Abstract
Tight regulation of the phosphatidylinositiol 3-kinase (PI3K) pathway is essential not only for normal immune system development and responsiveness, but also in the prevention of immunopathology. Indeed, unchecked activation of the PI3K pathway in T cells induces lymphoproliferation and systemic autoimmunity. Evaluating the importance of threshold levels of two key PI3K pathway phosphoinositol phosphatases, we previously reported that mice heterozygous for both Pten and SHIP develop a more rapid progression of a lymphoproliferative autoimmune syndrome than do Pten+\− mice. Investigating the basis for this difference, we now describe a quantitative and qualitative difference in the antibody responses of C57BL\6 Pten+\− SHIP+\− mice upon challenge with a T-dependent antigen. Suspecting that this phenotypic difference might be the result, at least in part, of a T-helper cell defect, an in vitro analysis of anti-CD3/interleukin (IL)-2-expanded CD4+ T cells was performed. After stimulation with anti-CD3, cells from mice heterozygous for both Pten and SHIP exhibited a striking increase in IL-4 secretion (> 10-fold), without a corresponding increase in T helper 2 (Th2) cell numbers being evident by intracellular staining for this cytokine. Modest increases were also seen for both IL-13 and IFN-γ. Perhaps in keeping with this abnormal in vitro cytokine profile, IgG1 serum levels were significantly elevated in young C57BL\6 Pten+\− SHIP+\− mice. Thus, the relative levels of Pten and SHIP appear to be key variables in CD4+ T-cell function, primarily via their ability to regulate IL-4 production.
Keywords: autoimmunity, cytokines, lipid mediators, T lymphocytes
Introduction
Tolerance induction is determined, in part, by intrinsic lymphocyte reactivity, which, in turn, is regulated by the balance of positive and negative signals emanating from lymphocyte antigen receptors.1 While the causes of autoimmunity are complex, involving a variety of pathogenic mechanisms and cell types, dysregulation of T-cell activation, differentiation and survival often plays a role in the genesis of autoimmunity (reviewed in ref. 2). T cells are inherently and necessarily autoreactive. They depend on the strength of extracellular signals, including differences in antigen avidity and affinity, to control not only intrathymic selection, but also the outcomes of responses to antigenic stimuli encountered in the periphery. It follows, then, that perturbations in the intracellular molecules which transduce and regulate the strength or duration of antigenic signals can potentially lead to immunosuppression and/or autoimmunity;2 for example, dysregulation of phosphatidylinositol-3 kinase (PI3K) signalling in murine T cells has been shown to favour the emergence of autoimmune phenotypes.3–5
The PI3K pathway has emerged as one of the key elements determining both T-cell reactivity and predisposition to autoimmunity. PI3K, activated by many different types of receptors in addition to those for antigens, catalyses the phosphorylation of phosphoinositol species in the plasma membrane. These second messengers induce the translocation of a variety of pleckstrin homology (PH) domain-containing molecules, leading to the activation of functionally diverse intracellular signalling pathways (reviewed in ref. 6). As evidence of its importance, deficiencies in PI3K signalling in cells of the immune system leads to immunodeficiency,7,8 while over-activity of PI3K can trigger a severe lymphoproliferative, autoimmune phenotype.3
The importance of the PI3K pathway in normal cell function has also been clearly demonstrated by studies in mice with disruptions of genes encoding critical negative regulators of this pathway, including the tumour suppressor gene, phosphatase with tensin homology (Pten), and the SH2-containing inositol phosphatase (SHIP).9–13 Pten, a ubiquitously expressed 3′ inositol phosphatase, acts to directly oppose PI3K by converting the product of the latter, PI3,4,5P3 (PIP3), into PI4,5P2·14 SHIP, restricted for the most part to the haematopoietic lineage, contains a 5′ phosphoinositol phosphatase domain that acts on the same substrate as Pten, to generate the putative second messenger, PI3,4P2·15 Together, these enzymes share the ability to negatively regulate pathways dependent on PIP3. However, they potentially differ in their functions with respect to pathways downstream of proteins having PH domains with specificity for PI3,4P2. SHIP–/– mice survive for several weeks or months after birth, but eventually succumb to a myeloproliferative syndrome, the main pathological features of which are splenomegaly and severe lung infiltration by macrophages and neutrophils.12,13SHIP–/– mice also show abnormal B-cell development, accompanied by an increased reactivity of their peripheral cells.16,17SHIP+/– mice appear phenotypically normal. Probably owing to the resistance of Pten–/– cells to pro-apoptotic stimuli, mice lacking Pten undergo embryonic lethality.9–11 Unlike SHIP heterozygotes, mice with disruption of a single Pten allele develop a progressive lymphoproliferative, autoimmune phenotype that becomes clinically overt by ≈ 6–9 months of age.18 The development of immunopathology in the latter was of particular interest, as it revealed a consequence of Pten haploinsufficiency and also hinted at a critical role for Pten levels in the regulation of threshold-dependent phenomena in the murine immune system. Further support for the notion that phosphoinositol phosphatase levels are important to immune system function was provided by our finding of an exacerbation of immunopathology when SHIP heterozygosity was superimposed onto a Pten+/– background.19
In view of the important role played by T cells in the pathogenesis of autoimmunity, and the importance of the PI3K pathway in this cell type, we extended our analysis of Pten+/– SHIP+/– mice. Specifically, we were searching for evidence of immune response abnormalities that might implicate CD4+ T-cell dysfunction in the process that leads to the accelerated development of lymphoproliferation, hypergammaglobulinaemia and autoimmunity in this compound heterozygote.
