Abstract
The role of specific B lymphocytes and T-cell populations in the control of experimental Echinococus multilocularis infection was studied in µMT, nude, T-cell receptor (TCR)-β–/–, major histocompatibility complex (MHC)-I–/– and MHC-II–/– mice. At 2 months postinfection, the parasite mass was more than 10 times higher in nude, TCR-β–/– and MHC-II–/– mice than in infected C57BL/6 wild-type (WT) mice, and these T-cell-deficient mice started to die of the high parasite load at this time-point. In contrast, MHC-I–/– and µMT mice exhibited parasite growth rates similar to those found in WT controls. These findings clearly point to the major role that CD4+ αβ+ T cells play in limiting the E. multilocularis proliferation, while CD8+ T and B cells appeared to play a minor role in the control of parasite growth. In the absence of T cells, especially CD4+ or αβ+ T cells, the cellular immune response to infection was impaired, as documented by the lack of hepatic granuloma formation around the parasite and by a decreased splenocyte responsiveness to concanavalin A (Con A) and parasite antigen stimulation. Surprisingly, in T-cell-deficient mice, the ex vivo expression of interferon-γ (IFN-γ) and other inflammatory cytokines (except for interleukin-6) were increased in association with a high parasite load. Thus, the relative protection mediated by CD4+ αβ+ T cells against E. multilocularis infection seemed not be IFN-γ dependent, but rather to rely on the effector's function of CD4+ αβ+ T cells. The local restriction of parasite germinal cell proliferation was reflected by a regulatory effect on the expression of 14-3-3 protein within the parasite tissue in T-cell-deficient mice. These results provide a strong indication that the CD4+ αβ+ T-cell-mediated immune response contributes to the control of the parasite growth and to the regulation of production of the parasite 14-3-3 protein in metacestode tissues.
Keywords: cell-deficient mice, cell-mediated immune response, Echinococcus multilocularis, T-cell deficiency, 14-3-3 protein
Introduction
Alveolar echinococcosis (AE) is a severe parasitic disease caused by the intrahepatic growth of the metacestode (larval) stage of Echinococcus multilocularis.1 Tumour-like growth of the metacestode results in clinical symptoms similar to hepatic carcinoma. The natural life cycle of E. multilocularis involves mainly rodents, but occasionally humans, as an intermediate host, both becoming infected through the ingestion of tapeworm eggs and the subsequent development of the metacestode (primary infection). Extensive experimental studies have been carried out in laboratory rodents infected via intraperitoneal inoculation of metacestode vesicles (secondary infection). The metacestode consists of an inner, germinal layer, and an outer, acellular carbohydrate-rich laminated layer (LL). Recently, it was shown that infected immuncompetent C57BL/6 mice failed to clear infection, even when infected with a minimal infectious dose consisting of a single E. multilocularis metacestode vesicle.2 An intact LL is required to allow the parasite to survive.2,3 Therefore, the LL appears to play an important role in protecting the parasite and allowing it to establish a chronic infection, while the germinal layer represents the living parasite tissue and is responsible for the infiltrating growth and proliferation of the parasite.1
Despite the failure of immune clearance upon secondary infection with parasite units protected by an intact LL, the involvement of cellular immunity in controlling the metacestode growth kinetics is strongly suggested by the intense granulomatous infiltration observed around the hepatic parasite lesions. This is observed in experimental mouse models,4,5 as well as in infected patients.6 As early as 1992, the group of Kamiya et al. presented evidence that athymic nude and severe combined immunodeficiency (SCID) mice exhibited a high susceptibility to infection, thus suggesting that the host immune response plays an important role in suppressing larval growth.7,8 Furthermore, the role of an immune response in the control of AE in human patients has been substantially demonstrated by the rapid, fatal outcome of the infection in a human immunodeficiency virus (HIV) co-infected immunodeficient patient.9 Nevertheless, the definitive biological significance of αβ+ T cells, CD4+ and CD8+ T cells, and of B cells, in modulating the growth behavior of E. multilocularis, is still poorly understood. Moreover, we do not know whether the immune response-mediated restriction of parasite growth is related to – or even based upon – affecting regulative molecule expression, especially within the proliferative parasite germinal layer. In this respect, recent molecular studies have focused on the 14-3-3 protein family of E. multilocularis, which putatively plays a role in the progressively infiltrative growth of the parasite.10 It is known that various members of the 14-3-3 protein family act as key molecules in the progression of cell differentiation and proliferation, cell cycle control and apoptotic cell death.11 An alternative expression of 14-3-3 genes is found in several neoplastic cell lines, which indicates that the 14-3-3 proteins are involved in the progressive growth of tumour cells.12,13 In E. multilocularis infection, the 14-3-3 gene is specifically hyper-expressed at the metacestode stage, the proteins predominantly localizing in the germinal layer.10 This high-level expression was associated with the fast progressive growth of E. multilocularis metacestodes, while in the slowly growing E. granulosus hydatid cyst, as in the adult stage of E. multilocularis, the expression of the 14-3-3 protein remained low.14
In the present study we used mutant mice, with defined T- and B-cell deficiencies, to investigate the relative importance of humoral and cellular immunity, and to specifically tackle the role of B cells, CD4+, CD8+ and αβ+ T cells, in modulating the course of an E. multilocularis infection. We further addressed the immune response in these mutant mice, as well as the relationship between specific immune deficiencies and respective 14-3-3 protein expression levels in the parasite tissue.
