Abstract
Jun N-terminal kinase (JNK) has been implicated in the pathogenesis of inflammatory diseases including asthma. We examined the effect of SP600125 (anthra [1,9-cd] pyrazol-6 (2H)-one), a novel inhibitor of JNK in a model of asthma. Brown-Norway rats were sensitized to ovalbumin and treated with SP600125 intraperitoneally (90 mg/kg in total). SP600125 inhibited allergen-induced, increased activity of phosphorylated c-jun but not of phosphorylated-MAPKAPK2, indicative of activation of p38 MAPK, in the lung. SP600125 inhibited macrophage (P < 0·04), lymphocyte (P < 0·05), eosinophil (P < 0·04) and neutrophil (P < 0·005) numbers in bronchoalveolar lavage. Eosinophil and T-cell accumulation in the airways, mRNA expression for interleukin-1β, tumour necrosis factor-β, interleukin-3, interleukin-4 and interleukin-5, serum levels of allergen-specific immunoglobulin E and bronchial hyperresponsiveness were not affected by SP600125. Selective inhibition of JNK reduced inflammatory cell egress into the airway lumen after single allergen exposure. The role of JNK mitogen-activated protein kinase activation may be limited in the pathogenesis of bronchial hyperresponsiveness after single allergen exposure.
Keywords: asthma: bronchial hyperresponsiveness, inflammation; enzymes: Jun N-terminal kinase; inhibitors: SP600125
Introduction
Asthma is a chronic inflammatory airway disease characterized by variable airflow obstruction and non-specific bronchial hyperresponsiveness.1 An imbalance between CD4+ T helper type 1 (Th1) and 2 (Th2) immune responses has been postulated as underlying the pathogenesis of asthma and bronchial hyperresponsiveness. Expression of the Th2-derived cytokines, particularly interleukin-4 (IL-4) and IL-5, is increased in the airways of patients with asthma, expressed by CD4+ T cells, while the expression of the Th1-derived cytokine interferon-γ is unchanged.2 In animal models, suppression or genetic deletion of Th2 cytokines, such as IL-4 and IL-5, prevents the development of airway inflammation and bronchial hyperresponsiveness;3,4 In addition, transfer of allergen-specific CD4+ Th2 cells into naïve recipient rats induces bronchial hyperresponsiveness and allergic inflammation, an effect reversed by allergen-specific Th1 cells partly through interferon-γ.5
Three major mitogen-activated protein kinase (MAPK) families that differ in their substrate specificity have been identified in vertebrates and have been implicated in the pathogenesis of inflammatory diseases such as asthma: Jun N-terminal kinase (JNK), extracellular regulating kinase (ERK) and p38 kinase.6 MAPKs phosphorylate selected intracellular proteins, including transcription factors, which subsequently regulate gene expression via transcriptional and post-transcriptional mechanisms. The JNK group MAPKs is activated by exposure of cells to a variety of stimuli, including cytokines, reactive oxygen species and environmental stress.7 JNK isoforms, encoded by three genes, phosphorylate specific sites (serine 63 and serine 73) on the amino-transactivation domain of c-jun following exposure to ultraviolet irradiation, growth factors or cytokines.6 JNK-1 and JNK-2 have been identified in the lungs, whilst JNK-3 has been localized to the brain.8 JNKs enhance the transcriptional activity of activator protein-1, which in turn activates a wide range of immunomodulatory genes9 including those implicated in the pathogenesis of asthma.10
There are several lines of evidence that implicate JNK in the pathogenesis of allergic inflammation. JNK is activated during costimulation of T cells11 and may contribute to the secretion of IL-2 and the proliferation of thymocytes.12 In addition, JNK is also activated during the polarization of CD4+ T cells into effector Th1 and Th2 cell populations.13 Cross-linking of the immunoglobulin E (IgE) receptor or ultraviolet irradiation, heat shock and hyperosmotic stimulation of the high-affinity IgE receptor (FcεRI) results in activation of JNK in mast cells.14 JNK may also play a role in the expression of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α)15 and in the transcription of E-selectin, essential for leucocyte adhesion and infiltration.16 These processes may be central to the development of bronchial inflammation and the expression of bronchial hyperresponsiveness.
