Abstract
Cytokines and their receptors represent key targets for therapeutic intervention. Ligands are being used to supplement cell numbers that become depleted as a result of disease (organ failure, infection) or subsequent disease treatments (i.e. chemotherapy). Conversely, the inhibition of target cell binding by cytokines is an established strategy for abrogating pathologic cellular activities common to many immunological diseases. Considerable effort in biomedical research is being focused on the cytokine families that play a dominant role in regulating immunity and then prioritizing each member for its therapeutic potential. Currently, the interleukin-2 (IL-2) family of cytokines is widely recognized for its central involvement in controlling lymphocyte function and is the most explored for medical utility. Collectively, these proteins (or their antagonists) are either marketed drugs or have received advanced testing for an impressive array of indications including cancer, infectious disease, transplantation, inflammation and allergic asthma. Here we review the current understanding of IL-21, the most recent member of this cytokine family to be discovered. As will be discussed, IL-21 shares many of the same attributes as its relatives in that it has broad immunoregulatory activity and can modulate both humoral and cell-mediated responses. Its ability to stimulate durable anti-tumour responses in mice defines one therapeutic indication that merits clinical development.
Keywords: anti-tumour responses, cell-mediated immunity, humoral immunity, interleukin-21, interleukin-2 family
Introduction
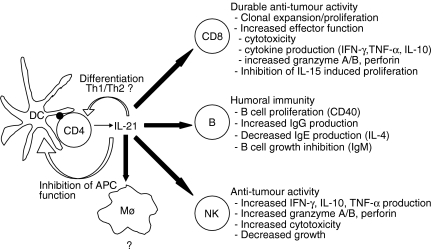
The cellular interactions that constitute a functional immune system require a communication network that includes cytokines and cytokine receptors. These proteins are produced in response to a large spectrum of stimuli that help protect the host from noxious substances and pathogenic organisms. Considerable attention over the years has been devoted to the interleukin-2 (IL-2) family of cytokines because of the central role it plays in the initiation and cessation of an immune response in mammals.1–4 Collectively, these cytokines (IL-2, IL-4, IL-7, IL-9, IL-13, IL-15 and IL-21) regulate leucocyte development and control a broad spectrum of processes that quantitatively and qualitatively shape acquired immune responses. Moreover, perturbations in the expression of these cytokines, or in the signals that they initiate, are associated with various pathologies including immunodeficiency, autoimmunity and atopic disease.3,4 IL-21, discovered using a strategy of ligand-receptor pairing,5,6 is probably the last member of the IL-2 family to be identified and possibly the least characterized. In general terms, IL-21 is a product of CD4+ T-helper cells that selectively modifies both humoral and cell-mediated immune responses through an interaction with various cell types including T cells, B cells, natural killer (NK) cells, and dendritic cells. More specifically, IL-21 is required for normal antibody responses in mice and it has a potent anti-tumour activity in a variety of mouse models. This review will focus on the activities of IL-21 as a T-helper cytokine and its regulation of an adaptive immune response (Fig. 1).
Figure 1.
IL-21 is a CD4+ T-helper cytokine. The expression of IL-21 following antigen-specific T-cell activation stimulates a number of predominant changes in an adaptive immune response. IL-21 has minimal effects on CD4+ T-cell proliferation and its regulation of Th1 versus Th2 differentiation needs further clarification. Its role in stimulating cell-mediated anti-tumour responses via enhancement of CD8+ T-cell and NK-cell differentiation is firmly established, as is its regulation of B-cell-mediated humoral immunity. Expression of IL-21R is low but detectable on monocytes and the affects of IL-21 on these cells remains to be established. IL-21R is also expressed on monocyte-derived dendritic cells where the inhibitory activity of IL-21 has been confirmed with immature dendritic cells but not activated dendritic cells.
Interleukin-21: a new member of the il-2 cytokine family
Analysis of the genetic and biochemical properties of IL-21 and the IL-21 receptor (IL-21R) clearly defines IL-21 as a member of the IL-2 family of cytokines that utilize the common γ-chain receptor subunit for signal transduction. The human IL-21 gene encodes a polypeptide precursor of 162 amino acids and a fully processed mature protein of 133 amino acids (∼15 000 MW). The primary sequence of IL-21 predicts a four-helix bundle conformation, typical for type I cytokines, and its closest homologues are IL-2 (13·7% identity) and IL-15 (20% identity). IL-21 shares two pairs of spatially conserved cysteine residues with IL-15, the more distal of each pair is also present in IL-2, IL-4 and granulocyte–macrophage colony-stimulating factor. The IL-21 gene is located on human chromosome 4q26-27, approximately 180 kilobases (kb) from the IL-2 gene. The human IL-15 gene lies distal to IL-2 and IL-21 and is located on chromosome 4q31. The similarities that these three cytokines share in gene structure, organization and location argue that they evolved via gene duplication events.