Materials and methods
Mice
Female, 8–10-week-old C57BL/6 mice and athymic nude mice (C57BL/6 background) were purchased from the Biotechnology & Animal Breeding Division, Füllinsdorf, Switzerland. The µMT mice were provided by Professor H. Hengartner (University of Zürich, Zürich, Switzerland). The major histocompatibility complex (MHC)-II I-Aβ–/– mice (selectively deficient for mature CD4+ T cells), the MHC-I β2-m–/– mice (deficient for CD8+ T cells) and the T-cell receptor (TCR)-β–/– mice (devoid of TCR-αβ T cells) were provided by Dr H. Mossmann (Max-Planck Institute for Immunobiology, Freiburg, Germany). All T- and B-cell-deficient mouse strains had been backcrossed to C57BL/6 (H-2b) mice. In all experiments, animals were matched for age and weight. All mice were raised, housed and treated according to the rules of the Swiss regulations for animal experimentation.
Parasite and infection of mice
The parasite isolate used in this study was derived from the cloned E. multilocularis isolate, KF5.2,3 The mutant mice with defined T- or B-cell deficiencies, and corresponding wild-type control mice, were injected intraperitoneally with either 100 freshly prepared metacestode vesicles suspended in 100 µl of RPMI-1640,3 or with one single vesicle per mouse.2 Control mice received an appropriate volume of RPMI-1640.
Histology
Infected mice were killed by exposure to CO2. Livers containing metacestode tissue were removed by dissection. Samples of livers with grossly visible white foci or parasite vesicles were fixed in 4% neutral-buffered formalin and embedded in paraffin. Haematoxylin and eosin (H & E)-stained slides were used for the assessment of inflammatory lesions by light microscopy.
Cell cultures and lymphocyte proliferation assays
Spleen cell suspensions were prepared from infected or non-infected mice, as described previously.3 Splenocytes were resuspended in RPMI-1640 containing 10% heat-inactivated fetal calf serum (FCS; Gibco, Basel, Switzerland), 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco). Spleen cells were cultured in 96-well round-bottom plates at 2 × 105 cells per well. Cells were stimulated with concanavalin A (Con A) (2 µg/ml; Sigma Chemical Co., Basel, Switzerland) for 72 hr, or with crude parasite VF-antigen (10 µg/ml of protein and 2·1 µg/ml of carbohydrate) for 96 hr, or were left unstimulated as negative controls. The antigens were of the same batches, as previously reported.3 Cells were pulsed with 1 µCi/well of [3H]thymidine (NEN, Boston, MA) and harvested 16–18 hr later. The results were calculated on the basis of mean counts per minute (c.p.m.) of quadruplicate wells. The set up of test parameters and test validation were as previously described.3
Semiquantification of cytokine transcripts by competitive reverse transcription–polymerase chain reaction (RT–PCR)
Total cellular RNA was isolated by the single-step guanidinium thiocyanate procedure using TRIZOL® Reagent (Gibco). RNAs were reverse transcribed and the cDNA used for competitive PCR, as described previously.15,16 In brief, constant amounts of cDNA (40 ng) were amplified using the different cytokine PCR primers.16 The competitor plasmids, pMUS and pNIL, were diluted fourfold, in nine dilution steps, from a concentration of 3·73 ng/ml (1 × 106 molecules, diluted up 2·5 × 105 to approximately 1 molecule) and added to the target DNA. Relative quantification of cDNA was carried out by calculating how many molecules of competitor were required at the beginning of the PCR to obtain equal amounts of target and competitor amplification products. The cDNA was first standardized to equal concentrations of the housekeeping gene, β2-microglobulin. PCR reactions were performed in 35 cycles, as follows: 20 seconds at 94°, 20 seconds at 56° and 30 seconds at 72°.
Immunoblotting
Expression of the gene encoding 14-3-3 was evaluated in E. multilocularis metacestodes isolated from C57BL/6 wild type (WT) and nude mice total parasite extracts, obtained as described previously.3 The protein concentration in parasite extracts was evaluated using the Bradford method.10 Total extracts were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) in 12·5% gels, in which four samples from each origin (i.e. from WT, nude, TCR-β–/– or MHC-II–/– mice) were loaded in homogeneous final concentrations of 10 µg of protein per lane for each individual sample. Subsequently, separated proteins were transferred onto nitrocellulose membranes. These were incubated with a polyclonal specific antibody against E. multilocularis 14-3-3, which was previously affinity-purified from an anti-E14t hyperimmune mouse serum on the recombinant E14t protein, as described previously.10 The immunoblot reaction was completed by incubation with an alkaline phosphatase-conjugated mouse IgG, and developed as described previously.10 The intensity of the immunoreactive-specific band, in each parasite extract, was measured by densitometry.
Results
Recovered parasite masses from T- and B-cell-deficient mice
To define the potential role of B cell, CD4+, CD8+ and αβ+ T cells in modulating the degree of resistance against E. multilocularis infection, the µMT, nude, MHC-II–/– (CD4-deficient), MHC-I–/– (CD8-deficient) and TCR-β–/– mice, as well as the WT C57BL/6 controls, were infected intraperitoneally with 100 vesicles (high dose) or with a single parasite vesicle (low dose). The metacestode tissues were isolated from the peritoneal cavity and from the liver at various time-points postinfection (p.i.). The total parasite mass was weighed after careful removal of any adjacent host tissue. As shown in Table 1, following infection with 100 vesicles, the parasite masses were comparable in µMT- and WT-infected mice at 1 month p.i. and not significantly increased at 2 months p.i. These data indicate that B cells and antibody production appear to play a minor role in the control of parasite growth. Moreover, the MHC-I–/– showed growth characteristics of E. multilocularis similar to those of the WT controls at 2 months p.i., as shown by comparable parasite masses.
