Abstract
Human serum amyloid P component (SAP) binds avidly to DNA, chromatin and apoptotic cells in vitro and in vivo. 129\Sv × C57BL\6 mice with targeted deletion of the SAP gene spontaneously develop antinuclear autoantibodies and immune complex glomerulonephritis. SAP-deficient animals, created by backcrossing the 129\Sv SAP gene deletion into pure line C57BL\6 mice and studied here for the first time, also spontaneously developed broad spectrum antinuclear autoimmunity and proliferative immune complex glomerulonephritis but without proteinuria, renal failure, or increased morbidity or mortality. Mice hemizygous for the SAP gene deletion had an intermediate autoimmune phenotype. Injected apoptotic cells and isolated chromatin were more immunogenic in SAP–\– mice than in wild-type mice. In contrast, SAP-deficient pure line 129\Sv mice did not produce significant autoantibodies either spontaneously or when immunized with extrinsic chromatin or apoptotic cells, indicating that loss of tolerance is markedly strain dependent. However, SAP deficiency in C57BL\6 mice only marginally affected plasma clearance of exogenous chromatin and had no effect on distribution of exogenous nucleosomes between the liver and kidneys, which were the only tissue sites of catabolism. Furthermore, transgenic expression of human SAP in the C57BL\6 SAP knockout mice did not abrogate the autoimmune phenotype. This may reflect the different binding affinities of mouse and human SAP for nuclear autoantigens and\or the heterologous nature of transgenic human SAP in the mouse. Alternatively, the autoimmunity may be independent of SAP deficiency and caused by expression of 129\Sv chromosome 1 genes in the C57BL\6 background.
Keywords: autoimmunity, glomerulonephritis, transgenics: knockouts
Introduction
Serum amyloid P component (SAP), named for its universal presence in amyloid deposits, is a normal plasma protein of the pentraxin family characterized by specific calcium-dependent ligand binding.1 In addition to its binding to all types of amyloid fibrils, which is responsible for its presence in amyloid deposits, SAP is also the only normal human plasma protein that shows specific calcium-dependent binding to DNA and chromatin under physiological conditions in vitro.2–4 Human SAP also binds in vivo to chromatin exposed by necrosis5 and to apoptotic cells,6 though not necessarily only to chromatin ligands.7 The binding of human SAP to native long chromatin in vitro is very avid; it displaces H1-type histones and thereby solubilizes the chromatin that is otherwise highly insoluble at physiological ionic strength.3 Furthermore this binding of human SAP stabilizes the chromatin and protects it from enzymatic degradation.8 These observations suggest that human SAP may have a role in the normal handling of exposed chromatin and of apoptotic cells in vivo.
We previously reported that C57BL/6 × 129/Sv SAP-deficient mice, created by targeted deletion of the SAP gene,9 spontaneously produce a wide range of antinuclear autoantibodies and develop significant immune complex glomerulonephritis although they suffer no morbidity or premature mortality.8 However, there are major effects of genetic background on the expression and effects of autoimmunity. For example, C1q deficiency that is associated with antinuclear antibody formation and glomerulonephritis in C57BL/6 × 129/Sv mice produces no such effects in pure-line C57BL/6 animals.10 We therefore backcrossed the SAP gene deletion into pure line C57BL/6 and 129/Sv mice and evaluated their respective autoimmune phenotypes, including in vivo handling of exogenous chromatin, and the effects of transgenic expression of human SAP. Our observations question the mechanism by which the SAP gene deletion causes loss of tolerance.
Materials and methods
Mice
The targeted SAP gene deletion produced in 129/Sv embryonic stem cells and initially evaluated in 129/Sv × C57BL/6 mice8,9 was backcrossed for six generations into pure-line C57BL/6 and 129/Sv mice. Mice were genotyped for the deletion as previously described.9 A cohort of 312 C57BL/6 mice, all housed and fed under identical standard conditions, was followed for 12 months. There were 103 wild-type mice (50 female), 104 hemizygous for the gene deletion (53 female) and 105 homozygous SAP–/– animals (55 female). All mice were tail bled 200 μl at 3, 6 and 9 months of age and the promptly separated sera were stored frozen at − 70°. At 12 months each mouse was transferred to a special cage for volumetric 24-h urine collection and then killed by exsanguination. After gross autopsy with visual assessment, the kidneys, liver, spleen, heart, lungs, large and small intestine, stomach, salivary gland and samples of skin were removed for histopathological examination. A cohort of 51 SAP–/– 129/Sv mice, housed and fed under identical standard conditions, were tail bled 200 μl at 6 and 12 months before terminal exsanguination at 18 months followed by removal of the kidneys for histopathological examination. A small cohort of 35 SAP–/– C57BL/6 mice, some of which were also carrying the human SAP transgene11,12 and with human SAP in their serum (assayed by electroimmunoassay13), were tail bled 200 μl at 6 months of age before terminal exsanguination at 12 months and removal of the kidneys for histological examination.
Autoantibody assays
Antinuclear autoantibodies (ANAs) producing homogeneous staining and autoantibodies to double-stranded DNA (dsDNA) were detected by immunofluorescence8 and sera with titres above 1/80 and above 1/20, respectively, were considered positive and were titrated to end-point. Autoantibodies to chromatin, single-stranded DNA (ssDNA) and histone, and also rheumatoid factor, were detected as previously described in assays standardized and calibrated with a single high titre pool of serum from MRL/Mp-lpr/lpr mice, except that immunoradiometric rather than enzyme-linked immunosorbent assay methods were used.8,14 All sera were assayed in triplicate and were considered positive when > 3 SD above the lower limit of detection; results are expressed relative to the standard pool which was assigned an arbitrary value of 100 units.