We determined the role played by JNK activation in allergic inflammation induced by single exposure to ovalbumin in ovalbumin-sensitized Brown-Norway rats17 by using SP600125, an anthrapyrazolone reversible ATP-competitive inhibitor of JNK.18
Materials and methods
Animals, sensitization procedures and allergen-exposure
Pathogen-free inbred male Brown-Norway rats (Harlan Olac Ltd. Bicester, UK) (200–250 g, 9–13 weeks old) were injected with 1 ml of 1 mg ovalbumin in 100 mg Al(OH)3 suspension in 0·9% (wt/vol) saline intraperitoneally on three consecutive days. Ovalbumin aerosol exposure (15 min; 1% ovalbumin) of rats was performed in a 6·5-L Plexiglas chamber connected to a DeVilbiss PulmonSonic nebulizer (model no. 2512, DeVilbiss Health Care, UK Ltd, Middlesex, UK) that generated an aerosol mist pumped into the exposure chamber by the airflow supplied by a small animal ventilator (Harvard Apparatus Ltd, Edenbridge, Kent, UK) set at 60 strokes/min with a pumping volume of 10 ml.
Protocol
Three groups of rats actively sensitized with ovalbumin (Grade V, salt-free) and Al(OH)3 were studied. Group 1 were sham-treated and saline-exposed animals (Saline group, n = 10): sensitized animals received vehicle for SP600125 (1·6 ml/dose of 10% ethanol, 15% Cremophor-EL, 30% PEG-400, 20% propylene glycol in sterile saline) by intraperitoneal injection 2 hr prior to saline aerosol (15 min) and 8 and 16–22 hr after (2 hr prior to measurements). Group 2 were sham-treated and ovalbumin-exposed animals (Ovalbumin group, n = 10). The procedures were the same as for group Saline above, except the aerosol exposure was with 1% ovalbumin aerosol. Group 3 were SP600125-treated and ovalbumin-exposed animals (SP600125 group, n = 10): The procedures were the same as for group ovalbumin, except that the compound SP600125 was used to treat animals (30 mg/kg in 1·6 ml vehicle, three doses over 2 days).
Sp600125
SP600125 (anthra [1,9-cd] pyrazole-6 (2H)-one) is a novel JNK inhibitor synthesized by the Signal Research Division of Celgene, Inc., San Diego, CA. SP600125 is active against JNK-1, -2 and -3 with a 50% activity inhibitory concentration (IC50) of 0·04–0·09 μm.18 SP600125 is selective for JNK over several related MAPKs such as ERK and p38, the IC50 is > 10 μm. According to the findings of a previous study in rats19 we used the dose of 30 mg/kg.
Measurement of bronchial responsiveness
Bronchial responsiveness was measured 18–24 hr after the final allergen challenge as previously described.20 Briefly, rats were monitored for airflow by whole body plethysmography with a pneumotachograph (Infodisp, Bordon, Hants, UK) connected to a transducer (Infodisp), transpulmonary pressure was measured via an oesophageal catheter and blood pressure was recorded via carotid artery catheterization (Infodisp). The signals from the transducers were analysed with infodisp software, which is programmed to calculate instantaneously pulmonary resistance (RL). Increasing half-log10 concentrations of acetylcholine were administered by inhalation for 45 breaths and lung resistance was measured. The concentration of acetylcholine required to increase baseline resistance to 200% (PC200) was determined by linear interpolation of log concentration–lung resistance curves.
Bronchoalveolar lavage and cell counting
This is described in detail elsewhere.21 Briefly, after an overdose of anaesthetic, rats were lavaged with a total of 20 ml 0·9% sterile saline via the endotracheal tube. Total cell counts, viability and differential cell counts from cytospin preparations stained by May–Grünwald–Giemsa stain were determined under an optical microscope (Olympus BH2, Olympus Optical Company Ltd, Tokyo, Japan). At least 500 cells were counted and identified as macrophages, eosinophils, lymphocytes and neutrophils according to standard morphology under × 400 magnification.
Collection of lung tissues
Rats were killed using an overdose of sodium pentobarbitone (500 mg/kg; intraperitoneally). The lungs were rapidly removed and insufflated with O.C.T. Tissue Tek™ mounting medium (Raymond A Lamb, London, UK) diluted 1 : 1 with phosphate-buffered saline. Regions of the left and right lung lobes were mounted on cork blocks with the main bronchi uppermost, snap-frozen in melting isopentane and stored at −25°.
Eosinophil major basic protein (MBP) and T-cell immunohistochemistry
Detailed methods have previously been described.17 Briefly, for the detection of eosinophils, cryostat sections were incubated with an IgG1 monoclonal antibody against human MBP, clone BMK-13 (Monosan, Uden, the Netherlands). After labelling with a biotinylated horse anti-mouse monoclonal secondary antibody, positively stained cells were visualized using the alkaline phosphatase–anti-alkaline phosphatase method. For staining of CD2+, CD4+, CD8+ T lymphocytes in tissue sections, sections were incubated with either mouse anti-rat CD2, CD4, or CD8 monoclonal antibody (pan T-cell markers, Pharmingen, Cambridge Bioscience, Cambridge, UK). After labelling with a biotinylated goat anti-mouse monoclonal secondary antibody, positively stained cells were visualized using the alkaline phosphatase–anti-alkaline phosphatase method. All sections were counterstained with Harris hamatoxylin (BDH, Poole, Dorset, UK) and mounted in Glycergel (Dako, Ely, Cambridgeshire, UK). Cellular influx around the five largest airways in each lung section was assessed as the number of positively stained cells in the bronchial submucosa expressed per mm of basement membrane.
