Abstract
Chemokine receptors are important in the entry of leucocytes into the inflammatory sites of systemic lupus erythematosus (SLE). CCR7+ and CCR7− memory T cells exert different functions in homing, cytokine production and cytotoxicity. To determine whether differential expression and functions of the CCR7 occur in SLE patients, we examined CCR3, CCR4, CCR5, CCR7 and CCR9 on CD4+ and CD8+ T cells from normal and SLE subjects. Flow cytometry, real-time quantitative reverse transcription polymerase chain reactions and Northern blotting were used to detect the expression of chemokine receptors and cytokines; a chemotaxis assay was used to detect their functions. CD4+ T-cell stimulation with syngeneic CCR7+ CD8+ CD45RO+ T cells and dendritic cells (including transwell chambers) was used to induce cytokine expression. We demonstrated that CCR7 was selectively, frequently and functionally expressed on CD8+ (94·8%) but not on CD4+ (16·1%) T cells from patients with active SLE, whereas this phenomenon was not seen in normal subjects and in those whose SLE was inactive. CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE, themselves T helper type 2 (Th2) biased, were inducers of Th2 bias in CD4+ T cells in a cell–cell contact manner in vitro, meanwhile, the cells from both normal subjects and those whose SLE was inactive drove CD4+ T cells into a regulatory T-cell-derived cytokine pattern. Our findings might provide new clues to understanding the functions of CCR7+ CD8+ CD45RO+‘central’ memory T cells in autoimmue diseases (such as SLE). We suggest that in the case of active SLE, CCR7+ central memory T cells were able to enter peripheral blood and inflammatory sites from secondary lymphoid organs, were continuously expressing CCR7, and interacted with dendritic cells and functioned as CCR7–‘effector’ memory T cells, which were described in normal humans.
Keywords: chemokine receptors: CCR7; systemic lupus erythematosus (SLE); T cells: memory cells, Th2 cells
Introduction
A considerable amount of data from genomic, structural and functional studies indicate the role of chemokine receptors in both normal physiology (e.g. organogenesis, haematopoiesis, angiogenesis and physiological lymphocyte trafficking) and disease, such as the pathological distribution of leucocytes in chronic inflammation.1 Systemic lupus erythematosus (SLE) is a prototypic spontaneous systemic autoimmune disease characterized by autoantibody formation, multiple organ damage, and diverse and variable clinical inflammatory manifestations.2 The chemokine receptor–ligand pair appears to be important in the entry of leucocytes into the inflammatory sites of SLE and the activation of leucocytes in the disease.3–7 The expression of BLC/CXCL13 is enhanced in the thymus and kidney in aged BWF1 mice that develop lupus nephritis.3 SLC/CCL21 preferentially chemoattracts splenic mature dendritic cells (DCs) from aged BWF1 mice, whereas SLC/CCL21, LARC/CCL20, RANTES/CCL5 and MIP-1/CCL3 selectively affect peripheral DCs.3 CCR4 is highly expressed on CD4+ T cells from patients with active SLE but not on those from healthy controls and SLE patients whose disease is inactive.6 The CCR4+ T cells preferentially migrate into the renal tissue of lupus nephritis.4 The serum concentrations of RANTES/CCL5 and MCP-1/CCL2 in SLE patients are significantly changed during progression of disease, indicating an interaction between SLE disease activity and the production of CC chemokines.8 T helper type 1 (Th1) and Th2 responses may play differential roles in the pathogenesis of lupus-associated tissue injury.9 Th1 and Th2 cells express distinct sets of chemokine receptors and differentially respond to chemokines,10 which are pivotal in the recruitment of infiltrating cells to the pathological lesions.4,5 However, there are some contradicting reports indicating that an imbalance toward Th1 predominance is associated with an acceleration of lupus-like autoimmune disease.11
CD45RO+ memory T cells can be divided into two subpopulations, based on the expression of CCR7. CCR7+ CD45RO+ central memory T cells (TCM) control homing to secondary lymphoid organs interacting with DCs. CCR7– CD45RO+ effector memory T cells (TEM) migrate into and exert their effector function at sites of inflammation.12–14 Some of the proliferating TCM differentiate into TEM cells, acquiring effector function and switching from expression of CCR7 to CCR5, providing a plausible mechanism for the maintenance of a polyclonal and functionally diverse repertoire of human CD4+ memory T cells.15 A sequence for differentiation of virus-specific memory CD8+ T cells is suggested, e.g. CCR5– CCR7+>CCR5+ CCR7CCR7+>CCR5+>CCR5CCR7+>CCR5+ CCR7–.16,17 However, the concept has been questioned, based on the results of similar responsiveness of CCR7CCR7+>CCR5+ TCM and CCR7– TEM from T-cell-receptor-transgenic mice to lymphocytic choriomeningitis virus.18
In the present study, we report that CCR7+ CD8+ CD45ROCCR7+>CCR5+ cells selectively and frequently appear in peripheral blood from patients with active SLE. The cells, being themselves Th2 biased, are inducers of Th2 bias in CD4CCR7+>CCR5+ T cells; the cells from normal subjects and those with inactive SLE drive CD4CCR7+>CCR5+ T cells into a regulatory T-cell pattern. In the case of active SLE, TCM cells are able to enter the peripheral blood and inflammatory sites from the secondary lymphoid organs, continuously expressing CCR7CCR7+>CCR5+ CD45ROCCR7+>CCR5+, and can interact with DCs and function as ‘effector’ memory T cells.
Materials and methods
Reagents and antibodies
Phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)- anti-CD4, and anti-CD8 monoclonal antibodies (mAbs) were purchased from DAKO (Glostrup, Denmark). All recombinant chemokines and cytokines, macrophage-derived chemokine (MDC/CCL22), secondary lymphoid tissue chemokine (SLC/6Ckine/CCL21), thymus-expressed chemokine (TECK/CCL25), and FITC-labelled mouse monoclonal anti-CCR3 (83101.111), anti-CCR5 (45548), anti-CCR7 (150503), anti-CCR9 (112509), purified anti-CXCR3 (49801.111), anti-CXCR4 (12G5), anti-interferon-γ (IFN-γ; K3.53), anti-interleukin-2 (IL-2; 5534.21), anti-IL-4 (3010.211), anti-IL-5 (9906.1), anti-IL-10 (23738.111), anti-transforming growth factor-1β (TGF-1β; 1D11), anti-CD11a (38), anti-CD11b (44), anti-CD18 (MEM 48), anti-CD29 (2B4), and anti-CD62L (4G8) mAbs were purchased from R & D Systems Europe Ltd. (Abingdon, UK). PE-labelled anti-CCR4 (1G1), purified anti-CD25 (M-A251), anti-CD45RO (UCHL-1), PerCP-labelled anti-HLA-DR (L243), and PerCP-labelled anti-CD69 (L78), purified anti-CLA (HECA-452), anti-CD49d (9F-10), and anti-CD49e (VC-5) mAbs were purchased from BD PharMingen (San Diego, CA).
Patients, normal subjects and cell purification
SLE patients who fulfilled at least four of the American Rheumatism Association 1982 revised criteria for SLE19 were included. The 37 SLE patients (35 female and two male) with a median age of 31·4 years (range 21–48 years) had a mean disease duration of 4·0 years (0·5–12 years). All patients were treated with prednisolone (mean 10·8 mg/day). Disease activity was assessed by a modified SLE Disease Activity Index (SLEDAI) score.20 The mean SLEDAI for the patients with active disease was 14·6 (range 3–29), for patients whose disease was inactive the SLEDAI was 0.20 All patients gave informed consent according to institutional guidelines. Patient information is given in Table 1. A total of 18 healthy volunteers (17 female and one male) with a median age of 30·5 years (range 18–48 years) served as controls. In the experimental procedure, the SLE patients were randomly selected for each assay to give sufficient numbers for statistical analysis. CD4+ and CD8+ T cells were purified from peripheral blood mononuclear cells (PBMC) from patients with SLE or from normal subjects. A positive selection procedure of anti-CD4 or anti-CD8 mAb coated Dynabeads M-450 (Dynal A/S, Dynal, Norway) was performed according to the manufacturer's instructions. The purity of the CD4+ and CD8+ T cells ranged from 94% to 99% as determined by flow cytometry. Ninety-nine per cent pure CCR7+ CD4+ CD45RO+ and CCR7+ CD8+ CD45RO+ T cells were obtained from PBMC from different subjects by fluorescence-activated cell sorting (FACS) assay.
Table 1.
