Abstract
CD4+ memory T cells (Tm) from rheumatoid arthritis peripheral blood (RAPB) or peripheral blood from normal donors produced IL-2, whereas fewer cells secreted IFN-γ or IL-4 after a brief stimulation. RAPB Tm contained significantly more IFN-γ producers than normal cells. Many rheumatoid arthritis (RA) synovial Tm produced IFN-γ alone (40%) and fewer cells produced IL-2 or IL-4. An in vitro model was employed to generate polarized T-helper (Th) effectors. Normal and RAPB Tm differentiated into both IFN-γ- and IL-4-producing effectors. RA synovial fluid (RASF) Tm demonstrated defective responsiveness, exhibiting diminished differentiation of IL-4 effectors, whereas RA synovial tissue (RAST) Tm exhibited defective generation of IFN-γ and IL-4 producers.
Keywords: CD4+ T-helper cells, cytokines, rheumatoid arthritis
Introduction
RA is an autoimmune disease that is characterized by persistent local and systemic inflammation [1,2,3]. Aggressive forms of RA are thought to be driven by T-lymphocyte activity, leading to the rapid erosion of bone and cartilage. Activated Tm are found in the perivascular regions of the inflamed synovium, and T cells accumulate in the synovial fluid [4,5,6]. It appears that memory T cells retain the potential to traffic through the synovium and lymphatics and recirculate in the blood, as evidenced by the increased numbers of activated memory T cells found in the blood of some RA patients [6,7,8]. It is not known, however, whether Tm migrate into the synovium and undergo activation and further differentiation, or whether activation occurs in regional lymph nodes before migration into the inflammatory site.
In vitro studies [9,10] have demonstrated that synovial CD4+ T cells proliferate poorly and produce decreased levels of IL-2 in response to mitogen or antigen, as compared with peripheral blood T cells from healthy individuals or RA patients. Although such findings suggest that synovial T cells might be partly anergic, more recent studies have indicated that the T cells found in the RA synovium were postactivated cells. Synovial T cells were activated as assessed by expression of CD40 ligand/CD154, and were also efficient helpers for B-cell immunoglobulin production [9,10,11,12,13]. Previous studies in animal models [14,15] suggested that a balance exists between immunoregulatory IFN-γ-producing Th1 cells and IL-4-producing Th2 cells. Dominance of either subset can result in a chronic disease state. Moreover, induction of Th2 cells in a Th1-mediated disease model of collagen-induced arthritis led to amelioration of autoimmune disease [16]. Thus, it is possible that, in RA, there is biased differentiation of IFN-γ-secreting proinflammatory Th1 CD4+ T cells, and insufficient differentiation of immunoregulatory IL-4-producing Th2 cells.
Human memory T cells acquire the ability to secrete IL-4 late during in vivo differentiation. Our previous studies and work by others have demonstrated that all of the in vivo differentiated IL-4-producing T cells reside within the mature memory (CD45RO+, CD27–) subset of CD4+ T cells [17,18,19]. Thus, early memory (CD45RO+, CD27+) CD4+ T cells cannot secrete IL-4 after a brief in vitro stimulation. Previous studies [17,18] suggested that early memory T cells represent an uncommitted precursor population, from which both IL-4-producing and IFN-γ-producing effector cells can be generated. The RA synovium appears to contain an increased number of phenotypically mature memory T cells as compared with the blood. However, the majority of memory T cells found in the synovium bear the phenotype of early Tm [7].
Therefore, the present study was undertaken to examine the cytokine effector status of synovial memory T cells and the functional status of immature memory cell precursors of cytokine-producing effector cells. For these experiments, Tm were isolated from the blood of normal subjects, and from RAPB, RASF and RAST. Intracellular cytokine expression was assessed by flow cytometry immediately after isolation and a brief in vitro stimulation, or after in vitro priming designed to generate IL-4-producing effector T cells.
The results suggest that a deficiency in the generation of adequate numbers of regulatory IL-4-producing effector cells in the synovium might be a contributing factor to the perpetuation of chronic inflammation.
Materials and methods
Antibodies and reagents
MAbs used included the following: anti-CD3 (OKT3; American Type Culture Collection, Rockville, MD, USA), anti-CD8 (OKT8; American Type Culture Collection), anti-CD16 (B73.1; generous gift of Dr G Trinchieri, The Wistar Institute, Philadelphia, PA, USA), anti-CD19 (Dako, Glostrup, Denmark), anti-CD45RO (UCHL1; Dako), and anti-CD45RA (2H4; gift of Dr C Morimoto, Boston, MA, USA) [17,18]. Anti-CD28 (28.2), Phycoerythrin (PE)-labeled anti-IL-2, PE-labeled anti-IL-4, and fluorescein isothiocyanate (FITC)-labeled anti-IFN-γ was from Pharmingen (San Diego, CA, USA). FITC-labeled anti-CD3, and PE-labeled or Quantum red-labeled anti-CD4 was from Sigma (St Louis, MO, USA). Recombinant IL-4 and recombinant IL-15 were from R&D Systems (Minneapolis, MN, USA). Neutralizing antihuman IFN-γ antibody was from Endogen (Cambridge, MA, USA). Culture medium contained RPMI1640 with penicillin G (200 U/ml), gentamicin (10 μg/ml), L-glutamine (0.3 mg/ml), and 10% normal human serum [17].
Patients
All patients had an established diagnosis of RA, as defined by the 1987 revised criteria of the American College of Rheumatology for the classification of RA. All patients had long-standing, active RA. All samples were obtained after informed consent, as approved by the UT Southwestern Institutional Review Board. The data shown were obtained from 14 RA patients (age range 29–78 years). Matching blood and synovial fluid were obtained from six patients. Peripheral blood alone was obtained from one patient and synovial fluid samples were obtained from three patients. Matching blood and synovial tissue samples were obtained from three patients at the time of surgery. An additional synovial tissue sample was obtained from one patient.
