Abstract
The effect of prenatal exposure to bisphenol A (BPA) on the immune system in mice was investigated. Virgin female mice were fed varying doses of BPA, on a daily basis, over a period of 18 days commencing on the day of pairing with stud males (day 0). On day 77, their male offspring of 8 weeks were immunized with hen egg lysozyme (HEL). Three weeks later, anti-HEL immunoglobulin G (IgG) in sera, and proliferative responses of spleen cells to the antigen, were measured. Anti-HEL IgG2a and interferon-γ (IFN-γ), secreted from splenic lymphocytes, were measured as indicators of T helper 1 (Th1) immune responses, while anti-HEL IgG1 and interleukin-4 (IL-4) were measured as indicators of Th2 responses. The results showed that fetal exposure to BPA was followed by significant increases in anti-HEL IgG as well as antigen-specific cell proliferation. Both Th1 responses (including anti-HEL IgG2a and IFN-γ production) and Th2 responses (including anti-HEL IgG1 and IL-4 production) were augmented by prenatal exposure to BPA, although the augmentation of Th1 responses appeared to be greater than that of Th2 responses. Two-colour flow cytometric analysis showed that mice exposed prenatally to BPA had 29% and 100% more splenic CD3+ CD4+ and CD3+ CD8+ cells, respectively, than control animals. Similar results were obtained from females whose mothers had consumed BPA during pregnancy. These results suggest that prenatal exposure to BPA may result in the up-regulation of immune responses, especially Th1 responses, in adulthood.
Keywords: bisphenol A, cytokine, fetal exposure, Th1, Th2
Introduction
Bisphenol A (BPA) is used in the manufacture of adhesives, building materials, optical lenses and plastic dental sealants. BPA is moderately soluble (120–300 mg/l at pH 7·0) and has been detected in food and water consumed by humans as well as animals.1 Gould et al.2 demonstrated that BPA was potent in activating the oestrogen receptor alpha, but 26-fold less so compared to oestrogen, suggesting that BPA is not only an oestrogen mimic but also may exhibit a mechanism of action similar to that of the sex hormone at the receptor. Therefore, considerable attention has focused on this environmental oestrogen-like chemical, which may affect reproductive organs. In fact, it was recently demonstrated that prenatal exposure of male mice to BPA significantly increased the size of the prostate3 and decreased sperm production.4
Oestrogen also plays a role in the immune system. For instance, previous studies have shown that oestrogen has stimulatory effects on humoral immune responses, but suppressive effects on cellular responses.5–8 More recent studies demonstrate that oestrogen increases the secretion of interferon-γ (IFN-γ) from splenic lymphocytes, which play a major role in regulating the function of all key immune cells.9,10 Therefore, it is of interest to determine whether BPA influences the immune system in a manner similar to oestrogen, although there is a report demonstrating that, in vitro, BPA decreases the substrate adherence capacity of antigen-presenting cells, including macrophages.11 BPA also increases the non-specific proliferation of spleen cells to the mitogen, concanavalin A, in vitro.12 However, few studies have shown a role for BPA in immune homeostasis in vivo. Therefore, we investigated the effect of exposure to BPA on antigen-specific antibody production and proliferative responses of lymphoid cells in mice. We also examined whether exposure to BPA modulated T helper 1 (Th1) immune responses, which play a role in cellular responses,13,14 as well as T helper 2 (Th2) immune responses, which are involved in humoral responses.15,16
In the present study we show that mice exposed to BPA during fetal life had greater antigen-specific IgG antibody production and proliferation of splenic lymphocytes in adulthood. Fetal exposure to BPA was also followed by augmentation of Th1 and, to a lesser extent, Th2 immune responses.
Materials and methods
Animals
Male and female DBA/1 J mice, 8 weeks of age, were used in all experiments. The animals were bred in the animal-breeding unit of Saga Medical School (Saga, Japan). They were maintained in a temperature- and light-controlled environment with free access to standard rodent chow and water.
Treatment with BPA
Varying doses, of 3, 30, 300 or 3000 µg/kg BPA (Sigma Chemical Co., St Louis, MO), were dissolved in ethanol and then in corn oil (Sigma). The final concentration of ethanol in corn oil was 0·5%. A 0·5-ml sample of BPA-containing corn oil was fed to virgin female mice, daily, for 17 days commencing on the day of pairing with stud males (day 0). As a control, 0·5 ml of corn oil containing 0·5% ethanol alone was given. Mating was permitted over a period of 24 hr and then females were separated from the males. Females usually delivered their litters on day 19 or day 20. Experiments were performed in accordance with the ethical guideline of Kobe Pharmaceutical University.