Clinical information for all patients included in this study*
| Organ involved** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex/age† | Dura.‡(year) | ANA§ | Anti-dsDNA§ | SLEDAI¶ | Ly | Th | CNS | GN | Sk | Ar | Type |
| 1 | F/30 | 0·5 | 2000 | 156 | 24 | + | + | – | + | + | – | Active |
| 2 | F/32 | 1·5 | 4000 | 17·5 | 3 | – | – | – | – | + | + | Active |
| 3 | F/36 | 2·5 | 8000 | 427 | 16 | + | + | – | + | – | – | Active |
| 4 | F/30 | 3·5 | 4000 | 7·5 | 5 | + | + | + | – | + | – | Active |
| 5 | F/19 | 1 | 8000 | 75 | 17 | – | + | + | + | – | – | Active |
| 6 | F/45 | 8 | 4000 | 185 | 29 | + | – | – | + | + | – | Active |
| 7 | F/27 | 3 | 8000 | 14·5 | 9 | + | + | – | – | + | – | Active |
| 8 | F/40 | 4 | 16000 | 90 | 23 | + | + | + | + | – | – | Active |
| 9 | F/24 | 2 | 8000 | 300 | 22 | – | – | + | + | – | – | Active |
| 10 | M/20 | 2·5 | 4000 | 114 | 15 | – | + | – | – | + | – | Active |
| 11 | F/38 | 4 | 2000 | 59 | 9 | + | – | – | – | – | – | Active |
| 12 | F/26 | 7 | 8000 | 75 | 12 | – | – | – | – | – | – | Active |
| 13 | M/31 | 2 | 2000 | 10·8 | 7 | + | + | – | – | + | + | Active |
| 14 | F/50 | 7 | 4000 | 69 | 29 | – | – | + | + | + | – | Active |
| 15 | F/48 | 4 | 2000 | 12 | 8 | + | – | – | + | + | – | Active |
| 16 | F/37 | 0·5 | 4000 | 90 | 19 | + | + | – | – | – | – | Active |
| 17 | F/32 | 2 | 2000 | 38 | 9 | – | – | – | + | + | – | Active |
| 18 | F/35 | 4 | 8000 | 11·5 | 6 | + | + | + | – | – | – | Active |
| 19 | F/30 | 0·5 | 16000 | 82 | 21 | + | – | + | + | + | – | Active |
| 20 | F/20 | 1 | 2000 | 48 | 17 | + | – | – | + | – | + | Active |
| 21 | F/31 | 4 | 1000 | 8 | 0 | – | – | – | – | – | – | Inactive |
| 23 | F/39 | 3 | 500 | 2·4 | 0 | + | – | – | + | – | – | Inactive |
| 24 | F/35 | 8 | 250 | 4·1 | 0 | + | – | – | – | + | – | Inactive |
| 25 | F/37 | 4 | 1000 | 1·4 | 0 | + | – | – | – | + | – | Inactive |
| 26 | F/22 | 2 | 1000 | 3·6 | 0 | – | – | – | + | + | – | Inactive |
| 27 | F/38 | 12 | 500 | <de. | 0 | + | – | – | – | – | – | Inactive |
| 28 | F/27 | 7 | 500 | 12 | 0 | – | – | – | – | + | – | Inactive |
| 29 | F/40 | 3 | 1000 | 4·5 | 0 | – | – | – | + | – | – | Inactive |
| 30 | F/45 | 5 | 500 | 5·5 | 0 | – | – | – | + | – | + | Inactive |
| 31 | F/21 | 8 | 250 | 2·5 | 0 | + | – | – | – | + | – | Inactive |
| 32 | F/25 | 2 | 500 | <de. | 0 | + | – | – | – | + | – | Inactive |
| 33 | F/29 | 6 | 250 | 4·5 | 0 | + | – | – | + | – | – | Inactive |
| 34 | F/26 | 9 | 250 | <de. | 0 | + | + | – | – | + | – | Inactive |
| 35 | F/25 | 6 | 500 | 2·4 | 0 | + | – | – | + | – | – | Inactive |
| 36 | F/28 | 8 | 1000 | 2·8 | 0 | + | – | – | – | – | – | Inactive |
| 37 | F/44 | 4 | 500 | 5 | 0 | – | – | – | + | + | – | Inactive |
The patients include those with active (SLEDAI score > 2) and inactive (SLEDAI score 0) SLE. The levels of CH50, C3 and C4 in sera, as well as proteinurea were determined but not listed for simplification. All the patients were receiving corticosteroid therapy, but stopped the treatment for 1 week prior to this study. None of the patients had any other autoimmune disease or infectious disease.
M = male; F = female.
Dura., duration of disease.
ANA, antinuclear antibodies expressed as the reciprocal of the titre; Anti-dsDNA, antibodies towards double-stranded DNA expressed as IU/ml; <de., undetectable level.
Systemic Lupus Erythematosus Disease Activity Index.
Ly, lymphocytopenia (≤1000 cells/μl); Th, thrombocytopenia; CNS, central nervous system; GN, glomerulonephritis; Sk, mucocutaneous lesions; Ar, Arthritis.
Flow cytometry
For detection of chemokine receptors (CCR3, CCR4, CCR5, CCR7, or CCR9), the purified CD4+ or CD8+ T cells, either isolated from patients with SLE or from normal controls, were incubated with a mouse anti-human FITC-labelled chemokine receptor (CCR4 PE-labelled) mAb at 5 μg/ml or 5 μg/ml matched isotype mouse antibody (DAKO), a mouse PE-labelled (or FITC-labelled) anti-human CD4 or CD8 (DAKO) mAb at 5 μg/ml, and other molecules on the cell surface (adhesion molecules and activation markers as indicated in Table 3) PerCP-labelled third antibody in phosphate-buffered saline (PBS; containing 2% bovine serum albumin and 0·1% sodium azide) for 20 min, followed by washing twice in staining buffer as previously described.21 All procedures were carried out at 4°. The analyses were performed with a flow cytometer (COULTER® XL, Coulter Corporation, Miami, FL). For intracellular cytokine immunofluorence staining as described elsewhere,22 the cells were washed twice in PBS and then fixed and permeabilized using IntraPrep® (Coulter-ImmunoTech, Miami, FL) according to the manufacturer's instructions. The cells were then incubated with the primary mouse anti-human cytokine mAb for 15 min at room temperature. Cells were washed twice and stained with FITC-conjugated goat anti-mouse antibodies for 15 min at room temperature. Cells were washed twice and stained with PE-labelled anti-human CD4 or CD8 (Dako) mAb for 15 min at 4°. Cells were washed and re-suspended in PBS containing 0·5% formaldehyde for FACS analysis.
Table 3.