Cell preparation
Mononuclear cells were isolated from synovial tissue by treating the synovial tissue with collagenase briefly at 37°C with constant shaking. The cells were washed, filtered through nylon mesh, and briefly adhered to plastic petri dishes at 37°C to deplete residual fibroblast and monocytes [7,9]. Mononuclear cells were obtained from blood or synovial samples of RA patients, or sex-matched and age-matched healthy adult individuals by ficoll density centrifugation. Cells were washed and incubated with neuraminidase-treated sheep red blood cells, and purified by negative selection panning as previously described [17]. The cells were incubated with anti-CD8, anti-CD19, B73.1, and 2H4 mAbs. After panning, the recovered cells were routinely greater than 99% CD3+, 95% CD4+, and 90% enriched for CD45RO+ cells.
Cell culture
T cells were immediately stimulated after isolation with ionomycin (0.5 μM; Sigma), phytohemagglutinin (2 μg/ml; Wellcome Diagnostics, Greenville, NC, USA), and phorbol myristate acetate (20 ng/ml; Sigma) in the presence of monensin (2 μM; Sigma) for 6 h and were fixed in paraformaldehyde for subsequent intracellular cytokine analysis. In some experiments, T cells were primed for 1 week before cytokine analysis [17]. T cells (5 ×105 cells/ml) were cultured in 96-well plates (Costar, Cambridge, MA, USA). Where indicated, wells were coated with anti-CD3 mAb (OKT3) before addition of T cells. Medium contained 10 U/ml recombinant IL-2, 5 ng/ml recombinant IL-15, 50 ng/ml recombinant IL-4, anti-CD28 mAb (0.5 μg/ml) and, where indicated, neutralizing anti-IFN-γ antibody (5 μg/ml). After 1 week in culture the cells were harvested and counted. The cells were washed, and incubated for 60 h in medium containing IL-2 (10 U/ml) and IL-15 (5 ng/ml) before restimulation. In preliminary experiments the presence of IL-4 during the rest phase did not increase the number of IL-4-producing cells detected on subsequent restimulation. Restimulation and intracellular cytokine analysis were carried out as described above for immediately stimulated T cells [17].
Flow cytometry
T cells (3 × 105) were stained with saturating amounts of directly conjugated mAb and analyzed on the fluorescence-activated cell sorter (FACScan; Becton Dickinson, San Jose, CA, USA). For intracellular staining of cytokines, cells were stimulated as described above. The cells were harvested, washed, fixed with paraformaldehyde, perme-abilized with saponin, and blocked with rodent serum. The cells were stained with directly conjugated mAbs to human cytokines (Pharmingen) and analyzed by flow cytometry [17]. Stimulated cells that were stained with irrelevant mAbs were used as controls. Analysis was carried out on 104 cells for each sample.
Statistical analysis
Statistically significant differences in the percentages of cells that expressed each cytokine were determined using the two-tailed Student's t-test, or the Mann–Whitney Rank Sum when appropriate. P < 0.05 was considered statistically significant.
Results
Enrichment of IFN-γ producers in freshly isolated RA synovial CD4+ CD45RO+ T cells
Initial experiments delineated cytokine production by freshly isolated CD4+ CD45RO+ T cells obtained from matching samples of RAPB and RASF. A representative sample is shown in Fig. 1 (RA patient1). The majority of peripheral blood T cells from RA patients secreted IL-2 alone, whereas fewer cells secreted IFN-γ alone or in combination with IL-2. By contrast, the CD4+ T cells isolated from the synovial fluid demonstrated a significant increase in the percentage of cells that produced IFN-γ alone or in combination with IL-2, whereas a decrease was observed in the percentage of cells that secreted IL-2 alone. Few cells from peripheral blood or synovial fluid produced IL-4.
Figure 1.
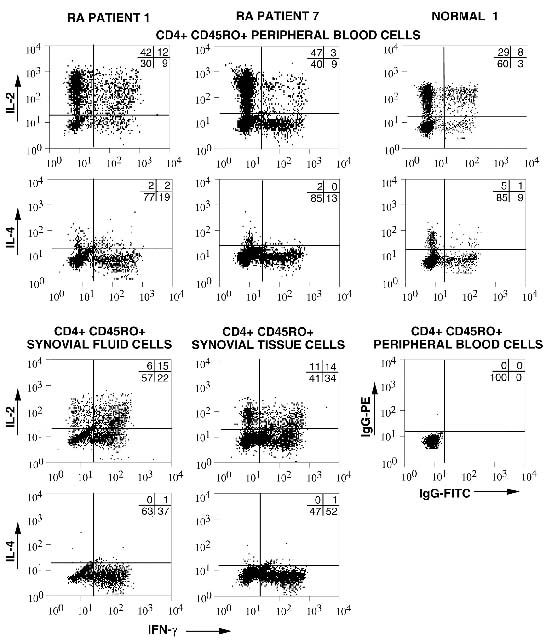
Representative cytokine profiles of Tm isolated from healthy donors and RA patients. CD4+, CD45RO+ T cells were isolated from the blood and synovial fluid of an RA patient (RA Patient 1), the blood and synovial tissue of an RA patient (RA Patient 7), and the blood of a healthy donor (Normal 1). Intracellular cytokines were assessed for peripheral blood and synovial cells from RA patients or blood of healthy donors. Cells were briefly stimulated for 6 h, fixed, permeabilized and stained with directly labeled control antibodies or anti-IFN-γ-FITC in combination with either PE-labeled anti-IL-2 or PE-labeled anti-IL-4, as indicated on the axes; 104 cells were collected for each analysis. The numbers shown represent the percentages of cells in each quadrant. Control staining with IgG-PE and IgG-FITC was carried out for each sample as shown (Normal 1) and the background was subtracted. Samples were obtained from matching RA blood and synovial fluid (n = 5), matching blood and synovial tissue (n = 3), and normal blood (n = 11). Statistical analysis of all samples evaluated for intracellular cytokines immediately after isolation is shown in Fig. 2.