Materials and methods
Mice
Pten+/– SHIP+/– mice, on a C57BL/6 background (n=7–8), were generated as previously described19 and were pathogenic virus antibody-free. All experiments on mice were performed in a barrier facility, in accordance with Canadian Council for Animal Care guidelines and a protocol approved by the University of Calgary Animal Care Committee.
Splenic CD4+ T-cell isolation
CD4+ T cells were positively selected using the Miltenyi Biotech magnetic separation system, as directed by the manufacturer (Miltenyi Biotech, Auburn, CA). Cells were plated at a density of 3–3·5 × 106 cells/ml in AIMV synthetic media (Gibco-BRL, Burlington, ON), with 3–6% interleukin (IL)-2 conditioned media, in 24-well plates precoated overnight with 0·5 µg/ml anti-CD3 (2C11; BD Pharmingen, San Jose, CA). After 48 hr of culture, cells were washed and plated in the absence of CD3, but in the presence of IL-2, for 5 days, with a 1 : 2 feeding of fresh media + IL-2 approximately every 2 days. Cells were used on day 8 after isolation.
Immunoblotting
Expanded CD4+ T cells from littermate Pten+/–, SHIP+/–, Pten+/– SHIP+/– and wild-type mice, on postisolation day 8, were enumerated and resuspended in fresh AIMV media. Then, 1 × 107 cells were lysed in phosphorylation solubilization buffer (consisting of 50 mm HEPES, 100 mm NaF, 10 mm Na4P2O7, 2 mm Na3VO4, 2 mm EDTA, 2 mm NaMoO4, 1% Triton-X-100) in the presence of a protease inhibitor cocktail (Roche Diagnostics, Laval, QC). Protein concentrations were determined by using the Bradford method-based assay (Bio-Rad, Mississauga, ON); 80 µg of total protein was separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to poly(vinylidine difluoride) (PVDF) membranes by electroblotting using a semidry transfer method. Filters were blocked, then probed overnight at 4° with either anti-Pten (Cell Signalling, Beverly, MA) or anti-SHIP (sc-8425; Santa Cruz, Santa Cruz, CA) and subsequently incubated with HRP-conjugated secondary antibody (Dako, Mississauga, ON). Proteins were detected by chemiluminescence (Amersham, San Francisco, CA), using a Fluor-S Max Multi Imager equipped with quantity one™ software (Bio-Rad Laboratories).
Immunizations
Six to seven female mice of each genotype, between the ages of 8–10 weeks, were selected for this study. Blood samples were taken on day 0, before immunization, by saphenous puncture. Mice were injected intraperitoneally with 20 µg of nitrophenylacetyl (NP)-Ficoll or 10 µg of NP-keyhole limpet haemocyanin (KLH) (Biosearch Technologies Inc., Novato, CA). Mice injected with NP-Ficoll were boosted with an additional dose of 20 µg of NP-Ficoll on day 7. A final blood sample was obtained from the mice on day 17, by vena cava puncture, immediately after killing. Mice injected with NP-KLH were boosted with an additional 10 µg of NP-KLH on day 10. A final blood sample was taken from the mice on day 21.
Enzyme-linked immunosorbent assays (ELISAs) for serum immunoglobulins, NP-specific antibody production and cytokines
ELISA quantification of serum immunoglobulin levels was performed, as previously described,19 using capture and biotinylated detection antibodies for immunoglobulin G1 (IgG1), immunoglobulin G2b (IgG2b) and immunoglobulin M (IgM) (BD Pharmingen). Concentrations were determined from curves generated from mouse immunoglobulin standards (BD Pharmingen). Cytokine ELISA on cell supernatants were performed using capture and detection antibodies and recombinant standards for IL-4, interferon-γ (IFN-γ) (both BD Pharmingen) and IL-13 (R & D Systems, Minneapolis, MN). For NP-specific antibody detection, 96-well plates were coated with 1 µg/ml NP-bovine serum albumin (BSA) (Biosearch Technologies Inc.) in phosphate-buffered saline (PBS) overnight at 4°. Serial dilutions of sera, performed in PBS + 10% fetal calf serum (FCS), were plated in duplicate. Dilutions ranged from 1 : 100–1 : 1000 for day 0 samples, to 1 : 20 000–1 : 2 560 000 for day 21 samples. Plates were washed and then incubated with 2 µg/ml biotin-conjugated secondary anti-IgM (BD Pharmingen), or with a 1 : 10 000 dilution of pan-anti-IgG (Jackson Immunoresearch, Westgrove, PA). Plates were developed with 3,3′,5,5′ tetramethyl benzidine (TMB) substrate, the reaction was stopped with 2 m H2SO4 and then the plates were read at 450 nm using a Multiscan ELISA reader and Multiscan software. Each plate contained blank wells, and the NP titres were defined as the dilution that gave an absorbance at 450 nm which was twice that of the background of the plate.
RNAse protection assay
Positively selected and expanded CD4+ T cells were plated in fresh AIMV media at a concentration of 1 × 107 cells/ml, 0·5 ml/well in a 24-well plate. Cells were either left unstimulated or were stimulated from 2 to 8 hr with 10 µg/ml soluble anti-CD3. At various time-points, cells were harvested and the supernatants reserved for ELISA and stored at −20°. RNA was extracted using TRIZOL, according to the manufacturer's instructions (Ambion, Austin, TX). Approximately 2 µg of RNA was utilized in the RNAse protection assay kit (Kit mCk-1; BD Pharmingen), according to the manufacturer's instructions and using P-33 as a radiolabel. Developed film was analysed by densitometry with the Fluor-S-Max system (Biorad) using Quantity One™ software. A loading control was achieved by normalizing to the L32 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) internal control bands, and values were expressed as fold-increase over wild-type levels at each time-point on any given day.