Detection of ovalbumin-specific IgE by enzyme-linked immunosorbent assay (ELISA)
Blood (2–3 ml) was left to clot for 1–2 hr at room temperature and centrifuged for 15 min at 1000 g. Serum was aspirated, aliquoted into Eppendorf tubes, and stored frozen at −20°. The assay was modified from that of Nonaka et al.22 Briefly, 96-well plates were coated overnight with MARE-1 mouse anti-rat IgE (mouse anti-rat IgE heavy chain, 1 mg/ml, 1/2000 dilution) in 0·5 μg/ml in carbonate bicarbonate buffer (ovalbumin buffer). A standard curve was constructed using doubling dilutions of standards (rat serum identified to be of high IgE titre) and samples were added and incubated for 2 hr. Biotinylated-ovalbumin (prepared using a EZ-Link Sulfo-NHS-LC-Biotinylation Kit; Pierce & Warriner, Chester, UK) was added at optimized concentration (∼10 μg/ml) and incubated for 90 min, followed by incubation for 45 min with 1 μg/ml streptavidin-alkaline phosphatase. Colour development was with 0·1 m substrate (p-nitro-phenyl phosphate enzyme) for up to 10 min. Antibody binding was determined from the absorbance at 405 nm. Titres were expressed as optical density (OD). A naïve control group of animals (n = 4) was also studied.
Western blot analysis
Immunoblot analysis was performed according to Laemmli.23 Frozen lung tissue samples were thawed in cold lysis buffer (1% Triton X-100, 1% sodium dodecyl sulphate (SDS), 1·5% deoxycholate, 20 mm Tris-base pH 7·4, 150 mm NaCl, 20 mm ethylene diaminetetraacetic acid, 2 mm phenylmethyl sulphonyl fluoride, 2 mm sodium orthovanadate, 20 μg/ml leupeptin, 200 μg/ml aprotinin, 10 mm sodium fluoride and 20 mm sodium pyrophosphate), homogenized with an Ultra-turrex and centrifuged at 13 000 g for 30 min at 4°. Supernatant was removed and centrifuged again. Lung supernatant lysates (50 μg protein) were mixed with sample buffer (62·5 mm Tris–HCl, 20% glycerol, 2% SDS, 10 mm 2-mercaptoethanol, 0·05% bromophenol blue), boiled for 5 min and stored at −70° until used for Western blot analysis.
Briefly, 50 µg of total lung protein per lane were separated through 8% denaturing-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in the following buffer (Tris 20 mm, pH 7·6, NaCl 140 mm and 0·1% Tween) and then incubated for 1 hr with monoclonal antibodies anti-non-phosphorylated c-jun (c-jun) (Cell Signalling Technology, Beverly, MA) and anti-phosphorylated c-jun (p-c-jun) (Cell Signalling Technology) as markers of JNK activity or polyclonal antibodies anti-phosphorylated-MAPKAPK2 (p-c-MAPKAPK2) (Cell Signalling Technology), a marker of p38 MAPK activity. The secondary antibody was horseradish peroxidase-conjugated sheep anti-mouse or anti-rabbit (diluted to 1: 10 000) and ECL reagent was used for detection. Positive controls were used to visualize the presence of potentially relevant differences in ovalbumin-exposed lungs. Each filter was reprobed with an anti-human α-actin (control for p-MAPKAPK-2) (Santa Cruz biotechnology, UK). The bands, which were visualized by autoradiography, were quantified using a densitometer with grab-it and gelworks software (UVP, Cambridge, UK).
RNase protection assays
Total RNA was isolated from approximately 100 mg of fresh lung tissue after homogenization in 1 ml of Ultraspec RNA solution (Biotecx, Houston, TX, USA) according to the manufacturer's instructions. 32P-labelled riboprobes (dUTP) were generated using an in vitro transcription kit and the rat cytokine multiprobe template set, which consists of probes for IL-1β, TNF-α, TNF-β, IL-2, IL-3, IL-4, IL-5, IL-10 and interferon-γ, according to the manufacturer's specifications. These antisense probes were hybridized with total RNA, then treated with a mixture containing RNase A + T1 from an RNase protection kit. The resulting hybrids were resolved on a 6% polyacrylamide-urea gel and analysed by autoradiography and quantified using a densitometer with grab-it and gelworks software (UVP).