Matching samples obtained from the peripheral blood and synovial tissue of RA patients were also assessed for cytokine expression (Fig. 1; RA patient 7). As was observed in the synovial fluid, memory T cells isolated from the synovial tissue contained a decreased percentage of IL-2-producing cells and an increased percentage of IFN-γ-producing cells. Thus, T cells isolated from the rheumatoid synovial tissue and fluid demonstrated a polarized Th1 cytokine profile.
As a control, the cytokine profiles of Tm from normal adult volunteers were examined (Fig. 1; normal1). As observed for blood from RA patients, the majority of cells produced IL-2 alone, whereas fewer cells produced the combination of IL-2 and IFN-γ. Relatively infrequent cells expressed a highly polarized phenotype by producing either IFN-γ alone or IL-4 alone.
A compilation of the frequency of cells that produced each cytokine from a number of donors is shown in Fig. 2. After a brief in vitro stimulation, the majority of Tm obtained from normal donors (n = 11) produced IL-2 alone (75 ± 4%; mean ± SEM). Fewer cells produced the combination of IL-2 and IFN-γ (14 ± 3%). Relatively infrequent cells expressed a highly polarized phenotype by producing either IFN-γ (6 ± 1%) or IL-4 alone (4 ± 1%). Less than 1% of the cells produced the combination of IFN-γ and IL-4.
Figure 2.
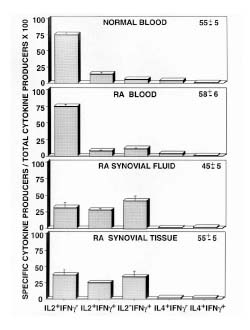
Frequency of cytokine producing cells in Tm subsets. Intracellular staining for cytokines was assessed for CD4+, CD45RO+ T cells that were immediately stimulated after isolation as described in Fig. 1; 104 cells were collected for each analysis of normal blood (n = 11), RAPB (n = 9), RASF (n = 8), and RAST (n = 4). The total number of cytokine-producing cells detected in each sample is shown in the upper right corner of each graph. The frequency of each subset of cytokine-producing cells is shown (mean ± standard error of the mean). The frequency was derived by using the total number of cytokine-producing cells as the denominator (× 100). Statistically significant differences are described in Results.
Cytokine secretion profiles of freshly isolated Tm obtained from RAPB (n = 9) also demonstrated that most secreted IL-2 alone (75 ± 3%), whereas fewer cells produced IL-2 in combination with IFN-γ (8 ± 1%). There was no significant difference between the normal T cells and RA T cells for these two groups. Of note, there was a significant (P < 0.05) increase in the percentage of cells that secreted IFN-γ alone compared with normal cells (11 ± 2% versus 6 ± 1%), whereas there was no difference between normal blood and RAPB in the number of cells that secreted IL-4 alone (4 ± 1%) or in the frequency of those rare cells that produced the combination of IL-4 and IFN-γ (1 ± 0%).
There was a significant decrease in the number of cells isolated from RASF (n = 8) that produced IL-2 alone (31 ± 9%) when compared with normal blood (P < 0.001) or RAPB (P < 0.001) T cells. Moreover, an increase was observed in the number of cells that secreted the combination of IL-2 and IFN-γ (27 ± 3%), which was significantly greater than the percentage of the same subset in normal blood (P < 0.01) or RAPB (P < 0.01). The percentage of RASF cells that produced IFN-γ alone (41 ± 8%) was also significantly increased over the percentage of such cells in normal blood (P < 0.002) or RAPB (P < 0.002) T cells. Significantly fewer cells produced IL-4 alone (1 ± 0%) as compared with normal blood (P < 0.05) or RAPB (P < 0.05) T cells, whereas no significant difference was observed in the number of cells that produced the combination of IL-4 and IFN-γ (2 ± 0%).
RAST Tm (n = 4) also contained a decreased percentage of IL-2-secreting cells (37 ± 9%), which did not differ significantly from that in the RASF, but did differ significantly from that in normal blood (P < 0.001) and RAPB (P < 0.001). The percentage of cells that produced both IFN-γ and IL-2 (25 ± 1%) did not differ significantly from that in RASF, but was significantly greater than the frequencies observed in normal blood (P < 0.05) or RAPB (P < 0.05). In RAST, 34 ± 9% of cytokine effectors secreted IFN-γ alone. This was not different from the percentage found in RASF, but was significantly greater than the percentage of IFN-γ-producing cells in normal blood (P < 0.005) or RAPB (P < 0.005). There was no significant difference in the number of synovial tissue cells that produced IL-4 alone (2 ± 1%) or IL-4 and IFN-γ (2 ± 0%), as compared with RASF, normal blood or RAPB.
Thus, T cells isolated from RAST and RASF demonstrated a decrease in IL-2-producing cells, with a concomitant increase in IFN-γ-producing cells when compared with normal blood and RAPB Tm.