Immunization with hen egg lysozyme (HEL)
On day 77, at 8 weeks of age, randomly selected five male or female offspring were immunized intraperitoneally (i.p.) with 100 µg of HEL (Sigma) dissolved in 100 µl of 0·9% NaCl.
Measurement of HEL-specific antibodies
Blood was collected on day 98, and sera were heat-inactivated at 56° for 30 min. Serum levels of immunoglobulin G (IgG), IgG1 and IgG2a, specific for HEL, were determined using an enzyme-linked immunosorbent assay (ELISA).17 In brief, 96-well flat-bottomed microtitre plates were incubated with 100 µl/well of HEL (100 µg/ml) at 37° for 1 hr and washed three times with phosphate-buffered saline (PBS). The wells were then blocked by incubation with 100 µl of PBS containing 1% ovalbumin (OVA; Sigma) at 37° for 1 hr. After washing, the plates were incubated with 100 µl of serially diluted serum samples. After 30 min at 37°, the plates were washed, and 100 µl/well of a 1 : 1000 dilution of rat anti-mouse IgG, IgG1 or IgG2a labelled with alkaline phosphatase (PharMingen, San Diego, CA) was added and incubated at 37° for 1 hr. After washing, 100 µl of 3 mmp-nitrophenylphosphate (Bio-Rad Laboratories, Hercules, CA) was added to each well, and the plates were incubated in the dark at room temperature for 15 min. Absorbance was then measured at 405 nm in a Titertec Multiscan spectrophotometer (EFLAB, Helsinki, Finland). A standard curve was run using hyperimmune anti-HEL, which was diluted from 1 : 50 down to extinction and was assigned an arbitrary value of 100 units. Test sera were then assayed at a 1 : 400 dilution and the number of arbitrary units of antibody they contained, relative to the standard, was calculated. The results were expressed as ELISA units at an absorbance (A) of 405 nm ± standard error of the mean (SEM).
Proliferation assay
Spleens were removed on day 98 and cell suspensions prepared.18 Erythrocytes in the cells were lysed with Tris–NH4Cl. A total of 5 × 106 cells/ml in 100 µl of RPMI-1640 (Flow Laboratories, Inc., McLean, VA), containing 1 mm glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 5 × 10−5 m 2-mercaptoethanol and 1% heat-inactivated autologous mouse serum, were added to each microwell followed by the addition of 100 µg/ml HEL. The cells were cultured for 72 hr. Each well was pulsed with 0·5 µCi [3H]thymidine, and the cells were cultured for a further 16 hr. The cultures were harvested onto fibreglass filters using a multiharvester and counted using standard liquid scintillation techniques.
Cytokine measurement
Spleen cell suspensions were prepared as described above. One millilitre of the above medium, containing a total of 5 × 106 cells, was cultured in 24-well tissue culture plates with 100 µg/ml HEL. Forty-eight hours later, supernatants were harvested and stored at −70° until assayed. Secretion of IFN-γ and interleukin-4 (IL-4) was quantified using sandwich ELISA techniques.19 In some experiments, spleen cells were cultured with 100 µg/ml OVA in order to examine the antigenic specificity of the effect of BPA on production of the cytokines. The ELISA kits for these cytokines were obtained from Funakoshi Co. (Tokyo, Japan).
Flow cytometric analysis
Six male or female mice were killed on day 77 and their spleens were removed. Splenic lymphocytes were incubated for 30 min at 4° with biotinylated rat anti-mouse CD4 monoclonal antibody (mAb) (GK1.5),20 anti-mouse CD8a-RED613™ (clone no. 53–6.7; Life Technologies, Gaithersburg, MD) and hamster anti-mouse CD3 mAb (145–2C11).21 Cells were washed three times with staining buffer (1% fetal calf serum and 0·1% NaN3 in PBS). Then, phycoerythrin (PE)-conjugated streptavidin (Vector Laboratories, Burlingame, CA) and fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-hamster IgG (H+L) (Caltag Laboratories, Burlingame, CA) were added and incubated for 30 min at 4°. A total of 1× 105 lymphocytes were analysed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) using cellquest software (Becton Dickinson). Dead cells were gated out from the analysis using forward- and side scatter.
