Abstract
Allergic atopic disorders, such as rhinitis, asthma, and atopic dermatitis, are the result of a systemic inflammatory reaction triggered by type 2 T helper (Th2) cell-mediated immune responses against ‘innocuous’ antigens (allergens) of complex genetic and environmental origin. A number of epidemiological studies have suggested that the increase in the prevalence of allergic disorders that has occurred over the past few decades is attributable to a reduced microbial burden during childhood, as a consequence of Westernized lifestyle (the ‘hygiene hypothesis’). However, the mechanisms by which the reduced exposure of children to pathogenic and nonpathogenic microbes results in enhanced responses of Th2 cells are still controversial. The initial interpretation proposed a missing immune deviation of allergen-specific responses from a Th2 to a type 1 Th (Th1) profile, as a result of the reduced production of interleukin-12 and interferons by natural immunity cells which are stimulated by bacterial products via their Toll-like receptors. More recently, the role of reduced activity of T regulatory cells has been emphasized. The epidemiological findings and the experimental evidence available so far suggest that both mechanisms may be involved. A better understanding of this question is important not only from a theoretical point of view, but also because of its therapeutic implications.
Keywords: hygiene hypothesis, immune deviation, immune suppression, Th1/Th2 cells, Treg cells
Introduction
The role of the type 2 T helper (Th2) cell-mediated immune response against ‘innocuous’ environmental antigens (allergens) in the immunopathogenesis of allergic atopy is well documented. An impressive body of experimental work supports the critical role of cytokines produced by Th2 cells, such as interleukin (IL)-4, IL-5, IL-9, and IL-13, in the initiation, maintenance and amplification of human allergic inflammation. This experimental evidence includes the identification of cytokines, chemokines, and transcription factors connected with Th2 responses in target organs of allergic subjects.1–3
The expression of the allergic phenotype is dependent upon the interaction between two major factors: a genetic predisposition and gene–environment interactions. The pattern of allergic inheritance is that of a complex polygenic disorder. The gene–environment interactions, however, heavily influence the development of atopic allergy, and several changes in lifestyle that have occurred over the past few decades are certainly responsible for the increased prevalence of allergy in Western countries. Recent epidemiological studies have clearly shown that modifications of the pattern of microbial exposure of children associated with Westernization represent a critical factor underlying the rising severity and prevalence of atopic disorders (the so-called ‘hygiene hypothesis’).4–7
If the ‘hygiene hypothesis’ is the most reasonable explanation for the ‘allergy epidemics’, its immunological basis is still controversial. The initial interpretations concluded that a lack of shifting of allergen-specific responses from the Th2 to the Th1 phenotype (i.e. missing immune deviation) was responsible.8,9 More recently, however, the importance of reduced activity of T regulatory (Treg) cells (reduced immune suppression) has been emphasized.10–12 In this article, I will try to discuss critically the respective relevance of these mechanisms in providing the immunological basis for the ‘hygiene hypothesis’.