Rheumatoid CD4+ T cells demonstrate defective responsiveness to differentiation signals in vitro
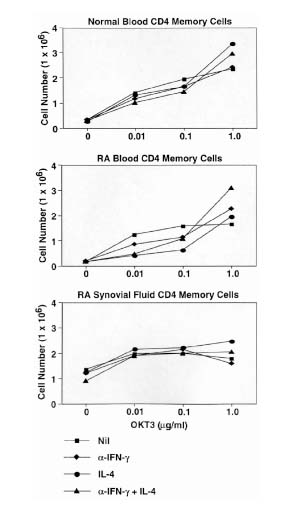
Peripheral blood and synovial Tm were than examined in an in vitro culture system in order to determine the relative capacity of differentiation signals to modulate cytokine production. Our previous studies with normal T cells [17] demonstrated that this system effectively generates polarized IFN-γ-producing or IL-4-producing effector cells. Fig. 3 shows the composite cytokine profiles of CD4+, CD45RO+ T cells obtained from cultures primed with anti-CD28 and cytokines in the absence or presence of anti-CD3 mAb. As seen in Fig. 3, stimulation of normal T cells after priming with anti-CD28 alone resulted in a significant decrease in the percentage of IL-2 producers (35% versus 75%) compared with cells immediately stimulated after isolation, and a significant increase in the percentages of IFN-γ (31% versus 6%) and IL-4 producers (17% versus 4%). After priming with a low dose of anti-CD3 (Fig. 3; middle panel), the percentage of IL-2 producers decreased further and the percentage of IFN-γ-secreting cells increased. As previously shown, the generation of IL-4-secreting cells was optimally induced by priming with anti-CD28 alone and was inhibited by costimulation with anti-CD3 mAb [17].
Figure 3.
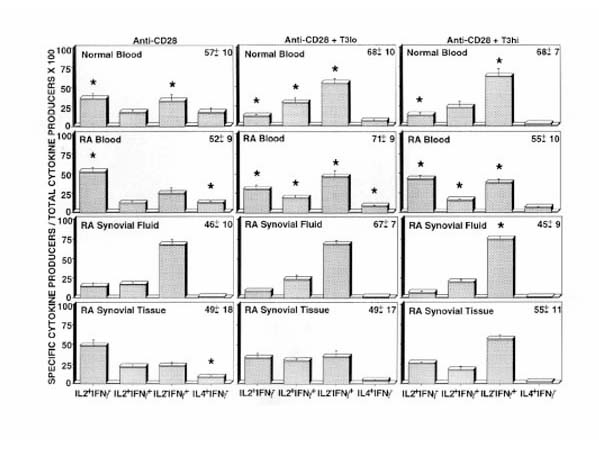
RA synovial memory T cells are deficient in IL-4-producing cells after in vitro priming. CD4+ memory effector T cells were placed into priming cultures to generate effector cells. Effector T cells were generated by priming in the presence of anti-CD28 mAb and cytokines, as described in Materials and methods, or with additional immobilized anti-CD3 mAb, OKT3, at the indicated concentrations (T3lo, 0.01 μg/ml; and T3hi, 1 μg/ml). After priming, the cells were harvested and rested in cytokines before restimulation to detect intracellular cytokines as described in Materials and methods; 104 cells were collected for each analysis. The data shown were derived from independent experiments; for normal donors (n = 4), RAPB (n = 4), RASF (n = 4), and RAST (n = 3), low-dose OKT3 was omitted in one experiment. The frequency of each subset of cytokine-producing cells is shown. The total number of cytokine-producing cells in each sample is shown in the upper right corner. *P < 0.05, versus freshly isolated samples shown in Fig. 2 (two-tailed Student's t-test).
T cells derived from RAPB demonstrated a distinct pattern of responsiveness. Whereas the percentage of IL-2-secreting cells decreased after in vitro priming with anti-CD28 as compared with freshly isolated cells (52% versus 75%), a greater decrease was observed after priming with low dose anti-CD3 (29%), and the cells were resistant to further modulation by higher concentrations of anti-CD3. Of note, the loss of IL-2 production by in vitro primed RA T cells was less marked than observed with normal T cells. An increased percentage of IFN-γ producers was observed after priming, and the peak response was achieved in the presence of low concentrations of anti-CD3 (45% versus 11%). Again, the induction of IFN-γ production in RAPB memory T cells was less than noted in normal blood. IL-4-producing cells were generated from RA memory T cells by stimulation with anti-CD28, and these were inhibited by the presence of anti-CD3 mAb. The percentage of IL-4 producers (12%) generated from RA memory T cells was less than was generated from normal T cells (17%).
T cells from RASF primed with anti-CD28 exhibited a decrease in the percentage of IL-2-producing cells (15% versus 31%) and an increase in the percentage of IFN-γ secreting cells (67% versus 41%) as compared with freshly isolated cells. Synovial fluid T cells were deficient in the ability to generate IL-4-producing cells (1%), which was in contrast to the blood. Whereas RAPB T cells were refractory to priming by high concentrations of anti-CD3 mAb, the synovial fluid T cells responded to anti-CD3 in a dose-dependent manner. T cells derived from synovial tissue were also relatively refractory to in vitro priming as compared with normal blood T cells. Synovial tissue T cells contained an increased percentage of IL-2 producers (48% versus 37%) and a small decrease in IFN-γ producers (23% versus 34%) after in vitro priming with anti-CD28. Importantly, IL-4-secreting cells (8% versus 2%) could be generated from synovial tissue memory T cells after in vitro differentiation, although in lesser numbers than from RAPB and normal blood. Priming in the presence of anti-CD3 reduced the percentages of IL-2-producing and IL-4-producing cells, while increasing the percentage of IFN-γ-secreting cells.