Histopathology
Tissues routinely processed for light and electron microscopy were reviewed blind by expert histopathologists. Glomerulonephritis was graded for the proportion of abnormally hypercellular glomeruli: 0 =< 25%; I = 25–50%; II = 51–90%; III => 90% as previously reported.8 Splenic lymphocytosis was scored for overall white pulp volume from 0 (none) to 3 (very abundant), white pulp coalescence from 0 (completely separate white pulp nodules) to 3 (totally coalescent white pulp), and red pulp lymphocytes from 0 (very rare) to 3 (numerous); and a total score of 4 or more was considered abnormal. Qualitative indirect immunohistochemical staining for mouse immunoglobulin G (IgG) and C3 in kidney cryostat sections was performed as reported previously.8
Renal function
Creatinine clearance was calculated from serum and urine creatinine concentrations (Olympus AU600, New York, NY). Albumin concentration in the 24-h urine specimens was determined by radial immunodiffusion, detection limit 50 μg/ml, using rabbit anti-mouse albumin (Biogenesis, Poole, UK) and mouse albumin standards (Sigma-Aldrich, Poole, UK) diluted in mouse urine.
Immunization
Mice were immunized by intramuscular injection into the thigh of chicken erythrocyte long chromatin,3 100 μg in solution in 50 μl of 10 mm Tris–HCl, pH 8·0, emulsified with an equal volume of Freund's complete adjuvant. After tail bleeds on days − 1, 14 and 28, all mice received a booster of the same dose of chromatin in Freund's incomplete adjuvant, and were then bled again on day 41 after the original injection before being killed by exsanguination on day 56. In other experiments mice received 4-weekly intravenous injections of 100 μl of a suspension of 108cells/ml of syngeneic apoptotic thymocytes in sterile phosphate-buffered saline, pH 7·4. Thymuses were removed from 6–8-week-old SAP–/– mice of the same strain as the recipients and cultured at 107 cells/ml in serum-free RPMI-1640 medium (Invitrogen Ltd, Paisley, UK) at 37° in 10% CO2 for 8 h to induce early apoptosis, detected by fluorescein isothiocyanate–annexin V (Immunotech, Marseilles, France) staining without coexistent propidium iodide staining.
Chromatin and nucleosome clearance and catabolism
Native long chromatin was prepared from chicken erythrocytes by limited digestion with staphylococcal nuclease as previously described.3 Mononucleosomes were obtained from long chromatin preparations after more extensive nuclease digestion by calcium-dependent precipitation of the residual long chromatin and oligonucleosome fragments. Integrity of the long chromatin and the quality of the nucleosome preparations were confirmed by agarose gel electrophoresis for DNA and sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for proteins, showing H1 as well as the other histones in the nucleosomes. After 125I radioiodination using N-bromosuccinimide,8,15 labelled long chromatin8,15 in 10 mm Tris–HCl, pH 8·0 was separated from unbound iodine by extensive dialysis against the same buffer containing a suspension of Amberlite IRA-420 anion exchange resin (BDH, Poole, UK). Nucleosomes labelled with 125I using N-bromosuccinimide were separated from unbound iodine by gel filtration on Sephadex G25 (PD10 column, Amersham Biosciences, Milton Keynes, UK). Incorporation of 125I and specific activity were determined by precipitation with 30% trichloroacetic acid. Tyramine cellobiose was synthesized, radiolabelled with 125I, and coupled covalently to nucleosomes using cyanuric chloride, as described elsewhere.16,17 The labelled material was separated from unbound [125I]tyramine cellobiose by Sephadex G25 gel filtration (PD10 column). Specific incorporation of 125I into the protein constituents of both the directly- and tyramine cellobiose-labelled preparations was confirmed by autoradiography after 8–18% reduced SDS–PAGE analysis. For in vivo studies of clearance of chromatin preparations, mice received potassium iodide in their drinking water for 48 h before the experiment to block thyroid uptake of free iodide. Whole body elimination was studied after intraperitoneal injection of 10 μg of 125I-labelled long chromatin containing 2 × 106 counts per minute (c.p.m.) into 13 female, age-and weight-matched C57BL/6 mice of each of the genotypes, SAP–/–, SAP+/– and SAP+/+. All mice were then counted immediately in a whole body gamma counter and again at 4 h and 7 h. Plasma clearance was studied in groups of 6–8-week-old age- and weight-matched female C57BL/6 SAP–/– and SAP+/+ mice after intravenous injection of 1 μg of either 125I-labelled long chromatin or 125I-labelled nucleosomes, corresponding to 100 000 c.p.m. and 400 000 c.p.m., respectively. Tail bleeds (50 μl) taken from each mouse 2 min after the injection and at 15, 30 and 90 min were collected into ethylenediaminetetraacetic acid-coated tubes. After centrifugation 25 μl of plasma was removed from each sample and precipitated with trichloroacetic acid to determine protein-bound radioactivity. The validity of the 125I-tyramine-cellobiose-labelled nucleosomes as representative of the clearance of directly labelled nucleosomes was established by showing in groups of three female wild-type C57BL/6 mice that both preparations were cleared from the plasma at the same rate throughout. The tissue sites of clearance and catabolism were then determined when the mice were killed and dissected at 24 h after intravenous injection of 125I-tyramine-cellobiose-labelled nucleosomes. After removal of as much blood as possible and of food and debris from the bowel, the liver, spleen, kidneys, heart, lungs, stomach, intestines, adrenal glands and the remaining carcass were each fixed in buffered formalin and counted individually. The liver and kidneys were then processed for dipping film histological autoradiography to identify cells containing radioactivity.
