 to actin cytoskeleton outside the IS. The kinase responsible for the activation of ERM proteins at the periphery of the IS is currently not known. It is unlikely that PKCθ acts on ERMs excluded from the IS as it is localized in the central zone of the IS. Rho is excluded from the IS, and thus it is possible that Rho-activated ROCK mediates ERM rephosphorylation. It is not known whether the tyrosine kinase Lck acts on ERM proteins during IS formation (see Fig. 2), but it is possible that Lck-mediated phosphorylation of the FERM domain104 could alter its interaction with ERM-binding receptors, such as CD43.
Recently, it has been reported that antibody-mediated TCR stimulation induces rapid dephosphorylation of ERM proteins via Rac and its exchange factor, Vav1.60 Dephosphorylation of ERM proteins in this context is thought to increase the deformability of the T cell, allowing closer interaction during T cell–APC conjugation. Rho and Rac could therefore act antagonistically to regulate ERM protein activity, where Rho induces ROCK activity and activation of ERM proteins and Rac induces phosphatase activity and inactivation of ERM proteins. The spatial distribution of Rac and Rho activity in a conjugating T cell might be similar to a migrating cell (active Rac in the IS, active Rho in the DPC) and could therefore reinforce the polarized distribution of activated ERM proteins.
Apoptosis
Fas-mediated apoptosis is crucial for T-cell selection in the thymus. Polarization of Fas (CD95) to the uropod of activated lymphocytes renders them susceptible to apoptosis.72 Ezrin has been shown to link Fas to the actin cytoskeleton, and ezrin (but not moesin) is required for Fas-mediated apoptosis. Indeed, efficient target cell killing by cytotoxic T lymphocytes requires ezrin and ICAM-2 localization to the uropod of the target cell.105 Microvillar collapse is a common early event in apoptosis, and Fas ligand (FasL) induction of microvillar collapse has been attributed to rapid dephosphorylation of ERM proteins.106 This may facilitate relocalization of Fas and ICAM-2 to the uropod. Crosslinking of ICAM-2 prevents Fas- or TNF-α-induced apoptosis, probably via tyrosine phosphorylation of ezrin and subsequent recruitment of PI3-kinase, a known promotor of cell survival.107,108 Interestingly, cross linking of ICAM-2 also induced ROCK-dependent phosphorylation of ezrin at the C-terminal threonine, suggesting an involvement of the Rho pathway in preventing apoptosis.108 Indeed, Rho, Rac and Cdc42 have all been shown to inhibit cytotoxic T lymphocyte- and Fas-induced apoptosis.109
Phagocytosis
Uptake of foreign particles/organisms by phagocytes is an actin-dependent process.110 Phagosomes are surrounded by polymerized actin, and ERM proteins associate with phagosomes.111,112 Destruction of internalized pathogens in phagosomes is mediated, in part, by NADPH oxidase-induced production of superoxide radicals. Rac is well known to associate with the NADPH oxidase upon cell stimulation, and to act together with members of the phox (phagocytic oxidase) family of proteins to activate the oxidase.113 The FERM domain of moesin can interact with p40phox and p47phox via their phosphoinositide-binding (PX) domains, and this interaction requires phosphoinositides.114 Rac is generally believed to dissociate from RhoGDI prior to NADPH oxidase activation,113 and thus it would be interesting to determine whether ERM proteins affect NADPH oxidase activity via RhoGDI or other mechanisms.
Conclusions and future perspective
The activation status of ERM proteins in leucocytes is highly dynamic and appears to be regulated positively by Rho and (possibly) negatively by Rac. Although further evidence is required to verify the negative effect of Rac, the antagonistic relationship between Rac and Rho during processes such as migration and adhesion89 is consistent with them having opposing effects on ERM proteins. Both positive and negative feedback loops probably exist between ERM proteins and Rho GTPases, and these could allow their precise tempo-spatial regulation during leucocyte responses such as migration and APC–T-cell interaction.