Data analysis
Data are presented as the mean ± SEM. For comparisons of different groups, Kruskall–Wallis test for analysis of variance was used. If the Kruskall–Wallis test for analysis of variance was significant, we applied Mann–Whitney U-test for comparison between two individual groups. The data were analysed using the graphpad™for windows statistical package. A P-value of less than 0·05 was considered significant.
Results
Bronchial responsiveness to ACh
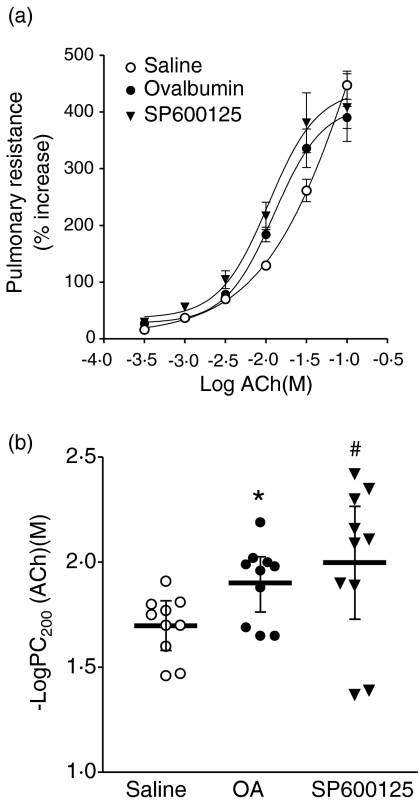
There was no significant difference in baseline lung resistance between the groups (data not shown). There was a leftward shift of the concentration–response curve of sensitized, ovalbumin-exposed rats compared to sensitized, saline-exposed rats, and SP600125-treated rats continued to show a similar leftward shift after allergen exposure (Fig. 1a). Sensitized, sham-treated and ovalbumin-exposed rats had a significant increase in mean −logPC200 compared to sensitized saline-exposed rats (P < 0·05, Fig. 1b). The drug SP600125 showed no effect on allergen-induced bronchial hyperresponsiveness, as reflected by the persistent leftward shift of acetylcholine concentration–lung resistance response curve and elevated PC200 compared to saline-exposed rats. In addition, in preliminary studies, we found that SP600125 had no effect on baseline responsiveness to acetylcholine in sensitized unexposed rats (data not shown).
Figure 1.
(a) Mean percentage increase in lung resistance to increasing concentrations of acetylcholine (ACh) for three groups of sensitized rats: sham-treated and saline aerosol exposed, n = 10; sham-treated and ovalbumin-aerosol-challenged, n = 10; treated with SP600125 and challenged with ovalbumin aerosol, n = 10. The concentration–response curves are significantly shifted leftward for both the Ovalbumin group and the SP600125 group, compared to the Saline group. (b) Mean −logPC200, the negative logarithm of the provocative concentration of ACh needed to increase baseline lung resistance by 200%, is shown for the three groups of rats as detailed in (a). Treatment with SP600125 did not alter allergen-induced increase in −logPC200. *P < 0·05 for Ovalbumin group or #P < 0·05 for the SP600125 group compared to the Saline group. Data are shown as mean ± SEM.
Inflammatory cell responses
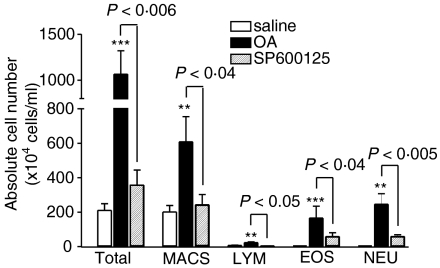
There was a significant increase in the numbers of total cells (P < 0·001), eosinophils (P < 0·001), macrophages (P < 0·01), lymphocytes (P < 0·01) and neutrophils (P < 0·01) recovered in the bronchoalveolar lavage fluid of sensitized rats exposed to ovalbumin compared to sensitized rats exposed to saline (Fig. 2). SP600125 significantly reduced the ovalbumin-induced increase in eosinophil (P < 0·05), macrophage (P < 0·05), lymphocyte (P < 0·05) and neutrophil counts (P < 0·005, Fig. 2).
Figure 2.