Bridges among innate immunity, adaptive immunity and immune regulation
It is now clear that many bacteria and viruses contain one or more components able to interact with molecular structures present on cells of the innate immunity, i.e. dendritic cells (DCs) and natural killer (NK) cells. These receptors are known as Toll-like receptors (TLRs) because of their similarity to the archetypal Drosophila Toll protein. So far, at least 10 distinct TLRs (TLR1-10) able to discriminate between diverse pathogen-associated molecular patterns (PAMPs), which then elicit pathogen-specific immune responses, have been identified.13 The interaction of PAMPs with TLRs results in the release of several cytokines, such as tumour necrosis factor (TNF)-α, interferon (IFN)-α and γ, and IL-1, IL-6, IL-10 and IL-12.13 The production of IL-12 promotes the development of the naïve Th cell into a type 1 Th (Th1) effector cell,14 because the interaction of this cytokine with the IL-12 receptor (IL-12R) results in the activation of the signal transducer and activator of transcription (STAT)-4,15 which is followed by the activation of another transcription factor, the protein T-box expressed in T cells (T-bet).16 T-bet binds to the promoter of the IFN-γ gene and induces the production of this cytokine.16 IFN-α produced by DCs, as well as IFN-γ produced by NK cells, also contributes to Th1 polarization.17,18 In contrast, when early IL-4 production occurs at the site of antigen presentation in the absence of IL-12 and IFN, the IL/IL-4R interaction on the naïve Th cell results in a cascade of different transcription factors, the most important being STAT6, c-maf, and GATA-3.19 The proto-oncogene c-maf binds to a maf response element (MARE) within the IL-4 proximal promoter. However, although c-maf is critical for high levels of IL-4 production, it is not sufficient for the initiation of IL-4 transcription. GATA-3 not only increases the transactivation of the IL-4 promoter, but also directly regulates IL-5 and IL-13 expression, and inhibits the production of IFN-γ.19 Thus, on the basis of present knowledge, it is likely that Th2 priming can occur either as a default pathway in the absence of TLR signalling by PAMPs or via a Th2-type activating receptor(s).
One of the already identified TLRs that allows Th2 differentiation is TLR2. Its ligand, Pam3Cys, is indeed able to suppress the expression of IL-12p70 and to divert the subsequent Th-cell response towards the Th2 profile,20 thus favouring the development of experimental asthma.21 TLR4 also seems to be involved in Th2 differentiation, but this is dependent on the ligand dose, inasmuch as inhalation of high lipopolysaccharide (LPS) doses promotes Th1 responses, whereas low doses favour Th2 priming.22
Th1 and Th2 cells represent polarized forms of the CD4+ Th cell-mediated immune response. Th1 cells produce IL-2, IFN-γ and TNF-β, without IL-4, IL-5, IL-9 and IL-13 production, cooperate with B cells for the production of immunoglobulin G 2a (IgG2a) antibodies in mice and IgG1 and IgG3 antibodies in humans, and activate phagocytic cells and CD8+ T cells, thus promoting cell-mediated immunity and cytotoxic T-cell responses.23,24 In contrast, Th2 cells produce IL-4, IL-5, IL-9 and IL-13 in the absence of IFN-γ and TNF-β production. Cytokines released by Th2 cells induce B cells to produce high amounts of IgG1 and IgE antibodies in mice and IgG4 and IgE in humans, promote the differentiation and growth of mast cells and eosinophils, and inhibit several phagocytic functions.23,24 Of note, while IL-4 inhibits the development of Th1 cells, IFN-γ inhibits the development of Th2 cells.23,24 Moreover, the expression of GATA-3, which allows the differentiation of Th2 cells, inhibits Th1 development; conversely, expression of T-bet, which allows the differentiation of Th1 cells, inhibits Th2 development.19 Thus, transcription factors expressed by, and cytokines released from, Th1 or Th2 cells exert a mutual antagonistic effect on the development of the other subset.