SAP ligand binding
Binding of 125I-labelled human and mouse SAP to native avian long chromatin, to avian mononucleosomes, and to single-stranded calf thymus DNA (Sigma, Poole, UK) that were immobilized in separate N-oxysuccinimide activated amine-binding polystyrene microtitre plates (Costar, Corning, NY), was evaluated using the same method previously reported for study of SAP binding to influenza virus.18
Results
Normal survival of SAP–\– C57BL\6 mice
There was no significant increase in mortality up to 12 months of age among SAP–/– mice, with eight deaths among 105 animals, compared to two of 104 SAP+/– animals and four of 103 wild-type SAP+/+ mice.
Spontaneous antinuclear autoimmunity in C57BL\6 SAP–\– mice
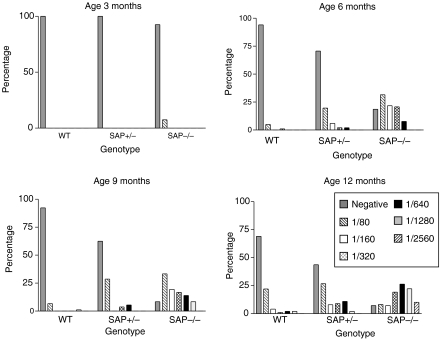
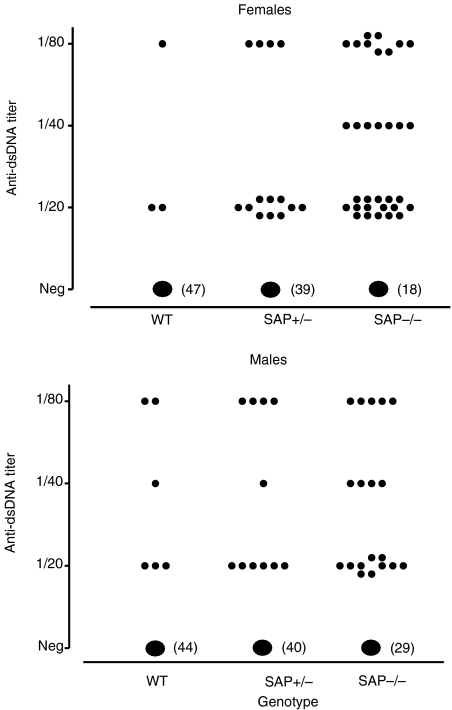
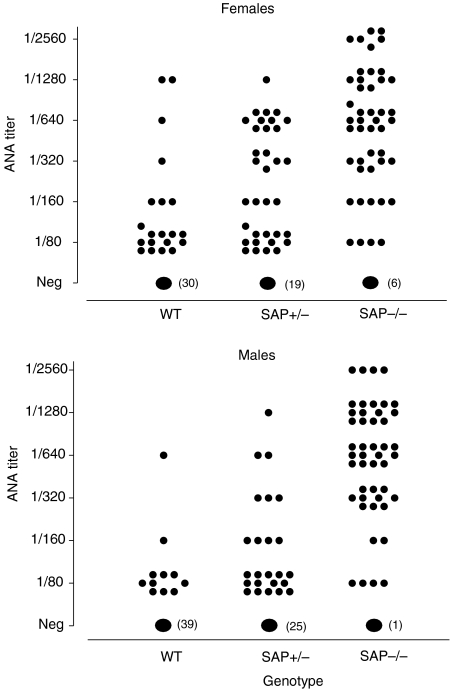
A small proportion of aging wild-type C57BL/6 mice are known to develop antinuclear autoantibodies spontaneously, mostly at low titre.19 In marked contrast, the SAP–/– C57BL/6 animals spontaneously developed a dramatically higher frequency of these autoantibodies and at significantly higher titre, with females generally being more affected (Figs 1–3; Table 1). SAP+/– mice showed an intermediate picture. Since SAP binds to the same chromatin nuclear antigens recognized by the various autoantibodies it was important to establish that SAP does not compete with the autoantibodies in the various assays used, and titres were therefore compared in SAP–/– serum before and after separate addition of 8·7 and 87 μg/l of isolated pure mouse SAP. In neither case was there any effect on the measured antibody titre, demonstrating that mouse SAP does not compete in these assays.
Figure 1.
Spontaneous development of homogeneous immunofluorescence staining pattern antinuclear autoantibodies (ANA) in C57BL/6 SAP knockout mice. ANA appeared in the SAP–/– group from age 3 months and increased in titre with increasing age in most mice. ANA in SAP+/– mice were intermediate in frequency and titre between the wild-type and SAP–/– groups.
Figure 3.
Anti-dsDNA autoantibody in C57BL/6 SAP–/–, SAP+/– and wild-type mice at age 12 months. Antibody frequency was highest in SAP–/– mice and intermediate in SAP+/– animals, compared to wild-type controls (χ2 test, males P = 0·003, females P = 0·001).
Table 1.
Immunological and histological abnormalities in SAP–/–, SAP+/–, and SAP+/+ C57BL/6 mice at age 12 months
| Anti-nuclear autoantibodies | Histology | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Homogeneous pattern immunofluoresce ncc(ANA) Positive n | Titre median (range) | Anti-dsDNA Positive n (%) | Anti-histone Titre median (range) | Anti-chromatin Titre median (range) | Anti-ssDNA Titre median (range) | Glomerulonephritis Positive n (%) | Spleen lymphocytosis Positive n (%) | |
| Female | |||||||||
| ″wild-type | 50 | 20 (40) | 0 (0–1/1280) | 3 (6) | 11·3 (2–48) | 3·0 (0–240) | 1·1(0–10) | 8 (16) | 15 (31) |
| ″SAP+/– | 53 | 34 (64) | 0 (0–1/1280) | 14 (26) | 15·1 (2–153) | 11·9 (0–98) | 2·7 (0–24) | 23 (43) | 29 (58) |
| ″SAP–/– | 51 | 45 (88) | 1/640 (0–1/2560) | 33 (65) | 16·7 (2–192) | 36·5 (0–365) | 3·8 (0–25·8) | 38 (75) | 30 (63) |
| P = 0·001 | P = 0·0001 | P = 0·001 | P = 0·1 | P = 0·0001 | P = 0·0001 | P < 0·0001 | P = 0·002* | ||
| Male | |||||||||
| ″wild-type | 50 | 11 (22) | 0 (0–1/640) | 6 (12) | 7·9 (2–24) | 2·5 (0–49) | 0 (0–7) | 0 (0) | 9 (20) |
| ″SAP+/– | 49 | 24 (49) | 0 (0–1/1280) | 9 (18) | 8·2 (2–81) | 4·5 (0–49) | 2·6 (0–24) | 10 (20) | 19 (39) |
| ″SAP–/– | 48 | 47 (98) | 1/640 (0–1/2560) | 19 (40) | 13·5 (2–36) | 38·8 (0–169) | 2·4 (0–22) | 13 (28) | 22 (49) |
| P = 0·001 | P = 0·001 | P = 0·003 | P = 0·009 | P = 0·0001 | P = 0·003 | P = 0·001* | P = 0·007* | ||
P-values for univariate differences between groups obtained by χ2 test, Fisher's exact test or Mann–Whitney U-test as appropriate.