Effect of SP600125 on the mean numbers of total cells (Tot), macrophages (Macs), eosinophils (Eos), lymphocytes (Lym) and neutrophils (Neu) in bronchoalveolar lavage fluid in groups of rats as specified in Fig. 1. Total cells, eosinophils, lymphocytes, and neutrophils were significantly increased in sensitized rats exposed to ovalbumin-aerosol. SP600125 treatment suppressed the increase in macrophages, lymphocytes, eosinophils and neutrophils. **P < 0·01 and ***P < 0·001 as compared to saline group; other P-values are as indicated. Data are shown as mean ± SEM.
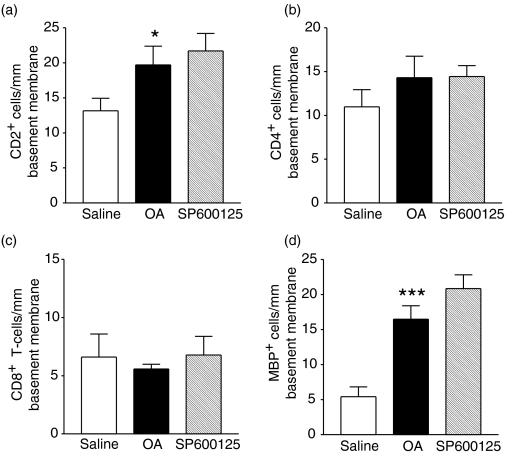
Allergen exposure of sensitized rats caused a significant increase in the airway submucosal infiltration of MBP+ eosinophils (P < 0·001) and CD2+ T lymphocytes (P < 0·05, Fig. 3). SP600125 did not alter the number of MBP+ eosinophils and T lymphocytes in the airway submucosa.
Figure 3.
Effect of SP600125 on eosinophil and T-lymphocyte subset (CD2+, CD4+ and CD8+) counts in airway submucosa per mm of basement membrane for groups as detailed in Fig. 1. Allergen challenge caused a significant increase in T lymphocytes expressing CD2+. (*P < 0·05 as compared to saline group), and in MBP + eosinophils (***P < 0·001 as compared to the saline group). Data are shown as mean ± SEM.
Ovalbumin sensitization and serum-specific IgE
Intraperitoneal administration of ovalbumin and adjuvant resulted in a significant increase in serum ovalbumin-specific IgE in all groups when compared to naïve animals (P < 0·01 and P < 0·05). Pre-treatment with SP600125 did not inhibit the allergen-induced increase in ovalbumin-specific IgE (Table 1).
Table 1.
Effect of ovalbumin sensitization and exposure, and treatment with SP600125 on serum ovalbumin-specific IgE levels
| Naïve (n=4) | Saline+ ovalbumin (n=10) | Ovalbumin (n=10) | Ovalbumin+ SP600125 (n=10), |
|---|---|---|---|
| 0·28 ± 0·07 | 0·57 ± 0·03 (**) | 0·48 ± 0·04 (*) | 0·51 ± 0·06 (*) |
Units: Optical Density (OD) at 405 nm. Saline = ovalbumin-sensitized, vehicle-treated, saline-challenged; Ovalbumin = ovalbumin-sensitized, vehicle treated, Ovalbumin-challenged; SP600125 = ovalbumin-sensitized, SP600125- treated, Ovalbumin-challenged. Data are presented as mean ± SEM.
P < 0·05
P<0·01 vs. naïve.
Cytokine expression in lungs
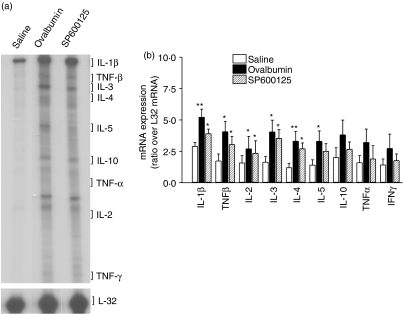
In sensitized rats, ovalbumin exposure induced a significant increase in mRNA expression for IL-1β (P < 0·01), TNF-β (P ≤ 0·05), IL-2 (P<0·05, n = 3, Fig. 4). SP600125 did not suppress the ovalbumin-induced increase in mRNA expression for IL-1β, TNF-β, IL-2, IL-3, IL-4 and IL-5.
Figure 4.
Effect of SP600125 on lung tissue Th2 cytokine gene expression. (a) RNA protection assay was performed on lung tissue homogenates of vehicle- and SP600125-treated rats (n = 3 for each). Representative lanes from each group are shown. (b) Each gel was analysed using densitometry and is shown as a ratio of L32 mRNA expression. The groups were as follows: Saline, ovalbumin-sensitized and saline challenged rats; ovalbumin, ovalbumin-sensitized and ovalbumin-challenged rats. SP600125 did not significantly reduce mRNA expression of Th1/Th2 cytokines. *P < 0·05; **P < 0·01 as compared to the saline group. Data are shown as mean ± SEM.