Table 1.
Infection of B- and T-cell deficient mice with a single vesicle or with 100 vesicles per intraperitoneal (i.p.) injection
| 100 vesicles | Single vesicle | ||
|---|---|---|---|
| PW, 1 month | PW, 2 months | PW, 2 months | |
| C57BL/6WT | 148·8 ± 53·0 | 1040 ± 648 | 25 ± 18·3 |
| µMT | 147·3 ± 29·3 | 1546 ± 524·8 | ND |
| Nude | 255·8 ± 178·2 | 14 840 ± 2728** | 3750 ± 456** |
| TCR-β KO | 802·2 ± 254·6* | 11 720 ± 984** | ND |
| MHC-II KO | 352·8 ± 107·9* | 13 750 ± 6125** | ND |
| MHC-I KO | ND | 1075 ± 375 | ND |
C57BL/6WT, B- and T-cell-deficient mice (five per group) were infected with a single vesicle or with 100 vesicles for a period of 1 or 2 months. The metacestodes were collected from the peritoneal cavity and liver, the parasite mass was determined for each individual mouse and the data presented refer to the mean of each group. Results from one out of three experiments with µMT and nude mice, and one out of two experiments concerning the other T-cell-deficient mice are listed, in order to document intratest variation, but to avoid intertest variation.
KO, knockout; MHC-, major histocompatibility complex; PW, parasite weight (in mg); TCR-, T-cell receptor.
P<0·05 (Students t-test);
P<0·01 (Students t-test).
Conversely, the parasite masses were significantly increased in TCR-β–/– and MHC-II–/– mice at 1 month p.i. At 2 months p.i., nude, TCR-β–/– and MHC-II–/– mice started to die as a result of very high parasite loads and diffuse dissemination to, and invasion into, adjacent host organs. The parasite masses in nude, TCR-β–/– and MHC-II–/– mice were significantly (more than 10-fold) increased when compared to those in C57BL/6WT mice. To exclude potential quantitative and qualitative intertest differences, which may occur after high-dose (100 vesicles) inoculation, mice were also infected with single vesicles (minimal dose) obtained from the same original culture. Following single parasite vesicle infection, nude mice exhibited parasite masses that were more than 15 times higher than those in WT mice at 2 months p.i. Together, these data proved the crucial role of T cells – especially CD4+ αβ+ T cells – in the acquired resistance to E. multilocularis infection.
Granuloma formation in E. multilocularis-infected T- and B-cell-deficient mice
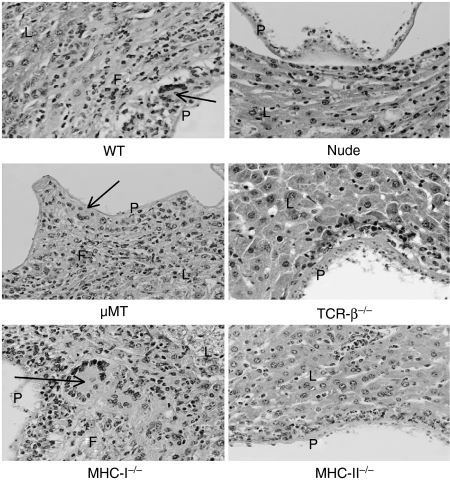
In all T-cell-deficient mice (15 nude mice, 10 TCR-β–/– and MHC-II–/– mice) a marked metastatic dissemination of the intraperitoneal metacestode into various different host organs was observed after only 1 month of infection, including a strong infiltration into the liver, while only six out of 15 C57BL/6 WT and µMT mice, and eight out of 10 MHC-I–/– mice, exhibited parasitic lesions in the liver. Some TCR-β–/– and MHC-II–/– mice had more than one parasitic lesion in the liver; thus, 13 sections from 10 TCR-β–/– mice and 12 sections from 10 MHC-II–/– mice could be investigated (Table 2). In livers of WT controls, a granulomatous inflammation with multinucleated giant cells and a marked fibrosis was consistently observed around parasite cysts by day 30 p.i. Granulomatous lesions also developed in µMT and MHC-I–/– mice (Fig. 1). In contrast, livers of E. multilocularis-infected nude, TCR-β–/– and MHC-II–/– mutants were virtually devoid of granulomatous lesions and fibrosis by day 30 (Fig. 1), and also day 60, p.i. (data not shown).
Table 2.
Granuloma formation in liver tissues of T- or B cell-deficient mice after infection with Echinococcus multilocularis
| No. of granuloma/section/mouse | Fibrosis/section/mice | |
|---|---|---|
| BL/6WT | 6/6/15 | 6/6/15 |
| µMT | 6/6/15 | 6/6/15 |
| Nude | 1/15/15 | 0/15/15 |
| TCR-β KO | 1/13/10 | 0/13/10 |
| MHC-II KO | 0/12/10 | 1/12/10 |
| MHC-I KO | 7/8/10 | 6/8/10 |
Sections of livers from C57BL/6 WT, B- and T-cell-deficient mice containing E. multilocularis metacestode. The periparasitic granuloma and fibrosis were detected by haematoxylin & eosin staining, and herewith presented as ‘positive’ number(s)/total number of section examined/number of mice in reference to the different groups.KO, knockout; MHC-, major histocompatibility complex; TCR-, T-cell receptor.