Under some conditions, the T-cell effector response (either Th1- or Th2-polarized) may become dangerous for the host and therefore needs to be controlled. Besides the mutual antagonism mentioned above, regulation is also carried out by the activity of other T-cell types, which have been named Treg cells.25 Treg cells are a highly heterogeneous family, which includes type 3 Th (Th3) cells, T regulatory 1 (Tr1) cells, and CD4+ CD25+ T cells. Th3 cells mainly produce transforming growth factor (TGF)-β and their regulatory function is attributable to a TGF-β-dependent mechanism,26 whereas Tr1 cells are mainly able to produce IL-10, with or without TGF-β.27 In contrast, CD4+ CD25+ T cells do not produce cytokines and act via a contact-dependent mechanism, which probably involves the activity of both membrane cytotoxic T lymphocyte-associated (CTLA)-4 and membrane TGF-β.25 Another feature of CD4+ CD25+ T cells is the expression of the products of the Foxp3 and glucocorticoid-induced TNFR-related (GITR) genes.28,29
In the light of the heterogeneity of the Treg cell family, a distinction between ‘natural’ and ‘adaptive’ Treg cells has recently been suggested.30 Natural Treg cells are generated in the thymus and normally function to prevent the activation of other self-reactive T cells that have the potential to develop into effector cells. These natural Treg cells act mainly via their contact with T cells or antigen-presenting cell (APC) in a cytokine-independent fashion. Similar to natural Treg cells, adaptive Treg cells also originate from the thymus, but then further differentiate and acquire their suppressive activity in the periphery under certain conditions of antigenic stimulation. Their expression of CD25 is variable and their mechanism of suppression is mediated by the production of inhibitory cytokines, such as IL-10 and TGF-β.30 Natural and adaptive Treg cells might function in different immunological settings, depending on the context of antigen exposure, the nature of the inflammatory response, and the TCR repertoires of the individual cells.30 The relationship between microbial stimulation of the TLR pathway and Treg cells is still unclear. However, it has been shown that microbial induction of the TLR pathway can also block the suppressive effect of CD4+ CD25+ Treg cells, allowing activation of pathogen-specific adaptive immune responses.31 Of note, stimulation of TLR2, the same TLR that has been found to be able to promote Th2 differentiation,20,21 also suppresses immunity against Candida albicans through induction of IL-10 and Treg cells.31 Thus, a complex network that is still to be fully elucidated seems to be operating among cells of the innate immunity, Th1 or Th2 effector cells, and Treg cells.
Can the hygiene hypothesis be better explained by missing immune deviation or by reduced immune suppression?
The possibility that the ‘hygiene hypothesis’ is the most reasonable explanation of the ‘allergy epidemics’ was strengthened by the demonstration that exposure of a pregnant mother and/or her son in the first year of life to microbial products released by farm animals, which is typical of the rural lifestyle, exerts an important protective role against the development of allergy as a consequence of the chronic stimulation of the TLRs expressed by cells of the innate immunity.7 The critical point now is to identify the immunological mechanism(s) by which the reduced microbial stimulation of TLRs on cells of the innate immunity in early life can result in easier and stronger Th2 responses to allergens. For some years, the most likely explanation has been that the reduction in microbial burden impairs the occurrence of immune deviation from Th2 to Th1, which usually takes place with increasing age. This means that reduced stimulation of TLRs on DCs and NK cells results in decreased production of cytokines, such as IL-12, IFN-α and IFN-γ, which not only promote the development of Th1 cells, but also antagonize the development of Th2 cells.23,24 Recently, however, an alternative view has emerged, which suggests the importance of reduced immune suppression rather than missing immune deviation. According to this alternative view, the lower microbial burden does not act by inducing a lower production of Th1-polarizing cytokines, but by decreasing the activity of Treg cells.10–12
Epidemiological findings: oversimplifications and omissions
The view that a lower microbial burden may favour increased prevalence of allergy by inducing lower activity of Treg cells is based largely on two major epidemiological observations: (i) that allergic diseases have a low prevalence in areas of the world characterized by diffuse and chronic helminth infections, which induce strong and persistent Th2 responses, but also high production of suppressive cytokines;10 and (ii) that the prevalence of insulin-dependent diabetes mellitus (IDDM), multiple sclerosis (MS) and inflammatory bowel diseases (IBD), which are thought to be Th1-dominated immune disorders, has also increased in the past few decades in developed countries.11 However, in my view, these observations have been misinterpreted or at least oversimplified. The fact that children from parasite-infested areas, such as Gabon, exhibit reduced risk for bronchial asthma and reduced prevalence of positive prick tests to allergens cannot be interpreted as a consequence of suppressive activity, resulting from high concentrations of IL-10 and TGF-β produced by Treg cells, down-regulating allergen-specific Th2 responses.10 Indeed, high proportions of the same children were found to possess in their serum house dust mite-specific IgE antibodies,10 suggesting that allergen-specific Th2 responses were increased rather than depressed. Thus, a more likely explanation is that allergen-specific IgE production in parasite-infested children is not suppressed, but rather that helminth infestations inhibit some late effector phase of the allergen-specific Th2 response.