Significance of wild-type vs. SAP –/– only.
Rheumatoid factor production in C57BL\6 SAP–\– mice
Rheumatoid factor was of interest in view of the possible role of chromatin–IgG complexes in activating rheumatoid-factor-producing B cells.20 Although rheumatoid factor titres were very modest in all mice, the SAP–/– animals, of both sexes grouped together, had significantly higher values at 1 year (median, 11·6; range, 0·0–30·2; units n = 100) than wild-type SAP+/+ animals (8·3; 0·0–22·1; n = 96), P = 0·0012 (Mann–Whitney test). The SAP+/– animals had intermediate values that were not significantly different from either the SAP knockouts or the wild-type controls (9·5; 0·0–37·8; n = 99). There were no significant differences between males and females within any genotype but the differences between SAP–/– and SAP+/+ mice were significant among both males (12·6; 0·0–30·2; n = 48 versus 8·2; 0·0–22·1; n = 49; P = 0·012) and females (10·4; 0–28·0; n = 52 versus 8·6; 0·0–21·9; n = 47; P = 0·036).
Histopathology in C57BL\6 SAP–\– mice
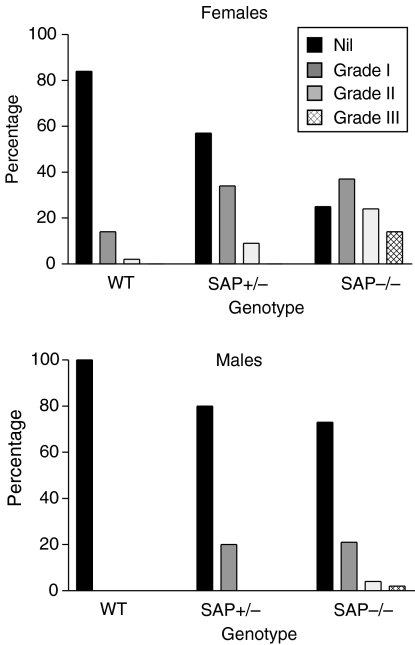
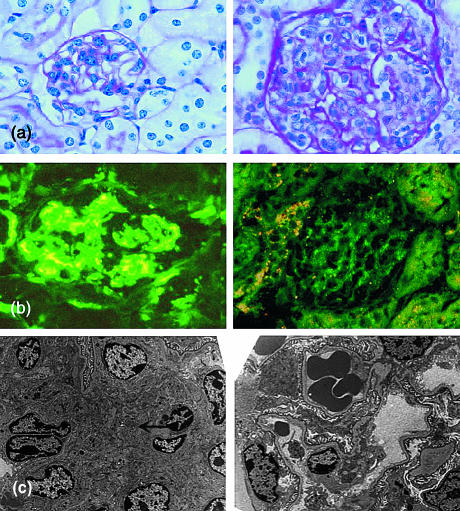
Significant histological abnormalities were present only in the kidneys and the spleen. Minor non-specific lymphoid infiltrates in the salivary glands, livers and occasionally in the lungs of some mice, were as previously reported in the C57BL/6 strain19 but with no differences between the sexes or the different SAP genotypes. In marked contrast there was a dramatically higher frequency and severity of proliferative, immune complex, glomerulonephritis in the kidneys of SAP–/– and SAP+/– compared to wild-type SAP+/+ mice, with the SAP–/– animals being more severely affected than the SAP+/– animals and the females very significantly more affected than males (Fig. 4). Severely affected glomeruli showed typical granular immunostaining for mouse IgG (Fig. 5) and C3, and there was also positive, though much less abundant, staining even in glomeruli with normal appearance (not shown). Electron microscopy showed the increased mesangial matrix and immune complex deposition characteristic of this type of nephritis (Fig. 5). There were significant associations between the titres of most of the various autoantibodies and presence of moderate or severe glomerulonephritis.
Figure 4.
Glomerulonephritis in C57BL/6 SAP–/–, SAP+/– and wild-type mice at age 12 months. Frequency and severity were greatest in the SAP–/– group with intermediate values in SAP+/– mice, and females notably more affected than males.
Figure 5.
Glomerulonephritis in C57BL/6 SAP–/– mice. (a) Right panel, typical proliferative glomerulonephritis in SAP–/– mouse showing increased mesangial matrix and marked hypercellularity with mononuclear cells in capillary loops; left panel, normal glomerulus from wild-type mouse (× 250, haematoxylin & eosin stain). (b) Left panel, immunofluorescence stain with anti-mouse IgG showing granular appearance typical of immune complex deposition; right panel, specificity control showing complete abolition of staining after absorption of the primary antibody with mouse IgG (× 250). (c) Left panel, electron micrograph of glomerulus from SAP–/– mouse showing mesangial expansion and immune complex deposition (arrowed); right panel, normal glomerulus from wild-type mouse (× 2000).