A similar argument can also be made for the evolution of the IL-21R gene, which in humans spans approximately 20 kb, is located on chromosome 16p11 and lies within 65 kb of IL-4RA.5 Human IL-21R encodes a 538 amino acid protein that shares a domain organization similar to IL-4Rα and a primary amino acid sequence related to IL-2RB (28% identity). The extracellular portion contains a single cytokine-binding domain with its conserved WSXWS motif and two paired cysteine residues. This region is followed by a transmembrane sequence and a relatively long intracellular domain, containing the conserved Box 1 and Box 2 elements that are important for signal transduction.5,7,8 The fact that the IL-21 receptor complex is a heterodimer, containing IL-21R and the γc-receptor subunit, is firmly established.9,10 These and other studies have shown that IL-21 binding stimulates activation of Janus kinase (JAK)1/JAK3 and the subsequent phosphorylation of signal transducer and activator of transcription-1 (STAT1), often considered a negative regulator of cell growth and survival, as well as STAT3 and STAT5, which suggests a role in promoting cell survival, cell-cycle progression and differentiation.9–12 In addition, IL-21 enhances STAT4 binding to the interferon-γ (IFN-γ) promoter.12 These results indicate that IL-21 shares more similarities in STAT phosphorylation with IL-2 and IL-15 than with IL-4, which predominantly utilizes STAT6-mediated signalling.
IL-21 IS A CD4+ T-HELPER CYTOKINE
Central to the theme that IL-21 helps shape adaptive immunity is its location of expression within the immune system and the cell types to which it binds. IL-21 gene expression appears to be restricted to activated CD4+ T cells, although very little is known about the physiological conditions that induce IL-21 transcription. Naive T cells have the flexibility to express different cytokines depending on the costimulatory signals they receive during activation and many retain this flexibility through extended cell division. The pathways that induce IL-21 gene expression and how the kinetics of this expression compares to the other T-helper cytokines, such as IL-2, IL-4 and IFN-γ, remains to be elucidated. Interestingly, IL-21 appears to be rarely expressed gene as its cDNA is practically undetectable in current mouse and human expressed sequence tag (EST) databases. In addition, IL-21 mRNA was found to be 100 times less prevalent than IL-2 mRNA in naïve T cells treated with phorbol 12-myristate 13-acetate + ionomycin for 14 hr (unpublished results). These results are only an indirect measure of IL-21 protein, but they suggest that IL-21 expression may be quite transient and stage-specific during T-cell differentiation. This is consistent with the report that IL-21 is induced, not during the activation of naïve T-cells, but only following secondary stimulation of differentiated T-helper type 2 (Th2) effector cells.13 This finding, if confirmed, has important implications with respect to defining IL-21 function. However, we find that Th1 and Th2 cells derived from both mouse and human cell cultures can readily express IL-21 (unpublished results). Whether IL-21 is expressed during additional stages of CD4+ T-cell differentiation (i.e. T-reg), in different types of T cells (i.e. NKT cells, γδ T cells) or by non-lymphocyte cells awaits further characterization. We have detected very slight expression of IL-21 in activated CD8+ cells using reverse transcription polymerase chain reaction but it remains to be determined whether this is analogous to the autocrine production of IL-2 that sustains CD8+ T-cell proliferation.14
Il-21 regulates t-cell proliferation and differentiation
The IL-2 family of cytokines exerts many overlapping and distinct functions that control T-cell biology.1,3,15,16 Collectively, these cytokines maintain naïve T-cell homeostasis and regulate the magnitude of clonal expansion following antigen activation by influencing rates of cell proliferation, activation-induced cell death and cell survival. In addition, these cytokines regulate T-cell differentiation, the balance between humoral and cell-mediated immunity and help ensure robust secondary immune responses through the establishment of memory. As expected for a member of this cytokine family, the data being generated from both in vitro and in vivo experimentation indicate that IL-21 is a potent T-cell stimulatory protein.