In order to best understand how and where ERM proteins are activated, identifying the kinase(s) and phosphatase(s) responsible for their regulation in vivo is a major goal for the future. In addition, the physiological relevance of N-terminal phosphorylation (for example at Y145 or T235) remains to be clarified, and there may be still other as-yet-unidentified phosphorylation sites. It is possible that ERM proteins have several stages of activation and that their ability to bind one protein better than another may rely on how many ‘critical activation residues’ are phosphorylated.
What also needs to be determined is whether ERM proteins can bind to one or more binding partners simultaneously, and whether they play a role in the adhesion receptor-induced activation of Rho GTPases. Finally, the analysis of leucocyte behaviour in mice lacking functional ERM proteins or Rho GTPases will provide further insight into their inter-relationship and physiological functions.
References
- 1.Sanchez-Madrid F, del Pozo MA. Leucocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leucocytes through the vessel wall and beyond. Thromb Haemost. 2003;90:598. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 3.Delon J, Stoll S, Germain RN. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol Rev. 2002;189:51. doi: 10.1034/j.1600-065x.2002.18906.x. [DOI] [PubMed] [Google Scholar]
- 4.Fais S, Malorni W. Leucocyte uropod formation and membrane/cytoskeleton linkage in immune interactions. J Leukoc Biol. 2003;73:556. doi: 10.1189/jlb.1102568. [DOI] [PubMed] [Google Scholar]
- 5.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 6.Cantrell DA. GTPases and T cell activation. Immunol Rev. 2003;192:122. doi: 10.1034/j.1600-065x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 7.Amieva MR, Furthmayr H. Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment, and cell–cell contacts. Exp Cell Res. 1995;219:180. doi: 10.1006/excr.1995.1218. [DOI] [PubMed] [Google Scholar]
- 8.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 9.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem. 1999;274:34507. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 10.Miller KG. A role for moesin in polarity. Trends Cell Biol. 2003;13:165. doi: 10.1016/s0962-8924(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 11.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 12.McClatchey AI. Merlin and ERM proteins: unappreciated roles in cancer development? Nat Rev Cancer. 2003;3:877. doi: 10.1038/nrc1213. [DOI] [PubMed] [Google Scholar]
- 13.Amieva MR, Wilgenbus KK, Furthmayr H. Radixin is a component of hepatocyte microvilli in situ. Exp Cell Res. 1994;210:140. doi: 10.1006/excr.1994.1021. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz-Albiez R, Merling A, Spring H, Moller P, Koretz K. Differential expression of the microspike-associated protein moesin in human tissues. Eur J Cell Biol. 1995;67:189. [PubMed] [Google Scholar]
- 15.Ramoni C, Luciani F, Spadaro F, Lugini L, Lozupone F, Fais S. Differential expression and distribution of ezrin, radixin and moesin in human natural killer cells. Eur J Immunol. 2002;32:3059. doi: 10.1002/1521-4141(200211)32:11<3059::AID-IMMU3059>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoshinaga-Ohara N, Takahashi A, Uchiyama T, Sasada M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp Cell Res. 2002;278:112. doi: 10.1006/excr.2002.5571. [DOI] [PubMed] [Google Scholar]
- 17.Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem. 1999;274:2315. doi: 10.1074/jbc.274.4.2315. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi S, Hata M, Fukumoto K, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31:320. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 19.Gary R, Bretscher A. Ezrin self association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gary R, Bretscher A. Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc Natl Acad Sci USA. 1993;90:10846. doi: 10.1073/pnas.90.22.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpen O. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumour suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112:895. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 22.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol. 2000;150:193. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270:31377. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- 24.Brown MJ, Nijhara R, Hallam JA, et al. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERMs which facilitates loss of microvilli and polarization. Blood. 2003;102:3890, 9. doi: 10.1182/blood-2002-12-3807. [DOI] [PubMed] [Google Scholar]