The phenotypical characterization of CCR7+-bearing CD4+ and CD8+ T cells from active SLE*
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Marker (%) | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ |
| CD3 | >99 | >99 | >99 | >99 | >99 | >99 | >99 | >99 |
| CD45RO | 53 | 97 | 46 | 94 | 45 | 92 | 56 | 96 |
| CD25 | 37 | 65 | 42 | 79 | 34 | 88 | 36 | 85 |
| CD69 | 36 | 92 | 39 | 89 | 32 | 95 | 25 | 86 |
| HLA-DR | 45 | 87 | 34 | 91 | 32 | 85 | 26 | 82 |
| CD62L | 28 | 93 | 47 | 95 | 52 | 98 | 28 | 92 |
| CD18 | 23 | 69 | 23 | 75 | 38 | 67 | 43 | 85 |
| CD11a | 56 | 76 | 65 | 45 | 52 | 65 | 43 | 54 |
| CD11b | 23 | 87 | 47 | 81 | 34 | 89 | 32 | 86 |
| CLA | 34 | 23 | 65 | 21 | 72 | 31 | 53 | 25 |
| CD29 | 33 | 41 | 32 | 24 | 31 | 23 | 38 | 35 |
| CD49d | 34 | 28 | 29 | 35 | 21 | 26 | 37 | 21 |
| CD49e | 27 | 36 | 32 | 43 | 28 | 31 | 27 | 37 |
| CXCR3 | 14 | 18 | 21 | 14 | 12 | 8 | 32 | 21 |
| CXCR4 | 2 | 4 | 3 | 6 | 10 | 15 | 8 | 2 |
*The data are phenotypes from each individual experiment in a total of four patients. The experiments were conducted in purified CD4+ or CD8+ T cells from patients with active SLE and gated CCR7+ cells.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) assay
All real-time quantitative RT-PCR reactions were performed as described elsewhere.23–25 Briefly, total RNA from purified CD4+ or CD8+ T cells (1 × 106, purity > 99%) was prepared by using a Quick Prep® total RNA extraction kit (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. RNA was reverse transcribed using oligo-dT(12–18) and Superscript II reverse transcriptase (Life Technologies, Grand Island, USA). The real-time quantitative PCR was performed in special optical tubes in a 96-well microtitre plate (Applied Biosystems, Foster City, CA) with an ABI PRISM® 7700 Sequence Detector System (Applied Biosystems). By using a SYBR® Green PCR Core Reagents Kit (Applied Biosystems, P/N 4304886), fluorescence signals were generated during each PCR cycle via the 5′-to 3′ endonuclease activity of AmpliTaq Gold24 to provide real-time quantitative PCR information. The sequences of the specific primers were as follows: CCR3 sense, 5′-TCTAAACCTTTGCAGCCACATTT-3′; CCR3 antisense, 5′-TTCCCGGCAAAGGAATAAACT-3′; CCR4 sense, 5′-ACTGTGGCTCCTCCAAATTT-3′; CCR4 antisense, 5′-CATGGTGGACTGCGTGTAAGA-3′; CCR5 sense, 5′-CATCATCCTCCTGACAATCGATAG-3′; CCR5 antisense, 5′-CCGTCCTGGCTTTTAAAGCA-3′; CCR7 sense, 5′-GCTCCAGGCACGCAACTTT-3′; CCR7 antisense, 5′-ACCACGACCACAGCGATGA-3′; CCR9 sense, 5′-CATTGACGCCTATGCCATGT-3′; CCR9 antisense, 5′-GACCTGGAAGCAGATGTCAATGT-3′; IFN-γ sense, 5′-GCTAAAACAGGGAAGCGAAAAA-3′; IFN-γ antisense, 5′-GGACAACCATTACTGGGATGCT-3′; IL-2 sense, 5′-TGCAAGGGACTCAGGTGATG-3′; IL-2 antisense, 5′-TGCTGCTTATTTAGGATACCTATTAACTCA-3′; IL-4 sense, 5′-CACAGGCACAAGCAGCTGAT-3′; IL-4 antisense, 5′-GCCAGGCCCCAGAGGTT-3′; IL-5 sense, 5′-ACGCAGTCTTGTACTATGCACTTTCT-3′; IL-5 antisense, 5′-AGAAGCATCCTCATGGCTCTGA-3′; IL-10 sense, 5′-GTGATGCCCCAAGCTGAGA-3′; IL-10 antisense, 5′-TCCCCCAGGGAGTTCACA-3′; TGF-β sense, 5′-TCAGAGCCACAAATCCTGAAAG-3′; TGF-β antisense, 5′-CACCAAGTGTACCCCGAAAGA-3′.
All unknown cDNAs were diluted to contain equal amounts of β-actin cDNA. The standards, ‘no template’ controls and unknown samples were added in a total volume of 50 μl per reaction. PCR conditions were 2 min at 50°, 10 min at 95°, 40 cycles with 15 seconds at 95°, 60 seconds at 60° for each amplification. Potential PCR product contamination was digested by uracil-N-glycosylase (UNG) since dTTP is substituted by dUTP.23 UNG and AmpliTaq Gold (PE Applied Biosystems) were applied according to the manufacturer's instructions.24,25
Northern blot assay
For mRNA detection (Northern blot), as previously described26 5 μg total RNA from each sample was electrophoresed under denaturing conditions, followed by blotting onto Nytran membranes, and cross-linked by UV irradiation as previously described.26 The target cDNA probes, labelled with α-[32P]dCTP, were obtained by PCR amplification of the sequence mentioned above from the total RNA taken from either PBMC from normal adults (for CCR3, CCR4 and CCR5) or thymocytes from the thymectomy specimens (for CCR7 and CCR9). The membranes were hybridized overnight with 1 × 106 counts/min/ml of 32P-labelled probe, followed by intensive washing with 0·2 × SSC (where 1 × SSC = 0·15 m NaCl, 0·015 m sodium citrate, pH 7·0) and 0·1% sodium dodecyl sulphate before being autoradiographed.
Chemotaxis assay
The chemotaxis assay was performed in a 48-well microchamber (Neuro Probe, Bethesda, MD).27 Briefly, chemokines were diluted in RPMI-1640 with 0·5% pooled human serum and placed in the lower wells (25 μl). Twenty-five microlitres of the cell suspension at 2 × 106 cells/ml was added to the upper well of the chamber, which was separated from the lower well by a 5-μm pore-size, polycarbonate, polyvinylpyrrolidone-free membrane (Nucleopore, Pleasanton, CA). The chamber was incubated for 60 min at 37° in an atmosphere containing 5% CO2. The membrane was then carefully removed, fixed in 70% methanol and stained for 5 min in 1% Coomassie Brilliant Blue. The cells that migrated and adhered to the lower surface of the membrane were counted under light microscopy. Approximately 6% of the cells will migrate spontaneously (known as migrating cells on negative control, MCNC).28 The results were expressed as a chemotactic index (CI), which was calculated as the ratio between the numbers of migrating cells in the sample and in the medium control,27 and with standard deviation (SD).
DC generation and T-cell stimulation
The DCs were generated from PBMCs as previously described.29 Briefly, monocytes were purified by positive sorting using anti-CD14 conjugated magnetic Dynabeads M-450 (Dynal A/S). The recovered cells (> 95% and < 99% CD14+ cells) were cultured at 3 × 105/ml in RPMI-1640 with 10% fetal calf serum supplemented with 25 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; R & D Systems) and 10 ng/ml IL-4 (R & D Systems) for 7 days. Maturation was induced by the addition of 1 μg/ml lipopolysaccharide (Sigma Chemicals, Co., St Louis, MO) and 50 ng/ml tumour necrosis factor-α (R & D System, Inc., Minneapolis, MN) for the last 40 h of cultures. For an optimal stimulation of freshly isolated CD4+ T-cell populations, mature syngeneic DCs were applied for stimulation.30 Freshly isolated CD4+ T cells (2 × 105 cells/well) were used for primary stimulation in the presence of optimal numbers of syngeneic DCs in 24-well plates (400 μl/well) for 2 days. This was followed by a secondary stimulation performed with the addition of different numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells, as indicated in the figure legends, for 4 days in the presence of tetanus toxoid (2·5 μg/ml). A total of 6 days after the onset of the primary culture, CD4+ T cells were harvested using a positive selection procedure of anti-CD4 mAb coated Dynabead M-450 assay, followed by real-time quantitative RT-PCR or an intracellular cytokine immunofluorence staining assay. In some cases, transwell experiments were performed in 24-well plates as described previously.31 Briefly, primary stimulation systems of CD4+ T cells in the presence of optimal numbers of syngeneic DCs (after 2 days) were placed in transwell chambers (Millicell, 0·4 μm; Millipore) in the presence of different numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells, as indicated in the figure legends, for 4 days in the presence of tetanus toxoid (2·5 μg/ml). After 4 days of culture, activated CD4+ T cells were harvested using Dynabeads prior to further investigation.
Results
Selective highly increased frequency of CCR7+ CD8+ CD45RO+ memory T cells in patients with active SLE
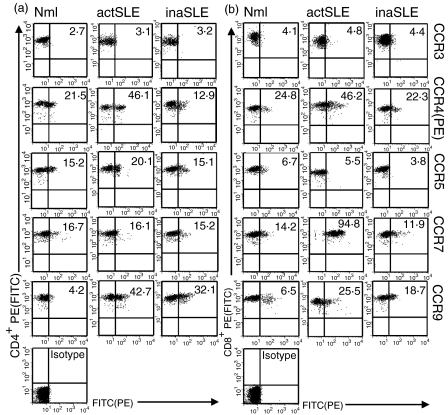
The expression levels of some chemokine receptors on CD4+ and CD8+ T-cell subsets from patients with active and inactive SLE (for patient information see Table 1) were investigated. The results from flow cytometric analyses in Fig. 1 documented that there was no difference of CCR3 and CCR5 expression on CD4+ and CD8+ T cells from patients with active SLE and inactive SLE, compared with that from normal subjects. Expression of CCR4 and CCR9 was moderately increased on both CD4+ and CD8+ T cells from both sets of SLE patients, which was in agreement with those who had reported that CCR4 was frequently expressed in T cells from SLE patients.4 Expression being 46·1% and 46·2% for CCR4; 42·7% and 25·5% for CCR9 on CD4+ and CD8+ T cells from the patients with active SLE, respectively; 12·9% and 23·3% for CCR4; 32·1% and 18·7% for CCR9 on CD4+ and CD8+ T cells from the SLE patients whose disease was inactive, respectively; and 21·5% and 24·8% for CCR4; 4·2% and 6·5% for CCR9 on CD4+ and CD8+ T cells from normal subjects, respectively. Interestingly, CCR7 was selectively and frequently expressed on CD8+ T cells from patients with active SLE. There were up to 94·8% of CCR7+ fractions in freshly isolated CD8+ T cells from patients with active SLE, but only 11·9% of CCR7+ CD8+ T cells in those from the patients with inactive SLE, and 14·2% CCR7+ cell fractions in CD8+ T cells from normal subjects. CCR7 was expressed at a low level in CD4+ T cells from all subjects (16·7%, 16·1% and 15·2%, normal, active SLE, inactive SLE, respectively). The data from a total of six experiments performed in each group of subjects on the CC chemokine receptors are presented in Table 2. The data from flow cytometry showed that almost all CCR7+ CD8+ T cells from patients with active SLE were CD45RO+(Table 3).