Like IL-2, IL-21 can enhance the clonal expansion of antigen-activated naïve T cells. This conclusion is based on studies that measured the costimulatory activity of IL-21 on both anti-CD3 stimulated human CD3+ CD45RA+ cells and mouse thymocytes,5 alloantigen-stimulated mouse splenocytes17 and phosphoantigen-stimulated Vγ9/Vδ2 T cells.18 Current data also indicate that IL-21 costimulates the proliferation of both primed naïve CD4+ and CD8+ T cells, although there may be differences in their relative responsiveness. For instance, IL-21 stimulated less than a two-fold increase in DNA synthesis in normal mouse splenic CD4+ T cells and ovalbumin-peptide-specific mouse transgenic DO11.10 CD4+ T cells (unpublished results). However, IL-21 has a much greater impact on the proliferation of CD8+ T cells. This was initially observed by the accelerated outgrowth of CD8+ CD4– cells in cultures of mouse thymocytes.5 Subsequent experiments have shown that IL-21 can stimulate cytomegalovirus-specific CD8+ CD45RA+ T cells to initiate numerous rounds of cell division19 and can enhance four- to six-fold the antigen-specific proliferation of T-cell receptor transgenic CD8+ OT-I T cells that recognize chick ovalbumin peptide SIINFEKL in the context of H-2Kb (unpublished results).
The costimulation of CD8+ T-cell proliferation by IL-21 has also been examined in vivo. Moroz et al.20 monitored the fate of adoptively transferred naïve OT-I cells in mice following the peritoneal injection of EG.7 thymoma cells that express the surrogate tumour antigen, ovalbumin. Injection of IL-21 protein clearly enhanced antigen responsiveness and clonal expansion of OT-I cells as measured by markers for T-cell activation, DNA synthesis/cell division and accumulation of cell numbers in peripheral lymphoid tissue. This animal model was also used to compare the relative effects of IL-21 with both IL-2 and IL-15 treatment.20 It was determined that IL-2 was more effective than IL-21 in stimulating OT-I cell numbers during the first 10 days after tumour exposure, while IL-15 had little effect relative to phosphate-buffered saline treatment in driving clonal expansion. After day 10, however, the numbers of OT-I cells dropped dramatically in IL-2-treated mice as a result of activation-induced cell death. This contraction in population size was not observed in the IL-21 treatment group, instead the numbers of transferred T cells remained elevated for up to 60 days as a result of increased cell survival, an effect that was also seen with IL-15. It was concluded that IL-21 shares features of both IL-2 and IL-15 with respect to its enhancement of cell proliferation and cell survival.
In addition to these effects, IL-21 has been shown to elicit several differentiative responses from T cells. For instance, IL-21 rapidly induced the genes encoding IL-12R, IL-18R, IFN-γ, IL-2Rα and the Th1-associated transcription factor T-bet in cultures of activated primary T-cells.12,21 IL-21 also synergized with IL-15 and IL-18 in stimulating IFN-γ gene expression in these same cultures. Wurster et al.13 reported that exposing naïve CD4 T cells to IL-21 under conditions that skew differentiation towards the Th1 phenotype actually inhibited IFN-γ production, although it had little effect on other Th1 cytokines such as tumour necrosis factor-α or IL-2. In these experiments, IL-21 reduced IL-12-induced phosphorylation of STAT4 and failed to modulate T-bet expression. This inhibitory effect on IFN-γ expression was lost if T cells had differentiated to the Th1 state prior to IL-21 exposure.13 It is well established that numerous lymphokines polarize CD4+ T-cell differentiation along a Th1/Th2 axis that then initiates humoral and cell-mediated immune responses, and cross-regulates the opposing subset' development and function. While more work is required to understand how IL-21 influences Th1 vs. Th2 development, the current data set clearly indicate that IL-21 can regulate both arms of an adaptive immune response.