Increased spleen white pulp volume, white pulp coalescence and red pulp lymphocytosis, were frequently observed in both female and male SAP–/– and SAP+/– mice and did not differ between these groups, but were very significantly more common than among the wild-type SAP+/+ controls (Table 1).
Normal renal function in C57BL\6 SAP–\– mice
Despite the frequency of histological glomerulonephritis in the SAP–/– and SAP+/– mice there was no reduction in creatinine clearance at 12 months of age, with both sexes in all groups having median clearance of 0·08–0·11 ml/min, and there was no significant albuminuria in any of the groups, all having less than 50 μg/ml.21
Enhanced autoimmune response of SAP–\– C57BL\6 mice to apoptotic cells
Intravenous injection of apoptotic syngeneic thymocytes has been reported to induce antinuclear autoantibodies in wild-type mice.22 Although none of our animals had any detectable antinuclear autoantibodies before the first immunization, the SAP–/– C57BL/6 mice, especially the females, rapidly produced these antibodies with significantly greater frequency and in higher titres than did the wild-type SAP+/+ controls, when bled on days 14, 28 (Table 2) and 42. Some of the female SAP–/– mice also developed glomerulonephritis (Table 2).
Table 2.
Response to immunization with apoptotic cells in SAP–/– and wild-type SAP+/+ C57BL/6 mice
| Positive,n (%) | ||||||
|---|---|---|---|---|---|---|
| n | ANA | Anti-dsDNA | Anti-chromatin | Anti-ssDNA | Glomerulonephritis | |
| Female | ||||||
| ″wild type | 20 | 1 (5) | 4 (20) | 9 (45) | 5 (25) | 0 (0) |
| ″SAP–/– | 26 | 18 (69) | 9 (35) | 20 (77) | 16 (62) | 9 (36) |
| P<0·0001 | P = 0·33 | P<0·04 | P<0·02 | P<0·003 | ||
| Male | ||||||
| ″wild type | 10 | 0 (0) | 2 (20) | 6 (60) | 2 (20) | 0 (0) |
| ″SAP–/– | 9 | 3 (33) | 4 (44) | 4 (44) | 2 (22) | 0 (0) |
| P = 0·09 | P = 0·35 | P = 0·36 | P = 1·0 | P = 1·0 | ||
P-values obtained by χ2 test or Fisher's exact test as appropriate.
Mice received 107 apoptotic syngeneic thymocytes in a single intravenous injection each week for 4 weeks. Autoantibodies were estimated on day 28 and glomerulonephritis on day 56.
Low frequency of spontaneous autoimmunity in 129\Sv SAP–\– mice
The 129/Sv strain does not spontaneously develop substantial autoimmunity and SAP deficiency did not significantly affect this. Glomerulonephritis was also relatively infrequent, with moderate involvement in one of 24 males at 1 year, and five of 19 females, two with severe grade, but there was no increased morbidity or mortality.
Failure to induce antinuclear antibodies by active immunization of 129\Sv mice
Immunization of 36 (16 female) 12-week-old SAP–/– pure-line 129/Sv mice and 41 (18 female) age- and weight-matched wild-type SAP+/+ 129/Sv controls, with avian long chromatin in complete Freund's adjuvant on day 0, followed by a booster in incomplete adjuvant on day 32, did not induce any ANA. Similarly, when 17 (eight females) 6–8-week-old SAP–/– 129/Sv mice and an identical group of matched SAP+/+ wild-type controls were immunized with apoptotic syngeneic thymocytes, there was no ANA response and no glomerulonephritis in any animal when they were all killed on day 56.
Plasma clearance and catabolism of long chromatin and nucleosomes
We have previously reported that in studies of large groups of mice, the whole body clearance of intravenously injected radiolabelled avian long chromatin was significantly retarded in SAP+/+ wild-type mice compared to SAP-deficient SAP–/– animals of the C57BL/6 × 129/Sv genetic background.8 These observations were not confirmed in the present studies of pure line C57BL/6 mice, either because of the effects of the strain difference or possibly because, as our further experience has revealed, the rates of clearance and catabolism of these chromatin preparations vary with their state of integrity or degradation. However, it is not possible to relate this to the form in which autologous chromatin is handled in vivo, and thus to reach physiologically relevant conclusions.
We also investigated here, for the first time, the plasma clearance of radioiodinated native long chromatin and found that in SAP–/– C57BL/6 mice the rapid early phase was slightly but significantly slower than in SAP+/+ wild-type animals. In two typical experiments with six mice per group there remained, 15 min after injection, respectively, mean (SD), 31% (9·5%) of initial activity compared to 13% (6·2%) (P < 0·004), and 29% (13·5%) compared to 15% (4·6%) (P < 0·05, Mann–Whitney U-tests). Thereafter there was no significant difference in residual activity between groups. We also studied the plasma clearance of radiolabelled nucleosome core particles, and with an intact undegraded nucleosome preparation, we found a significantly slower initial rate in SAP–/– than in SAP+/+ mice (n = 6 per group). Thus at 15 min there was, respectively, 36% (13·7%) of initial activity compared to 19% (6·5%) (P < 0·04) and at 30 min there was 24% (10·3%) compared to 10% (4·8%) (P < 0·03, Mann–Whitney U-tests). However in further studies with nucleosome preparations in which there was evidence of greater cleavage of the DNA, there was no significant difference in the rate of clearance between SAP–/– and SAP+/+ mice.