Figure 1.
Inflammatory reaction to hepatic infection with Echinococcus multilocularis metacestode (visualized by haematoxylin and eosin-stained histology) at day 30 postinfection. A marked granulomatous inflammation, with occasional multinucleated giant cells and a marked fibrosis, was detected around parasite cysts in C57BL/6 WT, µMT, and MHC-I–/– mice. Only a mild inflammatory reaction, with virtually no macrophages and no detectable fibrosis, was present in nude, TCR-β–/– or MHC-II–/– mice. The section numbers of each mouse strain analysed are listed in Table 2. L, intact liver tissue; F, fibrosis with variable numbers of neutrophilic and eosinophilic granulocytes; P, parasite laminated and germinal layer. The arrows indicate a multinucleated giant cell in a layer of macrophages adjacent to the parasite.
The most conspicuous lesions were detected in WT, µMT and MHC-I–/– mice (Fig. 1). The parasites were surrounded by massive layers of granulation tissue with numerous macrophages and multinucleated giant cells, numerous neutrophils and fewer lymphocytes, plasma cells and eosinophils. There was also a multifocal infiltration of neutrophils and eosinophils in the parenchyma and in the portal triads. A considerable number of lymphocytes and plasma cells were also present in the portal triads of MHC-I–/– and wild-type mice. No plasma cells were detected in µMT mice.
The most striking difference to the lesions described above was detected in TCR-β–/–, nude and MHC-II–/– mice, where virtually no granulomatous inflammation was detected. Exceptionally, a small zone of granulomatous inflammation with a few giant cells surrounded the parasite in the liver of one out of 15 sections from nude mice (Table 2), which may have been caused by incomplete deficiencies of T cells in nude mice. Furthermore, there were neither plasma cells nor eosinophils in the inflammatory infiltrate of TCR-β–/– mice. Finally, plasma cells were neither detected in nude nor in MHC-II–/– mice. Thus, our data confirm the essential role of CD4+ αβ+ T helper cells in the formation of peri-parasitic granuloma and point to a minor function of CD8+ T cells and B cells in the host–parasite interplay. Furthermore, the absence of parasite granuloma correlated with high parasite masses and more disseminated parasite foci in the liver of nude, TCR-β–/– and MHC-II–/– mutant mice, which confirms the important role of local granuloma formation in the control of parasite growth and dissemination.
Splenocyte proliferation in response to Con A and antigen stimulation in T- and B-cell-deficient mice
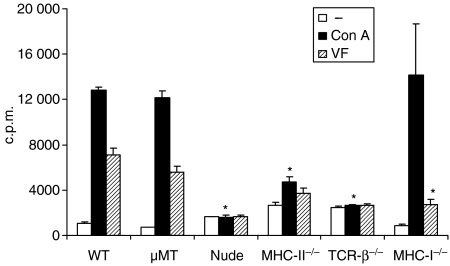
To assess the lymphocyte functions of different B- and T-cell-deficient mice and C57BL/6 WT mice – with or without E. multilocularis infection – the respective splenocytes were stimulated in vitro with Con A and parasite protein VF antigen. For these experiments, spleen cells were isolated from mice infected for 1 month. As shown in Fig. 2, the cellular proliferation in response to Con A and parasite-specific VF antigen was impaired in nude, MHC-II–/– and TCR-β–/– mice. In contrast, the cellular proliferation of µMT and MHC-I–/– mice, in response to Con A, was comparable to the responses of WT mice. The decreased proliferation in response to VF antigen stimulation in MHC-I–/– mice, similarly to the MHC-II–/– mice, indicated that the parasite antigen could stimulate both CD8+ and CD4+ T-cell proliferation.
Figure 2.
Lymphocyte proliferation upon stimulation in vitro with concanavalin A (Con A) and parasitic VF antigen. C57BL/6 WT, µMT, nude, TCR-β–/–, MHC-II–/– and MHC-I–/– mice (five mice per group) were infected intraperitoneally with 100 metacestode vesicles. The proliferative responses to Con A (black bars) and VF antigen (hatched bars) stimulation of pooled spleen cells from 1-month-infected mice were determined in two independent experiments providing identical results (data shown from one representative experiment). Cellular proliferation in response to both Con A and VF-antigen stimulation was impaired in nude, MHC-II–/– and TCR-β–/– mice when compared with WT mice. The proliferative response to VF-antigen stimulation was also impaired in MHC-I–/– mice. *Statistically significant difference between cell-deficient and WT mice.
Cytokine expression in the peritoneal exudate cells (PECs) of T-cell-deficient mice ex vivo
To further analyse the cell-mediated immune responses following E. multilocularis infection in T-cell-deficient mice, the cytokine expression at the infection locus (peritoneum) was analysed by semiquantitative RT–PCR.