Intracellular cytokine staining and apoptosis assay
For assessments of cell viability and apoptosis, expanded T cells, after 12 hr of stimulation, with or without soluble anti-CD3, were washed in ice-cold PBS and stained with 0·5 µg of propidium iodide and 5 µl of Annexin V (BD Pharmingen) for 15 min in the dark. Ungated samples were collected on a FACSCalibur (BD Pharmingen) and analysed using the flowjo program (TreeSstar, Ashland, OR). For intracellular staining, expanded T cells were stimulated with 10 µg/ml soluble anti-CD3 for 5 hr in the presence of Golgi Stop (BD Pharmingen). Intracellular staining was performed using the BD Pharmingen kit, according to the manufacturer's instructions, and permeabilized cells were stained with phycoerythrin (PE)-labelled anti-IL-4 or fluorescein isothiocyanate (FITC)-labelled anti-IFN-γ (BD Pharmingen). In each experiment, 10 000 ‘live events’, based on the forward- and side-scatter pattern, were collected for analysis.
Results
Pten+\− SHIP+\− mice demonstrate a significantly increased T-dependent antigenic response
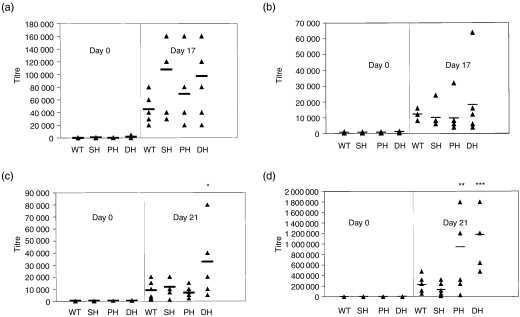
Our previous studies demonstrated the spontaneous elevation of serum immunoglobulin levels in older Pten+/–SHIP+/– mice with lymphadenopathy.19 Searching for evidence of an intrinsic abnormality that might have a bearing on the eventual development of hypergammaglobulinaemia, we therefore assessed humoral responses of young mice in response to defined antigenic challenges. Day 0 baseline titres for both KLH- and Ficoll-immunized groups showed no significant reactivity against NP-BSA in serum samples from any of the groups of preimmunized mice (Fig. 1a−1d). Titres of NP-specific antibodies in the serum of mice immunized and boosted with NP-Ficoll showed no significant differences in IgM or IgG responses between the genotypes (Fig. 1a,1b). In contrast, a comparison of the different groups immunized with the T-dependent antigen, NP-KLH, revealed a marginal yet statistically significant increase in the IgM responses of Pten+/– SHIP+/– mice over the other genotypes (P < 0·05, Fig. 1c). Pten+/– SHIP+/– IgG responses to NP-KLH were also significantly elevated over those of wild-type and SHIP+/– mice (P < 0·005), but these elevations were not significant when compared to Pten+/– samples. The latter were statistically increased over those from wild-type and SHIP+/– mice (P < 0·05) (Fig. 1d). These results demonstrate that Pten+/–, and Pten+/–SHIP+/– mice, in particular, are able to mount more vigorous humoral immune responses after immunization with a T-dependent antigen.
Figure 1.
Pten+/– SHIP+/– (phosphatase with tensin homology/SH2-containing inositol phosphatase) mice show an increased humoral response to the T-dependent antigen, nitrophenylacetyl (NP)-keyhole limpet haemocyanin (NP-KLH). Serum antibodies against NP-bovine serum albumin (NP-BSA) were assessed from six or seven mice of each genotype that had been challenged with NP-Ficoll or NP-KLH. Anti-NP titres [immunoglobulin M (IgM) and pan immunoglobulin G (IgG)] were determined by enzyme-linked immunosorbent assay (ELISA) on serially diluted serum samples. Bars indicate the average titre, and triangles represent individual mice. No differences were observed in either the IgM or IgG response to NP-Ficoll (a, b). However, statistically significant differences in IgM and IgG responses to NP-KLH were detected (c, d). *P < 0·05 compared with all genotypes, ***P < 0·005 compared with wild type and SHIP+/–, but not significant over Pten+/–. **P < 0·05 compared with wild type and SHIP+/–. WT, wild type; SH, SHIP+/–; PH, Pten+/–; DH, Pten+/– SHIP+/–.
Pten and SHIP protein levels are reduced in T cells from heterozygous and doubly heterozygous mice
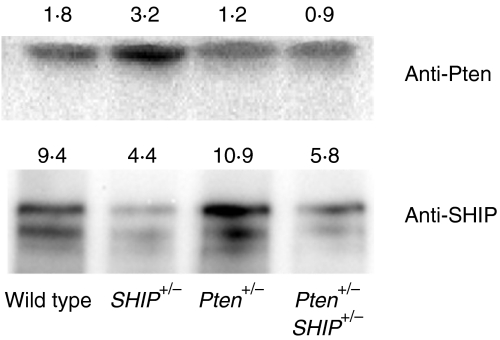
Although both Pten and SHIP are expressed in T cells,20,21 a reduction in the level of these proteins in cells from heterozygous mice has not been demonstrated. While the loss of one co-dominant allele can lead to a reduction of ≈ 50% in the level of the encoded protein, the possibility of allelic compensation remains. Thus, before carrying out a phenotypic analysis of in vitro-expanded CD4+ T-cell populations, it was important to assess Pten and SHIP protein levels in lysates prepared from these cells. Figure 2 demonstrates reduced levels of Pten and SHIP in lysates prepared from Pten+/– and Pten+/– SHIP+/– cells, and SHIP+/– and Pten+/– SHIP+/– cells, respectively. Furthermore, no change in the levels of Pten and SHIP, either before or after stimulation with anti-CD3, was observed as a result of activation of the T-cell receptor (anti-TCR) (data not shown).