Il-21 is required for normal humoral immunity
CD4+ T-helper cells regulate acquired antibody responses by stimulating B-cell activation, clonal expansion and maturation. Key effector molecules delivered by T cells that synergize in driving B-cell proliferation and antibody production include CD40 ligand22,23 and a variety of cytokines including IL-4 and IL-2.24 IL-21 is also critical for normal humoral immunity. IL-21R is initially expressed on B-lineage cells coincident with the appearance of B220+ immunoglobulin M (IgM)+ cells within the bone marrow and is readily detectable on peripheral B cells. IL-21 is not mitogenic by itself, but synergizes with anti-CD40 antibody treatment to dramatically enhance primary B-cell proliferation in vitro.5 IL-21 also stimulated IgG1 and IgG2a production in cultured mouse splenocytes treated with lipopolysaccharide (LPS).25 Conversely, IgG1 production was reduced in cultured B cells isolated from IL-21R–/– mice and the synthesis of antigen-specific IgG1, IgG2b and IgG3 antibodies was significantly impaired following immunization of IL-21R–/– animals.26 Moreover, mice doubly deficient for both IL-21R and IL-4 have a severely compromised IgG response.26 These results highlight the co-operation of IL-21 with IL-4 in regulating antibody production.
In contrast to this co-operation, IL-21 can also antagonize IL-4 function. For instance, IL-21 prevents the proliferation of human B cells cultured with anti-IgM antibodies plus IL-45 and overrides the anti-apopotic effect of IL-4 on primary mouse B cells.27 In addition, IL-21 can inhibit IL-4 regulation of antibody isotype switching, particulary with respect to IgE production,25 while immunizing IL-21R–/– animals stimulated a 20–50-fold increase in antigen-specific IgE titres relative to normal mice.26 Evidence linking this phenotype to a defect in B-cell function, rather than to a failure in T-cell help, includes the observations that T-helper cytokine responses appeared normal in IL-21R–/– mice, that IL-21R–/– CD4+ T cells were normal with respect to Th1 and Th2 differentiation, and that IgG1 and IgE production was normal in IL-21R–/– B cells cultured with IL-4 but not with IL-21.26 Additionally, Suto et al.25 established that IL-21 abrogates LPS + IL-4 induction of IgE synthesis in cultured B cells and that the inhibition of isotype switching by IL-21 occurred at the level of Cε transcription. Interestingly, IL-21 administration in a mouse asthma model effectively reduced titres of antigen-specific IgE and IgG1 antibodies, as well as symptoms of airway hypersensitivity including eosinophil recruitment. This has suggested to some that IL-21 therapy may benefit IgE-dependent atopic disease.
Il-21 regulates cell-mediated immunity and the clearance of tumours
In the course of a cell-mediated immune response CD4+ T-helper cells stimulate the differentiation of effector CD8+ T cells and NK cells, both of which readily respond to IL-21 in vitro. For instance, the stimulation of CD8+ T-cell proliferation by IL-21 (see above) is accompanied by the induction of various cytokines such as IFN-γ and tumour necrosis factor-α, synthesis of perforin and granzyme B, and acquisition of cytolytic activity (ref. 17 and unpublished results). In addition, combination of IL-21 with IL-2 or IL-15 significantly enhanced cytokine production and cytotoxic T-lymphocyte activity in alloantigen-stimulated mouse lymph node cells and ovalbumin-primed transgenic OT-I cells. Thus, IL-21 can co-operate with additional cytokines in generating potent killer T cells. IL-21 also induces a central memory phenotype in antigen-treated human γδ T cells.18 Further studies are needed to address the type of memory CD8+ T cells stimulated by IL-21 and how these cells compare to those generated with IL-15.