Effect of anti-IFN-γ antibody on the generation of IL-4-secreting effector cells from RA synovial fluid T cells
In order to determine whether cytokine production by rheumatoid T cells was resistant to modulation in the in vitro priming cultures because of the secretion of IFN-γ, the in vitro priming was also carried out in the presence of anti-IFN-γ antibody. As shown in Fig. 4, the addition of increasing concentrations of a neutralizing antibody to IFN-γ had little effect on the generation of cytokine-producing cells from either normal synovial fluid or RASF CD4+ T cells. Then we examined whether the combined effects of anti-IFN-γ antibody and IL-4 could influence the effector phenotype of cells isolated from the synovium. As shown in Table 1, Tm were isolated from matching RAPB and RASF, and were cultured under conditions that optimized the generation of IL-4-secreting effector cells [17]. RAPB Tm generated a marked increase in the percentage of IL-4-producing cells when primed with the combination of IL-4 and anti-IFN-γ antibody. In contrast, T cells from the synovial fluid of the same patient generated virtually no IL-4-producing cells and few IL-2-producing cells, despite supplemental IL-4 and anti-IFN-γ antibody.
Figure 4.
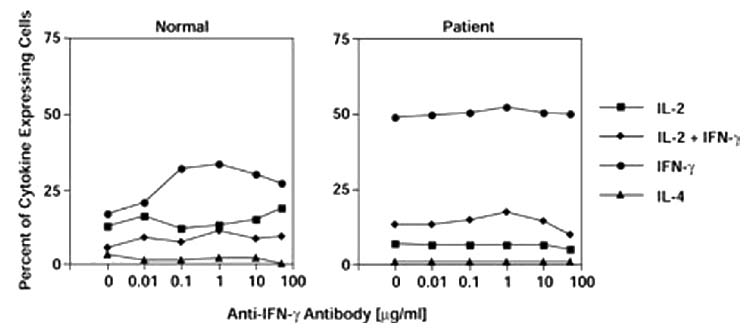
In vitro generation of effector cells in the presence of anti-IFN-γ antibody. CD4+ memory effector T cells obtained from the peripheral blood of a healthy donor (normal) or from RASF (patient) were stimulated as described in Fig. 3 with immobilized anti-CD3 mAb (OKT3, 0.01 μg/ml), anti-CD28, and cytokines. In addition, cultures were carried out in the presence of increasing concentrations of a neutralizing anti-IFN-γ antibody as indicated. After priming, the cells were washed, rested for 2.5 days and restimulated with mitogen to assess intracellular cytokines; 104 cells were collected for each analysis. The data are representative of at least two experiments. No significant difference was observed in the presence of anti-IFN-γ antibody (P > 0.05).
Table 1.
Deficient induction of IL-4 producers from synovial precursors
| Percentage of cytokine-producing cells | ||||||||
| RAPB | RASF | |||||||
| Addition | IL-2 | IL-2 + IFN-γ | IFN-γ | IL-4 | IL-2 | IL-2 + IFN-γ | IFN-γ | IL-4 |
| Nil | 19 | 24 | 34 | 2 | 4 | 27 | 60 | 0 |
| Anti-IFN-γ | 23 | 25 | 28 | 1 | 7 | 33 | 47 | 0 |
| IL-4 | 32 | 23 | 25 | 14 | 5 | 29 | 49 | 0 |
| Anti-IFN-γ + IL-4 | 41 | 16 | 20 | 23 | 4 | 23 | 54 | 0 |
CD45RO+, CD4+ memory effector T cells were obtained from RA matching peripheral blood and synovial fluid. T cells were cultured for 1 week with OKT3 (0.01 μg/ml) and anti-CD28, as described in Materials and methods. Where indicated, the cultures contained saturating concentrations of anti-IFN-γ antibody and/or IL-4. Afterward, the cells were washed, rested for 2.5 days, and restimulated for 6 h with mitogen to assess intracellular cytokines by flow cytometry; 104 cells were analyzed for each sample. The percentage of cytokine-expressing cells for each sample is shown. Similar results were obtained in at least two independent experiments. No significant difference (P > 0.05) was observed in the percentage of synovial IL-4-producing effector cells in the absence or presence of anti-IFN-γ antibody and/or IL-4.
As a control, cell growth during the priming cultures was monitored in the presence or absence of antibody to IFN-γ and IL-4. As shown in Fig. 5, synovial T-cell growth was not inhibited by the addition of anti-IFN-γ antibody or IL-4. These data suggest that RAPB Tm have the capacity to generate IL-4-producing or IFN-γ-producing effector cells, whereas memory T cells from RASF are inhibited in their capacity to become IL-4-producing effector cells. Further studies indicated that anti-CD28 mAb and cytokines enhanced synovial fluid Tm growth approximately fourfold above the initial input cell number. Synovial fluid T cells cultured in medium alone underwent a fourfold reduction in cell number, whereas cells cultured with cytokines alone increased in number by an average of twofold (data not shown). These data further support the conclusion that synovial fluid T cells were responsive to signals delivered by the combination of anti-CD28 and cytokines.
Figure 5.
T-cell growth in the presence of IL-4 and anti-IFN-γ antibody. T cells (0.5 × 106/sample) were cultured in the presence or absence of IL-4, anti-IFN-γ antibody, or both as described in Table 1. After priming, the cells were harvested and counted to assess cell growth, as described in Materials and methods. Similar results were obtained in at least two experiments. No significant difference (P > 0.05) was observed when the cells were cultured in the presence of IL-4 alone or in combination with anti-IFN-γ antibody.
Discussion
RAST and RASF were deficient in IL-4-secreting cells as compared with RAPB after a brief in vitro stimulation. Thus, mature IL-4-secreting effector cells were found to be decreased in the RA synovium but not in the blood.
