Abstract
Antigen-presenting cells, including dendritic cells, monocytes and macrophages, produce members of the interleukin-12 (IL-12) family that are important in initiating and maintaining cell-mediated immune responses. These include IL-12p35 and p19 that dimerize with IL-12p40 to form IL-12 (also termed IL-12p75) and IL-23, respectively, and Epstein–Barr virus-induced gene 3 (EBI3) protein (a protein related to IL-12p40), that forms a dimer with p28, termed IL-27. Intestinal epithelial cells, which are the initial site of contact between the host and enteric pathogens, can act as antigen-presenting cells, and are known to express mediators important in inflammatory and immune responses. In the current studies, we hypothesized that intestinal epithelial cells express members of the IL-12 family, which can function as an early signalling system important in mucosal immunity. Using in vitro and in vivo model systems of human intestinal epithelium, we demonstrate the regulated expression of EBI3, IL-12p35 and p19 by human intestinal epithelial cells. However, intestinal epithelial cells do not coexpress IL-12p40 or p28 that are required to generate heterodimeric IL-12p75, IL-23 and IL-27. To the extent that IL-12p35, p19 and EBI3 cannot form IL-12p75, IL-23 or IL-27 heterodimers in intestinal epithelial cells, these data suggest that those cells may express other, currently unknown, molecules that can associate with EBI3, IL-12p35 and/or p19 or, alternatively, intestinal epithelial cells may release IL-12-related molecules that by themselves, or in combination with other molecules in the mucosal microenvironment, mediate biological activities.
Keywords: Epstein–Barr virus-induced gene-3, intestinal epithelial cells, IL-12-related molecules, IL-12p35, p19, p28, intestinal xenograft
Introduction
The single layer of intestinal epithelial cells that lines the intestinal tract is an initial site of contact between the host and ingested microbial pathogens. Moreover, intestinal epithelial cells can function as antigen-presenting cells1 and produce cytokines, chemokines and other immune mediators that are important in signalling the onset of mucosal immune and inflammatory responses.2–8
Members of the interleukin (IL)-12 family of proteins are produced by antigen-presenting cells including monocytes, macrophages and dendritic cells9–14 and have an important role in the development of T helper 1 (Th1)-polarized CD4+ T-cell responses. The prototypic member of this family, IL-12p75, is a heterodimeric cytokine that contains two independently regulated subunits, IL-12p40 and IL-12p35.9,10,15,16 IL-12p75 stimulates interferon-γ (IFN-γ) production by T and natural killer (NK) cells and has a key role in the induction and maintenance of Th1 immune responses.17–21 Additional members of the IL-12 family of proteins share significant homology with IL-12p35 or IL-12p40. For example, p19 is related to IL-12p35 and forms a heterodimer with IL-12p40, termed IL-23.22 IL-23 in humans is expressed by activated dendritic cells and has biological activities similar to as well as distinct from those of IL-12p75.22
Epstein–Barr virus (EBV)-induced gene-3 (EBI3) encodes a 34 000 MW glycoprotein that is structurally related to IL-12p40.23 Like IL-12p40, EBI3 is encoded by mRNA with a 3′ untranslated Alu repeat sequence, lacks a membrane anchoring motif, and is predicted to be secreted.23 EBI3 is expressed by human B lymphoblast cell lines, placental syncytiotrophoblasts and extravillous trophoblasts, activated dendritic cells, and by macrophage-like cells in the lamina propria of the human colon in patients with ulcerative colitis.23–26 As part of a cDNA microarray analysis, we discovered that EBI3 mRNA was expressed by human colon epithelial cell lines.27 Initially, EBI3 was reported to non-covalently complex with IL-12p35 to form an EBI3/p35 heterodimer whose biologic function is as yet unknown.28 More recently, EBI3 was found to heterodimerize with an IL-12p35-related molecule, p28, to form the cytokine IL-27·29 Human IL-27 stimulates the clonal expansion of naive CD4+ T cells and, in synergy with IL-12, increases IFN-γ production by T cells.29 As shown in studies of EBI3-deficient mice, EBI3 and/or EBI3-dependent molecules also have a regulatory role in the induction of IL-4-mediated Th2 immune responses and the development of Th2-mediated inflammation in vivo, that appear to be mediated through their role in regulating the function of NK T cells.30
The key role of intestinal epithelium in interfacing with microbial pathogens in the human intestinal mucosa coupled with the recognition of EBI3 as an IL-12p40-related molecule prompted us to hypothesize that the intestinal epithelium could be an important site for the production of IL-12-related molecules. In the present study we used in vitro and in vivo models, to examine the regulated expression of EBI3 by human intestinal epithelium and to determine if intestinal epithelial cells express other IL-12-related molecules. We report that three IL-12-related molecules, EBI3, IL-12p35, and p19, but not their known respective dimerization partners, p28 and IL-12p40, are differentially regulated and expressed by human intestinal epithelial cells.