Histology
The thymus and spleen were removed on day 77 (as described above) and fixed in 4% formalin, followed by embedding in paraffin. The organs were then sectioned at 4 µm and stained with haematoxylin and eosin.
Statistics
To analyse data statistically, the Mann–Whitney U-test was used as a non-parametric statistical method because sample sizes in our experiments were small; therefore, the normality obtained was poor.
Results
Effects of exposure to BPA on pregnancy, gender ratios and body weights
Female mice were administered, per os (p.o.), 3, 30, 300 or 3000 µg/kg BPA, daily, for 18 days, commencing on the day of pairing with males (day 0). The number of pregnant mice was counted on day 18, and the gender ratio of litters was examined on day 49. At the end of the experiments (day 98), body weights were measured. The results are shown in Table 1. There were no significant differences in pregnancy rates, gender ratios, or body weights between controls or BPA-treated mice.
Table 1.
Effects of bisphenol A (BPA) on pregnancy, gender ratios and body weights in mice
| BPA (µg/kg) | No. of pregnant mice* | No. of newborn mice† | Body weight (g)‡ |
|---|---|---|---|
| 0 | 8/12 | 23/22 | 23·2 ± 0·37 |
| 3 | 9/12 | 25/20 | 22·8 ± 0·29 |
| 30 | 7/12 | 18/15 | 23·0 ± 0·31 |
| 300 | 7/12 | 20/18 | 22·9 ± 0·33 |
| 3000 | 8/12 | 19/21 | 23·5 ± 0·40 |
Female mice were administered the indicated doses of BPA, orally, on a daily basis over a period of 18 days, commencing on the day of pairing with males (day 0). The number of pregnant mice and gender ratios of newborn mice were examined on days 18 and 49, respectively. Body weights were measured on day 98, at the end of the experiment.
No. of pregnant mice/no. of total female mice mated.
Male/female.
Results are expressed as mean ± standard error of the mean (SEM) of five male littermates selected randomly.
Production of anti-HEL IgG in mice exposed to BPA during fetal life
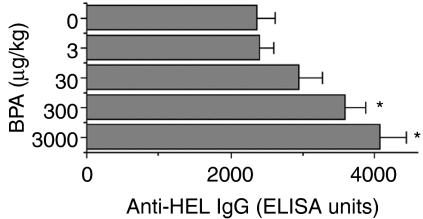
To investigate the effect of fetal exposure to BPA on antigen-specific IgG production, on day 77 male litters (at the age of 8 weeks) were immunized with HEL and, 3 weeks later, anti-HEL IgG were measured in sera. The results showed that production of anti-HEL IgG was increased, in a dose-related manner, in mice fetally exposed to BPA (Fig. 1). The level of antigen-specific antibodies in the group exposed to 3000 µg/kg BPA was up to 72% higher than found in controls. Similarly to that observed in males, female offspring also had a higher production of anti-HEL IgG at higher levels of BPA (data not shown).
Figure 1.
Effect of fetal exposure to bisphenol A (BPA) on production of anti-hen egg lysozyme (HEL) immunoglobulin G (IgG). Female mice were administered per os (p.o.) the indicated doses of BPA, daily over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, male littermates of 8 weeks were immunized with HEL and, 3 weeks later, anti-HEL IgG in sera was measured as described in Materials and methods. Values represent the mean ± standard error of the mean (SEM) of five mice. ELISA, enzyme-linked immunosorbent assay. *P < 0·05 versus control (0 µg/kg BPA).
Proliferative responses of spleen cells to HEL in mice exposed to BPA during fetal life
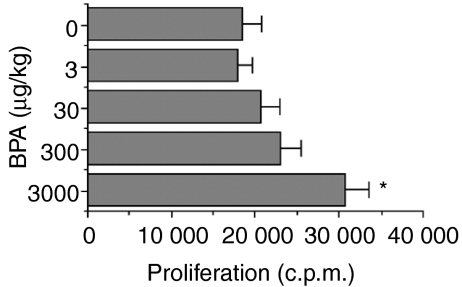
Proliferative responses of spleen cells to HEL were determined in male mice exposed to BPA during fetal life. As shown in Fig. 2, significantly enhanced proliferation (65%) of splenic lymphocytes was observed in the animals exposed to 3000 µg/kg BPA compared to controls. Female offspring also showed a similar pattern of cell proliferation (data not shown).