Figure 2.
Reduced levels of Pten (phosphatase with tensin homology) and SHIP (SH2-containing inositol phosphatase) protein in Pten+/– SHIP+/– T cells. Whole-cell lysates from expanded CD4+ T cells of the different genotypes were examined for Pten and SHIP levels by immunoblotting. Numbers represent densitometric values obtained for Pten and SHIP protein bands from independent immunoblots, and the results are representative of lysates from two independent sets of littermate control mice.
Pten+\− SHIP+\− T cells respond to stimulation with anti-CD3 by producing greater amounts of IL-4, IL-13 and IFN-γ, at both RNA and protein levels
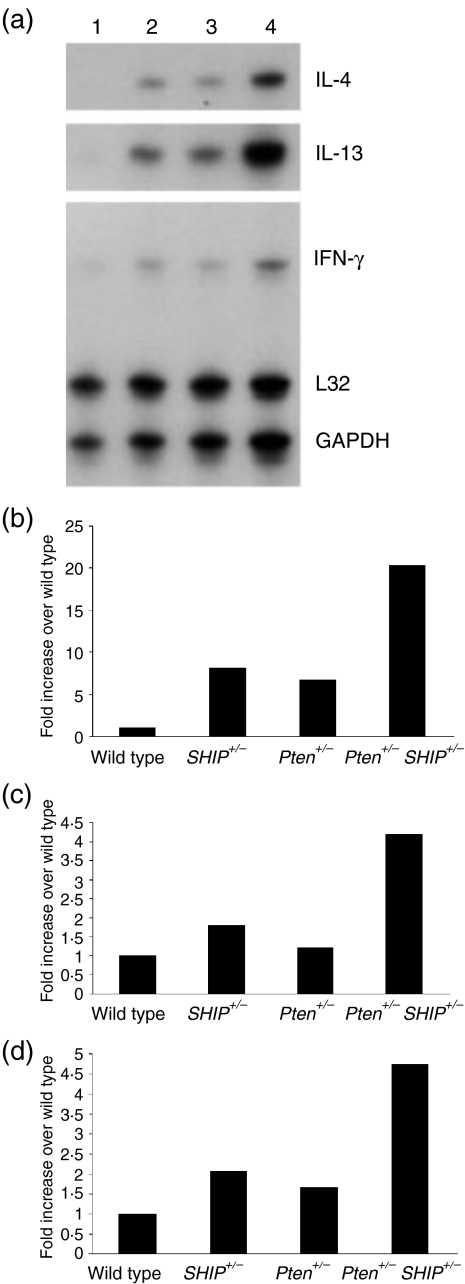
The augmented humoral response to a T-dependent antigenic challenge suggested that T-helper cell function might be abnormal in Pten+/– SHIP+/– mice. We thus hypothesized that CD4+ T-cell cytokine production might be altered as a result of compound heterozygosity for Pten and SHIP. Using an RNAse protection assay template that allowed quantification of nine individual cytokines (IL-4, IL-5, IL-13, IL-10, IL-2, IL-6, IL-15, IL-9 and IFN-γ), we examined the pattern of cytokines produced by expanded T-cell populations following stimulation with anti-CD3. In three independent experiments we observed increases in IL-4, IL-13 and IFN-γ transcripts in RNA samples from Pten+/– SHIP+/– cells, as compared to cells from the other genotypes (Fig. 3). The data shown represent typical results obtained 8 hr poststimulation, and the relative increases were calculated versus wild-type cytokine RNA levels. While there was some variation in the time-point at which cytokines reached their maximum level after stimulation (data not shown), increases were most often observed at the 8-hr time-point. No consistent differences in the levels of transcripts of any of the other cytokines were observed.
Figure 3.
Increased levels of transcripts for specific cytokines in Pten+/– SHIP+/– (phosphatase with tensin homology/SH2-containing inositol phosphatase) T cells. Expanded CD4+ T cells were stimulated with 10 µg/ml soluble anti-CD3, and RNA levels were analysed by using the RNAse protection assay (a) (lane 1, wild type; lane 2, SHIP+/–; lane 3, Pten+/–; lane 4, Pten+/– SHIP+/–). Quantification was carried out by densitometry using quantity one™ software, with values normalized using endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts as the internal control. Pten+/– SHIP+/– T cells show increases in (b) interleukin-13 (IL-13) (c) interleukin-4 (IL-4) and (d) interferon-γ (IFN-γ) transcripts after 8 hr of stimulation. These results were from one representative experiment of three conducted on T cells isolated from individual mice of each genotype.