Materials and methods
Cytokines and antibodies
Recombinant human (rh)IL-1α, rhIL-4, rhIL-12, and rhIL-13 were from Pepro Tech (Rocky Hill, NJ), recombinant human tumour necrosis factor-α) (rhTNF-α) was from R & D Systems (Minneapolis, MN), and rhIFN-γ was from BioSource International (Camarillo, CA). MG-132, control mouse ascites fluid (from mice bearing the NS-1 myeloma) and rabbit IgG were from Sigma Chemical (St. Louis, MO). Affinity-purified rabbit anti-human EBI3 and mouse monoclonal antibodies to EBI3 (20H11 and 23H4) were as previously reported.23,25 Donkey anti-rabbit immunoglobulin, sheep anti-mouse immunoglobulin, and streptavidin–horseradish peroxidase (HRP) were from Amersham Pharmacia Biotech (Piscataway, NJ). Biotinylated anti-mouse immunoglobulin and streptavidin–HRP (LSAB2 System) were from DAKO Corporation (Carpinteria, CA).
Intestinal epithelial cell lines
The human colon adenocarcinoma cell lines HT-29 (ATCC HTB 38) and Caco-2 (ATCC HTB 37) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and 2 mm l-glutamine. T84 cells were grown in 50% DMEM/50% Ham's F12 medium supplemented with 5% newborn calf serum.3 To obtain polarized epithelial monolayers, Caco-2 cells were grown on microporous collagen-coated filter inserts (0·4 µm pore size; Transwell; Costar, Cambridge, MA) for ∼14 days, at which time a mean transepithelial resistance of 350 Ω°m2 was established.
Bacterial infection of cell lines
HT-29 and Caco-2 human colon epithelial cells were grown to confluence in six-well plates and infected with Salmonella enteritica serovar Dublin strain Lane (S. dublin)3 at a multiplicity of infection (MOI) of 100 for 1 hr to allow bacterial invasion to occur, after which extracellular bacteria were removed by washing, as described before.6 Cells were subsequently incubated in the presence of 50 µg/ml of the non-membrane-permeant antibiotic gentamicin to kill remaining extracellular bacteria.
Adenovirus constructs and adenovirus infection
Confluent HT29 cells in six-well tissue culture plates were infected with recombinant adenovirus containing an IκBα-AA superrepressor (Ad5-IκB A32/36) or the Escherichia coliβ-galactosidase gene (Ad5-LacZ) as we described before.7,31 After infection, adenovirus was removed by washing, fresh medium containing serum was added, and cells were incubated for an additional 12 hr before stimulation with proinflammatory cytokines.
Human intestinal xenografts
Human intestinal xenografts were generated as described before.6,32–36 Briefly, human fetal intestine, gestational age 16–20 weeks (Advanced Biosciences Resources, Alameda, CA) was transplanted subcutaneously onto the backs of C57BL/6 SCID mice. Intestinal xenografts were allowed to mature for at least 10 weeks after transplantation, at which time the epithelium, which is strictly of human origin, is fully differentiated.35 For cytokine stimulation of the xenografts, mice carrying mature xenografts were injected i.p. with 1 µg of rhIL-1α and 10 µg rhIFN-γ in 200 µl phosphate-buffered saline (PBS), or with 200 µl of PBS alone as a control. For infection, xenografts were injected intraluminally with ∼5 × 107 of attenuated aroA aroC S. typhi in DMEM/F12 medium in a 100-µl volume. Xenografts were removed 6 hr after cytokine injection or infection and were frozen in liquid nitrogen for RNA isolation and fixed in formalin for immunohistology.
Reverse transcription (RT)–polymerase chain reaction (PCR)
Total cellular RNA was extracted using an acid guanidinium-phenol-chloroform method (TRIzol Reagent; Gibco-BRL Life Technologies, Grand Island, NY) and treated with ribonuclease-free deoxyribonuclease (Stratagene, La Jolla, CA). RT– PCR was performed as described before.3 The following primers were used to amplify a 225 bp fragment of IL-12p35: Sense primer 5′-TTC ACC ACT CCC AAA ACC TGC-3′; antisense primer 5′-GAG GCC AGG CAA CTC CCA TTA G-3′. The primers used to amplify a 151 bp fragment of p19 were: Sense primer 5′-TTC CCC ATA TCC AGT GTG GAG-3′; antisense primer 5′-TCA GGG AGC AGA GAA GGC TC-3′, and the primers used to amplify a 132 bp fragment of p28 were: Sense primer 5′-ATC TCA CCT GCC AGG AGT GAA-3′; antisense primer 5′-TGA AGC GTG GTG GAG ATG AAG-3′. Primers for the amplification of human EBI3, human IL-12p40, and human β-actin mRNA were as we have described before.3,27 The amplification profile for EBI3 was 35 cycles of 45 s denaturation at 95° and 2·5 min annealing and extension at 63°. IL-12p40, p28 and p19 were amplified for 35 cycles and β-actin was amplified for 28 cycles under the same conditions, except the annealing temperatures were 65° for IL-12p40, 66° for p19, and 72° for β-actin, respectively. The amplification profile for IL-12p35 was 35 cycles of 1 min denaturation at 94°, 1 min annealing at 57°, and 1 min extension at 72°.