- 25.Ng T, Parsons M, Hughes WE, et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietromonaco SF, Simons PC, Altman A, Elias L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J Biol Chem. 1998;273:7594. doi: 10.1074/jbc.273.13.7594. [DOI] [PubMed] [Google Scholar]
- 27.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura N, Oshiro N, Fukata Y, et al. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells. 2000;5:571. doi: 10.1046/j.1365-2443.2000.00348.x. [DOI] [PubMed] [Google Scholar]
- 29.Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol. 1998;141:409. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hishiya A, Ohnishi M, Tamura S, Nakamura F. Protein phosphatase 2C inactivates F-actin binding of human platelet moesin. J Biol Chem. 1999;274:26705. doi: 10.1074/jbc.274.38.26705. [DOI] [PubMed] [Google Scholar]
- 32.Yang HS, Hinds PW. Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol Cell. 2003;12:269. doi: 10.1016/s1097-2765(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 33.Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, Studzinski GP. Expression of the neuronal cyclin-dependent kinase 5 activator p35Nck5a in human monocytic cells is associated with differentiation. Blood. 2001;97:3763. doi: 10.1182/blood.v97.12.3763. [DOI] [PubMed] [Google Scholar]
- 35.Yonemura S, Matsui T, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115:2569. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 36.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate PIP(2) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000;151:1067. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamada K, Shimizu T, Yonemura S, Tsukita S, Hakoshima T. Structural basis of adhesion-molecule recognition by ERM proteins revealed by the crystal structure of the radixin–ICAM-2 complex. Embo J. 2003;22:502. doi: 10.1093/emboj/cdg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamada K, Shimizu T, Matsui T, Tsukita S, Hakoshima T. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 2000;19:4449. doi: 10.1093/emboj/19.17.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shcherbina A, Bretscher A, Kenney DM, Remold-O'Donnell E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 1999;443:31. doi: 10.1016/s0014-5793(98)01674-3. [DOI] [PubMed] [Google Scholar]
- 41.Yan B, Calderwood DA, Yaspan B, Ginsberg MH. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J Biol Chem. 2001;276:28164. doi: 10.1074/jbc.M104161200. [DOI] [PubMed] [Google Scholar]
- 42.Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signalling pathway. J Cell Biol. 1996;135:37. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotani H, Takaishi K, Sasaki T, Takai Y. Rho regulates association of both the ERM family and vinculin with the plasma membrane in MDCK cells. Oncogene. 1997;14:1705. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- 44.Mackay DJ, Esch F, Furthmayr H, Hall A. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 46.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 47.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 48.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- 49.Guillemot JC, Kruskal BA, Adra CN, et al. Targeted disruption of guanosine diphosphate-dissociation inhibitor for Rho-related proteins, GDID4: normal haematopoietic differentiation but subtle defect in superoxide production by macrophages derived from in vitro embryonal stem cell differentiation. Blood. 1996;88:2722. [PubMed] [Google Scholar]
- 50.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 51.Hamada K, Seto A, Shimizu T, Matsui T, Takai Y, Tsukita S, Hakoshima T. Crystallization and preliminary crystallographic studies of RhoGDI in complex with the radixin FERM domain. Acta Crystallogr D Biol Crystallogr. 2001;57:889. doi: 10.1107/s090744490100556x. [DOI] [PubMed] [Google Scholar]
- 52.Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP–Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 53.Hancock JF, Hall A. A novel role for RhoGDI as an inhibitor of GAP proteins. EMBO J. 1993;12:1915. doi: 10.1002/j.1460-2075.1993.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K, Sasaki T, Mammoto A, et al. Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. O. Oncogene. 1998;16:3279. doi: 10.1038/sj.onc.1201874. [DOI] [PubMed] [Google Scholar]