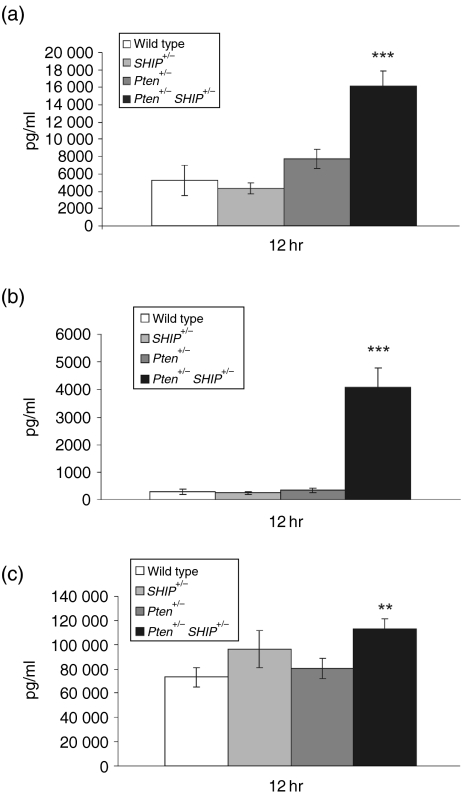
To determine whether increases in transcripts were reflected at the protein level, cytokine production in the cell cultures was quantified by ELISA. Analysis of supernatants from four independent experiments revealed significant increases in the amounts of IL-4 and IL-13 protein in the supernatant of Pten+/– SHIP+/– cells (Fig. 4a,4b, P <0·001 over all genotypes). These increases, relative to wild-type levels, were approximately 14-fold and threefold, for IL-4 and IL-13 protein secretion, respectively. Thus, the increase in IL-13 over IL-4, seen in the RNAse protection studies, was not reflected by the increased translation or secretion of this cytokine. We also detected a 1·5-fold increase in IFN-γ secretion in Pten+/– SHIP+/– supernatants compared to those of wild-type cells, and this augmented production was significant compared to wild-type and Pten+/– supernatants (Fig. 4c, P < 0·05, not significant over SHIP+/–). These results demonstrated, at both the transcript and protein levels, that cultures of Pten+/– SHIP+/– cells produced greater quantities of three key T-helper cell cytokines following stimulation with anti-CD3, with the increase in IL-4 protein production being particularly impressive.
Figure 4.
Increased cytokine secretion by Pten+/– SHIP+/– (phosphatase with tensin homology/SH2-containing inositol phosphatase) T cells. Expanded CD4+ T cells were stimulated with 10 µg/ml soluble anti-CD3, and cytokine protein levels were measured by enzyme-linked immunosorbent assay (ELISA). Pten+/– SHIP+/– T-cell cultures contained significantly increased concentrations of (a) interleukin-13 (IL-13) and (b) interleukin-4 (IL-4) at 12 hr poststimulation. (c) Levels of interferon-γ (IFN-γ) were only modestly increased. The data are the combined results of four or five independent experiments performed on cells from individual mice, with each data point assessed in duplicate. Error bars represent the standard error of the mean (SEM) from all readings. ***P < 0·001 compared with all genotypes; **P < 0·05 compared with Pten+/– and wild type only.
Increased cytokine production was not attributable to the increased survival or altered percentages of polarized T cells
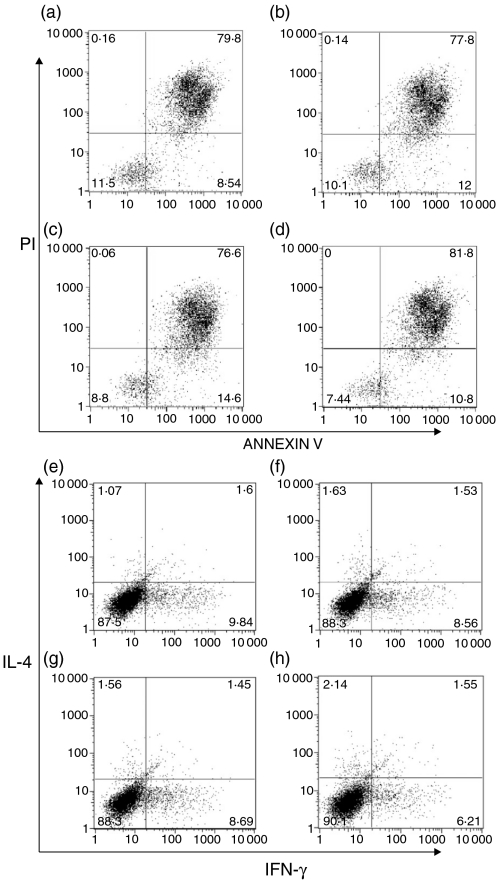
The ability of PI3K pathway activity to increase cellular survival has been well characterized, primarily as a function of Akt activation (reviewed in ref. 6). Having previously demonstrated modest increases in Akt phosphorylation in T cells from Pten+/– SHIP+/– mice,19 it was considered possible that increased survival of cells might be a factor contributing to the differences in cytokine concentrations in the supernatants. To investigate this, we examined the viability of expanded T cells from each of the different genotypes, both with and without anti-CD3 stimulation, using Annexin V/propidium iodide (PI) staining and flow cytometry. We found that cell viability did not differ between the genotypes, either with no stimulation (results not shown) or after 12 h of stimulation (Fig. 5a−5d). Thus, it was not possible to attribute increased cytokine production to an increased survival of Pten+/– SHIP+/– cells.
Figure 5.