IL-21 was initially identified for its ability to stimulate the maturation of human CD56+ CD16+ NK cells from bone marrow-derived progenitor cells.5 Subsequent experiments have established that IL-21 is a potent regulator of NK-cell terminal differentiation. Freshly isolated human NK cells express IL-21R5 and rapidly respond to IL-21 as measured by STAT phosphorylation, IFN-γ production and increased cytolytic activity (ref. 5 and unpublished result). While IL-21 has no apparent effect on freshly isolated mouse NK cells, it enhances differentiation significantly following costimulation with polyI:C or cytokines like IL-2, IL-15, or IL-18.12,17,28 Included in this response is a dramatic change in cell morphology and surface marker expression, increased cytokine expression, and enhanced cytolytic activity.12,17,28,29
Induction by IL-21 of CD8+ T-cell and NK-cell differentiation argues that it could be used to augment cell-mediated immune responses in mice. This concept was tested and confirmed in several mouse tumour models that used various methods to deliver IL-21. Transfection of tumour cells with IL-21 expression plasmids established that IL-21 prevents the growth of Colon 26 colon carcinoma and AsPC-1 pancreatic carcinoma,30,31 B16F1 melanoma and MathA fibrosarcoma,32 Rlmale1 lymphoma33 and TS/A adenocarcinoma.34 In addition, hydrodynamics-based gene delivery to mouse liver established the anti-tumour activity of IL-21 against MCA205 fibrosarcoma and B16 melanoma35 as well as against renal carcinoma and DA3 mammary carcinoma.28 The effector cell types mediating this anti-tumour activity included CD8+ T cells and NK cells, although their relative contribution varied between experiments. The mechanism by which these cells cleared tumour included a requirement for perforin and increased cytolytic activity28,32 and in one case, expression of IFNγ-dependent CXC chemokines.34 In another experiment, Moroz et al.20 compared the relative anti-tumour activity of IL-2, IL-15 and IL-21 in mice implanted with syngeneic E.G7 thymoma tumour cells. Durable cures were achieved in ∼30% of mice injected with IL-21 protein, whereas no mice survived following treatment with IL-2 or IL-15. The anti-tumour activity of IL-21 correlated with the accumulation of tumour-specific CD8+ T cells that possessed increased cytolytic activity and that persisted in lymphoid tissues for several weeks. Elimination of these cells abrogated mouse survival and abolished the induction of memory by IL-21 as measured by the ability to reject subsequent tumour challenge. Collectively, these experiments show that IL-21 can be used to alter cell-mediated anti-tumour responses.
Inhibitory activities of il-21
While IL-21 is immunostimulatory in many respects, it also has inhibitory activities, suggesting that it may down-regulate elements of an immune response. For instance, IL-21 antagonizes IL-4 function in vitro and in vivo,5,25,26 inhibits delayed-type hypersensitivity in mice,13 prevents IFN-γ expression,13 and stimulates robust expression of IL-10 in CD4+ T cells, CD8+ T cells and NK cells (ref. 28 and unpublished data). IL-21 can also inhibit proliferation and survival of B cells stimulated with LPS or anti-IgM antibodies,5,27 prevent IL-15 induced proliferation of memory CD8+ T cells17 and significantly decrease the proliferation and survival of NK cells cultured with IL-2 or IL-15.17,28 Another intriguing finding demonstrated that IL-21 could down-regulate major histocompatibilty complex class II expression on monocyte-derived dendritic cells, reduce their priming of CD4 and CD8 T-cell proliferation, and stimulate antigen uptake (ref. 36 and unpublished result). Transfer of these cells failed to induce antigen-specific contact hypersensitivity. In addition, IL-21 blocked the LPS-induced maturation of dendritic cells in vitro.37 These results suggest that IL-21 may keep dendritic cells in an immature state as measured by increased antigen uptake and diminished antigen presentation.
The immunoregulatory activities of T cells and the cytokines they produce, such as IL-2, IL-4, IL-10, IFN-γ and transforming growth factor-β, co-ordinate the initiation and cessation of an immune response. Their activities guarantee host protection from the environment by achieving the appropriate balance between humoral and cell-mediated effector pathways, but they also provide negative feedback control to maintain self-tolerance. The ability of IL-21 to both enhance and inhibit cellular function is consistent with the regulatory activities of the other members of the IL-2 family. Further investigation is needed to fully elucidate the significance of its immunoregulatory activity. However, despite its effects there is no indication that IL-21 is strictly required for the establishment and maintenance of self-tolerance since IL-21–/– and IL-21R–/– animals appear healthy and fail to acquire spontaneous inflammatory diseases similar to IL-2–/– or IL-10–/– mice (ref. 17,26 and unpublished results).
Conclusions
IL-21 is a newly described cytokine that has potent immunoregulatory activity. Genetic and biochemical analyses establish its membership to the IL-2 family of cytokines, its utilization of a receptor complex containing the γc-receptor subunit, and its activation of the JAK/STAT signalling cascade. As a CD4+ T-helper cytokine, IL-21 modulates the proliferation and differentiation of T cells, B cells, NK cells, and dendritic cells. It regulates normal humoral immunity by stimulating IgG antibody responses and antagonizing IgE production. IL-21 is also a potent regulator of cell-mediated immunity and directs cytotoxic T lymphocytes and NK cell effector activity in the clearance of tumours from mice. Collectively, IL-21 controls the strength and duration of an adaptive immune response and its use in a clinical setting may prove efficacious for the treatment of cancer and infectious disease.