Real-time PCR
Real-time PCR was performed using an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). Each reaction contained 25 µl of 2× SYBR Green Master Mix (containing 200 µm dATP, dGTP, and dCTP; 400 µm dUTP; 2 mm MgCl2, 0·25 units uracil N-glycosylase, and 0·625 units Amplitaq Gold DNA polymerase), 25 pmol each of sense and antisense primers, and 2 µl of cDNA in a final volume of 50 µl. IL-12p35, p19 and p28 primers were as described above, EBI3 primers were: sense primer 5′-AGC ACA TCA TCA AGC CCG AC-3′; antisense primer 5′-GCT CCC TGA CGC TTG TAA CG-3′, and β-actin primers were: sense primer 5′-CAA AGA CCT GTA CGC CAA CAC-3′; antisense primer 5′-CAT ACT CCT GCT TGC TGA TCC-3′. Reactions were incubated at 50° for 2 min followed by 95° for 10 min. The amplification profile was 15-s denaturation at 95° followed by 1 min annealing at 60° for EBI3 and p28, at 57° for IL-12p35, at 59° for p19, and at 62° for β-actin for a total of 40 cycles. Amplification of the expected single products was confirmed on 1% agarose gels stained with ethidium bromide. Data analysis used sequence detection system software provided by the manufacturer where ΔRn was calculated using the equation ΔRn=(Rn+) − (Rn–) where Rn+ is the fluorescence signal of the product and Rn– is the fluorescence signal of the baseline emission. Ct is the cycle number at which the ΔRn crosses threshold. Fold changes in EBI3, IL-12p35, and p19 mRNA expression were determined as: fold change = 2ΔCt where ΔCt=(Ct EBI3/p35/p19 control − Ct actin control) −(Ct EBI3/p35/p19 stimulated − Ct actin stimulated).
Immunohistology
Immunohistology was performed using sections from formalin-fixed paraffin embedded normal human colon and small intestinal biopsies, and from human colon and small intestinal xenografts. Sections were deparaffinized, washed with PBS, and pretreated with 3% H2O2 in water for 5 min at room temperature. Sections were then stained with monoclonal mouse anti-human EBI3 antibody as ascites fluid (IgG2aκ, 1 : 100 dilution) and mouse myeloma ascites fluid as a control, followed by staining with a biotinylated anti-mouse immunoglobulin and streptavidin–HRP using the DAKO LSAB2 System. Sections were developed with 3,3-diaminobenzidine (DAB Peroxidase Substrate Kit, Vector Laboratories, Inc., Burlingame, CA), which yields a brown-coloured product. Before mounting, sections were counterstained with haematoxylin and eosin.
Immunoblot analysis
Supernatants from confluent epithelial cell monolayers in six-well plates were harvested and concentrated fivefold using a Centricon YM-3 filter (Millipore Co., Bedford, MA). Equivalent volumes of concentrated supernatant were size-fractioned on denaturing, non-reducing 10% polyacrylamide minigels (Mini-PROTEAN II; Bio-Rad Laboratories), and electrophoretically transferred to nitrocellulose membranes (0·1 µm pore size). Specific protein was detected using rabbit antihuman EBI3 (1 : 200 dilution) followed by incubation for 1 h with biotinylated donkey anti-rabbit immunoglobulin G (IgG; 1 : 1000 dilution), after which streptavidin–HRP was applied. Specifically bound peroxidase was detected by enhanced chemiluminescence (ECL System; Amersham Pharmacia Biotech) and exposure to X-ray film (XAR5; Eastman Kodak Company, Rochester, NY) for 5–15 min.
All studies involving human subjects and animal protocols were approved by the UCSD Committee on Human Subjects and the UCSD Animal Subjects Program.
Results
Constitutive and regulated EBI3, IL-12p35, p19, p28 and IL-12p40 mRNA expression in human intestinal epithelial cell lines
To determine the capacity of intestinal epithelium to produce IL-12-related molecules, we first evaluated expression of the six known members of the IL-12 family in a cell culture model of human intestinal epithelium. EBI3 is known to dimerize with an IL-12p35 like molecule, p28, to form IL-2729 and was reported to dimerize also with IL-12p35 in placental trophoblasts.28 As shown by qualitative (Fig. 1a) and real time RT–PCR (Fig. 2a), HT-29 human colon epithelial cells constitutively expressed EBI3 mRNA and EBI3 mRNA levels were markedly increased when cells were stimulated with IL-1α or TNF-α. Further, S. dublin infection up-regulated EBI3 mRNA levels, consistent with our observations from cDNA microarray analyses.27 In contrast, EBI3 was minimally, if at all, affected by stimulation of cells with IFN-γ although IFN-γ markedly potentiated TNF-α or IL-1α stimulated EBI3 mRNA expression.