Figure 2.
Effect of fetal exposure to bisphenol A (BPA) on proliferative responses of spleen cells to hen egg lysozyme (HEL). Female mice were administered per os (p.o.) the indicated doses of BPA, daily over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, male littermates of 8 weeks were immunized with HEL and, 3 weeks later, proliferative responses of spleen cells to HEL were measured as described in the Materials and methods. Values represent the mean ± standard error of the mean (SEM) of five mice. *P < 0·05 versus control (0 µg/kg BPA). c.p.m., counts per minute.
Production of anti-HEL IgG2a and IgG1 in mice exposed to BPA during fetal life
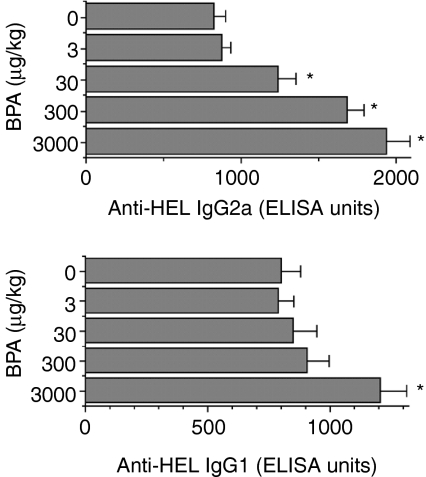
Figure 3 shows the results of the effect of prenatal exposure to BPA on anti-HEL IgG2a and IgG1 production in males that are T helper 1 (Th1) and T helper 2 (Th2) cell-dependent, respectively.22,23 Treatment with 30 µg/kg BPA was followed by a significant increase in anti-HEL IgG2a. The increase in this antibody isotype was further accelerated, up to 134%, by higher doses of BPA. The production of anti-HEL IgG1 was also significantly augmented by BPA, but only by the highest dose (3000 µg/kg) (51% increment). Similar increases in anti-HEL IgG2a and IgG1 were observed in female offspring (data not shown).
Figure 3.
Effect of fetal exposure to bisphenol A (BPA) on the production of anti-hen egg lysozyme (HEL) immunoglobulin G 2a (IgG2a) and immunoglobulin G1 (IgG1). Female mice were administered per os (p.o.) the indicated doses of BPA, daily over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, male littermates of 8 weeks were immunized with HEL and, 3 weeks later, anti-HEL IgG2a and IgG1 in sera were measured as described in the Materials and methods. Values represent the mean ± standard error of the mean (SEM) of five mice. ELISA, enzyme-linked immunosorbent assay. *P < 0·05 versus control (0 µg/kg BPA).
Secretion of IFN-γ and IL-4 in mice exposed to BPA during fetal life
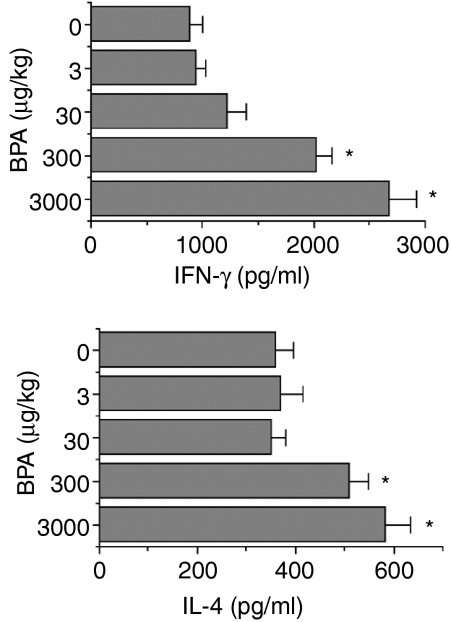
To investigate the effect of prenatal treatment with BPA on the secretion of Th1 and Th2 cytokines,24,25 the levels of IFN-γ (Th1 cytokine) and IL-4 (Th2 cytokine), secreted from spleen cells, were measured in male offspring (Fig. 4). Exposure to BPA was followed by a dose-related increase in IFN-γ. Compared with control mice, IFN-γ secretion in mice exposed to 3000 µg/kg BPA was increased by ≈ 200%. Significant augmentation (up to 62%) of IL-4 was also seen in mice prenatally treated with 300 or 3000 µg/kg of BPA. Augmented secretion of IFN-γ and IL-4 was observed when spleen cells were cultured with HEL, but not with the irrelevant antigen, OVA (Table 2), suggesting that the augmenting effect of BPA on Th1 and Th2 cytokine secretion was antigen-specific. Similarly to males, females also showed a higher secretion of the Th1 and Th2 cytokines following exposure to BPA (data not shown).