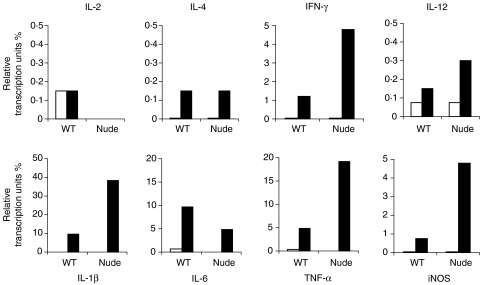
The parasite infection localized primarily in the peritoneum, which resulted in a significant cellular infiltration (predominantly Mac-1+ cells) into the peritoneum.3,6 We analysed the cytokine expression, in recovered PECs ex vivo, of uninfected and 1-month-infected nude and C57BL/6 WT control mice (Fig. 3). Consistent with the findings of previous studies, parasite infection resulted in an increased expression of interleukin (IL)-4 and inflammatory cytokines, including interferon-γ (IFN-γ), IL-1β, IL-6, tumour necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS) in C57BL/6 mice. As expected, the PECs of T-cell-deficient mice did not express the T-cell cytokine, IL-2. IL-4 expression was similar in infected nude and C57BL/6 mice. Interestingly, the expression of IL-12 and IFN-γ in the PECs of infected nude mice was significantly increased in association with an increased expression of the inflammatory cytokines IL-1β, TNF-α and iNOS, when compared to those of infected WT mice. The PECs of infected MHC-II–/– and TCR-β–/– mice also expressed increased levels of IFN-γ, IL-12, IL-1β, TNF-α and iNOS when compared with infected C57BL/6 WT control mice (data not shown). These data indicate a non-T-cellular source of IFN-γ and IL-4 in T-cell-deficient nude mice following an E. multilocularis infection, and an increased inflammatory response in T-cell-deficient mice in association with a higher parasite load.
Figure 3.
Cytokine mRNA levels in peritoneal exudate cells (PECs) – isolated from uninfected (white bars) and 1-month-infected (black bars) C57BL/6 WT and nude mice (five mice per group) – were determined by semiquantitative competitive reverse transcription–polymerase chain reaction. Cytokine transcripts were standardized to the levels of β2-microglobulin transcripts and quantified by using fourfold dilutions of the competitive plasmid, pMUS. The results are presented as relative transcription units (cytokine mRNA levels/β2-microglobulin mRNA level). Data show one representative out of three independent experiments with nude mice. Identical patterns were found in one experiment performed with MHC-II–/– and one with TCR-β–/– mice. c.p.m., counts per minute.
Evaluation of 14-3-3 expression in E. multilocularis metacestodes recovered from WT and T-cell-deficient mice
Immune control of E. multilocuaris metacestode proliferation might include the regulative manipulation of parasite protein synthesis within the germinative metacestode compartment. In this respect, we addressed the expression levels of 14-3-3 protein as a putative marker for the parasite growth kinetics in T-cell-deficient versus WT mice. Semiquantification was approached by measuring the specific band intensity by immunoblot in several parallel samples. For comparative purposes, an arbitrary value of 1·0 was assigned to the intensity corresponding to the specific 14-3-3 band in the parasite isolated from the WT mouse number 1. The results shown in Fig. 4 demonstrate that the metacestodes isolated from nude mice contained approximately double the amount of 14-3-3 protein than those isolated from WT mice. TCR-β–/– mice exhibited a similar increase in 14-3-3 protein expression when compared with WT mice (data not shown).
Figure 4.
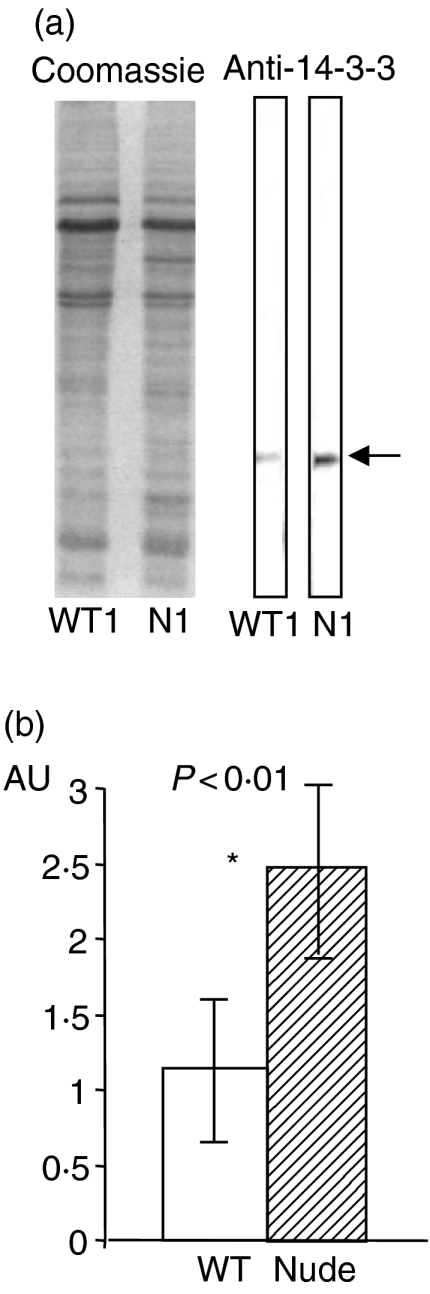
The Coomassie-stained gel and immunoblot (a) represent one out of four samples for WT and nude (N) mice, respectively. The arrow indicates the 14-3-3 specific reactivity in the immunoblot. Identical results were achieved with samples collected from infected MHC-II–/– and TCR-β–/– mice (data not shown). Immunoblotting was performed with affinity-purified mouse anti(rec)14-3-3 immunoglobulin and in vivo-expressed 14-3-3 protein. Immunoblot results were semiquantitatively assessed by densitometry (b), bars representing the mean value ± 2 standard deviations (SD) of AU (arbitrary antibody units) of samples from four WT and four nude mice. *Statistically significant differences (P < 0·01).