There are at least three major mechanisms by which IL-4 production might be inhibited in the RA synovium. One mechanism could be through the selective recruitment of IFN-γ-producing effector cells. For example, it has been suggested that the T cells found in the RA synovium have been selectively recruited on the basis of expression of chemokine receptors [20]. Thus, T cells found in the rheumatoid synovium express CCR5, whereas T cells found at sites of parasitic infection express CCR4 and CCR3 [20,21]. However, the role of chemokine receptor expression in the recruitment of T cells to these sites is unknown. This possibility cannot be the full explanation of the current observations in light of the finding that synovial tissue T cells had the capacity to differentiate into IL-4-secreting effector cells in in vitro cultures. Thus, some synovial cells remained responsive to IL-4- and anti-CD28-mediated signaling in the priming cultures. A second mechanism for suppression of IL-4 production in the rheumatoid synovium could be through regulatory signals received in the local microenvironment. This remains a likely possibility because studies in murine models have shown that only the most highly differentiated T cells obtain a polarized cytokine secretion profile that cannot be altered by external stimuli [22]. A third possibility is that precursor cells of IL-4 producers rapidly migrate out of the synovium, perhaps being unresponsive to retention signals.
Although the exact mechanism that is operative in the RA synovium remains to be elucidated, our ongoing studies are focused on determining the phenotype and response defect of these highly polarized Th cells.
Tm isolated from rheumatoid blood or synovium contained increased numbers of cells with the capacity to produce IFN-γ after a brief in vitro stimulation, compared with the same subset isolated from the periphery of normal donors. A strong mitogenic stimulation was employed in order to obtain the maximum cytokine-secreting potential of the effector population. We have previously demonstrated that staining for intracellular cytokines correlated with cytokine secretion [17]. However, intracellular cytokine analysis proved to be a more sensitive method for detecting cytokines, such as IL-2 and IL-4, and also made it possible to determine the percentage of cells producing each. It should be noted that longer incubations did not result in a substantial increase in the number of cytokine-secreting cells detected in this assay. These studies corroborate previous studies that demonstrated that synovial T cells are enriched in IFN-γ-producing cells on initial isolation [11]. The results suggest that these memory cells appear to have undergone biased differentiation into Th1 cells, perhaps as a result of signals received in the synovial microenvironment. The small increase in the number of IFN-γ-producing cells found in rheumatoid blood may well reflect the recirculation of memory T cells previously activated in the blood [7,23].
Tm were used for these studies because it has been shown [7,9] that the majority of CD4+ cells in the RA synovium are of the memory subset. Therefore, a potential concern was that the in vitro priming protocol might select for cells that are already committed to IFN-γ or IL-4 production, rather than generate new effector cells. In humans, however, highly polarized effector T cells arise late in the differentiation pathway [17,18,19]. Whereas early memory T cells, identified by the CD45RO+, CD27+ phenotype, have the potential to secrete IL-2 and IFN-γ, these cells cannot produce IL-4 and few produce IFN-γ in the absence of IL-2 [17,19]. Only mature memory cells, identified by the presence of CD45RO and the loss of CD27 cell-surface expression, have the capacity to secrete IFN-γ or IL-4 alone. We have previously shown that the in vitro priming protocol used in the present study induces the differentiation of early uncommitted CD4+, CD27+ memory T cells into IL-4-producing effector cells [17]. Thus, it was hypothesized that the uncommitted early CD4+, CD27+ memory cells that were isolated from the RA synovium or blood maintained the capacity to differentiate into IL-4-producing effector cells. The majority of T cells that are found in the RA synovium belong to the early memory subset [7]. Therefore, the defective generation of IL-4 producers from RA synovial fluid under conditions that induced IL-4-producing effector cells from blood memory T cells suggested that the rheumatoid microenviroment played an important role in selecting or modifying the precursor effector memory population.
CD28 is expressed by most human T cells and is thought to be critical for T-cell differentiation. CD28 is also important during T-cell receptor-mediated activation, IL-2 production, and the prevention of anergy [24]. Recent studies [25,26,27] have indicated that there were increased numbers of CD4+, CD28– T cells in the periphery and synovium of RA patients. Therefore, the lack of expression of CD28 on RA CD4+ T cells represented a potential explanation for the abnormal memory T-cell differentiation observed in RA blood and tissue and the deficiency in IL-4-producing effector cells from the synovial fluid after in vitro priming. However, CD4+, CD28– cells are a minor subset of T cells, representing less than 3% of peripheral CD4+ T cells from the most chronically active RA patients versus less than 1% from healthy control individuals [26], and are therefore unlikely to account for the broad defect in memory T-cell differentiation found here. We have observed that CD4+, CD28– cells in the periphery represent a unique subset that is restricted to the CD4+, CD27– population (Davis L, unpublished observation). As noted above, although there were increased numbers of well-differentiated CD4+, CD27– cells in the RA synovium, these cells represented the minority of Tm [7]. Therefore, the inability to modulate effector cell responses in RASF samples could not be explained by the absence of CD28. Moreover, RASF T cells were capable of cell growth in response to anti-CD28 mAbs and cytokines. This finding is in agreement with previous studies [28] demonstrating that the CD28 signaling pathway was intact in RA synovial CD4+ T cells.
The present studies also demonstrated that synovial fluid Tm lacked the capacity to generate IL-4-secreting effector cells in numbers similar to those found in the periphery. Our previous studies [17] demonstrated that IL-4 was required during the in vitro priming cultures to generate IL-4-producing effector cells from non-IL-4-producing early memory precursor cells. Therefore, one concern in the current studies was that RA T cells might require exogenous IL-4 to generate IL-4-producing effector cells.