Protein kinase activation
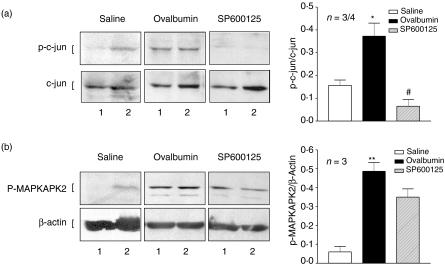
Increased expression of phosphorylated-c-jun (p-c-jun) was detected in lung tissue isolated from ovalbumin-stimulated sensitized animals as shown by Western blot analysis (P < 0·05, Fig. 5a). Pre-treatment with SP600125 (30 mg/kg) abolished p-c-jun expression in ovalbumin-stimulated sensitized animals (P < 0·05). Western blot analyses of phosphorylated MAPKAPK2 (p-MAPKAPK2) showed increased expression in lung tissue isolated from ovalbumin-stimulated sensitized animals (P < 0·01, Fig. 5b). SP600125 did not alter the expression of p-MAPKAPK2 indicative of the selectivity of SP600125 for JNK over the p38 protein kinase pathway.
Figure 5.
Effect of SP600125 on the phosphorylation of c-jun and MAPKAPK2. Western blot analysis of c-jun and phospho-c-jun (a) and p-MAPKAPK2 and β-actin expression (b) in lung tissue isolated from sensitized rats exposed to saline (saline) or to ovalbumin (ovalbumin) or to ovalbumin and pretreated with SP600125 (SP600125). Phospho-c-jun expression was observed in sensitized and ovalbumin-exposed rats and was inhibited in SP600125- treated rats. The p-MAPKAPK2 expression was increased in sensitized and ovalbumin-exposed rats and was not inhibited in SP600125-treated rats. β-actin was included as a control. A representative example of two out of three experiments for each group is shown (labelled 1 or 2). The mean density of bands from three rats in each group is shown on the right-hand side of the panel. *P < 0·05, **P < 0·01 as compared to group saline. #P < 0·05 as compared to group Ovalbumin. Data are shown as mean ± SEM.
Discussion
Using a novel inhibitor of JNK, SP600125, we evaluated the role of JNK in a rat model of allergic asthma. Pretreatment with SP600125 attenuated bronchoalveolar lavage eosinophil, neutrophil, macrophage and lymphocyte accumulation induced by single allergen challenge. Eosinophil and T-cell accumulation in the airways, mRNA expression for IL-1β, TNF-β, IL-2, IL-3, IL-4 and IL-5, allergen-specific IgE levels and bronchial hyperresponsiveness were not affected by SP600125. We detected increased phospho-c-jun expression and phospho-MAPKAPK2 expression in the lungs of allergen-exposed animals, and SP600125 abolished phospho-c-jun expression and not phospho-MAPKAPK2 expression, indicating that this compound inhibited JNK activity selectively over p38 activity.
SP600125 is a reversible ATP-competitive inhibitor with >20-fold selectivity over a range of kinases and enzymes, and has been shown to dose-dependently inhibit the phosphorylation of c-jun, the expression of inflammatory genes and prevent the activation and differentiation of primary human CD4 cell cultures.18 In an in vivo adjuvant arthritis model in the rat, inhibition of metalloproteinase expression and joint destruction was observed at doses of 30 mg/kg, with evidence of suppression of JNK activation in the synovium.19 Based on this previous study in the rat, we chose a dose of 30 mg/kg for our current study, and showed that at this dose in the rat, there was inhibition of phospho-c-jun protein levels in the lungs following allergen exposure of sensitized rats.
Enhanced expression of mRNA expression of the Th2 cytokines, IL-3, IL-4, and IL-5 together with IL-2 following single allergen challenge has been demonstrated. SP600125 treatment resulted in a nonsignificant inhibition of these cytokines. Inhibition of IL-2 production from Th1 and Th2 cells has been observed in vitro at an IC50 of 5 μm which is two-log-fold higher than that observed for the inhibition of JNK.18 The shift in IC50 between biochemical (JNK) and cell assays is the result of the increase in ATP concentration in the cell. The lack of significant suppression of IL-4 and IL-5 particularly by SP600125 is consistent with the persistence of airway eosinophilia and of the elevated serum levels of ovalbumin-specific IgE. IL-5 is important in the terminal differentiation and prolonged survival of eosinophils24 and the administration of anti-IL-5 monoclonal antibody prevents allergen-induced airway eosinophilia in animal models25 while IL-5 gene knock-out mice fail to recruit eosinophils in response to allergen.4 IL-4 is important in the isotype switch of IgG to IgE production in B cells.26 Ligation of CD40, also involved in B-cell activation and differentiation, by soluble gp39 (CD40 ligand) leads to the activation of the JNK pathway in B cells.27 However, inhibition of JNK alone was insufficient in altering CD40-mediated IgE production,27 indicating that CD40 signalling may involve more than the activation of JNK sequential protein kinase pathways. In addition, in our model, we administered the JNK inhibitor following sensitization procedures, and therefore this would be unlikely to affect the increase in ovalbumin-specific serum IgE following sensitization.