Discussion
The participation of immunological events in the control of AE has been strongly supported by the unusually rapid proliferation of the parasite in immunodeficient SCID mice as well as in an AE patient co-infected with HIV.8,9 Immunosuppressive therapy after liver transplantation in AE patients may favour larval growth.17 However, the role of individual cell subsets of the immune system in the control of parasite growth is still unknown. By using genetically mutant mice, deficient in specific B- or T-cell subsets, we demonstrated that the CD4+ αβ+ T-cell-mediated cellular immunity appears to be essential for the resistance against E. multilocularis infection.
B-cell and antibody-mediated responses are important in protection, especially against extracellular bacteria. During infection with an extracellular metazoan parasite, such as E. multilocularis, the respective contributions of B cells and antibodies have not yet been clearly determined.1 Immunocompetent patients and mice with AE both respond to infection with a marked multiclonal production of antibodies against protein and carbohydrate antigens.3,18 To date, parasite-specific antibodies have not been shown to exhibit any direct restricting role on the growth of an established metacestode. In the present experiments, the absence of functional B cells, and thus antibody production, in µMT mice, appeared to render them no more susceptible to parasite proliferation than WT mice, which confirmed the minor role of B cells and antibody production in the defence against secondary infection with intact metacestode vesicles. However, if the mature metacestode loses its protection by the shielding carbohydrate-rich laminated layer, rendering the germinal layer accessible to, i.e. antibodies, then the immune system will regain the potential to kill the parasite.2
It has been shown that elevated numbers of activated CD8+ T cells are present in the peripheral blood during the chronic state of infection.19 Crude unfractionated protein antigen pulsed with dendritic cells can stimulate autologous CD8+ lymphocyte proliferation in vitro.20 Our proliferation data in MHC-I-deficient mice also indicated that the parasite antigen (VF) stimulated CD8+ T-cell proliferation. However, the functional role of these multiclonally proliferating and activated CD8+ T cells have not yet been elucidated. On the other hand, the CD8+ lymphocytes may contribute to the immunsuppression phenomenon investigated in AE.21 There was a definite increase in the expression of IL-10 in CD8+ lymphocytes from AE patients.22 Our present data point to a minor role of CD8+ T cells in the murine immune defence against secondary infection with intact metacestode vesicles. The LL, with its impressive high-molecular-weight carbohydrate matrix, may present a natural barrier preventing the direct cytotoxic effect of CD8+ T cells. The potential bilateral effects of CD8+ lymphocytes (immune protective versus suppressive effect) in AE need to be further characterized.
In contrast to MHC-I–/– mice, the TCR-β–/– and MHC-II–/– (CD4-deficient) animals were highly susceptible to infection, similarly to nude mice. These data highlight the crucial role that CD4+ αβ+ T cells seem to play during the immunological control of parasite growth. Usually, the IFN-γ-dependent T helper 1 cell-mediated mechanisms contribute to the control of infection with intracellular pathogens.15 Based on in vitro and some in vivo studies, T helper 2-dominated immunity is associated with increased susceptibility to disease, while T helper 1 cell activation is assumed to induce protective immunity in AE.23–26 Surprisingly, E. multilocularis infec-tion resulted in an increased expression of IL-12 and IFN-γ in peritoneal cells of T-cell-deficient mice (i.e. in those cells that have the potential to directly contact the parasite). The IFN-γ signalling was associated with an increased inflammatory response, reflected by elevated levels of TNF-α, IL-1β and iNOS. These findings indicate that T cells do not represent the major source of IFN-γ during infection with E. multilocularis in T-cell-deficient mice. IFN-γ is well known to be produced by T cells and natural killer (NK) cells. Some recent studies indicated that antigen-presenting cells, include dendritic cells and macrophages, are potent IFN-γ-producing cells.27,28 The potential cellular source of IFN-γ in T-cell-deficient mice will have to be addressed in future experiments. Moreover, the high parasite load found in nude, MHC-II–/– and TCR-β–/– mice, together with a strong inflammatory response, suggests that the failure of immunological control in T-cell-deficient mice is not dependent on the IFN-γ signalling pathway and the type 1 inflammatory cytokine production. These findings are similar to those found in Babesia microti infections, where the CD4+ αβ+ T-cell-mediated immunity, but not IFN-γ, were essential for resolution of the pathogen.29 In fact, in humans, the treatment of late-stage AE with IFN-γ was not found to be particularly successful.30 The protective effect of IFN-γ treatment, prior to infection, was not as strong as seen during treatment with IL-1225 and IFN-α31 in mice. In previous investigations we showed that the strong inflammatory responses, especially the elevated production of iNOS, were acting against the protective mechanisms produced during murine E. multilocularis infection.32,33 This seems to oversimplify the complex situation observed in vivo by easily linking T helper 1-mediated inflammatory cytokine production with protection during the chronic stage of AE infection. The important protective effect of type 1 cytokines (IL-12, IFN-γ, IFN-α, TNF-α) may occur at a very early innate stage of infection.26