References
- 1.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 2.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory and effector memory CD4+ T cells. Pathol Biol (Paris) 2003;51:64–6. doi: 10.1016/s0369-8114(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 3.Di Santo JP, Kuhn R, Muller W. Common cytokine receptor gamma chain (gamma c) -dependent cytokines: understanding in vivo functions by gene targeting. Immunol Rev. 1995;148:19–34. doi: 10.1111/j.1600-065x.1995.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelly J, Leonard WJ. Immune deficiencies due to defects in cytokine signaling. Curr Allergy Asthma Rep. 2003;3:396–401. doi: 10.1007/s11882-003-0073-y. [DOI] [PubMed] [Google Scholar]
- 5.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 6.Foster D, Parrish-Novak J, Fox B, Xu W. Cytokine-receptor pairing: accelerating discovery of cytokine function. Nat Rev Drug Discov. 2004;3:160–70. doi: 10.1038/nrd1305. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M, Narazaki M, Hibi M, Yawata H, Yasukawa K, Hamaguchi M, Taga T, Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci USA. 1991;88:11349–53. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurney AL, Wong SC, Henzel WJ, de Sauvage FJ. Distinct regions of c-Mpl cytoplasmic domain are coupled to the JAK-STAT signal transduction pathway and Shc phosphorylation. Proc Natl Acad Sci USA. 1995;92:5292–6. doi: 10.1073/pnas.92.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–31. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 11.Bennett F, Luxenberg D, Ling V, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–18. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 12.Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–9. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 13.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–77. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topp MS, Riddell SR, Akatsuka Y, Jensen MC, Blattman JN, Greenberg PD. Restoration of CD28 expression in CD28– CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med. 2003;198:947–55. doi: 10.1084/jem.20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:3810–15. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4(Suppl. 3):S161–7. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–69. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 18.Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human gamma-delta T cells towards distinct memory phenotypes. Cell Immunol. 2002;218:1–6. doi: 10.1016/s0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 19.Van Leeuwen EM, Gamadia LE, Baars PA, Remmerswaal EB, Ten Berge IJ, Van Lier RA. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8(+) T cells. J Immunol. 2002;169:5838–43. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 20.Moroz A, Eppolito C, Nelson A, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to acheive durable tumor immunity: Comparative evaluation of IL-2,IL-15 and IL-21. J Immunol. 2004. in press. [DOI] [PubMed]
- 21.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–5. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 22.Durie FH, Foy TM, Masters SR, Laman JD, Noelle RJ. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994;15:406–11. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 23.Jumper MD, Nishioka Y, Davis LS, Lipsky PE, Meek K. Regulation of human B cell function by recombinant CD40 ligand and other TNF-related ligands. J Immunol. 1995;155:2369–78. [PubMed] [Google Scholar]
- 24.Murray R. Physiologic roles of interleukin-2, interleukin-4, and interleukin-7. Curr Opin Hematol. 1996;3:230–4. doi: 10.1097/00062752-199603030-00011. [DOI] [PubMed] [Google Scholar]
- 25.Suto A, Nakajima H, Hirose K, et al. Interleukin-21 prevents antigen-induced IgE production by inhibiting germline Cɛ transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–73. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki KR, Spolski CG, Feng C-F, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 27.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–18. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 28.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–58. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 29.Sivori S, Cantoni C, Parolini S, Marcenaro E, Conte R, Moretta L, Moretta A. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol. 2003;33:3439–47. doi: 10.1002/eji.200324533. [DOI] [PubMed] [Google Scholar]
- 30.Ugai S, Shimozato O, Kawamura K, Wang YQ, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther. 2003;10:187–92. doi: 10.1038/sj.cgt.7700552. [DOI] [PubMed] [Google Scholar]
- 31.Ugai S, Shimozato O, Wang L, et al. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and – independent antitumor effects. Cancer Gene Ther. 2003;10:771–8. doi: 10.1038/sj.cgt.7700630. [DOI] [PubMed] [Google Scholar]
- 32.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–15. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 33.Kishida T, Asada H, Itokawa Y, et al. Interleukin (IL) -21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther. 2003;8:552–8. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- 34.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–7. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Tschoi M, Spolski R, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–22. [PubMed] [Google Scholar]
- 36.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–8. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 37.Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Ruckert R. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol. 2003;121:1379–82. doi: 10.1046/j.1523-1747.2003.12603.x. [DOI] [PubMed] [Google Scholar]