Figure 1.
Chemokine receptor distribution. Double-colour flow cytometric analysis of the distribution of chemokine receptors as indicated on CD4+ (a) or CD8+ (b) T cells from normal subjects (Nml), patients with active SLE (actSLE) and those with inactive SLE (inaSLE). The CD4+ and CD8+ T cells were freshly isolated and stained as described in the Materials and methods. The graphs in bottom lefthand corner in the panels are isotype controls. The numbers in the graphs are percentages of chemokine receptor-positive cells as indicated. The data are from a single experiment, which was representative of at least six similar experiments performed. The demarcations for analysis of chemokine receptor-positive cells were defined within 1·5–2% of negative controls according to the manufacturer's recommendation. To simplify, only isotype antibody controls for CCR7 staining on CD4+ and CD8antibody controls for CCR7 staining on CD4+ T cells from patient with active SLE were shown, but all chemokine receptor-positive cells were gated according to simultaneous isotype antibody control staining. (a), CCR4(b), CCR5(c), CCR7(d) and CCR9(e) in freshly isolated CD4antibody controls for CCR7 staining on CD4+ (black bars) and CD8antibody controls for CCR7 staining on CD4++ (grey bars) T cells from normal subjects (Mnl) and patients with active SLE (actSLE) or inactive SLE (inaSLE). The procedure for quantitative RT-PCR amplification was described in the Materials and methods. Statistically significant differences as compared with normal controls are indicated (*P<0·01). The illustrated data were mean values (± SD) of six experiments.
Table 2.
CC chemokine receptor expression on CD4+ and CD8+ T cells from distinct subjects*
| Normal† | actSLE† | inaSLE† | ||||
|---|---|---|---|---|---|---|
| CCR(%)‡ | CD4+ | CD8+ | CD4+ | CD8+ | CD4+ | CD8+ |
| CCR3 | 3·2±1·2 | 5·3±1·2 | 3·8±1·8 | 4·7±1·9 | 5·4±2·7 | 5·1±2·2 |
| CCR4 | 23±8 | 25±12 | 47±10 | 45±11 | 17±9 | 23±7 |
| CCR5 | 25±9 | 7·3±5·2 | 27±10 | 6·9±2·9 | 16±5 | 10±3·2 |
| CCR7 | 16±11 | 26±9 | 17±9 | 93±11 | 18±6 | 19±13 |
| CCR9 | 5·6±4·5 | 6·3±4·8 | 43±18 | 33±14 | 33±10 | 20±11 |
*The data in the table were the mean values ± SD from a total of six experiments performed in each group.
†The experiments were conducted in purified CD4+ or CD8+ T cells from normal subjects (Normal), patients with active SLE (actSLE) and inactive SLE (inaSLE).
‡The data shown are percentages of CC chemokine receptor-positive cells.
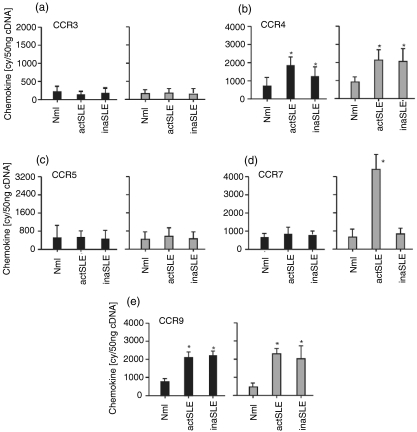
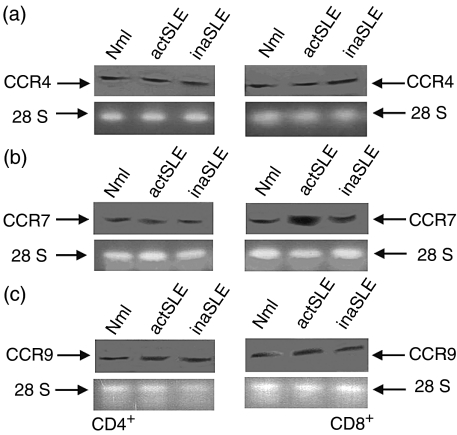
To confirm the observation mentioned above, a real time quantitative RT-PCR assay was performed to detect the expressions of different chemokine receptors at mRNA level. The results in Fig. 2(a) and Fig. 2(c) showed that CCR3 mRNA and CCR5 mRNA were indistinguishably expressed at low levels in freshly isolated CD4+ and CD8+ T cells from the normal subjects, and those with active and inactive SLE. Expression of CCR4 and CCR9 mRNA was moderately increased in both CD4+ and CD8+ T cells from the SLE patients, compared with that from normal subjects (Fig. 2b,e). According to the amplification of the standard DNA template with a housekeeping gene (β-actin) (2·0 × 104 copies), there were approximately 5·3 × 103 ± 1·1 × 103 and 8·1 × 102 ± 0·8 × 102 copies for mRNA CCR7 in CD8+ T cells from the patients with active and inactive SLE, respectively, whereas there were only approximately mRNA CCR7 6·7 × 102 ± 0·9 × 102 copies in the cells from the normal subjects (Fig. 2d). There were approximately 6·7 × 102 ± 0·6 × 102, 7·1 × 102 ±0·7 × 102 and 8·1 × 102 ± 0·7 × 102 copies for mRNA CCR7 in the CD4+ T cells from the normal subjects, active SLE, and inactive SLE patients, respectively (Fig. 2d). The data for CCR3, CCR4, CCR5 and CCR9 mRNA expression in distinct subjects are shown in Fig. 2(a–c,e). A linear relationship between CTand log starting quantity of standard DNA template or target cDNAs was detected (data not shown). In all experiments the correlation coefficients were approximately 0·95–0·99. The same pattern of CCR4, CCR7 and CCR9 mRNA expression in CD4+ and CD8+ T cells from the normal subjects, and the SLE patients was confirmed by Northern blot (Fig. 3a–c). Obviously, CCR7 mRNA expression in CD8+ T cells from patients with active SLE was highly and selectively up-regulated. It was shown that comparable total RNA amounts from different cells were added (Fig. 3, lower panels).
Figure 2.
Chemokine receptor mRNA. The real-time quantitative detection of RT-PCR for mRNA of CCR3 (a), CCR4 (b), CCR5 (c), CCR7 (d) and CCR9 (e) in freshly isolated CD4+ (black bars) and CD8+ (grey bars) T cells from normal subjects (Mnl) and patients with active SLE (actSLE) or inactive SLE (inaSLE). The procedure for quantitative RT-PCR amplification was described in the Materials and methods. Statistically significant differences as compared with normal controls are indicated (*P < 0·01). The illustrated data were mean values (± SD) of six experiments.
Figure 3.
Chemokine receptor mRNA. Northern blot of CCR4 (a), CCR7 (b) and CCR9 (c) mRNA in freshly isolated CD4+ (left panels) CD8+ (right panels) T cells from normal subjects (Mnl) and patients with active SLE (actSLE) or inactive SLE (inaSLE). Total RNA from different cells as indicated were isolated, electrophoresed and blotted as described in the Materials and methods. The hybridization signals for CCR4, CCR7, or CCR9 mRNA in CD4+ or CD8+ T cells from different subjects are shown in the upper panels. The 28S rRNAs in the lower panels confirm that comparable amounts of total RNA were loaded. The illustrated data are from a single representative experiment of six performed.
To investigate further the observations described above, the phenotype of CCR7-bearing CD4+ and CD8+ T cells from patients with active SLE was investigated. Table 3 shows that a large majority of CCR7-bearing CD8+ T cells were CD45RO+, indicating that they were CCR7+ CD8+ CD45RO+ memory T cells. The results also showed that some of the activation markers (such as CD25, CD69, CD62L, CD18 and CD11b) on CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE were highly up-regulated, compared with the CCR7-bearing CD4+ T cells. For instance, CD25 was expressed at a very high level (65–85%), this phenomenon could be because the cells were abnormally activated during active SLE disease. Another explanation could be that the cells were activated by the positive selection procedure using the Dynabead assay. The exact mechanism will be subjected to further investigations. The phenomenon was not seen in the patients with inactive SLE or in the normal subjects (data not shown).