Despite the increased IFN-γ signalling-mediated stronger inflammatory responses in nude, TCR-β–/– and MHC-II–/– mice, the absence of T cells, especially CD4+ αβ+ T cells, resulted in an impaired cellular immune response, reflected by a decreased lymphocyte proliferative response and impaired periparasitic granuloma formation in the liver. Granulomatous reactions, however, are essential to limit locally the growth of infectious agents and for the successful elimination of various pathogens. For E. multilocularis, cellular infiltration and fibrosis in periparasitic granuloma may contribute to limiting the parasite proliferation and dissemination to other sites. In fact, in addition to a high parasite mass, the parasite dissemination into the liver and other adjacent organs took place at an earlier stage in nude, TCR-β–/– and MHC-II–/– mice when compared with WT and µMT mice. Furthermore, the lack of a granulomatous inflammation in TCR-β–/– and MHC-II–/– mice indicated that αβ+ and CD4+ T cells were essential for mounting a granulomatous inflammatory response. In one out of 15 sections from nude mice, and one out of 13 sections from TCR-β–/– mice, we found a weak granulomatous inflammation surrounding the parasite. Nude mice are not always completely T-cell deficient and γδ T cells may play an auxiliary compensatory role in granuloma formation.34
Finally, we had shown previously that the infiltrative growth of the metacestode is based upon a progressive proliferation of the germinal layer, which could be potentially associated with the relative over-expression of the parasite 14-3-3 protein at this developmental stage.10 We therefore addressed the question of whether the expression level of the 14-3-3-gene is higher in the highly proliferating metacestode status encountered in nude, MHC-II–/– and TCR-β–/– mice, when compared to the WT mouse, using similar quantities of metacestode tissue masses. We found an increased relative 14-3-3 expression in the immune-deficient mouse strains, and in all strains the effect was also associated with a high parasite load. Claiming that the observed higher expression of 14-3-3 in T-cell-deficient mice also reflects a more active germinal cellular proliferation of the metacestode tissue, we consequently need to study the underlying mechanisms as to how T cells alter 14-3-3 expression. This may be associated with altered periparasitic physiological conditions, including granuloma and fibrosis. We are planning to search for the actual molecular instruments responsible for regulating the parasite 14-3-3 expression.
In summary, our data, obtained with T-cell-deficient mice, demonstrate the crucial role that CD4+ αβ+ T-cell-mediated cellular immune responses can play in the protection against secondary E. multilocularis infection, while CD8+ T and B cells appeared to be less important. The protective, or growth-suppressing, effect mediated by CD4+ αβ+ T cells was revealed not to be dependent on the IFN-γ signalling pathway, but rather to be associated with local granuloma formation in the periparasitic hepatic host tissue and the regulation of the 14-3-3 protein expression in the metacestode tissue.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant no. 31-63615·00), the Interreg II-project no. BWA 30·027 and the EU EchinoRisk-project QLK2-CT-2001–01995 (BBW no. 00·0586–1).
References
- 1.Gottstein B, Hemphill A. Immunopathology of echinococcosis. Chem Immunol. 1997;66:177–208. doi: 10.1159/000058670. [DOI] [PubMed] [Google Scholar]
- 2.Gottstein B, Dai WJ, Walker M, Stettler M, Muller M, Hemphill A. An intact laminated layer is important for the establishment of secondary Echinococcus multilocularis infection. Parasitol Res. 2002;88:822–8. doi: 10.1007/s00436-002-0659-7. [DOI] [PubMed] [Google Scholar]
- 3.Dai WJ, Hemphill A, Waldvogel A, Ingold K, Deplazes P, Mossmann H, Gottstein B. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of alphabeta+ CD4+ T cells. Infect Immun. 2001;69:6074–83. doi: 10.1128/IAI.69.10.6074-6083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresson-Hadni S, Liance M, Meyer JP, Houin R, Bresson JI, Vuitton D. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:378–83. doi: 10.1111/j.1365-2249.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emery I, Liance M, Deriaud D, Vuitton D, Houin R, Leclerc C. Characterization of T-cell immune responses of Echinococcus multilocularis infected C57BL/6 mice. Parasite Immunol. 1996;18:463–72. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 6.Vuitton DA, Bresson-Hadni S, Laroche L, Kaiserlian D, Guerret-Stocker S, Bresson JL, Gillet M. Cellular immune response in Echinococcus multilocularis infection in humans. II. Natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:67–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Playford MC, Kamiya M. Immune response to Echinococcus multilocularis infection in the mouse model. Jpn J Vet Res. 1992;40:113–30. [PubMed] [Google Scholar]
- 8.Playford M, Ooi HK, Oku Y, Kamiya M. Secondary Echinococcus multilocularis infection in severe combined immunodeficient (scid) mice: biphasic growth of the larval cyst mass. Int J Parasitol. 1992;22:975–82. doi: 10.1016/0020-7519(92)90056-q. [DOI] [PubMed] [Google Scholar]
- 9.Sailer M, Soelder B, Allerberger F, Zaknun D, Feichtinger H, Gottstein B. Alveolar echinococcosis of the liver in a six-year-old girl with acquired immunodeficiency syndrome. J Pediatr. 1997;130:320–3. doi: 10.1016/s0022-3476(97)70364-0. [DOI] [PubMed] [Google Scholar]