Figure 4.
Effect of fetal exposure to bisphenol A (BPA) on the secretion of T helper 1 (Th1) and T helper 2 (Th2) cytokines. Female mice were administered per os (p.o.) the indicated doses of BPA, daily over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, male littermates of 8 weeks were immunized with HEL and, 3 weeks later, secretion of interferon-γ (IFN-γ) and interleukin-4 (IL-4) by spleen cells was measured as described in the Materials and methods. Values represent the mean ± standard error of the mean (SEM) of five mice. *P < 0·05 versus control (0 µg/kg BPA).
Table 2.
Antigen-specific augmentation of interferon-γ (IFN-γ) and interleukin-4 (IL-4) secretion by bisphenol A (BPA)
| Antigen | BPA (µg/kg) | IFN-γ (pg/ml) | IL-4 (pg/ml) |
|---|---|---|---|
| HEL | 0 | 637 ± 46 | 262 ± 18 |
| HEL | 3000 | 2294 ± 182* | 485 ± 32* |
| OVA | 0 | 820 ± 57 | 326 ± 37 |
| OVA | 3000 | 762 ± 85 | 288 ± 32 |
Female mice were administered the indicated doses of BPA, orally, on a daily basis over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, male littermates of 8 weeks were immunized with hen egg lysozyme (HEL) and, 3 weeks later, spleen cells were cultured with either HEL or ovalbumin (OVA), as described in the Materials and methods. Values represent the mean ± standard error of the mean (SEM) of five mice.
P < 0·05 versus control (0 µg/kg BPA).
Expression of CD3, CD4 and CD8 molecules on splenic lymphocytes from mice exposed to BPA during fetal life
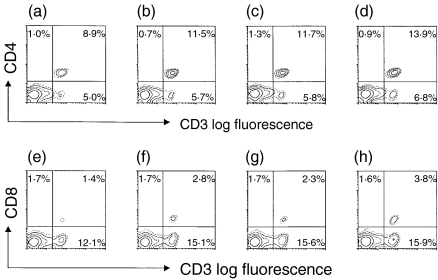
The expression of CD3, CD4 and CD8, on the splenic lymphocytes of male and female mice exposed prenatally to BPA, was examined. As shown in Fig. 5, both genders had increased numbers of CD3+ CD4+ and CD3+ CD8+ cells. Males had 29 and 100% more CD3+ CD4+ and CD3+ CD8+ cells, respectively, while females had 19 and 94% more CD3+ CD4+ and CD3+ CD8+ cells, respectively.
Figure 5.
Two-colour flow cytometric analysis of the expression of CD3, CD4 and CD8 on splenic lymphocytes from mice exposed to bisphenol A (BPA) during fetal life. Female mice were administered per os (p.o.) the indicated doses of BPA, daily over a period of 18 days, commencing on the day of pairing with males (day 0). On day 77, splenic lymphocytes from male (a, b, e, f) or female (c, d, g, h) littermates of 8 weeks exposed to control (a, c, e, g) and BPA (b, d, f, h) were stained with anti-mouse CD4, CD8, and CD3 monoclonal antibodies, as described in the Materials and methods.
Histology
There were no significant differences, regarding histological changes in the spleen and thymus, between the control group and the group treated prenatally with BPA (data not shown).
Discussion
The present study demonstrates that exposure to the endocrine disrupter, BPA, during fetal life may result in augmentation of antigen-specific immune responses in adulthood, as 8-week-old offspring of female mice fed BPA during pregnancy, when subsequently immunized with HEL showed an increased production of anti-HEL IgG as well as proliferation of splenic cells to the antigen. There are a number of studies showing that BPA is biologically active, for instance: treatment of adult rats with BPA suppresses P450-dependent monooxygenase activity in the liver microsomes;26 BPA lowers serum levels of cholesterol and stimulates proliferation of human breast cancer cells;27 and exposure of male mouse fetuses to BPA increases the size of the prostate3 and decreases sperm production.4 With respect to the effect of BPA on the immune system, in vitro studies demonstrate that BPA modulates the substrate adherence capacity of antigen-presenting cells, including macrophages,14 and increases the non-specific proliferation of spleen cells to the mitogen, concanavalin A.12 However, few studies show the in vivo effect of fetal exposure to BPA on antigen-specific immune responses.