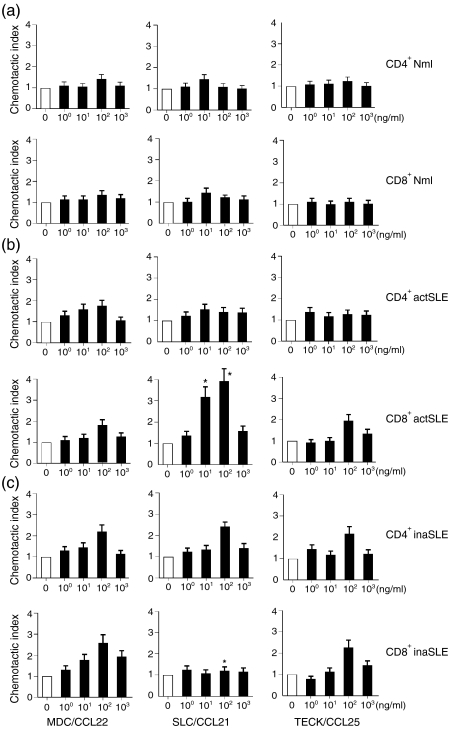
Next, the abilities of MDC/CCL22 (a ligand for CCR4), SLC/CCL21 (a ligand for CCR7) and TECK/CCL25 (a ligand for CCR9) to induce chemotaxis of CD4+ and CD8+ T cells from different subjects were examined. The results in Fig. 4(a) showed that the three chemokines mentioned above did not induce significant chemotactic migration of CD4+ and CD8+ T cells from normal subjects. The results in Fig. 4(c) showed that the three chemokines induced significant, but moderately chemotactic, migration of CD4+ and CD8+ T cells from patients with inactive SLE. Except for the CD8+ T cells towards SLC/CCL21, the chemokines induced typical bell-shaped, dose-dependent, moderate chemotaxis response curves. The maximum CIs were 2·1 ± 0·25, 2·2 ± 0·36, 1·9 ± 0·37, 2·4 ± 0·44, 1·2 ± 0·25 and 2·5 ± 0·21 (Fig. 4c). The results in Fig. 4(b) showed that SLC/CCL21 selectively induced significant chemotactic migration of CD8+ T cells from patients with active SLE, whereas MDC/CCL22 and TECK/CCL25 induced insignificant chemotactic migration of CD4+ and CD8+ T cells from these patients. The maximum CIs were 1·8 ± 0·35, 1·5 ± 0·17, 1·4 ± 0·13, 1·5 ± 0·22, 3·8 ± 0·42 and 1·6 ± 0·36 (Fig. 4b). Spontaneous migration negative control (known as MCNC) of different CD4+ and CD8+ T cells was approximately 6% of total cells added, indicating by CI = 1 (Fig. 4). To confirm SLC/CCL21 via CCR7, we used anti-CCR7 mAb to block the active SLE CD8+ T-cell chemotactic activity towards SLC/CCL21. The anti-CCR7 mAb could completely block the chemotaxis of the cells towards SLC/CCL21 (data not shown), whereas it had no any effect on the chemotaxis of the cells towards MDC/CCL22 (data not shown). The isotype antibody had no blocking effect at all (data not shown). The checkerboard chemotaxis assays confirmed that enhanced motility of CD8+ T cells from patients with active SLE towards the chemokine (SLC/CCL21) was the result of chemotaxis, but not chemokinesis (data not shown).32
Figure 4.
Chemotaxis analysis. The migration of freshly isolated CD4+ or CD8+ T cells from normal subjects (Nml) and patients with active SLE (actSLE) or inactive SLE (inaSLE) towards MDC/CCL22, SLC/CCL21 and TECK/CCL25. All results were determined as described in the Materials and methods and expressed as a Chemotactic Index (CI ± SD), based on triplicate determinations of chemotaxis on each concentration of chemokine applied. The applied chemokine concentrations are indicated as ng/ml. The open bars indicate spontaneous migration towards negative (medium) control (known as MCNC; CI = 1) in each experiment. Statistically significant differences as compared with normal controls are indicated (*P < 0·01). The illustrated data are from a single representative experiment of eight performed.
CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE were Th2 biased
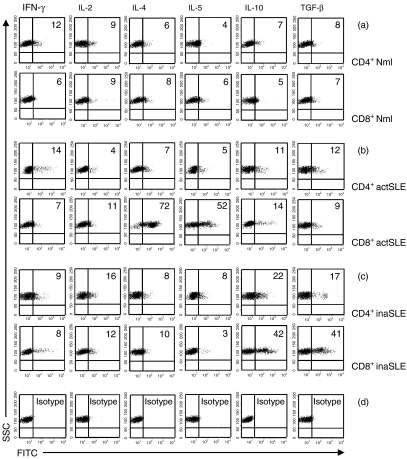
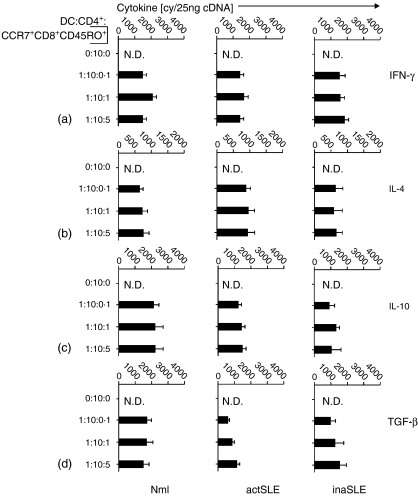
To characterize further CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE, we investigated the expression of cytokines in CCR7+ CD4+ CD45RO+ and CCR7+ CD8+ CD45RO+ T cells from different subjects. CCR7+ CD4+ CD45RO+ and CCR7+CD8+ CD45RO+ T cells from normal subjects expressed low levels of Th1 (IFN-γ and IL-2), Th2 (IL-4 and IL-5) and regulatory T cell (Tr)-derived (IL-10 and TGF-1β) cytokines (Fig. 5a). Interestingly, Th2 (IL-4 and IL-5) cytokines were selectively and highly expressed in purified and unstimulated CCR7+ CD8+ CD45RO+ T cells from patients with active SLE (72% and 52%), but not in CCR7+ CD4+ CD45RO+ T cells (7% and 5%) (Fig. 5b). Tr-derived (IL-10 and TGF-1β) cytokines were moderately expressed in CCR7+ CD4+ CD45RO+ and CCR7+CD8+ CD45RO+ T cells from inactive SLE patients (22% and 42%; 17% and 41%, respectively) (Fig. 5c).
Figure 5.
Intracellular cytokine analysis. Intracellular Th1- (IFN-γ and IL-2), Th2- (IL-4 and IL-5) and Tr-derived (IL-10 and TGF-β) cytokine detection by flow cytometry as indicated. The purified cells were CCR7+ CD4+ CD45RO+ or CCR7+ CD8+ CD45RO+ T cells obtained by FACS sorting assay from normal subjects (Mnl)(a) and patients with active SLE (actSLE) (b) or inactive SLE (inaSLE)(c) as described in the Materials and methods. The graphs in (d) are isotype controls. The numbers listed in the figure are in percentage detected with intracellular cytokine assay as described in the Materials and methods. The illustrated data are from a single representative experiment of five performed.
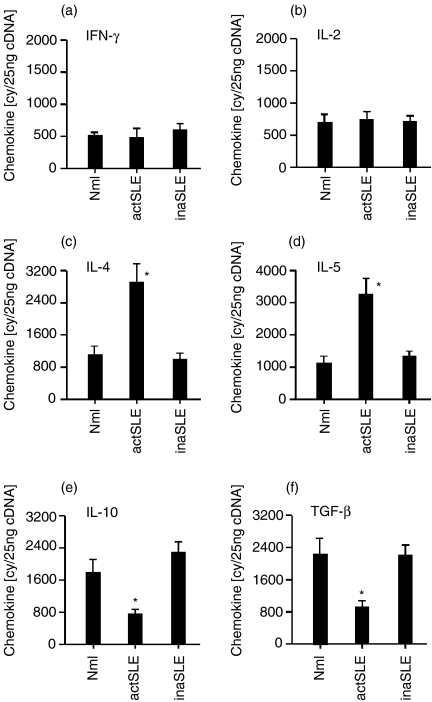
To confirm the observation mentioned above, we conducted the real-time quantitative RT-PCR assay to detect the expressions of different cytokines. The results in Fig. 6(a,b) show that IFN-γ mRNA and IL-2 mRNA were indistinguishably expressed at low levels in freshly isolated CCR7+ CD8+ CD45RO+ memory T cells from all subjects. Expression of IL-10 mRNA and TGF-β mRNA was significantly lower in CCR7+ CD8+ CD45RO+ memory T cells from the patients with active SLE, compared with those from normal subjects and patients whose SLE was inactive (Fig. 6e,f). There were approximately 3·0 × 103 and 3·4 × 103 copies for IL-4 and IL-5 mRNA in CCR7+ CD8+ CD45RO+ memory T cells from the patients with active SLE, respectively, whereas only approximately 1·0 × 103, 9·1 × 102, for IL-4 mRNA; 1·0 × 103 and 1·1 × 103 copies in the cells from the normal subjects and patients with inactive SLE, respectively (Fig. 6c,d), showing that Th2 cytokines (IL-4 and IL-5) were significantly and selectively increased in CCR7+ CD8+ CD45RO+ memory T cells from the patients with active SLE. The same pattern of Th1, Th2 and Tr-derived mRNA expressions in CCR7+ CD8+ CD45RO+ memory T cells from different subjects was confirmed by Northern blot showing that IL-4 and IL-5 mRNA expression in CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE was highly and selectively up-regulated (data not shown). Notably, there were some inconsistent results on IL-10 and TGF-β from controls and patients with inactive SLE by flow cytometry and real-time RT-PCR presenting in Figs 5(a,c) and 6(e,f). This discrepancy might be because these data were from individual representatives. However, overall the data from the flow cytometry and real-time RT-PCR showed corresponding trends.