Increased cytokine production by Pten+/– SHIP+/– (phosphatase with tensin homology/SH2-containing inositol phosphatase) cells was not attributable to differences in survival or spontaneous polarization of T-cell populations. AnnexinV/propidium iodide (PI) staining, followed by fluorescence-activated cell sorter (FACS) analysis, revealed no difference in survival between genotypes after stimulation with anti-CD3: (a) wild type; (b) SHIP+/–; (c) Pten+/–; and (d) Pten+/– SHIP+/–. Intracellular staining for interleukin-4 (IL-4) and interferon-γ (IFN-γ) was performed on CD4+ T cells stimulated with anti-CD3: (e) wild type; (f) SHIP+/–; (g) Pten+/–; and (h) Pten+/– SHIP+/–. The percentages of cells producing IL-4 and IFN-γ did not differ significantly between genotypes, and the Pten+/– SHIP+/– cells did not appear to be polarized towards a greater proportion of T helper 2 (Th2) cells, implying increased cytokine production on a per-cell basis. The results shown represent one experiment of five independent experiments carried out.
Given the elevation in levels of IL-4 and IL-13, it was important to establish whether a genotype-dependent increase in spontaneous polarization towards a Th2 phenotype was occurring. Figure 5(e)−5(h) illustrates results typical of at five independent experiments using an intracellular staining method to detect IL-4 and IFN-γ. There was no significant difference in the percentage of IL-4- or IFN-γ-producing cells between the different genotypes. Thus, the increased production of cytokines by Pten+/– SHIP+/– cells could not be accounted for by an abnormality of T-cell polarization. Instead, it argued for an increase in cytokine production by cells of this genotype on a ‘per cell’ basis.
Increased levels of IgG1 in young Pten+\− SHIP+\− mice
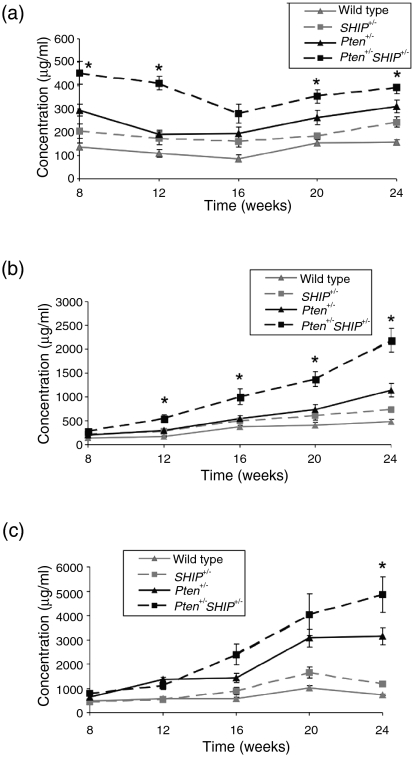
Given the ability of purified Pten+/– SHIP+/– T cells to generate increased levels of IL-4 and IL-13 in response to stimulation with the TCR, we sought evidence for expression of this phenotype in vivo. IL-4 is involved in class switching to IgG1 and immunoglobulin E (IgE),22 and IL-13 might play a role in IgE switching in mice,23 while IFN-γ induces IgG2a. Using ELISA we examined immunoglobulin isotypes in the serum of 8-week-old mice, an age well in advance of any evidence of lymphoproliferative disease. At this time-point, the levels of IgG1 in Pten+/–SHIP+/– mice were significantly elevated compared to all the other genotypes (Fig. 6a). Increases in IgE, which were significant versus SHIP+/– and wild-type mice, were also seen, although these were not significant when compared to Pten+/– samples (data not shown). Serum IgG2a levels were variable within each group and did not show any significant differences between the genotypes (data not shown). Interestingly, regular monitoring of isotype-specific serum immunoglobulin levels in individual mice up to 24 weeks of age revealed that IgG1 did not increase significantly over time. In contrast, other isotypes, such as IgM and IgG2b, continued to increase over time (Fig. 6a−6c). The pattern of spontaneous immunoglobulin isotypes in young mice was in keeping with the ability of anti-CD3-stimulated Pten+/– SHIP+/– T cells to produce greater amounts of IL-4 and IL-13 in vitro.
Figure 6.
Increased levels of serum immunoglobulin G1 (IgG1) in Pten+/– SHIP+/– (phosphatase with tensin homology/SH2-containing inositol phosphatase) mice. (a) Spontaneous increases in circulating serum IgG1 levels were detected in the sera of 8-week-old Pten+/– SHIP+/– mice, as compared to the other genotypes, and this pattern remained steady and elevated over time. (b) Serum levels of circulating immunoglobulin M (IgM) were not significantly increased over all genotypes at the 8-week point, but steadily increased, becoming significant at later time-points. (c) Levels of circulating immunoglobulin G2b (IgG2b) did not achieve statistical significance compared to all genotypes until the 24-week time-point. Values were representative of sera from at least nine animals per genotype, assessed in duplicate. Error bars represent the standard error of the mean (SEM) of the duplicate readings. *P < 0·05 compared with all genotypes.