An additional concern was that IFN-γ-producing effector cells might inhibit potential IL-4 precursors from differentiating or expanding in these cultures, although previous studies [29,30,31] demonstrated that IFN-γ indirectly affected the generation of IL-4-secreting cells through the activities of antigen-presenting cells. It should be noted that antigen-presenting cells were depleted from the memory T-cell population before priming in the present studies, and therefore this possibility was only a minor concern.
Both the addition of IL-4 to the priming cultures and the presence of anti-IFN-γ antibody ensured that potential IL-4-secreting precursor populations had optimal conditions for differentiation. Blocking IFN-γ activity during in vitro priming with a neutralizing anti-IFN-γ antibody had little effect on the number of IFN-γ-secreting effector cells detected on subsequent restimulation. Moreover, the availability of IFN-γ had little impact on the generation of either subset. Thus, the inability to generate IL-4-producing effector cells from RASF precursor cells was not simply explained by the presence of IFN-γ-producing effector cells or the lack of a subset of fully differentiated IL-4-producing T cells in the initial memory cell population. Recent studies [32] have suggested that there is an intrinsic defect in the development of IL-4-producing effector cells in RA patients. Therefore, it is possible that the apparent disordered differentiation of effector cells becomes more marked at inflammatory sites such as in the rheumatoid synovium [23,32].
It is interesting to note that both IL-2 production and IL-4 production appear to be downregulated in RASF T cells. Previous studies [9] have shown that mature memory (CD45RO+, CD27–) CD4+ T cells isolated from the blood have the same capacity as early memory (CD45RO+, CD27+) CD4+ T cells to produce IL-2. In the RA synovium, IL-2 appears to be downregulated [9]. It should be noted that IL-2 production was not decreased in T cells obtained from osteoarthritic joints [9]. In addition, Tm obtained from other tissues, such as inflamed tonsil, expressed levels of IL-2 that were similar to those in blood (Davis L, unpublished observation). Therefore, migration into tissue per se does not induce downregulation of IL-2 production. Recent studies [33] have attributed the lack of IL-2 production by synovial CD4+ T cells to a defect in the T-cell receptor-mediated signal transduction cascade. However, in the present studies we made use of stimuli that bypassed the early steps in the T-cell receptor-mediated signaling cascade and found that there was defective IL-2 production in cells stimulated immediately on isolation from the RA synovium, whereas these same cells were completely competent for IFN-γ production. Thus, it is interesting to speculate that IL-2 and IL-4 are downregulated in the rheumatoid synovium by some active repressor mechanism induced by the synovial microenvironment. Although recent studies have indicated that redox balance alterations were critical in determining whether cells can produce IL-2 [33,34], it is not known whether this influences IL-4 production, or has it been determined whether readjustment of redox balance in RA synovial T cells could correct the deficiency in IL-4.
Few studies have used similar techniques to assess cytokine production in blood, synovial tissue, and synovial fluid obtained from RA patients. Immunohistology is routinely carried out on synovial tissue sections, whereas enzyme-linked immunosorbent assays are employed to determine cytokine profiles of serum and synovial fluid [9,11,12,13,31]. Therefore, it has remained difficult to determine the potential impact of these compartments on T-cell function in rheumatoid inflammation. In the current studies, RA T cells from all three compartments produced increased amounts of IFN-γ on immediate stimulation. However, the cytokine profiles diverged when assessed after in vitro priming. Whereas, synovial tissue Tm were deficient in IL-4 producers compared with matching blood, these cells retained the ability to generate IL-4-producing effector cells. In this regard, recent studies [31] have suggested that synovial tissue cells were selectively responsive to IL-12 produced in the synovium, because IL-12 induced increased production of IFN-γ, but not IL-2 and IL-4. Those studies support the hypothesis that the synovial microenvironment may play a role in skewing T cells toward a Th1 phenotype. The finding that IL-4-producing effector cells could be generated from synovial tissue T cells once removed from that environment suggests that all of the cells were not yet terminally polarized to Th1-like effector cells. Importantly, synovial fluid T cells appeared to have been more affected by the synovial microenvironment than synovial tissue T cells because, regardless of the in vitro priming conditions, these cells yielded increased percentages of IFN-γ producers and were deficient in IL-4 producers.
The present data suggest that synovial fluid T cells are those that have passed through the synovium, whereas synovial tissue T cells are a mixed population of recently migrated cells and those that have been retained in the synovium. Whether the inability of synovial fluid memory T cells to generate IL-4 producers is the result of activation, differentiation, or prolonged exposure to the synovial microenvironment, the data clearly indicate that a majority of synovial fluid memory T cells appear to be polarized IFN-γ effector cells.
In summary, the present studies show that RA blood and synovial T cells contain increased numbers of polarized IFN-γ effector cells. RA synovial T cells also demonstrated a deficiency in the ability to generate IL-4-producing effector cells. The diminished ability to generate IL-4 effector cells from synovial T cells in vitro suggests that the rheumatoid microenvironment alters T-cell effector function and thereby perpetuates the chronic inflammatory disease state.
Acknowledgments
Acknowledgements
The authors wish to thank the doctors and staff who participated in the acquistion of the patient samples. We would like to express special gratitude to Dr Richard E Jones (Dallas, Texas) for some of the samples. We would like to thank Angie Mobley of the UT Southwestern Dallas cell analysis facility for technical support for the flow cytometer. This work was supported by the Arthritis Foundation and National Institutes of Health grant AR45293.