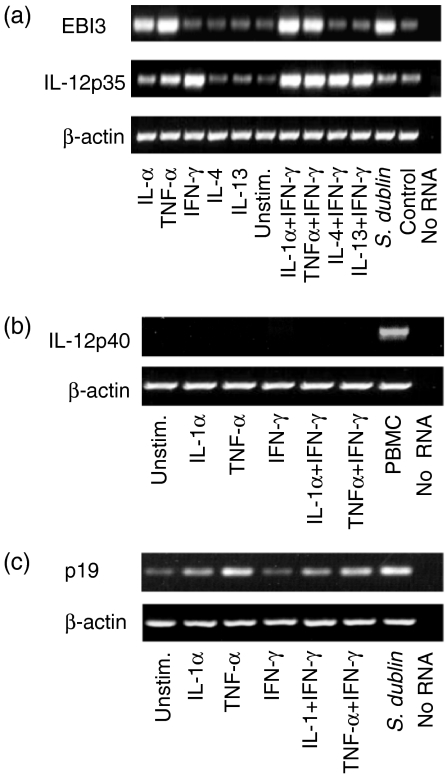
Figure 1.
Regulation of EBI3, IL-12p35, IL-12p40 and p19 mRNA expression in human intestinal epithelial cell lines. HT-29 cells grown to confluence were stimulated for 8 h with the indicated cytokines (20 ng/ml, except 40 ng/ml for IFN-γ), infected with Salmonella dublin, or not stimulated or infected (control lanes) (a) RT–PCR for EBI3, IL-12p35 (35 cycles) and β-actin (28 cycles) (b) RT–PCR for IL-12p40 (c) RT–PCR for p19. Results are from a representative experiment. Similar results were obtained in three independent experiments. In the lane labelled ‘no RNA’, RNA was omitted from RT reaction and PCR amplification. Similar results were found for Caco-2 and T84 intestinal epithelial cell lines.
Figure 2.
Increased epithelial EBI3 and IL-12p35 mRNA expression in response to cytokine stimulation or bacterial infection. HT-29 cells were stimulated with the indicated cytokines or infected with S. dublin. Controls were left unstimulated or uninfected for the same duration. EBI3 and IL-12p35 mRNA levels were assayed by real time PCR 8 and 24 hr after cytokine stimulation or infection, respectively. Values are fold-increase ± SEM, n = 3, of stimulated or infected cultures relative to matched control cultures.
HT-29 cells also constitutively expressed IL-12p35 mRNA, but in contrast to EBI3, IL-12p35 mRNA was markedly up-regulated by IFN-γ stimulation, but not IL-1α or TNF-α, although those cytokines increased IFN-γ-stimulated levels of IL-12p35 mRNA (Figs 1a and 2b). If EBI3 produced by colon epithelial cells forms dimers within the cell with IL-12p35, we predicted that the up-regulated expression of those subunits in response to stimulation with proinflammatory cytokines (i.e. TNF-α and IFN-γ) might follow a similar time course. This was not the case as EBI3 mRNA levels were maximal by 8 hr after cytokine stimulation and returned towards baseline by 12–15 hr whereas IL-12p35 mRNA levels increased 12–15 hr after stimulation, reached maximal levels at 24 hr and returned towards baseline at 48 hr.
Intestinal epithelial cells did not express IL-12p40 mRNA constitutively or in response to cytokine stimulation3 (Fig. 1b), although in controls IL-12p40 was expressed by human PBMC (Fig. 1b). Similarly, HT-29 and Caco-2 intestinal epithelial cells did not express p28 constitutively or in response to TNF-α, IL1α, or IFN-γ stimulation, as assessed by qualitative and real time PCR, although p28 was constitutively expressed by human peripheral blood mononuclear cells (data not shown). Nonetheless, HT-29 cells constitutively expressed mRNA for p19 (Fig. 1c), a dimerization partner of IL-12p40. p19 mRNA expression in HT-29 cells was up-regulated by IL-1α and TNF-α stimulation as well as by S. dublin infection (Fig. 1c). By real-time PCR, IL-1α, TNF-α and S. dublin induced a fourfold, 20-fold and sevenfold increase in p19 transcripts, respectively, compared to unstimulated controls. Similar results were found using Caco-2 cells, a second human colon epithelial cell line (data not shown). Taken together, these studies indicate that human colon epithelial cells express and can regulate the expression of EBI3, IL-12p35 and p19, but do not express the currently known dimerization partners for those molecules that result in the generation of biologically active IL-12, IL-23 and IL-27.