Figure 6.
Cytokine mRNA. The real-time quantitative detection of RT-PCR for mRNA of Th1- [IFN-γ, (a); IL-2, (b)], Th2- [IL-4, (c); IL-5, (d)] and Tr-derived [IL-10, (e); TGF-β, (f)] cytokines in freshly isolated CCR7+ CD8+ CD45RO+ T cells from normal subjects (Nml) and patients with active SLE (actSLE) or inactive SLE (inaSLE) obtained by FACS sorting assay as described in the Materials and methods. The procedure for quantitative RT-PCR amplification was also described in the Materials and methods. The data are representative of six similar experiments conducted. Statistically significant differences as compared with normal controls are indicated (*P<0·01).
CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE are inducers of Th2 bias in CD4+ T cells
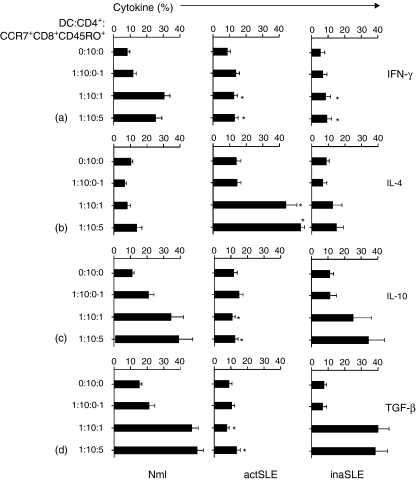
To characterize further the function of CCR7+CD8+ CD45RO+ memory T cells from different subjects, cells were cocultured with syngeneic CD4+ T cells in the presence of mature syngeneic DCs, and subsequently the cytokine expression levels in CD4+T-cell subsets were investigated. The results from flow cytometric analyses in Fig. 7 documented that CCR7+CD8+ CD45RO+ memory T cells from normal subjects could significantly drive CD4+ T cells into expression of Th1 cytokines (IFN-γ; 30%) in a concentration-dependent manner, whereas the cells from patients with both active SLE and inactive SLE did not show this ability (12% and 9%, respectively) (Fig. 7a). A similar phenomenon was observed for IL-2 expression (data not shown). In contrast, CCR7+ CD8+ CD45RO+memory T cells from the patients with active SLE could significantly drive CD4+ T cells into expression of Th2 cytokines (IL-4; 50%), whereas the cells from the normal subjects as well as those from patients with inactive SLE did not show such ability (11% and 10%, respectively) (Fig. 7b). A similar phenomenon was also observed for IL-5 expression (data not shown). Interestingly, CCR7+ CD8+ CD45RO+ memory T cells from normal subjects and patients with inactive SLE could significantly drive CD4+ T cells into expression of Tr1 cytokines (IL-10 and TGF-1β) in a concentration-dependent manner (39% and 45%; 37% and 39%, respectively), whereas the cells from patients with active SLE did not show this ability (10% and 11%, respectively) (Fig. 7c,d).
Figure 7.
Intracellular cytokine analysis. Intracellular Th1- (IFN-γ) (a), Th2- (IL-4) (b) and Tr-derived (IL-10 and TGF-β) (c, d) cytokine detection by flow cytometry as indicated. The CD4+ T cells were purified from normal subjects (Nml) and patients with active SLE (actSLE) or inactive SLE (inaSLE). The CD4+ T cells were then cocultured with optimal numbers of mature syngeneic DCs and with the indicated numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells in the presence of tetanus toxoid as described in the Materials and methods. CD4+ T cells were then harvested using Dynabeads as described in the Materials and methods. The numbers listed in the figure are in percentage detected with intracellular cytokine assay as described in Materials and methods. The illustrated data are from a single representative experiment of five performed. Statistically significant differences as compared with normal controls are indicated (*P<0·01).
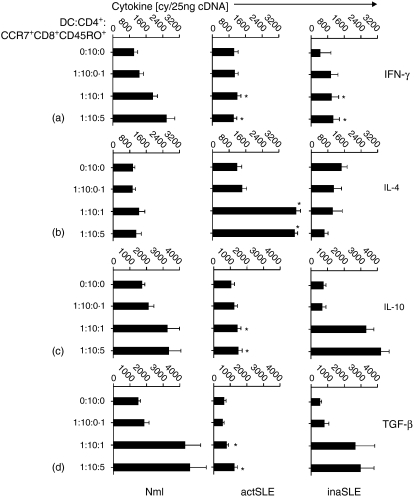
The observation described above was confirmed using the real-time quantitative RT-PCR assay at mRNA level. CCR7+ CD8+ CD45RO+ memory T cells from normal subjects could significantly drive CD4+ T cells into expression of Th1 cytokine (IFN-γ) in a concentration-dependent manner (2·5 × 103 copies), whereas the cells from the patients with active or inactive SLE did not show this ability (8·1 × 102 and 8·0 × 102 copies, respectively) (Fig. 8a). A similar phenomenon was observed for IL-2 expression (data not shown). In contrast, CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE could significantly drive CD4+ T cells into expression of a Th2 cytokine (IL-4; 3·9 × 103), whereas the cells from the normal subjects as well as those from patients with inactive SLE did not show this ability (9·2 × 102 and 8·3 × 102 copies, respectively) (Fig. 8b). Similar phenomena were observed for IL-5 expression (data not shown). CCR7+ CD8+ CD45RO+ memory T cells from normal subjects and patients with inactive SLE could significantly drive CD4+ T cells into expression of Tr1 cytokines (IL-10 and TGF-1β) in a concentration-dependent manner (3·1 × 103 and 4·5 × 103 copies; 4·2 × 103 and 2·8 × 103 copies, respectively), whereas the cells from patients with active SLE did not show this ability (1·2 × 103 and 1·3 × 103 copies, respectively) (Fig. 8c,d).
Figure 8.
Cytokine mRNA. The real-time quantitative detection of RT-PCR for mRNA of Th1- (IFN-γ) (a), Th2- (IL-4) (b) and Tr-derived (IL-10 and TGF-β) (c, d) cytokines in stimulated CD4+ T cells from normal subjects (Nml) and patients with active SLE (actSLE) or inactive SLE (inaSLE). The purified CD4+ T cells were then cocultured with optimal numbers of mature syngeneic DCs and with the indicated numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells in the presence of tetanus toxoid, CD4+ T cells were then harvested using Dynabeads as described in the Materials and methods. The procedure for quantitative RT-PCR amplification was described in the Materials and methods. The data are representative of six similar experiments conducted. Statistically significant differences as compared with normal controls are indicated (*P<0·01).