The exposure of pregnant mice to BPA was also related to increases in both anti-HEL IgG2a and IgG1 in their offspring, although the increase in anti-HEL IgG2a appeared to be greater than that of anti-HEL IgG1 specific for the antigen. As IgG2a and IgG1 production are dependent on Th1 and Th2 cells,22,23 respectively, these results suggest that exposure to BPA during fetal life facilitates Th1 and, to a lesser extent, Th2 responses in adulthood. This appears to be supported by the increased secretion of both the Th1 cytokine, IFN-γ,24 and the Th2 cytokine, IL-4,25 in mice exposed prenatally to BPA; however, the increase in IFN-γ was greater than that of IL-4. To our knowledge, this is the first demonstration showing that treatment of pregnant mice with BPA resulted in increases in Th1 and, to a lesser extent, Th2 immune responses in their offspring.
BPA is potent in activating the oestrogen receptor alpha (ERalpha)2 through which oestrogen has immunomodulatory effects.5–8 It has also been shown that ERalpha is expressed on splenic T cells in mice.28 Therefore, one may conclude that BPA plays a direct role in the systemic immune system in adult mice. However, this is unlikely because, in our studies, BPA was fed to pregnant mice but not to their adult offspring. The immune system in fetuses exposed to BPA would not have been established when BPA was fed to their mother during pregnancy. Therefore, it appears to be reasonable to speculate that the augmentation of immune responses by fetal exposure to BPA might be a result of the effect of BPA on the development of the immune system.
Our two-colour flow cytometric analysis showed that fetal exposure to BPA was followed by an increase in CD4+ T cells in the adult mouse spleen. This indicates that at least one of the developing organs responsible for immune responses in adulthood, and influenced permanently by prenatal exposure to BPA, was the spleen. Holladay et al.28 has demonstrated that fetal liver cells, enriched for prelymphoid cells, contain potentially significant levels of receptors for the synthetic oestrogen, diethylstilbesterol. This suggests that BPA could also show oestrogenic activity, through the oestrogen receptor, in fetuses if they were exposed to this oestrogen-like chemical. The increase in number of CD4+ T cells also suggests that this subset of T cells might have played, at least in part, a role in the augmentation, by exposure to BPA, of antigen-specific immune responses, including anti-HEL IgG, IgG2a and IgG1 production associated with the enhanced secretion of IFN-γ and IL-4. Production of antibodies to soluble proteins, such as HEL, is, in general, CD4+ T-cell dependent. Unexpectedly, our studies also showed that the offspring of mice who were fed BPA during pregnancy had a greater number of CD8+ T cells than control mice, and that the increase in number of CD8+ T cells was greater than that observed for CD4+ T cells. This suggests that CD8+ T cells in the offspring play a role, in the augmentation by BPA, of production of HEL-specific antibodies and the two cytokines measured. In addition to CD4+ and CD8+ T cells, other cell types, including monocytes, B cells and natural killer (NK) cells, might also have played a role, in BPA-exposed mice, in augmenting the immune responses, as relative proportions (but not absolute numbers) of T-cell subsets were determined in the present study.
Holladay et al.29 also previously demonstrated that prenatal exposure to diethylstilbesterol resulted in the loss of cortical thymic lymphocytes. In contrast, there were no histological changes in the thymus or spleen of mice exposed to BPA during fetal life. The difference in the histology results reported by Holladay et al. and those of the present study may occur because BPA is 26-fold less potent in activating the oestrogen receptor compared to oestrogen.2 BPA should exert its activity as an oestrogenic agonist when little oestrogen is present. In contrast, BPA may function as an antagonist of oestrogen in the presence of considerable amounts of the sex hormone. In our studies, BPA might have played a role as an antagonist, but not as an agonist, at the oestrogen receptor, as pregnant females fed BPA normally have high levels of oestrogen in serum, although other unknown mechanisms might exist in terms of its effect on the developing immune system.