One possibility is that helminths induce a high production of IgG4 which interacts with allergen, thus preventing its binding to the IgE antibodies present on mast cells and the subsequent release of mediators. This mechanism has been demonstrated in children with high exposure to cat allergens and has been named the ‘modified Th2 response’.32 A still more likely possibility is the ‘IgE blocking hypothesis’.33 Both the positivity of prick tests and clinical allergy require efficient cross-linking of high-affinity IgE receptors (FcεRI) on mast cells and basophils. At least two FcεRI-bound IgE molecules must capture a single allergen to induce mediator release. Helminth infestations are consistently associated with highly polyclonal IgE production, which is not specific for parasite antigens. If IgE, for which no antigen is available, saturates mast cells and blocks the binding of specific IgE directed either to parasite antigen or to environmental allergens, it could inhibit degranulation and immediate hypersensitivity.34
Indeed, in a preliminary study, it was recently shown that basophils from a healthy Dutch donor sensitized with serum of Gabonese mite-sensitized children do not readily release histamine in response to mite allergen. Compared to sensitization with serum from Dutch mite-sensitized children, around 1000-fold higher concentrations of allergen were needed. When basophils originating from Gabonese children (n = 2) were used, a similar discrepancy was observed. Co-sensitization with Gabonese and Dutch serum samples resulted in inhibition of histamine release observed after sensitization with Dutch serum alone (R. Von Ree and M. Yazdanbakhsh, Leiden, Netherlands, personal communication). These data clearly demonstrate that helminth infestations do not suppress allergen-specific IgE production, and therefore they do not suppress allergen-specific Th2 responses, but rather impair the allergen-induced release of mediators by mast cells and basophils through the production of very high concentrations of nonallergen-specific IgE.
Similarly, the purported equivalence of the increased prevalence of allergy on the one hand and MS, IBD and IDDM on the other11 is inappropriate. The reported increased prevalence of MS is indeed highly questionable, for different reasons. Firstly, the study quoted by Bach11 to support the existence of the MS increase35 was limited to the city of Sassari in Sardinia, while in the same study no increase in the prevalence of MS was found in another Italian city, Ferrara.35 In addition, a more recent report has shown that the MS prevalence in Sardinia was significantly increased in rural, genetically ‘archaic’ areas, where the Westernization process had been less pronounced, but not in the clean areas of the same city, suggesting an opposite role for hygiene in the increased prevalence of MS.36
Secondly, studies performed in other European countries reported increased prevalence of MS between 1941 and 1961, but that the prevalence of the disease fell in the following two decades,37 at the time when the prevalence of allergy was increasing. Thirdly, the improvement of diagnostic accuracy, such as the use of nuclear magnetic resonance, makes it impossible to make accurate comparisons of the prevalence of MS in the past few decades. The difficulty in interpreting the results of epidemiological studies on IBD and in making meaningful comparisons between different reports has already been underlined.38
Finally, and most importantly, the comparison between the increased prevalence of atopic allergy and IDDM in the past few decades in rich countries11 is also questionable. Atopic allergy is indeed a chronic and often systemic inflammatory process, which currently affects a high proportion of the population, whereas in IDDM, which is a much rarer disease, the inflammatory process is limited to the pancreatic β cells and rapidly ends with their destruction, thus resulting in a lifelong metabolic, rather than immune-mediated, disease. Thus, it is incorrect to suggest that the prevalence of both Th1- and Th2-dominated diseases are increased, on the basis of this comparison, as IDDM cannot be reasonably considered as an opposite pole to allergy. In any case, the possibility cannot be excluded that, while the increased prevalence of allergy attributable to reduced microbial burden is the result of a missing immune deviation, the possible increase in the prevalence of IDDM may reflect different mechanisms, such as the reduced protection by bacterial heat shock proteins sharing T-cell epitopes with target self antigens.39
There are epidemiological data, which are usually neglected, that allow the opposite conclusions to those reported in the above-mentioned reviews10–12 to be drawn. For example, patients with rheumatoid arthritis or MS, which are clearly chronic, Th1-polarized, autoimmune disorders, exhibit a lower prevalence of allergic diseases.40–43 Of note, the reduced prevalence of allergic disease in patients with MS was found to be associated with enhanced IL-12 production.44 More importantly, it has been shown that Th1-mediated renal disorders, such as proliferative glomerulonephritis, are increasing in poor countries, whereas Th2-mediated renal diseases, such as ‘minimal change’ and IgA nephropathy, are strongly increasing in rich countries.45 This latter epidemiological finding is clearly in favour of missing immune deviation rather than reduced immune suppression. Thus, the results of epidemiological studies do not allow us to conclude whether missing immune deviation or reduced immune suppression has an exclusive or prevalent role as an explanation for the hygiene hypothesis.