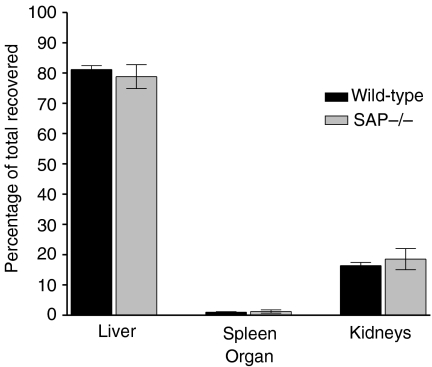
The cellular and tissue sites of catabolism of nucleosomes were investigated, using the tyramine-cellobiose-trapped catabolism method16 after first establishing that the rates of plasma clearance of directly radiodinated and tyramine-cellobiose-labelled nucleosomes were the same. At 24 h after intravenous injection there was no residual activity in blood and the major sites of clearance and retention, indicating catabolism of the labelled nucleosomes, were the liver and kidneys (Fig. 6). The carcass contained mean (SD), 16·5% (9·8%) of the activity, the spleen and intestine 0·9% (0·3%) and 0·7% (0·1%), respectively; the remaining organs each contained less than 0·5%. There were no significant differences between SAP–/– and SAP+/+ mice. Autoradiography showed that the cellular sites of nucleosome catabolism were hepatocytes, Kupffer's cells and renal parenchymal cells, and there was no apparent difference between SAP–/– and SAP+/+ mice.
Figure 6.
Localization of catabolism of nucleosomes in C57BL/6 SAP–/– and wild-type mice. Major sites were the liver and kidneys, with no significant difference between wild-type and SAP knockout mice. Values shown are per cent of activity recovered excluding the carcass; eight mice per group.
Anti-nuclear autoimmunity in SAP–\– C57BL\6 transgenic for human SAP
In small-scale preliminary experiments we investigated whether transgenic expression of human SAP affected the autoimmune phenotype of C57BL/6 mice that were either SAP-deficient or of the wild-type, SAP+/+, background. A cohort of 35 mice comprising 13 SAP–/– animals (seven female) and 22 SAP–/– human SAP transgenic animals (12 female) were bled at 6 and 12 months of age. The median (range) baseline serum concentration of human SAP (μg/ml) in mice carrying the transgene was significantly higher in the females, 93 (62–126) than in the males, 23 (22–62; P = 0·0001, Mann–Whitney U-test). However, the pattern of development and the final titres at 12 months of all the various antinuclear autoantibodies, and also the presence and severity of glomerulonephritis, were not significantly different between the groups, regardless of the expression of human SAP, and closely resembled the phenotype seen in the much larger cohort of SAP–/– C57BL/6 mice reported above.
When female SAP–/– mice that were transgenic for human SAP were immunized with apoptotic syngeneic thymocytes, their antinuclear autoantibody responses and subsequent incidence of glomerulonephritis were similar to those seen in non-transgenic SAP–/– mice and were significantly greater than matched SAP+/+ wild-type C57BL/6 controls. There were no detectable autoantibodies in any animal before the first injection but significant differences had emerged by day 14 and the typical results at day 28 are shown in Table 3, together with the eventual frequency of glomerulonephritis.
Table 3.
Response to immunization with apoptotic cells in SAP–/– mice, SAP–/– mice transgenic (tg) for human SAP, and wild-type SAP+/+ C57BL/6 mice
| Positive, n (%) | ||||||
|---|---|---|---|---|---|---|
| n | ANA | Anti-dsDNA | Anti-chromatin | Anti-ssDNA | Glomerulo- nephritis | |
| Wild-type | 10 | 0 (0) | 0 (0) | 2 (20) | 3 (30) | 0 (0) |
| SAP–/– | 16 | 12 (75) | 2 (12) | 11 (69) | 13 (81) | 8 (50) |
| SAP–/– human SAP transgenic | 16 | 11 (69) | 1 (6) | 8 (50) | 12 (75) | 13 (81) |
| P: SAP–/– & wild type | 0·0002 | 0·5 | 0·04 | 0·02 | 0·009 | |
| P: SAP–/– human SAP tg & wild-type | 0·0008 | 1·0 | 0·2 | 0·04 | 0·0001 | |
| P: SAP–/– & SAP–/– human SAP tg | 1·0 | 1·0 | 0·47 | 1·0 | 0·13 | |
P-values obtained by χ2 test or Fisher's exact test as appropriate.
Mice received 107 apoptotic syngeneic thymocytes in a single intravenous injection each week for 4 weeks. Autoantibodies were estimated on day 28 and glomerulonephritis on day 56.
Binding of mouse and human SAP to DNA and chromatin
When 125I-radiolabelled pure human and mouse SAP was offered separately to immobilized ssDNA, long chromatin, or nucleosomes, notably more human than mouse SAP was bound in each case. With DNA, 26–29% of the offered human SAP was bound but there was no detectable binding of mouse SAP. With long chromatin, 3·4–5·6% of the offered human SAP was bound but just 0·1–0·2% of the mouse SAP. With nucleosomes, 6·1–9·6% of human SAP bound and 1·8–2·4% of the offered mouse SAP.
Discussion
Pure line C57BL/6 mice homozygous for targeted deletion of the SAP gene had a distinctive phenotype of spontaneous antinuclear autoimmunity that developed progressively from 3 months onwards, and included both typical immune complex glomerulonephritis and abnormal lymphoproliferative spleen histology. These features were more marked in females than males, as in human systemic lupus erythematosus, but there was no abnormal proteinuria, no morbidity and no early mortality. This is consistent with evidence that the discrete genetic loci responsible for the propensity to develop autoantibodies are different from those that permit expression of clinical autoimmune disease.23–25 Pure-line C57BL/6 SAP+/– mice, hemizygous for the SAP gene deletion, produce less SAP9 and developed an intermediate phenotype.