EBI3 is an nuclear factor (NF)-κB target gene
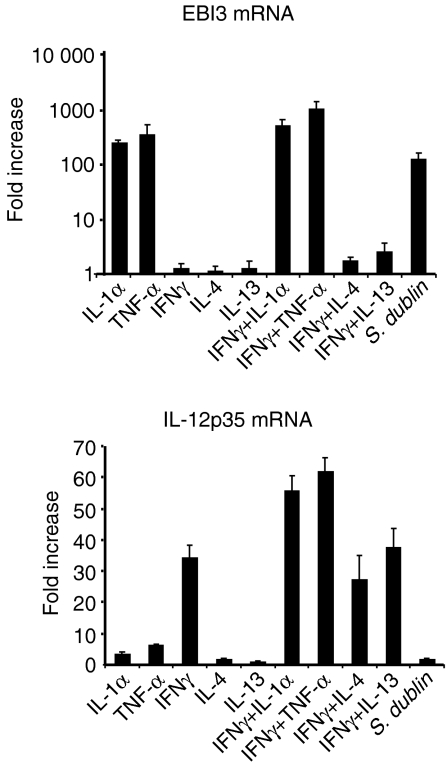
Given the novel observation of EBI3 expression by human intestinal epithelium, in contrast to the complete absence of constitutive or induced expression of its homologue, IL-12p40, further studies focused on the mechanism of EBI3 regulation in intestinal epithelial cells in vitro and in vivo. Because both IL-1α and TNF-α up-regulate EBI3 mRNA expression and both are known to activate cellular signalling pathways that activate the transcription factor NF-κB, we tested whether the up-regulated expression of EBI3 depended on the activation of NF-κB. We assessed this initially using the proteosome inhibitor MG-132 as a pharmacologic inhibitor of NF-κB activation. MG-132 treatment of cells before IL-1α or TNF-α stimulation significantly inhibited cytokine-induced EBI3 mRNA expression (Fig. 3) irrespective of whether or not cells were additionally stimulated with IFN-γ. In contrast, MG-132 did not inhibit IFN-γ induction of IL-12p35 mRNA, irrespective of IL-1α or TNF-α stimulation. Because pharmacological inhibitors are not always specific, in further studies we also used a genetic approach to block NF-κB activation. HT-29 cells were infected with a recombinant adenovirus that expresses a mutant IκBα protein (Ad5IκB-A32/36) with serine-to-alanine substitutions at positions 32 and 36 and acts as a superrepressor of NF-κB activation or with control adenovirus expressing LacZ.31 As shown in Fig. 3, EBI3 mRNA expression in response to TNFα stimulation was markedly inhibited in Ad5IκB-A32/36-infected cells compared to cells infected with control adenovirus, as assessed by real time PCR.
Figure 3.
Effect of NF-κB inhibition on EBI3 and IL-12p35 mRNA expression. (a) HT-29 cells grown to confluence were treated with the proteasome inhibitor MG-132 (50 µm) for 30 min prior to stimulation with IL-1α or TNF-α (20 ng/ml) alone or in combination with IFN-γ (40 ng/ml) or left unstimulated (unstim.), followed by RT–PCR analysis for EBI3, IL-12p35, and β-actin mRNA. (b) HT-29 cells left uninfected, infected with control adenovirus (AdLacZ), or adenovirus expressing mutant IκBα as a superrepressor (AdSR) were left unstimulated or stimulated with TNF-α or IFN-γ, after which EBI3 and IL-12p35 mRNA expression was determined by real time PCR. Values are expressed as fold induction relative to cells that were not virus infected. Similar results were obtained in 3 repeated experiments.
Polarized epithelial cell secretion of EBI3
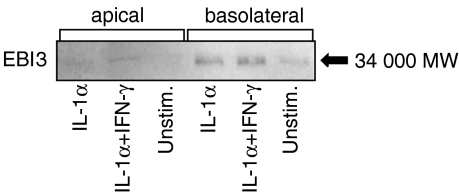
To determine if agonist-stimulated EBI3 expression was accompanied by increased EBI3 protein secretion, intestinal epithelial cells were left untreated or were stimulated with cytokines, after which protein levels in culture supernatants were assayed by immunoblot analysis. Since intestinal epithelial cells in vivo are morphologically and functionally polarized, we modelled the polarized state in vitro by growing Caco-2 cells as polarized monolayers in transwells and assayed EBI3 secreted into the apical and basolateral transwell chambers. As shown in Fig. 4, IL-1α stimulation markedly increased EBI3 secretion, which was little, if at all, further increased by costimulation with IFN-γ. Moreover, consistent with secretion in the physiologic basolateral direction, EBI3 was mainly secreted into the basolateral chamber. Non-polarized HT-29 cells stimulated with IL-1α, but not IFN-γ also secreted EBI3 (data not shown).
Figure 4.
EBI3 protein secretion in response to proinflammatory cytokines. Polarized Caco-2 cells were stimulated with IL-1α (20 ng/ml) and IFN-γ (40 ng/ml) alone or in combination added to the basal and apical wells of a transwell chamber, or left unstimulated. Apical and basal chamber supernatants were harvested after 16 hr, and assayed for EBI3 protein by immunoblot analysis.