BPA is an essential component of epoxy resins used in the lacquer lining of metal food cans and in dental sealants, and has been detected in food and water.1 It was previously shown that adult humans (of 50 kg in weight) ingest ≈ 0·15 mg (3 µg/kg body weight) of BPA, each day, from food and water. It has also been shown that nearly 1 mg (20 µg/kg body weight) of BPA is swallowed during the first hour after application of a plastic dental sealant.30,31 In our studies, 3–3000 µg/kg BPA were fed to pregnant mice. It was found that proliferation of spleen cells to HEL, and anti-HEL IgG1 production in their offspring, were significantly augmented by 3000 µg/kg BPA, which is 150–1000-fold higher than the doses to which humans are exposed. Production of anti-HEL IgG and IgG2a, IFN-γ, and IL-4 were increased by 300 µg/kg BPA, which is still 15–100-fold higher than the doses ingested by humans. However, the production of anti-HEL IgG2a was enhanced by the relatively low dose of 30 µg/kg BPA, which is only 10-fold higher than the dose ingested daily by humans and close to that of the amount of BPA swallowed following application of a plastic dental sealant. Furthermore, mice used in our studies were exposed to BPA (up to ≈ 1 mg per mouse) for only 18 days during their fetal life, while humans ingest BPA over a much longer time-period. We recently found that exposure of adult mice to BPA also augmented antigen-specific immune responses, including Th1 and Th2 responses.32 Similarly, it was also recently reported that feeding adult mice BPA was followed by an enhanced secretion of IFN-γ33 as well as IL-4.34 Thus, it appears to be undeniable that at least part of the immune system, especially Th1 responses, may be modulated by either prenatal or postnatal exposure to the environmental oestrogen-like chemical, BPA.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan, and by the Kobe Pharmaceutical University Collaboration Fund and Science Research Promotion Fund of the Japan Private School Promotion Foundation.
Abbreviations
- BPA
bisphenol A
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- HEL
hen egg lysozyme
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL-4
interleukin-4
- OVA
ovalbumin
- PBS
phosphate buffered saline
- Th1
T helper 1
- Th2
T helper 2
References
- 1.Staples CA, Dorn PB, Klecka GM, O'Block ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36:2149–73. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 2.Gould JC, Leonard LS, Maness SC, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–14. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 3.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Heath Perspect. 1997;105:70–6. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Indust Health. 1998;14:239–60. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 5.Paavonén T, Andersson LC, Adlercreutz H. Sex hormone regulation of in vitro immune response. estradiol enhances human B cell maturation via inhibition of supressor T cells in pokeweed mitogen stimulated culture. J Exp Med. 1981;154:1935–45. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holdstock G, Chastenay BF, Krawitt EL. Effects of testosterone, estradiol and progestrone on immune regulation. Clin Exp Immunol. 1982;47:449–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Ablin RJ, Bruns GR, Guinan P, Bush IM. The effect of estrogen on the incorporation of H-thymidine by PHA-stimulated human peripheral blood lymphocytes. J Immunol. 1974;113:705–10. [PubMed] [Google Scholar]
- 8.Seaman WE, Blackman MA, Gindlast TD. Beta-estradiol reduced natural killer cells in mice. J Immunol. 1978;121:2193–7. [PubMed] [Google Scholar]
- 9.Karpuzogle-Sahin E, Hissong BD, Ahmed SA. Interferon-γ levels are upregulated by 17-β-estradiol and diethylstilbestrol. J Reproduc Immunol. 2001;52:113–27. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 10.Karpuzogle-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ahmed SA. Effects of long-term estrogen treatment on IFN-γ, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–17. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- 11.Segura JJ, Jimenez-Rubio A, Pulgar R, Olea N, Guerrero JM, Calvo JR. In vitro effect of the resin component bisphenol A on substrate adherence capacity of macrophages. J Endod. 1999;25:341–4. doi: 10.1016/S0099-2399(06)81168-4. [DOI] [PubMed] [Google Scholar]