Our findings in SAP knockout mice contrast remarkably with the absence of any increased antinuclear autoimmunity in pure-line C57BL/6 mice carrying targeted deletion of the C1q gene.10 Deficiency of C1q in C57BL/6 × 129/Sv mice is associated with immune complex glomerulonephritis and reduced glomerular clearance of apoptotic bodies, together with homogeneous staining pattern ANAs, but little antichromatin or anti-DNA autoantibody.26 The genetic background is thus critically important in enabling phenotypic expression of impaired tolerance to nuclear antigens.
The important role of genetic background was emphasized by the absence of significantly increased autoimmunity in pure-line 129/Sv mice carrying the SAP gene deletion. The wild-type 129/Sv strain shows much less spontaneous antinuclear autoimmunity than the C57BL/6, and SAP deficiency did not affect this. Even when the SAP–/– 129/Sv mice were immunized either with avian chromatin or apoptotic thymocytes, they did not produce any ANA. In marked contrast, such intravenous injections induced ANA, anti-DNA responses and glomerulonephritis with significantly greater frequency and titre in SAP–/– C57BL/6 mice than in wild-type SAP+/+ C57BL/6 controls.
We sought to establish more firmly the role of SAP deficiency itself in causation of the phenotype by preliminary experiments with a small cohort of SAP–/– mice expressing transgenic human SAP. Interestingly, despite abundant production of human SAP, these mice continued to exhibit the same autoimmunity phenotype as the SAP–/– mice without the human SAP transgene. Thus they spontaneously produced antinuclear autoantibodies and developed glomerulonephritis as they aged, and they also responded more vigorously than wild-type mice to immunization with apoptotic thymocytes. Although more extensive studies in a larger cohort are in progress to substantiate these observations, the apparent failure of human SAP to reconstitute the wild-type phenotype may reflect the significant differences that exist between the mouse and human proteins. For example, we have previously reported the notably higher affinity of human SAP than mouse SAP for binding to amyloid,27 to influenza virus18 and to low molecular weight ligands.28 Here we compared their binding to nuclear ligands, albeit unphysiologically immobilized by covalent attachment to plastic, and found that human SAP bound much more avidly than did mouse SAP to DNA, long chromatin and nucleosomes. Another possibility is that human SAP may not interact appropriately with soluble murine molecules or cellular receptors that are required for proper functioning of mouse SAP in its autologous environment, and may thus not properly abrogate the autoantigenicity of nuclear ligands.
A further possible explanation is that the phenotype of the SAP–/– mice is not caused by deficiency of mouse SAP but by another effect of the targeted deletion of the mouse SAP gene. The gene was deleted by homologous recombination in embryonic stem cells from the 129/Sv strain and in both the original 129/Sv × C57BL/6 cross and in pure-line C57BL/6 mice reported here, the fragment of 129/Sv chromosome 1 genomic material is present within a partly or wholly C57BL/6 genomic environment. In addition the mouse SAP gene is situated within the well-known sle1 locus that controls the propensity to antinuclear autoantibody formation.29 Even though mapping studies show that the SAP gene itself is not responsible for the sle1 locus,30 and we know that our targeted deletion did not include any other gene,9 the possibility remains that the homologous recombination event may have had functional consequences in this genomic region that are expressed as the autoimmunity phenotype of the SAP-deficient mice, as we noted in a caveat in our original report.8 It is also possible that the phenotype could even be caused by the presence of unaltered 129/Sv genes from this key region of chromosome 1 operating in the C57BL/6 genomic background. Congenic breeding studies to investigate this mechanism are currently in progress (Dr M Botto, personal communication). Transgenic introduction of the C57BL/6 mouse SAP gene into C57BL/6 SAP–/– mice, so that mouse SAP is expressed together with homozygosity for the targeted 129/Sv SAP gene deletion, would also delineate the specific role of SAP itself, if any.
We previously found that whole body clearance of radiolabelled avian long chromatin, a global measure comprising clearance, catabolism and urinary elimination of radioactive breakdown products, was accelerated in SAP–/– 129/Sv × C57BL/6 mice.8 We postulated that the known capacity of SAP to stabilize its macromolecular ligands and protect them from degradation, and its powerful antiopsonic effect31 could both slow the elimination of chromatin and reduce its immunogenicity.8 In the present work in pure-line C57BL/6 mice we could not detect any effect on whole body clearance of long chromatin, either because of the strain difference or because of differences in the chromatin preparations. The rates of plasma clearance and breakdown of chromatin preparations are very sensitive to even subtle variations in the integrity of the chromatin that are very difficult to prevent and control, or even detect before use in vivo. However, in looking at clearance from the circulation alone, rather than combined with subsequent catabolism and excretion of degradation products, we found a slower initial rate with both long chromatin and nucleosome core particles in the SAP-deficient animals compared to SAP+/+ wild-type control C57BL/6 mice. Although nucleosome core particles are the form of chromatin that is released into the circulation by autologous cell death, rather than either long chromatin or naked DNA, interpretation of these findings in physiological terms must be cautious as avian chromatin may be handled differently than the autologous murine counterpart.
We also formally examined for the first time the tissue sites of nucleosome catabolism in vivo, using the trapped catabolism method with tyramine cellobiose as a non-degradable, lysosome-marking, covalent tag.16,17 Directly radioiodinated proteins cannot reveal accurately their sites of catabolism because of the rapid diffusion of labelled amino acids out of the catabolizing cell. Most of the nucleosomes were removed from the circulation in the liver and broken down in both Kupffer's cells and hepatocytes, with the remainder being destroyed in the kidney parenchymal cells. Only traces were found in the spleen and there were no differences between wild-type and SAP-deficient mice. Thus if murine SAP does have an anti-immunogenic effect in relation to clearance or breakdown of chromatin antigens it must be at a more subtle level than differential tissue or cellular distribution.
Figure 2.