We also found an increased expression of the proinflammatory cytokines IL-1β, TNF-β and to a lesser extent, TNF-α, after allergen challenge. It is interesting that JNK activation in sensitized, unexposed rats showed some background level of JNK activity. In preliminary studies, we found that SP600125 in these rats had no effect on bronchial responsiveness and on bronchoalveolar lavage cells, but the background expression of IL-1β and TNF-β may be the resultant effect of the background JNK activity. After SP600125 administration in sensitized and ovalbumin-exposed rats, there was a trend towards a reduction in the expression of these cytokines, particularly the activator protein-1-dependent cytokines interleukin-1α and TNF-α. Inhibition of TNF-α from lipopolysaccharide-activated monocytes in vitro was achieved at a higher IC50 of 10 μm18 indicating that a higher dose of SP600125 may be needed in vivo to achieve inhibition of pro-inflammatory cytokine expression. Alternatively, the transcriptional regulation of these cytokines may be dependent on other transcription factors such as NFκB28 in addition to activator protein-1. Huang et al. have previously shown that a joint activator protein-1 and nuclear factor-kB inhibitor, SP100030 inhibited the mRNA expression of IL-2, IL-5 and IL-10 in the lungs of allergen-exposed rats.20 These differences observed with SP100030 compared to SP600125, may result from the ability of SP100030 to inhibit NFκB, in addition to activator protein-1.
SP600125 inhibited the recruitment of inflammatory cells into the bronchoalveolar lavage fluid without affecting the number of cells in the airway submucosa. One explanation for this effect could be through the inhibition of epithelial expression of adhesion molecules. Inflammatory cells adhere to airway epithelium in their egress into the airway lumen. In human endothelial cell lines, TNF-induced E-selectin transcription was dependent on the activation of JNK.16,29 Other adhesion molecules such as α4-integrin and VCAM-1 may also be regulated by JNK and these may have important roles in the antigen-induced recruitment of T cells and eosinophils during ovalbumin-induced airway inflammation.30 However, because tissue eosinophilia was not affected by SP600125, we presume that the inhibitory effect of SP600125 was selective for epithelial and not for the airway endothelial expression of adhesion molecules. Our data highlight the fact that migration through the epithelium is JNK-dependent, while migration through the endothelium is not.
The lack of effect of JNK inhibition on bronchial hyperresponsiveness induced by single allergen exposure to ovalbumin raises the issue as to whether other MAPK pathways are involved. There are very few in vivo data. An inhibitor of p38 MAPK, SB203580, was shown to be ineffective in inhibiting eosinophil or neutrophil recruitment in bronchoalveolar lavage fluid in rats.31 However, in vitro studies indicate that both p38 and ERK MAPK play an important role in eosinophil differentiation, cytokine production, and deganulation.32 More recently, we have shown that SP600125 was able to inhibit bronchial hyperresponsiveness and tissue eosinophilia in the chronic exposure rat model;33 this is likely to be the result of the inhibitory effect of SP600125 on the structural remodelling changes in the airways induced by chronic exposure, such as the airway smooth muscle proliferation. The current study is more in line with the increasing concept that eosinophils may not be important in the pathogenesis of bronchial hyperresponsiveness. In summary, selective inhibition of JNK with SP600125 had no effect on mucosal inflammation and bronchial hyperresponsiveness, but reduced inflammatory cell egress into the airway lumen after single allergen exposure. JNK MAPK activation plays a very limited role in the pathogenesis of allergic inflammation following single allergen exposure.
Acknowledgments
This study was supported by a Wellcome Trust Grant. We thank Signal Research Division of Celgene Inc. for the provision of SP600125.