Discussion
The finding that Pten heterozygosity augmented the IgG response to the T-dependent antigen, NP-KLH, is novel, and this same property may play a role in the spontaneous age-dependent hypergammaglobulinaemia that occurs in these mice.18 The increased IgG and IgM responses of Pten+/–SHIP+/– mice to this same antigen is suggestive of an even greater level of responsiveness, and may be in keeping with the spontaneous increases in IgM and various IgG isotypes that we observed previously in this compound heterozygote.19 The responses of Pten+/–, and especially Pten+/–SHIP+/–, mice towards the NP-KLH, but not the T-independent antigen, could be interpreted as implying that reduced levels of inositol phosphatases exert their impact at the level of T- and B-cell interactions. There is already considerable evidence showing that dysregulation of the PI3K pathway in T cells alone can induce autoimmunity in vivo. For instance, expression of a constitutively active mutant PI3K under the control of a T-cell-specific gene promoter resulted in lymphadenopathy and autoimmune glomerulonephritis in transgenic mice.3 Similarly, expression of a constitutively active protein kinase B (PKB) mutant under the control of the CD2 promoter in transgenic mice resulted in lymphadenopathy and autoantibody production.5 Lastly, in a T-cell-specific conditional knockout of Pten, autoreactivity and increased cytokine production by CD4+ cells was associated with the development of autoantibodies and hypergammaglobulinaemia.4 One caveat of the former study was that it was carried out in the context of B cells that were heterozygous for Pten, and for this reason the potential contribution of B-cell dysfunction to this process could not be excluded. Interestingly, and probably owing to altered B-cell developmental fate determination, conditional deletion of Pten in B cells was not associated with autoimmune phenomena.24,25 While our in vitro studies revealed abnormalities of Pten+/– SHIP+/– T-cell function, the possibility of an alteration of B-cell responsiveness to T-cell help was not examined in this study and therefore cannot be excluded as a factor in the in vivo phenotype we observe. Given that abnormalities in B and/or T cells can lead to the development of lupus-like disease in other murine models,26 the potential role of an intrinsic defect of Pten+/– SHIP+/– B cells would be interesting to explore.
The increase in IL-13 and IL-4 protein secretion by Pten+/– SHIP+/– T cells in vitro was consistent with the observation that young Pten+/– SHIP+/– mice (with no signs of the lymphoproliferative syndrome that develops many months later) showed significant spontaneous increases in the levels of circulating IgG1. In contrast to IgG1, the levels of IgM and another class-switched isotype, IgG2b, in Pten+/– SHIP+/– mice increased steadily over time, correlating with the increasing levels of immunopathology seen in these animals. Consistent with the very modest increase in IFN-γ production by the T cells, significant genotype-specific differences in IgG2a were not seen in either the serum from the 8-week-old or older mice.19
The lack of T helper 2 (Th2) skewing of stimulated populations of Pten+/– SHIP+/– T cells, as seen by flow cytometry, suggested that the greater amounts of cytokine produced were the result of increased protein production on a per cell basis. As our FACS analysis did not reveal an upward shift in intracellular IL-4 staining intensity in Pten+/– SHIP+/– cells, it suggested that increased duration of secretion may have accounted for the higher levels of IL-4 seen in the ELISA. Given our hypothesis that an abnormality in the level and/or half-life of the PIP3 second messenger was responsible for the phenotypes we observed, quantification of this species, both before and after stimulation of primary T cells, was of great interest. Indeed, this was attempted by several methods, and while a trend of increased PIP3 levels was observed in Pten+/– SHIP+/– T cells, variability between samples prevented us from obtaining statistically significant data (results not shown). PIP3 levels are, in general, technically difficult to quantify in primary cells and this difficulty was compounded by the fact that the cells of interest only had partial deficiencies of the two phosphoinositol phosphatases of interest. Additionally, studies of samples derived from the entire stimulated CD4+ cell population failed to show consistent differences in the activation and kinetics of phosphorylation of members of a number of signalling pathways with the potential to impact cytokine expression levels, including phospho-STAT6, phospho-p42/44 MAPK and phospho-p38MAPK, and phospho-GSK-3 (data not shown). It is, however, conceivable that subtle changes in immediate-early signalling events could translate into amplified downstream signals. The significant differences in downstream events, such as the increased cytokine RNA and protein secretion seen in this study, may support this hypothesis. An alternative explanation for the increased cytokine production we have observed also lies in the possibility of an alteration in message stability, a factor recently suggested to account for the strain differences in cytokine production between C57BL/6 and DBA/2 mice.27
There is evidence that SHIP is able to regulate phosphoinositol metabolism in T cells, as Jurkat cells deficient for both Pten and SHIP had much higher levels of PIP3 than did control cells lacking only Pten.28 Furthermore, reintroduction of SHIP into Jurkat cells increased PI3,4P2 levels and decreased the membrane localization of PKB/Akt. Despite these studies, the functional role of SHIP in T cells has remained elusive. The in vivo role of SHIP was previously studied via the generation of SHIP–/–RAG1–/– mice, which yielded lymphocytes deficient for this phosphatase.21 Several different assays of T-cell function revealed no differences from wild-type T cells, except for a slight increase in the numbers of CD4+ cells in the SHIP–/– mice. In contrast, primary cells from SHIP–/– mice revealed increased chemotaxis by CD4+ and CD8+ thymocytes, as well as CD4+ splenic T cells, indicating that the ability to detect phenotypic differences depended on the specific assay used.29 Our in vitro study of primary CD4+ T cells, however, provides evidence of a novel, functional role for SHIP in the regulation of T-cell cytokine production, and IL-4 secretion in particular. The assays we employed revealed that SHIP heterozygosity alone had no effect on T-cell behaviour; however, when combined with Pten haploinsufficiency, the T-cell cytokine response was greatly altered. Thus, we propose that Pten and SHIP probably co-operate in the regulation of CD4+ T-cell function, specifically by augmenting IL-4 production, apparently on a ‘per cell’ basis, in these C57BL/6 background mice. It would be interesting to study these responses in mice carrying conditional mutants of SHIP in T cells, to further define the role of this gene in this particular cell type. Furthermore, a comparison of SHIP-deficient T cells with those conditionally null for Pten might allow dissection of functional differences stemming from alterations in PIP3 versus PI3,4P2 intracellular pools.