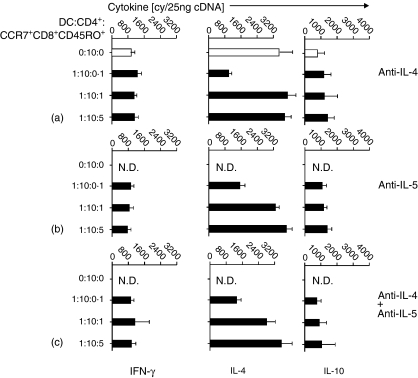
To elucidate the how the CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE induce the Th2 cytokine bias in syngeneic CD4+ T cells while the same cells from normal subjects and patients with inactive SLE drive syngeneic CD4+ T cells into a Tr 1 cell pattern, an analysis of the properties of the cells in transwell stimulations was performed in comparison with original conditions. The semi-permeable transwell membrane prevents direct cell–cell contact between the responsive CD4+ T cells and the CD8+ memory T cells. In contrast to the findings mentioned above, CCR7+ CD8+ CD45RO+ memory T cells from different subjects lost the ability to induce polarization of syngeneic CD4+ T cells (Fig. 9). These data strongly suggested that our findings on the direction of memory T-cell function was dependent on cell–cell contact. Since we found that CCR7+ CD8+ CD45RO+ memory T cells from patien+0ts with active SLE were Th2 cytokine producers, we applied IL-4 and/or IL-5 blocking antibody in the coculture system. The cytokine mRNA results in Fig. 10 showed that neither anti-IL-4 nor anti-IL-5 nor anti-IL-4 + anti-IL-5 mAb(s) blocked the ability of CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE to induce Th2 cytokine bias of syngeneic CD4+ T cells, supporting the observation that directing function of CCR7+ CD8+ CD45RO+ memory T cells was cell–cell contact dependent.
Figure 9.
Cytokine mRNA. The real-time quantitative detection of RT-PCR for mRNA of Th1- (IFN-γ) (a), Th2- (IL-4) (b) and Tr-derived (IL-10 and TGF-β) (c, d) cytokines in stimulated CD4+ T cells from normal subjects (Mnl) and patients with active SLE (actSLE) or inactive SLE (inaSLE). The purified cells were CD4+ T cells were then cocultured with optimal numbers of mature syngeneic DCs and with the indicated numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells in a transwell manner (⊃) in the presence of tetanus toxoid, following the procedure of CD4+ cell purification as described in the Materials and methods. The procedure for quantitative RT-PCR amplification was described in the Materials and methods. The data are representative of six similar experiments conducted. N.D., not determined.
Figure 10.
Cytokine mRNA. The real-time quantitative detection of RT-PCR for mRNA of Th1- (IFN-γ), Th2- (IL-4) and Tr-derived (IL-10) cytokines in stimulated CD4+ T cells from patients with active SLE. The purified cells, CD4+ T cells, were then cocultured with optimal numbers of mature syngeneic DCs in the presence of tetanus toxoid and with indicated different numbers of purified syngeneic CCR7+ CD8+ CD45RO+ memory T cells in the presence of anti-IL-4 (a), anti-IL-5 (b), or anti-IL-4 + anti-IL-5 (c) mAb(s), followed a procedure of CD4+ cell purification as described in the Materials and methods. Open bars indicate coculture without mAb. The procedure for quantitative RT-PCR amplification was described in the Materials and methods. The data are representative of five similar experiments conducted. N.D., not determined.
Discussion
Chemokine receptors are differently expressed on naive and activated T cells.33,34 Naive T cells express CCR7, a receptor for CCL19 and CCL21 that are produced by stromal cells in the T-cell zone of the spleen, lymph nodes and Peyer's patches.33–35 CCL19 and CCL21 are able to induce migration of naive T cells to T-cell areas of lymphoid organs in search of antigen presented by DCs.36,37 In normal humans, TCM cells express CCR7 and represent a non-polarized, antigen-experienced cell population that lacks immediate effector cell functions. TEM cells have been down-regulated by CCR7 and are capable of immediately producing cytokines after antigen recognition.12 In contrast to that, immediate IFN-γ production has been observed in CD4+ CCR7+ memory T cells after mitogen/ionomycin stimulation.38 Among antigen-specific CD8+ T cells, IFN-γ-producing cells have been found both in CCR7– and CCR7+ subsets of HIV-infected subjects.39 In mice, differentiated IFN-γ-producing Th1 cells exhibit strong reactivity toward CCR7 ligands in vitro, whereas Th2 cells do not respond.40 The majority of circulating effector T cells migrate efficiently toward CCR7 ligands and are able to re-circulate through secondary lymphoid tissues, similar to naive T cells.41 CCR7 is down-regulated in CD8+ T cells activated in vivo by lymphocytic choriomeningitis virus infection, and effector T cells accumulated in the red pulp but failed to enter white pulp areas in the spleen.42 CCR7– and CCR7+ memory T cells generated in vivo by a viral infection differed neither in cytokine production nor in cytolytic activity.18 A substantial number of CCR7+ memory T cells are isolated from the livers of virus-infected recipient mice.18 These conflicting data in humans and mice do not fit the current concept of central and effector memory cells, which was originally proposed in normal humans,12 indicating that CCR7+ memory T cells have the capacity to perform immediate effector cell functions both in humans and mice.
To understand the auto-activation of T cells under the condition of SLE, we have focused on the expression of several chemokine receptors and the interaction between these receptors and their ligands on CD4+ and CD8+ T cells during active and inactive disease. We demonstrate that CCR7 is selectively, high frequently and functionally expressed in CD8+ T cells from patients with active SLE. Interestingly, the frequency of CCR7+ CD8+ CD45RO+ T cells is correlated with the activity of the SLE. The numbers of CCR7+ CD8+ CD45RO+ T cells are significantly decreased during disease remission. To our knowledge, this is the first report on up-regulated frequency of CCR7+ CD8+ CD45RO+ T cells in human active SLE, and is the first direct evidence of the increased biological activity (chemotaxis) of active SLE CCR7+CD8+ CD45RO+ T cells induced by CC chemokine SLC/CCL21. A rather complex picture is beginning to take shape of how active SLE CCR7+ CD8+ CD45RO+ T cells selectively transmigrate through lymphoid and non-lymphoid tissue to make contact with antigen-presenting cells and provide local inflammation to cause pathophysiological events under continuous interaction with chemokines and cytokines.
What are the functions of these up-regulated CCR7+ CD8+ CD45RO+ T cells in active SLE? The imbalance between Th1 cells and Th2 cells has been proposed to be associated with the pathogenesis of SLE. The production of Th2 cytokines is increased, while Th1 cytokines are decreased in patients with active SLE.9 Th2 cells may play a pivotal role in the development of auto-antibodies in SLE patients. However, some reports have contradicted this supposition, indicating that an imbalance toward Th1 predominance is associated with an acceleration of lupus-like autoimmune disease.11 Th1 and Th2 responses may play differential roles in the pathogenesis of lupus-associated tissue injury.43 In humans with lupus, IFN-γ is present in the kidneys of patients with severe lupus glomerulonephritis and enhances production of IFN-γ by peripheral blood cells of most severe SLE patients, compared with individuals with milder renal disease.20,44 In mice with lupus, more conflicting data have been presented. Th1 cytokines play a dominant role in SLE pathogenesis.45 Disease severity in MRL-Fas1pr mice is linked to the presence of the Th1 cytokines IFN-γ and IL-12.46–49 IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone BWF1 mice.50 Transgenic expression of IFN-γ in the epidermis of non-autoimmune mice leads to the development of inflammatory skin disease resembling lupus erythematosus and the severe immune complex-mediated glomerulonephritis.51,52 Expression of an IFN-γR Fc fusion protein can retard lupus development and disease progression in MRL-Faslpr mice.53 However, we have demonstrated that CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE are Th2 biased, whereas CCR7+ CD8+ CD45RO+ T cells from patients whose SLE is inactive are selectively expressed Tr-derived cytokines. CCR7+ CD8+ CD45RO+ memory T cells from patients with active SLE induce a Th2 bias in the CD4+ T cells in a cell–cell contact manner, whereas the cells from patients with inactive SLE drive Tr-derived cytokine expression in CD4+ T cells. These data indicate that only subsets of T cells from patients with active SLE are Th2 biased. However, they are inducers of Th2 bias for other subsets of T cells. This mechanism may be important to explain some differences in the current literature. One question remains: what is the actual mechanism for CCR7+ CD8+ CD45RO+ T cells from patients with active SLE to induce Th2 bias in CD4+ T cells in a cell–cell contact manner?