References
- 1.Chung KF. Role played by inflammation in the hyperreactivity of the airways in asthma. Thorax. 1986;41:657–62. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–36. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model [see comments] J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang TJ, Macary PA, Eynott P, et al. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol. 2001;166:207–17. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 6.Davis RJ. Signal transduction by the c-Jun N-terminal kinase. Biochem Soc Symp. 1999;64:1–12. doi: 10.1515/9781400865048.1. [DOI] [PubMed] [Google Scholar]
- 7.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Miller BS, Rosenzweig SA, Bhat NR. Activation of C-jun N-terminal kinase/stress-activated protein kinase in primary glial cultures. J Neurosci Res. 1996;46:114–21. doi: 10.1002/(SICI)1097-4547(19961001)46:1<114::AID-JNR14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–39. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–57. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during co-stimulation of T lymphocytes. Cell. 1994;77:727–36. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 12.Nishina H, Bachmann M, Oliveira-dos-Santos AJ, et al. Impaired CD28-mediated interleukin 2 production and proliferation in stress kinase SAPK/ERK1 kinase (SEK1)/mitogen-activated protein kinase kinase 4 (MKK4)-deficient T lymphocytes. J Exp Med. 1997;186:941–53. doi: 10.1084/jem.186.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Hartman SE, Holland PM, Cooper JA, Kawakami T. Multiple signaling pathways for the activation of JNK in mast cells: involvement of Bruton's tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J Immunol. 1998;161:1795–802. [PubMed] [Google Scholar]
- 15.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 16.Min W, Pober JS. TNF initiates E-selectin transcription in human endothelial cells through parallel TRAF-NF-kappa B and TRAF-RAC/CDC42-JNK-c-Jun/ATF2 pathways. J Immunol. 1997;159:3508–18. [PubMed] [Google Scholar]
- 17.Haczku A, Moqbel R, Sun J, et al. T-cell subsets and activation in bronchial mucosa of sensitised Brown-Norway rats after single allergen exposure. Immunol. 1995;85:598–603. [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Z, Boyle DL, Chang L, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TJ, Adcock IM, Chung KF. A novel transcription factor inhibitor, SP100030, inhibits cytokine gene expression, but not airway eosinophilia or hyperresponsiveness in sensitized and allergen-exposed rat. Br J Pharmacol. 2001;134:1029–36. doi: 10.1038/sj.bjp.0704344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haczku A, Chung KF, Barnes PJ, Kay AB, Moqbel R. Airway hyperresponsiveness, elevation of serum IgE and activation of T cells in Brown-Norway rats. Immunol. 1995;85:598–603. [PMC free article] [PubMed] [Google Scholar]
- 22.Nonaka T, Mitsuhashi H, Takahashi K, Sugiyama H, Kishimoto T. Effect of TEI-9874, an inhibitor of immunoglobulin E production, on allergen-induced asthmatic model in rats. Eur J Pharmacol. 2000;402:287–95. doi: 10.1016/s0014-2999(00)00530-6. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson CJ. Interleukin-5, eosinophils and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 25.Hamelmann E, Oshiba A, Loader J, et al. Antiinterleukin-5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. Am J Respir Crit Care Med. 1997;155:819–25. doi: 10.1164/ajrccm.155.3.9117011. [DOI] [PubMed] [Google Scholar]
- 26.Snapper CM, Finkelman FD, Paul WE. Regulation of IgG1 and IgE production by interleukin-4. Immunol Rev. 1988;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakata N, Patel HR, Terada N, Aruffo A, Johnson GL, Gelfand EW. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J Biol Chem. 1995;270:30823–8. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- 28.Liu SF, Haddad E-B, Adcock IA, et al. Inducible nitric oxide synthase after sensitisation and allergen challenge of Brown-Norway rat lung. Br J Pharmacol. 1997;159:1241–6. doi: 10.1038/sj.bjp.0701242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Cesaris P, Starace D, Starace G, Filippini A, Stefanini M, Ziparo E. Activation of Jun N-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor alpha leads to intercellular adhesion molecule-1 expression. J Biol Chem. 1999;274:28978–82. doi: 10.1074/jbc.274.41.28978. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–54. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escott KJ, Belvisi MG, Birrell MA, Webber SE, Foster ML, Sargent CA. Effect of p38 kinase inhibitor, SB 203580, on allergic airway inflammation in the rat. Br J Pharmacol. 2000;131:173–6. doi: 10.1038/sj.bjp.0703605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adachi T, Choudhury BK, Stafford S, Sur S, Alam R. The differential role of extracellular signal-regulated kinases and p38 mitogen-activated protein kinase in eosinophil functions. J Immunol. 2000;165:2198–204. doi: 10.4049/jimmunol.165.4.2198. [DOI] [PubMed] [Google Scholar]
- 33.Eynott PR, Nath P, Leung SY, Adcock IM, Bennett BL, Chung KF. Allergen-induced inflammation and airway epithelial and smooth muscle cell proliferation: role of Jun N-terminal kinase. Br J Pharmacol. 2003;140:1371–80. doi: 10.1038/sj.bjp.0705569. [DOI] [PMC free article] [PubMed] [Google Scholar]