Anti-nuclear autoantibody (ANA) in C57BL/6 SAP−/−, SAP+/− and wild-type mice at age 12 months. In both sexes, ANA frequency (χ2 test, P = 0·001) was highest in SAP−/− mice and intermediate in SAP+/− animals, compared to wild-type controls, and ANA titres were higher in the SAP−/− mice group compared to the other two (Mann–Whitney U-test, P = 0·0001).
Acknowledgments
This work was supported by Medical Research Council Programme Grant G7900150 and a Wolfson Foundation grant to M.B.P., and by a Wellcome Trust Clinical Research Fellowship to J.D.G. We thank Dr M. Botto for undertaking the backcross of the SAP gene deletion into C57BL/6 mice, Dr Botto and Prof. M.J. Walport for helpful discussions, Prof. H.T. Cook for the ‘blind’ scoring of renal histology, Prof. A. Dhillon and Ms K. Savage for help in examination of autoradiography slides, and Mrs E. Jones for expert preparation of the manuscript.
Abbreviations
- ANA
antinuclear autoantibody
- dsDNA
double-stranded DNA
- SAP
serum amyloid P component
- ssDNA
single-stranded DNA
References
- 1.Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E. Amyloid P component. A critical review. Amyloid: Int J Exp Clin Invest. 1997;4:274–95. [Google Scholar]
- 2.Pepys MB, Butler PJG. Serum amyloid P component is the major calcium-dependent specific DNA binding protein of the serum. Biochem Biophys Res Commun. 1987;148:308–13. doi: 10.1016/0006-291x(87)91111-9. [DOI] [PubMed] [Google Scholar]
- 3.Butler PJG, Tennent GA, Pepys MB. Pentraxin–chromatin interactions: serum amyloid P component specifically displaces H1-type histones and solubilizes native long chromatin. J Exp Med. 1990;172:13–18. doi: 10.1084/jem.172.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepys MB, Booth SE, Tennent GA, Butler PJG, Williams DG. Binding of pentraxins to different nuclear structures. C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol. 1994;97:152–7. doi: 10.1111/j.1365-2249.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breathnach SM, Kofler H, Sepp N, Ashworth J, Woodrow D, Pepys MB, Hintner H. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989;170:1433–8. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hintner H, Booker J, Ashworth J, Auböck J, Pepys MB, Breathnach SM. Amyloid P component binds to keratin bodies in human skin and to isolated keratin filament aggregates in vitro. J Invest Dermatol. 1988;91:22–8. doi: 10.1111/1523-1747.ep12463283. [DOI] [PubMed] [Google Scholar]
- 7.Familian A, Zwart B, Huisman HG, Rensink I, Roem D, Hordijk PL, Aarden LA, Hack CE. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001;167:647–54. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- 8.Bickerstaff MCM, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 1999;5:694–7. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 9.Botto M, Hawkins PN, Bickerstaff MCM, et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nature Med. 1997;3:855–9. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol. 2002;168:2538–43. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 11.Iwanaga T, Wakasugi S, Inomoto T, et al. Liver-specific and high-level expression of human serum amyloid P component gene in transgenic mice. Dev Genet. 1989;10:365–71. doi: 10.1002/dvg.1020100504. [DOI] [PubMed] [Google Scholar]
- 12.Yamamura K-I, Tashiro F, Yi S, Wakasugi S, Araki S, Maeda S, Shimada K. Transgenic mouse model for human genetic diseases. Mol Reprod Dev. 1993;36:248–50. doi: 10.1002/mrd.1080360222. [DOI] [PubMed] [Google Scholar]
- 13.Pepys MB, Dash AC, Markham RE, Thomas HC, Williams BD, Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978;32:119–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mather SJ, Ward BG. High efficiency iodination of monoclonal antibodies for radiotherapy. J Nucl Med. 1987;28:1034–6. [PubMed] [Google Scholar]
- 16.Pittman RC, Carew TE, Glass CK, Green SR, Taylor CRJ, Attie AD. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation. Biochem J. 1983;212:791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchinson WL, Noble GE, Hawkins PN, Pepys MB. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo. J Clin Invest. 1994;94:1390–6. doi: 10.1172/JCI117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbert J, Hutchinson WL, Carr J, Ives J, Jakob-Roetne R, Yamamura K, Suzuki M, Pepys MB. Influenza virus infection is not affected by serum amyloid P component. Mol Med. 2002;8:9–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi Y, Utsuyama M, Kurashima C, Hirokawa K. Spontaneous development of organ-specific autoimmune lesions in aged C57BL/6 mice. Clin Exp Immunol. 1989;78:120–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 21.Robson MG, Cook HT, Botto M, et al. Accelerated nephrotoxic nephritis is exacerbated in C1q-deficient mice. J Immunol. 2001;166:6820–8. doi: 10.4049/jimmunol.166.11.6820. [DOI] [PubMed] [Google Scholar]
- 22.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis. Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–65. [PubMed] [Google Scholar]
- 24.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–72. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan C, Morel L, Yang P, Watanabe H, Croker B, Gilkeson G, Wakeland EK. Genetic dissection of lupus pathogenesis: a recipe for nephrophilic autoantibodies. J Clin Invest. 1999;103:1685–95. doi: 10.1172/JCI5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins PN, Myers MJ, Epenetos AA, Caspi D, Pepys MB. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. J Exp Med. 1988;167:903–13. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepys MB, Herbert J, Hutchinson WL, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–9. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- 29.Morel L, Mohan CYuY, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–28. [PubMed] [Google Scholar]
- 30.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–92. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noursadeghi M, Bickerstaff MCM, Gallimore JR, Herbert J, Cohen J, Pepys MB. Role of serum amyloid P component in bacterial infection: protection of the host or protection of the pathogen. Proc Natl Acad Sci USA. 2000;97:14584–9. doi: 10.1073/pnas.97.26.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]