References
- Lipsky PE, Davis LS. The central involvement of T cells in rheumatoid arthritis. The Immunologist. 1998;6:121–128. [Google Scholar]
- Harris ED., Jr Rheumatoid arthritis: pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Van Boxel JA, Paget SA. Predominantly T cell infiltration in rheumatoid synovial membranes. N Engl J Med. 1975;293:517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Kingsley G, Haskard D, Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988;18:1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Iannone F, Corrigall VM, Kingsley GH, Panayi GS. Evidence for the continuous recruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol. 1994;24:2706–2713. doi: 10.1002/eji.1830241120. [DOI] [PubMed] [Google Scholar]
- Cush JJ, Lipsky PE. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988;31:1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Kohem CL, Brezinschek RI, Wisbey H, Tortorella C, Lipsky PE, Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim, CD27- memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:844–854. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- Potocnik AJ, Kinne R, Menninger H, Zacher J, Emmrich F, Kroczek RA. Expression of activation antigens on T cells in rheumatoid arthritis patients. Scand J Immunol. 1990;31:213–224. doi: 10.1111/j.1365-3083.1990.tb02762.x. [DOI] [PubMed] [Google Scholar]
- Thomas R, McIlraith M, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992;35:1455–1465. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- Matthews N, Emery P, Piling D, Akbar A, Salmon M. Subpopulations of primed T helper cells in rheumatoid arthritis. Arthritis Rheum. 1994;36:603–607. doi: 10.1002/art.1780360505. [DOI] [PubMed] [Google Scholar]
- Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenberg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- Miltenburg AM, Vanlaar JM, de Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Quayle AJ, Chomarat P, Miossec P, Kjeldsen J, Forre O, Natvig JB. Rheumatoid inflammatory T-cell express mostly Th1 but also Th2 and mixed (Th0-like) cytokine patterns. Scand J Immunol. 1993;38:75–82. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Das MP, Nicholson LB, Greer JM, Kuchroo VK. Autopathogenic T helper cell type 1 (Th1) and protective Th2 clones differ in their recognition of the autoantigenic peptide of myelin proteolipid protein. J Exp Med. 1997;186:867–876. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers WP, Beyer JC, Knowles DP. Type 1 and type 2 cytokine gene expression by viral gp135 surface protein-activated T lymphocytes in caprine arthritis-encephalitis lentivirus infection. J Virol. 1997;71:6259–6263. doi: 10.1128/jvi.71.8.6259-6263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LK, Tang B, Rosloniec EF, Stuart JM, Chiang TM, Kang AH. Characterization of a peptide analog of a determinant of type II collagen that suppresses collagen-induced arthritis. J Immunol. 1998;161:3589–3595. [PubMed] [Google Scholar]
- Schulze-Koops H, Lipsky PE, Davis LS. Human memory T cell differentiation into Th2-like effector cells is dependent on IL-4 and CD28 stimulation and inhibited by TCR ligation. Eur J Immunol. 1998;28:2517–2529. doi: 10.1002/(SICI)1521-4141(199808)28:08<2517::AID-IMMU2517>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tortorella C, Schulze-Koops H, Thomas R, Splawski JB, Davis LS, Picker LJ, Lipsky PE. Expression of CD45RB and CD27 identifies subsets of CD4+ memory T cells with different capacities to induce B cell differentiation. J Immunol. 1995;155:149–162. [PubMed] [Google Scholar]
- De Jong R, Brouwer M, Hooibrink B, Van der Pouw-Kraan T, Miedema F, Van Lier RAW. The CD27- subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- Gerber BO, Zanni MP, Uguccioni M, Loetscher M, MacKay CR, Pichler WJ, Yawalker N, Baggiolini M, Moser B. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- Carter LL, Swain SL. From naive to memory: development and regulation of CD4+ T cell responses. Immunol Res. 1998;18:1–13. doi: 10.1007/BF02786509. [DOI] [PubMed] [Google Scholar]
- Davis LS, Lipsky PE. Disordered differentiation of memory T cells in rheumatoid arthritis. Rev Rheum. 1998;65:291–296. [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;114:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Goronzy JJ, Weyand CM. CD4+CD7-CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- Namekawa T, Wagner UG, Goronzy JJ, Weyand CM. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum. 1998;41:2108–2116. doi: 10.1002/1529-0131(199812)41:12<2108::AID-ART5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maurice MM, van der Voort EAM, Leow A, Levarht N, Breedveld FC, Verweij CL. CD28 co-stimulation is intact and contributes to prolonged ex vivo survival of hyporesponsive synovial fluid T cells in rheumatoid arthritis. Eur J Immunol. 1998;28:1554–1562. doi: 10.1002/(SICI)1521-4141(199805)28:05<1554::AID-IMMU1554>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Morita Y, Yamamura M, Nishida K, Harada S, Okamoto H, Inoue H, Ohmoto Y, Modlin RL, Makino H. Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum. 1999;41:306–314. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Skapenko A, Wendler J, Lipsky PE, Kalden JR, Schulze-Koops HS. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J Immunol. 1999;163:491–499. [PubMed] [Google Scholar]
- Gringhuis SI, Maurice MM, Leow A, van der Voort EAM, Huizinga TWJ, Breedveld FC, Verweij CL. Displacement of LAT from the plasma membrane is associated with hyporesponsiveness of synovial T lymphocytes in rheumatoid arthritis [abstract]. Arthritis Rheum. 1998;41(suppl 9):S188. [Google Scholar]
- Maurice MM, Nakamura H, van der Voort EAM, van Vliet AI, Staal FJT, Tak P-P, Breedveld FC, Verweij CL. Evidence for the role of an altered redox state in hyporesponsiveness of synovial T cells in rheumatoid arthritis. J Immunol. 1997;158:1458–1465. [PubMed] [Google Scholar]