- 12.Jontell M, Hanks CT, Bratel J, Bergenholtz G. Effects of unpolymerized resin components on the function of accessory cells derived from the rat incisor pulp. J Dent Res. 1995;74:1162–7. doi: 10.1177/00220345950740050401. [DOI] [PubMed] [Google Scholar]
- 13.Fong TA, Mossmann TR. The role of IFN-gamma in delayed type hypersensitivity mediated by Th1 clones. J Immunol. 1989;143:2887–93. [PubMed] [Google Scholar]
- 14.Mosmann TR, Coffman RL. Th1 and Th2 cells. Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 15.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge makedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–13. [PubMed] [Google Scholar]
- 17.Yoshino S, Ohsawa M. Effect of a monoclonal antibody against interleukin-4 on the induction of oral tolerance in mice. Eur J Pharmacol. 1997;336:203–9. doi: 10.1016/s0014-2999(97)01244-2. [DOI] [PubMed] [Google Scholar]
- 18.Yoshino S. Treatment with an anti-IL-4 monoclonal antibody blocks suppression of collagen-induced arthritis in mice by oral administration of type II collagen. J Immunol. 1998;160:3067–71. [PubMed] [Google Scholar]
- 19.Yoshino S, Sagai M. Enhancement of collagen-induced arthritis in mice by diesel exhaust particles. J Pharmacol Exp Ther. 1999;290:524–9. [PubMed] [Google Scholar]
- 20.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–51. [PubMed] [Google Scholar]
- 21.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–8. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnstein HJ, Abbas AK. In vivo role of interleukin-4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993;177:457–63. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isakson PC, Pure E, Vitetta S, Krammer PH. 1982. T cell-derived B cell differentiation factor(s): effect on the isotype switch of murine B cells. J Exp Med. 1982;155:734–48. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamantstein TR, Eckert HD, Kupier-Wglinski JW. 1988. Reversal by interferon-gamma of inhibition of delayed type hypersensitivity induction by anti-CD4 or anti-interleukin 2 receptor (CD25) monoclonal antibodies. Evidence for the physiological role of the CD4+ TH1+ subset in mice. Eur J Immunol. 1988;18:2101–6. doi: 10.1002/eji.1830181237. [DOI] [PubMed] [Google Scholar]
- 25.Mu H-H, Sewell WA. Regulation of DTH and IgE responses by IL-4 and IFN-g in immunized mice given pertussis toxin. Immunology. 1994;83:639–45. [PMC free article] [PubMed] [Google Scholar]
- 26.Hanioka N, Jinno H, Nishimura T, Ando M. Suppression of male-specific cytochrome P450 isoforms by bisphenol A in rat liver. Arch Toxicol. 1998;72:387–94. doi: 10.1007/s002040050518. [DOI] [PubMed] [Google Scholar]
- 27.Dodge JA, Glasebrook AL, Magee DE, Phillips DL, Sato M, Short LL, Bryant HU. Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rats. J Steroid Biochem Mol Biol. 1996;59:155–61. doi: 10.1016/s0960-0760(96)00104-5. [DOI] [PubMed] [Google Scholar]
- 28.Sakazaki H, Ueno H, Nakamuro K. Estrogen receptor alpha in mouse splenic lymphocytes: possible involvement in immunity. Toxicol Lett. 2002;133:221–9. doi: 10.1016/s0378-4274(02)00203-5. [DOI] [PubMed] [Google Scholar]
- 29.Holladay JD, Blayllock BL, Comment CE, Heindel JJ, Fox WM, Korach KS, Luster MI. Selective prothymocyte targeting by prenatal diethylstilbesterol exposure. Cell Immunol. 1993;152:131–42. doi: 10.1006/cimm.1993.1273. [DOI] [PubMed] [Google Scholar]
- 30.Olea N, Pulgar R, Pérez P, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect. 1995;103:608–12. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effect of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and Th1 and Th2 immune responses in mice. Br J Pharmacol. 2003;138:1271–6. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JY, Park HY, Lee JW, Jung IO, Choi KH, Kim K, Cho KH. Evaluation of the immune response following exposure of mice to bisphenol A. induction of Th1 cytokine and prolactin by BPA exposure in the mouse spleen. Arch Pharm Res. 2002;25:946–53. doi: 10.1007/BF02977018. [DOI] [PubMed] [Google Scholar]
- 34.Lee MH, Chung SW, Kang BY, Park J, Lee CH, Hwang SY, Kim TS. Enhanced interleukin-4 production in CD4+ T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca2+ Immunol. 2003;109:76–86. doi: 10.1046/j.1365-2567.2003.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]