CD4+ CD25+ T cells have been shown to be potent regulatory cells in humans.54 CD4+ CD25+ T cells activate in vitro to suppress the proliferation of CD4+ CD25– T cells in a cell contact-dependent manner.55 Their effects in vivo appear to depend on IL-10 and/or TGF-β expression.56 Very little is known about the trafficking behaviour of CD4+ CD25+ T cells or the site where they exert their regulatory activity in vivo. CD4+ CD25+ T cells can be easily found in secondary lymphoid organs. CD4+ CD25+ splenocytes express high levels of CCR5 and are attracted by activated antigen-presenting cells that express MIP-1.57 Meanwhile, CD4+ CD25+ T cells from human peripheral blood selectively express CCR8 and CCR4 and show a strong chemotactic response to MDC and thymus- and activation-regulated chemokine (TARC), suggesting that CD4+ CD25+ T cells may be attracted to inflamed tissues to regulate or prevent autoimmune disease.58 CD4+ CD25+ CD62L+ splenocytes express CCR7 at high levels and migrate toward lymphoid chemokines SLC/CCL21 and ELC/CCL19, whereas CD4+ CD25+ CD62L– splenocytes preferentially express CCR2, CCR4 and CXCR3 and migrate toward inflammatory chemokines. CD4+ CD25+ CD62L+, but not CD4+ CD25+ CD62L–, splenocytes delay transfer of diabetes.59 The mechanism of action by which CD4+ CD25+ T cells prevent the development of autoimmune disease is not completely understood. Our results that Tr-derived cytokines are significantly expressed in CCR7+ CD8+ CD45RO+ T cells from patients with inactive SLE, which drive CD4+ T cells into expression of Tr-derived cytokines, indicate an interesting phenomenon that Tr cells may play an important role in SLE remission. On the other hand, it also raises an interesting question how Tr-derived cytokines function during that remission.
Generally, our studies suggest that there may be a broad spectrum of central and effector memory T-cell subsets in response to different physiological and pathophysiological conditions. In some autoimmue diseases, for instance active SLE, CCR7+ CD8+ CD45RO+‘central’ memory T cells are able to enter into peripheral blood and inflammatory sites from secondary lymphoid organs, to express CCR7+ CD8+ CD45RO+ continuously, and to interact with DCs and function as CCR7– CD8+ CD45RO+‘effector’ memory T cells, which have been described in normal humans. Although this pattern corresponded roughly with the depiction of ‘effector memory’12 the picture here is somewhat more complex. Compared with the normal condition, instead of the proliferating TCM differentiating into TEM cells, TCM cells acted directly as effectors, switching other subsets of T cells in cell–cell contact manner from one Th1 (or Th0) polarization to Th2, providing a plausible mechanism for the maintenance of polyclonal autoantibody production and over-functionally diverse repertoire of human CD4+ T cells. Based on the results in the present study on the correlation between frequency, and abnormal function of CCR7+ CD8+ CD45RO+ T cells and activity of the disease, we suggest that the expression frequency and some functions of CCR7+ CD8+ CD45RO+ T cells could be used as a marker for SLE activity.
Acknowledgments
This work was supported by the National Science Foundation of China (no. 39870674), a special grant from the Personnel Department of Wuhan University, China, Science Foundation of Anhui Province, China (no. 98436630), and Education and Research Foundation of Anhui Province, China (no. 98JL063).
Abbreviations
- act(ina)SLE
active (inactive) systemic lupus erythematosus
- BLC
B lymphocyte chemokine
- CI
chemotactic index
- CXCL (CC)
CXC (CC) chemokine ligand
- CXCR (CC)
CXC (CC) chemokine receptor, DC, dendritic cell
- LARC
liver and activation-regulated chemokine
- MCNC
migrating cells on negative control
- MDC
macrophage-derived chemokine
- MIP-3β
macrophage inflammatory protein-3β
- SLC
secondary lymphoid tissue chemokine
- SLEDAI
SLE disease activity index
- TARC
thymus- and activation-regulated chemokinem
- TECK
thymus-expressed chemokine
- Tr cells
regulatory T cells
References
- 1.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 2.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa S, Sato T, Abe M, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada M, Yagita H, Inoue H, et al. Selective accumulation of CCR4+ T lymphocytes into renal tissue of patients with lupus nephritis. Arthritis Rheum. 2002;46:735–40. doi: 10.1002/art.10112. [DOI] [PubMed] [Google Scholar]
- 5.Furuichi K, Wada T, Sakai N, et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am J Nephrol. 2000;20:291–9. doi: 10.1159/000013603. [DOI] [PubMed] [Google Scholar]
- 6.Hase K, Tani K, Shimizu T, Ohmoto Y, Matsushima K, Sone S. Increased CCR4 expression in active systemic lupus erythematosus. J Leukoc Biol. 2001;70:749–55. [PubMed] [Google Scholar]
- 7.Ishikawa S, Nagai S, Sato T, Akadegawa K, Yoneyama H, Zhang YY, Onai N, Matsushima K. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur J Immunol. 2002;32:1881–7. doi: 10.1002/1521-4141(200207)32:7<1881::AID-IMMU1881>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko H, Ogasawara H, Naito T, et al. Circulating levels of beta-chemokines in systemic lupus erythematosus. J Rheumatol. 1999;26:568–73. [PubMed] [Google Scholar]
- 9.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunol Rev. 1995;144:157–93. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1644–8. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 13.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 15.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–9. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol. 2002;168:2225–32. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 17.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–50. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 18.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7– memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–41. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 21.Jinquan T, Quan S, Jacobi HH, et al. CXC chemokine receptor 3 expression on CD34+ hematopoietic progenitors from human cord blood induced by granulocyte–macrophage colony-stimulating factor: chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood. 2000;96:1230–8. [PubMed] [Google Scholar]
- 22.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–8. [PubMed] [Google Scholar]
- 23.Heid CA, Stevens J, Livak KJ, William PM. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 24.Kruse N, Pette M, Toyka K, Rieckmann P. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Meth. 1997;210:195–203. doi: 10.1016/s0022-1759(97)00188-9. [DOI] [PubMed] [Google Scholar]
- 25.Jinquan T, Quan S, Jacobi HH, et al. Expression of the nuclear factors of activated T cells in eosinophils: regulation by IL-4 and IL-5. J Immunol. 1999;163:21–4. [PubMed] [Google Scholar]
- 26.Sica A, Saccani A, Borsatti A, et al. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–74. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinquan T, Frydenberg J, Mukaida N, et al. Recombinant human growth regulated oncogene-α induces T lymphocyte chemotaxis; A process regulated via interleukin-8 receptors by IFN-γ, TNF-α, IL-4, IL-10 and IL-13. J Immunol. 1995;155:5359–68. [PubMed] [Google Scholar]
- 28.Jinquan T, Larsen CG, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. 1993;151:4545–51. [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+) CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137:387–470. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 35.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 36.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 38.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–17. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 40.Randolph DA, Huang G, Carruthers CJ, Bromley LE, Chaplin DD. The role of CCR7 in TH1 and TH2 cell localization and delivery of B cell help in vivo. Science. 1999;286:2159–62. doi: 10.1126/science.286.5447.2159. [DOI] [PubMed] [Google Scholar]
- 41.Debes GF, Hopken UE, Hamann A. In vivo differentiated cytokine-producing CD4(+) T cells express functional CCR7. J Immunol. 2002;168:5441–7. doi: 10.4049/jimmunol.168.11.5441. [DOI] [PubMed] [Google Scholar]
- 42.Potsch C, Vohringer D, Pircher H. Distinct migration patterns of naive and effector CD8 T cells in the spleen: correlation with CCR7 receptor expression and chemokine reactivity. Eur J Immunol. 1999;29:3562–70. doi: 10.1002/(SICI)1521-4141(199911)29:11<3562::AID-IMMU3562>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 43.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunol Rev. 1995;144:157–93. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 44.Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus–like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng SL, Moslehi J, Craft J. Roles of interferon- and interleukin-4 in murine lupus. J Clin. Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas C, Ryffel B, Le Hir M. IFN-γ is essential for the development of autoimmune glomerulonephritis in MRL/lpr mice. J Immunol. 1997;158:5484–91. [PubMed] [Google Scholar]
- 48.Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, Kelley VR. IL-12 drives IFN-γ-dependent autoimmune kidney disease in MRL-Faslpr mice. J Immunol. 1999;163:6884–9. [PubMed] [Google Scholar]
- 49.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–7. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haas C, Ryffel B, Le Hir M. IFN-γ receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160:3713–8. [PubMed] [Google Scholar]
- 51.Seery JP, Carroll JM, Cattell V, Watt FM. Antinuclear autoantibodies and lupus nephritis in transgenic mice expressing interferon in the epidermis. J Exp Med. 1997;186:1451–9. doi: 10.1084/jem.186.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seery JP. IFN-γ transgenic mice: clues to the pathogenesis of systemic lupus erythematosus? Arthritis Res. 2000;2:437–40. doi: 10.1186/ar124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawson BR, Prud'homme GJ, Chang Y, Gardner HA, Kuan J, Kono DH, Theofilopoulos AN. Treatment of murine lupus with cDNA encoding IFN-γR/Fc. J Clin Invest. 2000;106:207–15. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:F41–6. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Read S, Powrie F. CD4+ regulatory T cells. Curr Opin Immunol. 2001;13:644–9. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 57.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 58.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses 1-selectin and high levels of CCR7. J Immunol. 2002;169:2461–5. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]