- 10.Siles-Lucas M, Felleisen RS, Hemphill A, Wilson W, Gottstein B. Stage-specific expression of the 14-3-3 gene in Echinococcus multilocularis. Mol Biochem Parasitol. 1998;91:281–93. doi: 10.1016/s0166-6851(97)00208-9. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe MB. How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513:53–7. doi: 10.1016/s0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- 12.Stavridi ES, Chehab NH, Malikzay A, Halazonetis T. Substitutions that compromise the ionizing radiation-induced association of p53 with 14-3-3 proteins also compromise the ability of p53 to induce cell cycle arrest. Cancer Res. 2001;61:7030–3. [PubMed] [Google Scholar]
- 13.Takihara Y, Matsuda Y, Hara J. Role of the beta isoform of 14-3-3 proteins in cellular proliferation and oncogenic transformation. Carcinogenesis. 2002;21:2073–7. doi: 10.1093/carcin/21.11.2073. [DOI] [PubMed] [Google Scholar]
- 14.Siles-Lucas M, Nunes CP, Zaha A. Comparative analysis of the 14-3-3 gene and its expression in Echinococcus granulosus and Echinococcus multilocularis metacestodes. Parasitology. 2001;122:281–7. doi: 10.1017/s0031182001007405. [DOI] [PubMed] [Google Scholar]
- 15.Dai WJ, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5297–304. [PubMed] [Google Scholar]
- 16.Kopf M, Brombacher F, Kohler G, et al. IL-4-deficient BALB/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–36. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bresson-Hadni S, Koch S, Beurton I, et al. Primary disease recurrence after liver transplantation for alveolar echinococcosis: long-term evaluation in 15 patients. Hepatology. 1999;30:857–64. doi: 10.1002/hep.510300426. [DOI] [PubMed] [Google Scholar]
- 18.Gottstein B, Wunderlin E, Tanner I. Echinococcus multilocularis: parasite-specific humoral and cellular immune response subsets in mouse strains susceptible (AKR, C57B1/6J) or ‘resistant’ (C57B1/10) to secondary alveolar echinococcosis. Clin Exp Immunol. 1994;96:245–52. doi: 10.1111/j.1365-2249.1994.tb06549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manfras BJ, Reuter S, Wendland T, Kern P. Increased activation and oligoclonality of peripheral CD8(+) T cells in the chronic human helminth infection, alveolar echinococcosis. Infect Immun. 2002;70:1168–74. doi: 10.1128/IAI.70.3.1168-1174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenne L, Arrighi JF, Sauter B, Kern P. Dendritic cells pulsed with unfractionated helminthic proteins to generate antiparasitic cytotoxic T lymphocytes. Parasite Immunol. 2001;23:195–201. doi: 10.1046/j.1365-3024.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 21.Kizaki T, Kobayashi S, Ogasawara K, Day NK, Good RA, Onoe K. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J Immunol. 1991;147:1659–66. [PubMed] [Google Scholar]
- 22.Kilwinski J, Jenne L, Jellen-Ritter A, Radloff P, Flick W, Kern P. T lymphocyte cytokine profile at a single cell level in alveolar Echinococcosis. Cytokine. 1999;11:373–81. doi: 10.1006/cyto.1998.0432. [DOI] [PubMed] [Google Scholar]
- 23.Emery I, Liance M, Leclerc C. Secondary Echinococcus multilocularis infection in A/J mice: delayed metacestode development is associated with Th1 cytokine production. Parasite Immunol. 1997;19:493–503. doi: 10.1046/j.1365-3024.1997.d01-162.x. [DOI] [PubMed] [Google Scholar]
- 24.Liance M, Ricard-Blum S, Emery I, Houin R, Vuitton DA. Echinococcus multilocularis infection in mice: in vivo treatment with a low dose of IFN-gamma decreases metacestode growth and liver fibrogenesis. Parasite. 1998;5:231–7. doi: 10.1051/parasite/1998053231. [DOI] [PubMed] [Google Scholar]
- 25.Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 26.Vuitton DA. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop. 2003;85:119–32. doi: 10.1016/s0001-706x(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 27.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–8. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzue K, Asai T, Takeuchi T, Koyasu S. In vivo role of IFN-gamma produced by antigen-presenting cells in early host defense against intracellular pathogens. Eur J Immunol. 2003;33:2666–75. doi: 10.1002/eji.200323292. [DOI] [PubMed] [Google Scholar]
- 29.Clawson MI, Paciorkowski N, Rajan T, et al. Cellular immunity, but not gamma interferon, is essential for resolution of Babesia microti infection in BALB/c mice. Infect Immun. 2002;70:5304–6. doi: 10.1128/IAI.70.9.5304-5306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenne L, Kilwinski J, Radloff P, Flick W, Kern P. Clinical efficacy of and immunologic alterations caused by interferon gamma therapy for alveolar echinococcosis. Clin Infect Dis. 1998;26:492–4. doi: 10.1086/516316. [DOI] [PubMed] [Google Scholar]
- 31.Godot V, Harraga S, Podoprigora G, Liance M, Bardonnet K, Vuitton DA. IFN alpha-2a protects mice against a helminth infection of the liver and modulates immune responses. Gastroenterology. 2003;124:1441–50. doi: 10.1016/s0016-5085(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 32.Dai WJ, Gottstein B. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology. 1999;97:107–16. doi: 10.1046/j.1365-2567.1999.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai WJ, Waldvogel A, Jungi T, Stettler M, Gottstein B. Inducible nitric oxide synthase deficiency in mice increases resistance to chronic infection with Echinococcus multilocularis. Immunology. 2003;108:238–44. doi: 10.1046/j.1365-2567.2003.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1998;25:3525–8. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]