Studying the impact of gene heterozygosity on T-cell function and autoimmunity represents an important approach when contemplating the relevance of mouse models to human disease, as mutations that lead to decreased function are more common than biallelic gene inactivations. While the latter can reveal critical functions of the encoded molecules, the absence of a protein can potentially alter developmental and differentiation programmes of cells, and may even select for the up-regulation of compensatory proteins or pathways. The potential importance of synergistic heterozygosity has been recently recognized for human metabolic disorders,30 prompting the consideration of this phenomenon in the development of other multigenic traits, possibly including human autoimmune diseases. In this study we have shown that compound heterozygosity for two genes encoding PI3K pathway regulators induces both CD4+ T-cell dysfunction, manifested by excess cytokine production, and an augmented humoral response to a T-dependent antigen.
Acknowledgments
The authors thank J. Miller, C. Pereira and C. Huang for excellent technical assistance and J. Gorday and S. Chan for animal maintenance. This study was supported by a grant from the Canadian Institutes for Health Research. F.R.J. was the recipient of Alberta Heritage Foundation for Medical Research (AHFMR) Medical Scientist and Canada Research Chair awards; and J.L.M. was the recipient of an AHFMR Studentship award.
References
- 1.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–71. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi PS. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–38. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- 3.Borlado LR, Redondo C, Alvarez B, et al. Increased phosphoinositide 3-kinase activity induces a lymphoproliferative disorder and contributes to tumor generation in vivo. Faseb J. 2000;14:895–903. doi: 10.1096/fasebj.14.7.895. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki A, Yamaguchi MT, Ohteki T, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–34. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 5.Parsons MJ, Jones RG, Tsao MS, Odermatt B, Ohashi PS, Woodgett JR. Expression of active protein kinase B in T cells perturbs both T and B cell homeostasis and promotes inflammation. J Immunol. 2001;167:42–8. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–2. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 8.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–7. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 9.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina K, Ellenson LH, Nemes A, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–8. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A, de la Pompa JL, Stambolic V, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–78. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 12.Helgason CD, Damen JE, Rosten P, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–20. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–91. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie NR, Downes CP. PTEN. The down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–95. doi: 10.1016/s0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 15.Krystal G, Damen JE, Helgason CD, et al. SHIPs ahoy. Int J Biochem Cell Biol. 1999;31:1007–10. doi: 10.1016/s1357-2725(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 16.Brauweiler A, Tamir I, Dal Porto J, Benschop RJ, Helgason CD, Humphries RK, Freed JH, Cambier JC. Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5′ inositol phosphatase (SHIP) J Exp Med. 2000;191:1545–54. doi: 10.1084/jem.191.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helgason CD, Kalberer CP, Damen JE, Chappel SM, Pineault N, Krystal G, Humphries RK. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity: aberrant development and enhanced function of B lymphocytes in ship-/- mice. J Exp Med. 2000;191:781–94. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/- mice. Science. 1999;285:2122–5. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 19.Moody JL, Pereira CG, Magil A, Fritzler MJ, Jirik FR. Loss of a single allele of SHIP exacerbates the immunopathology of Pten heterozygous mice. Genes Immun. 2003;4:60–6. doi: 10.1038/sj.gene.6363903. [DOI] [PubMed] [Google Scholar]
- 20.Gjorloff-Wingren A, Saxena M, Han S, et al. Subcellular localization of intracellular protein tyrosine phosphatases in T cells. Eur J Immunol. 2000;30:2412–21. doi: 10.1002/1521-4141(2000)30:8<2412::AID-IMMU2412>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Shalaby F, Jones J, Bouchard D, Dumont DJ. The SH2-containing inositol polyphosphate 5-phosphatase, ship, is expressed during hematopoiesis and spermatogenesis. Blood. 1998;91:2753–9. [PubMed] [Google Scholar]
- 22.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie GJ, Emson CL, Bell SE, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 24.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–94. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Kaisho T, Ohishi M, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–67. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 27.Butler NS, Monick MM, Yarovinsky TO, Powers LS, Hunninghake GW. Altered IL-4 mRNA stability correlates with Th1 and Th2 bias and susceptibility to hypersensitivity pneumonitis in two inbred strains of mice. J Immunol. 2002;169:3700–9. doi: 10.4049/jimmunol.169.7.3700. [DOI] [PubMed] [Google Scholar]
- 28.Freeburn RW, Wright KL, Burgess SJ, Astoul E, Cantrell DA, Ward SG. Evidence that SHIP-1 contributes to phosphatidylinositol 3,4,5-trisphosphate metabolism in T lymphocytes and can regulate novel phosphoinositide 3-kinase effectors. J Immunol. 2002;169:5441–50. doi: 10.4049/jimmunol.169.10.5441. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, Hangoc G, Cooper S, Helgason CD, Yew S, Humphries RK, Krystal G, Broxmeyer HE. Altered responsiveness to chemokines due to targeted disruption of SHIP. J Clin Invest. 1999;104:1751–9. doi: 10.1172/JCI7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vockley J, Rinaldo P, Bennett MJ, Matern D, Vladutiu GD. Synergistic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol Genet Metab. 2000;71:10–8. doi: 10.1006/mgme.2000.3066. [DOI] [PubMed] [Google Scholar]