Regulated expression of EBI3 and IL-12p35 in human intestinal xenografts
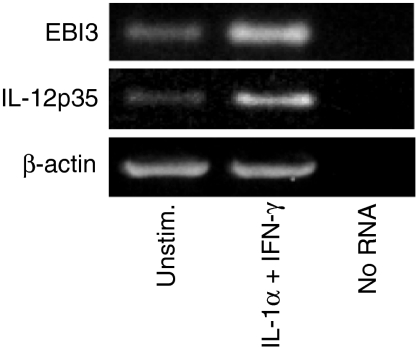
To determine if EBI3 and IL-12p35 are also expressed and regulated by human colon epithelium in vivo, we used a human intestinal xenograft model. The xenografts have an intact epithelium that is strictly human in origin, and lack a commensal bacterial flora.37 Mice with mature human colon or small intestinal xenografts were injected intraperitoneally with rhIL-1α and rhIFN-γ in combination or with PBS as a control, after which the xenografts were harvested and EBI3 and IL-12p35 mRNA expression was assessed. EBI3 and to a lesser extent IL-12p35 were constitutively expressed in control xenografts, and were markedly up-regulated by IL-1α and IFN-γ injection (Fig. 5).
Figure 5.
Expression of EBI3 and IL-12p35 mRNA in human fetal intestinal xenografts. Severe combined immune deficiency mice carrying human fetal colon xenografts were injected intraperitoneally with PBS or IL-1α (1 µg) and IFN-γ (10 µg). After 6 hr, xenografts were removed and total xenograft RNA was used for RT–PCR analysis of EBI3, IL-12p35, and β-actin mRNA expression. In the lane labeled ‘no RNA’, RNA was omitted from RT reaction and PCR amplification
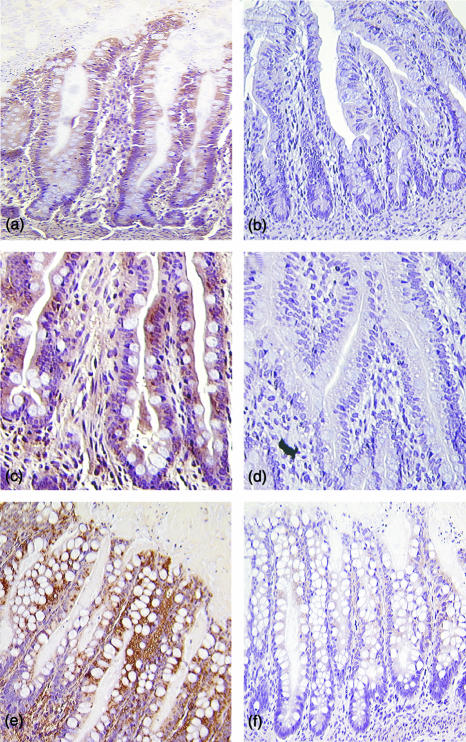
To determine if the up-regulated expression of EBI3 mRNA in the xenografts was accompanied by increased epithelial production of EBI3, small intestinal xenografts from mice injected with rhIL-1α and rhIFN-γ were immunostained for EBI3. As shown in Fig. 6, epithelium of control xenografts constitutively expressed EBI3 (Fig. 6a) and epithelial EBI3 staining was markedly increased in xenografts from the cytokine injected mice (Fig. 6c). In additional experiments, small intestinal and colon xenografts were infected intraluminally with S. typhi and harvested 6 hr after infection. EBI3 immunostaining was markedly increased in response to infection as shown for a colon xenograft in Fig. 6(e). These data demonstrate the regulated expression and production of EBI3 by human intestinal epithelium in vivo.
Figure 6.
Immunohistochemical detection of EBI3 in human intestinal xenografts. Xenografts were immunostained using murine monoclonal antibody to EBI3 (a, c and e) or control mouse ascites (panels b, d and f). (a,b) Small intestine from control xenografts; (c,d) small intestine from xenografts of mice were injected with IL-1α (1 µg) and IFN-γ (10 µg) as in Fig. 5; (e,f) colon from xenografts infected with S. typhi. Original magnification × 200.
Immunostaining of EBI3 in normal human colon and small intestine
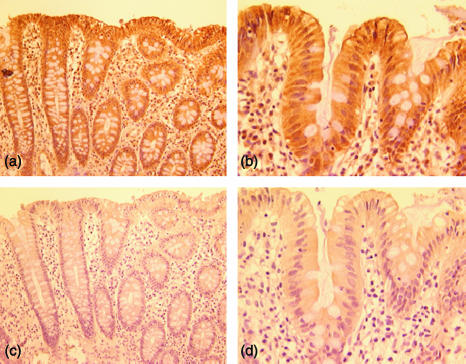
To determine if EBI3 protein is constitutively present in human intestinal epithelial cells in vivo, sections from normal human colon and small intestinal biopsies were immunostained for EBI3. As shown in Fig. 7(a, b), EBI3 was expressed in normal human colon and small intestine and its expression was predominately observed in epithelial cells.
Figure 7.