- 55.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 56.Shaw RJ, Henry M, Solomon F, Jacks T. RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts. Mol Biol Cell. 1998;9:403. doi: 10.1091/mbc.9.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaiskunaite R, Adarichev V, Furthmayr H, Kozasa T, Gudkov A, Voyno-Yasenetskaya TA. Conformational activation of radixin by G13 protein alpha subunit. J Biol Chem. 2000;275:26206. doi: 10.1074/jbc.M001863200. [DOI] [PubMed] [Google Scholar]
- 58.Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 59.Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Ialpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 60.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell–APC conjugation. Nat Immunol. 2004;5:272, 9. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 61.Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- 63.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ–domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 64.Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na–H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell. 2000;6:1425. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 65.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na–H exchanger NHE1. J Cell Biol. 2002;159:1087. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Itoh K, Sakakibara M, Yamasaki S, et al. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J Immunol. 2002;168:541. doi: 10.4049/jimmunol.168.2.541. [DOI] [PubMed] [Google Scholar]
- 67.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol. 1998;140:885. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granes F, Berndt C, Roy C, Mangeat P, Reina M, Vilaro S. Identification of a novel Ezrin-binding site in syndecan-2 cytoplasmic domain. FEBS Lett. 2003;547:212. doi: 10.1016/s0014-5793(03)00712-9. [DOI] [PubMed] [Google Scholar]
- 69.Serrador JM, Vicente-Manzanares M, Calvo J, et al. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J Biol Chem. 2002;277:10400. doi: 10.1074/jbc.M110694200. [DOI] [PubMed] [Google Scholar]
- 70.Romero IA, Amos CL, Greenwood J, Adamson P. Ezrin and moesin co-localize with ICAM-1 in brain endothelial cells but are not directly associated. Brain Res Mol Brain Res. 2002;105:47. doi: 10.1016/s0169-328x(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 71.Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sanchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J Cell Biol. 1997;138:1409. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parlato S, Giammarioli AM, Logozzi M, et al. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 2000;19:5123. doi: 10.1093/emboj/19.19.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lozupone F, Lugini L, Matarrese P, et al. Identification and relevance of the CD95-binding domain in the N-terminal region of Ezrin. J Biol Chem. 2003;279:9199, 207. doi: 10.1074/jbc.M305561200. [DOI] [PubMed] [Google Scholar]
- 74.Finnerty CM, Chambers D, Ingraffea J, Faber HR, Karplus PA, Bretscher A. The EBP50–moesin interaction involves a binding site regulated by direct masking on the FERM domain. J Cell Sci. 2004;117:1547. doi: 10.1242/jcs.01038. [DOI] [PubMed] [Google Scholar]
- 75.Urzainqui A, Serrador JM, Viedma F, et al. ITAM-based interaction of ERM proteins with Syk mediates signalling by the leucocyte adhesion receptor PSGL-1. Immunity. 2002;17:401. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 76.Moores SL, Selfors LM, Fredericks J, Breit T, Fujikawa K, Alt FW, Brugge JS, Swat W. Vav family proteins couple to diverse cell surface receptors. Mol Cell Biol. 2000;20:6364. doi: 10.1128/mcb.20.17.6364-6373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signalling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755. [PubMed] [Google Scholar]
- 78.Brenner B, Gulbins E, Busch GL, Koppenhoefer U, Lang F, Linderkamp O. L-selectin regulates actin polymerization via activation of the small G-protein Rac2. Biochem Biophys Res Commun. 1997;231:802. doi: 10.1006/bbrc.1997.6191. [DOI] [PubMed] [Google Scholar]
- 79.Brenner B, Weinmann S, Grassme H, Lang F, Linderkamp O, Gulbins E. L-selectin activates JNK via src-like tyrosine kinases and the small G-protein Rac. Immunology. 1997;92:214. doi: 10.1046/j.1365-2567.1997.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bretscher A. Purification of the intestinal microvillus cytoskeletal proteins villin, fimbrin, and ezrin. Methods Enzymol. 1986;134:24. doi: 10.1016/0076-6879(86)34072-2. [DOI] [PubMed] [Google Scholar]
- 81.Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem. 1998;273:34663. doi: 10.1074/jbc.273.52.34663. [DOI] [PubMed] [Google Scholar]