Immunohistochemical detection of EBI3 in normal colon and small intestine. Normal human colon (a and c) and small intestine (b and d) were immunostained using a murine monoclonal antibody to EBI3 (20H11) (a and b) or control mouse ascites (c and d). Original magnification: (a and c) ×200; (b and d) ×400. Similar results were obtaining using monoclonal antibody 23H4 (not shown).
Discussion
In this study we tested the hypothesis that human intestinal epithelial cells produce IL-12-related molecules, a property that is generally attributed mainly to macrophages and dendritic cells.9–14,22,24,29 Our underlying rationale was that human intestinal epithelial cells can function as antigen-presenting cells and, in response to proinflammatory signals or microbial infection, produce cytokines and chemokines that are important for signalling mucosal immune and inflammatory responses.2,3,5,7,8,31,38 Using a spectrum of in vitro and in vivo model systems, we report herein on the constitutive and regulated expression by human intestinal epithelial cells of three IL-12-related molecules; EBI3, IL-12p35 and p19. EBI3 is an IL-12p40-related molecule known to dimerize with p28 to form the cytokine IL-27,29 IL-12p35 is a dimerization partner of IL-12p40 that is required to form IL-12p70 and may also complex with EBI3,28 whereas p19 is an IL-12p35-related molecule that forms a heterodimer with IL-12p40 to produce the cytokine IL-23.22
Human colon epithelial cells in vitro and in vivo constitutively expressed EBI3 and EBI3 expression was up-regulated by proinflammatory mediators (e.g. IL-1α and TNF-α) that are released by lymphoid cells and monocytes in the intestinal mucosa during acute and chronic inflammatory reactions. These same proinflammatory mediators up-regulate the production of a group of chemokines by intestinal epithelial cells, which in turn can chemoattract cells that mediate mucosal innate and acquired mucosal immune responses (e.g. the CXC chemokines CXCL-8/IL-8 and CXCL1/GROα that chemoattract neutrophils, and the CC-chemokines CCL2/MCP-1, CCL20/MIP-3α, and CCL22/MDC that chemoattract monocytes, dendritic cells and a population of T cells, respectively.5,7,8 Further, like many of those chemokine genes7,8,31 EBI3 expression by intestinal epithelial cells was rapidly up-regulated by proinflammatory stimuli, and consistent with its up-regulated expression in response to IL-1α and TNF-α, was shown by pharmacological and genetic approaches to function as a NF-κB target gene. Those results suggest a potential role for EBI3 in the early host response to inflammation or infection. Although EBI3 is known to dimerize with p28 to generate IL-27 that functions in regulating Th1 type responses29 intestinal epithelial cells do not produce p28 and therefore are not a source of IL-27. In addition, EBI3 may form a non-covalently bound heterodimer with IL-12p3526,28 but no function is currently known for possible EBI3/p35 heterodimers.
We found that intestinal epithelial cells express IL-12p35 mRNA. Although the only known potential pairing partner for IL-12p35 in intestinal epithelial cells was EBI3, the kinetics of regulated expression and the response to proinflammatory stimuli of EBI3 and IL-12p35 differed markedly. Thus, IL-12p35 expression was up-regulated mainly by IFN-γ rather than TNF-α or IL-1α, and its expression was markedly delayed relative to that of EBI3. Such data suggest a lack of coordinate regulation of IL-12p35 and EBI3 in intestinal epithelial cells. Further, IL-12p35 is known to be poorly, if at all, secreted in the absence of dimerization to a secreted molecule like IL-12p40 or EBI328,39 and, relevant to its possible binding to EBI3 in the extracellular environment, we note that little EBI3 was shown to complex with IL-12p35 in solution.28 Whereas placental tissue produces both EBI3 and IL-12p35, significant levels of secreted EBI3/p35 complexes could not be detected in placental culture explants.26 Moreover, we did not detect EBI3/p35 heterodimers in supernatants of the intestinal epithelial cell lines studied herein by immunoblot analysis (not shown), although we cannot exclude their presence in amounts below the detection limit of the assay.