- 82.Yonemura S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM) -binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol. 1999;145:1497. doi: 10.1083/jcb.145.7.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dwir O, Kansas GS, Alon R. Cytoplasmic anchorage of L-selectin controls leucocyte capture and rolling by increasing the mechanical stability of the selectin tether. J Cell Biol. 2001;155:145. doi: 10.1083/jcb.200103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snapp KR, Heitzig CE, Kansas GS. Attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is essential for leucocyte rolling on P-selectin. Blood. 2002;99:4494. doi: 10.1182/blood.v99.12.4494. [DOI] [PubMed] [Google Scholar]
- 86.Ivetic A, Deka J, Ridley A, Ager A. The cytoplasmic tail of L-selectin interacts with members of the ezrin-radixin-moesin (ERM) family of proteins: cell activation-dependent binding of moesin but not ezrin. J Biol Chem. 2002;277:2321. doi: 10.1074/jbc.M109460200. [DOI] [PubMed] [Google Scholar]
- 87.Roberts AW, Kim C, Zhen L, et al. Deficiency of the haematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 88.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116:4695. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 89.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 90.Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- 91.Xu J, Wang F, Van Keymeulen A, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 92.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 93.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 95.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil–neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leucocyte accumulation of P-selectin in vitro. J Clin Invest. 1996;98:1081. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leucocyte rolling in venules. J Exp Med. 2003;197:1355. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 98.Miletic AV, Swat M, Fujikawa K, Swat W. Cytoskeletal remodeling in lymphocyte activation. Curr Opin Immunol. 2003;15:261. doi: 10.1016/s0952-7915(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 99.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell–dendritic cell interactions in lymph nodes. Science. 2002;296:1873. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 100.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 101.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 102.Allenspach EJ, Cullinan P, Tong J, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 103.Roumier A, Olivo-Marin JC, Arpin M, et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 104.Autero M, Heiska L, Ronnstrand L, Vaheri A, Gahmberg CG, Carpen O. Ezrin is a substrate for Lck in T cells. FEBS Lett. 2003;535:82. doi: 10.1016/s0014-5793(02)03861-9. [DOI] [PubMed] [Google Scholar]
- 105.Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 106.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139:749. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:7300. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perez OD, Kinoshita S, Hitoshi Y, Payan DG, Kitamura T, Nolan GP, Lorens JB. Activation of the PKB/AKT pathway by ICAM-2. Immunity. 2002;16:51. doi: 10.1016/s1074-7613(02)00266-2. [DOI] [PubMed] [Google Scholar]
- 109.Subauste MC, Von Herrath M, Benard V, Chamberlain CE, Chuang TH, Chu K, Bokoch GM, Hahn KM. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J Biol Chem. 2000;275:9725. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- 110.Greenberg S, Burridge K, Silverstein SC. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1990;172:1853. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desjardins M, Celis JE, van Meer G, Dieplinger H, Jahraus A, Griffiths G, Huber LA. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194. [PubMed] [Google Scholar]
- 112.Defacque H, Egeberg M, Habermann A, et al. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000;19:199. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- 114.Wientjes FB, Reeves EP, Soskic V, Furthmayr H, Segal AW. The NADPH oxidase components p47 (phox) and p40 (phox) bind to moesin through their PX domain. Biochem Biophys Res Commun. 2001;289:382. doi: 10.1006/bbrc.2001.5982. [DOI] [PubMed] [Google Scholar]
- 115.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1998;273:21893. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 116.Luciani F, Molinari A, Lozupone F, et al. P-glycoprotein–actin association through ERM family proteins: a role in P-glycoprotein function in human cells of lymphoid origin. Blood. 2002;99:641. doi: 10.1182/blood.v99.2.641. [DOI] [PubMed] [Google Scholar]