p19 mRNA was also expressed and regulated by intestinal epithelial cells and its known dimerization partner, like IL-12p35, is IL-12p40. Notably, the regulated expression of p19 and IL-12p35 differed, suggesting they may have different functions. Thus p19 paralleled EBI3 in its response to IL-1α and TNF-α suggesting a more likely role in acute inflammatory responses, whereas IL-12p35 was minimally responsive to those cytokines but markedly up-regulated by IFN-γ, perhaps pointing to a more prominent role in cell-mediated immune responses. As we also reported before3 intestinal epithelial cells do not express IL-12p40 and as a corollary cannot produce either IL-12p75 or IL-23, as those cytokines, like IL-27, are formed intracellularly by heterodimerization of their respective subunits, and are secreted as preformed heterodimers.22,29,39 The expression of p19mRNA in the absence of IL-12p40 expression is not limited to intestinal epithelial cells, as TNF-α-activated endothelial cells and some Th1- and Th2-polarized T cells also were noted to express p19 in the apparent absence of IL-12p40.22
Proinflammatory mediators produced in the intestinal mucosa can differentially regulate the production of IL-12-related molecules by intestinal epithelial cells. It is surprising then, that intestinal epithelial cells, in contrast to human airway epithelial cells40 do not express the IL-12p40 dimerization partner of IL-12p35 or p19 that results in the production of IL-12p75 and IL-23, or p28 that pairs with EBI3 to generate the functional cytokine IL-27. It appears that intestinal epithelial cells are programmed to respond to host inflammatory and environmental stimuli by up-regulating the expression of IL-12-related molecules, but not those that are known to lead to the generation of functional cytokines that influence the development of Th1-type immune responses. It is possible that EBI3, IL-12p35 and/or p19 can be released into the mucosal microenvironment by intestinal epithelial cells and act, by themselves or in combination with other molecules, to mediate biological activities. Alternatively, it should be considered that intestinal epithelial cells might produce still undiscovered pairing partners for one or more of those IL-12 related molecules.30,41
Acknowledgments
We thank J. R. Smith for technical support and Dr N. Varki for assistance with immunostaining studies. This work was supported by NIH grants DK 35108 and DK 58960. C.M. was supported by the Deutsche Forschungsgemeinschaft (MA 2247/1-1). L.E. was supported in part by a Research Grant from the Crohn's and Colitis Foundation of America.
References
- 1.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–8. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 2.Eckmann L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines. regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 3.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 5.Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–23. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 6.Eckmann L, Stenson WF, Savidge TC, Lowe DC, Barrett KE, Fierer J, Smith JR, Kagnoff MF. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin H synthase-2 expression and prostaglandin E2 and F2α production. J Clin Invest. 1997;100:296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1217–26. doi: 10.1152/ajpgi.2001.280.6.G1217. [DOI] [PubMed] [Google Scholar]
- 8.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–9. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 9.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes. selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–50. [PubMed] [Google Scholar]
- 10.Snijders A, Hilkens CM, van der Pouw Kraan TC, Engel M, Aarden LA, Kapsenberg ML. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]
- 11.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–55. [PubMed] [Google Scholar]
- 13.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 14.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 15.Bette M, Jin SC, Germann T, Schafer MK, Weihe E, Rude E, Fleischer B. Differential expression of mRNA encoding interleukin-12 p35 and p40 subunits in situ. Eur J Immunol. 1994;24:2435–40. doi: 10.1002/eji.1830241026. [DOI] [PubMed] [Google Scholar]
- 16.Cassatella MA, Meda L, Gasperini S, D'Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 18.Chan SH, Kobayashi M, Santoli D, Perussia B, Trinchieri G. Mechanisms of IFN-γ induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J Immunol. 1992;148:92–8. [PubMed] [Google Scholar]
- 19.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- 21.Yap G, Pesin M, Sher A. Cutting edge. IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–31. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 22.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–53. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto SI, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. Identification of genes specifically expressed in human activated and mature dendritic cells through serial analysis of gene expression. Blood. 2000;96:2206–14. [PubMed] [Google Scholar]
- 25.Christ AD, Stevens AC, Koeppen H, Walsh S, Omata F, Devergne O, Birkenbach M, Blumberg RS. An interleukin 12-related cytokine is up-regulated in ulcerative colitis but not in Crohn's disease. Gastroenterology. 1998;115:307–13. doi: 10.1016/s0016-5085(98)70197-0. [DOI] [PubMed] [Google Scholar]
- 26.Devergne O, Coulomb-L'Hermine A, Capel F, Moussa M, Capron F. Expression of Epstein–Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol. 2001;159:1763–76. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckmann L, Smith JR, Housley MP, Dwinell MB, Kagnoff MF. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J Biol Chem. 2000;275:14084–94. doi: 10.1074/jbc.275.19.14084. [DOI] [PubMed] [Google Scholar]
- 28.Devergne O, Birkenbach M, Kieff E. Epstein–Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–6. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4 (+) T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwenhuis EE, Neurath MF, Corazza N, et al. Disruption of T helper 2-immune responses in Epstein–Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci USA. 2002;99:16951–6. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999;163:1457–66. [PubMed] [Google Scholar]
- 32.Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–83. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent F, Eckmann L, Savidge TC, Morgan G, Theodos C, Naciri M, Kagnoff MF. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–73. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurent F, Kagnoff MF, Savidge TC, Naciri M, Eckmann L. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2α production. Infect Immun. 1998;66:1787–90. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savidge TC, Morey AL, Ferguson DJ, Fleming KA, Shmakov AN, Phillips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–71. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 36.Seydel KB, Li E, Swanson PE, Stanley SL., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–9. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–63. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dwinell MB, Lugering N, Eckmann L, Kagnoff MF. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology. 2001;120:49–59. doi: 10.1053/gast.2001.20914. [DOI] [PubMed] [Google Scholar]
- 39.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med. 2001;193:339–51. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–12. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]