In vivo studies
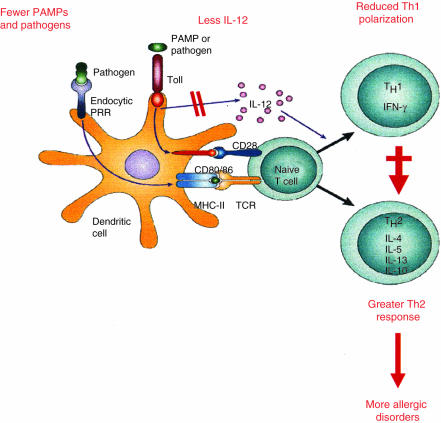
Several years ago, we showed that cytokines, such as IL-12 and the IFNs, not only induce Th1 differentiation but are also able to shift, at least in vitro, the allergen-specific Th2 response to a less polarized, or even Th1-polarized, phenotype.14,17,18 Subsequently, we found that a similar effect was also induced by double-stranded (ds) RNAs, such as polyinosinic:polycytidilic acid.46 More recently, a Th1-switching activity by single-stranded RNA has also been reported.47 Similarly, microbial CpG-containing oligodeoxynucleotides (ODNs) in vitro mixed to, or conjugated with, allergens promote the switch of the allergen-specific response from Th2 to Th1 by inducing the production of high amounts of Th1-polarizing cytokines, such as the IFNs and IL-12, by cells of the innate immunity.48,49 Of note, IL-12 produced by DCs in response to CpG-containing ODNs was also found to be able to induce IFN-γ production by established allergen-specific Th2 effectors by up-regulating their expression of the IL-12R β2 chain.50 Similar results were subsequently obtained by using synthetic adjuvants, such as imidazoquinolines, which induce IL-12 production by DCs and IFN-γ production by NK cells.51 Thus, an impressive series of experimental data suggests that allergen-specific T-cell responses can be shifted, at least in vitro, from Th2 to Th1 by microbial products or other compounds that favour the production of IL-12 and IFNs by cells of the innate immunity, thus strongly supporting the concept that many bacterial and viral infections that stimulate DCs and NK cells may not only promote Th1 polarization but also inhibit the development of Th2 cells. The effect of reduced microbial stimulation on the Th2/Th1 balance (missing immune deviation) is summarized in Fig. 1.
Figure 1.
The hypothesis of missing immune deviation as an explanation for the increased prevalence of allergy as a consequence of improved hygiene. The reduced microbial burden during childhood caused by Westernized lifestyles results in decreased stimulation of innate immune cells and reduced production of IL-12, which is the most important Th1-polarizing cytokine. Under these conditions, the adaptive immune response to ‘innocuous’ environmental antigens (allergens) is shifted towards a prevalent Th2 response (missing immune deviation).
In vivo studies
The results of in vivo experiments based on the administration of cytokines or the injection of infectious agents are more controversial.52 Promising results were obtained from murine asthma models in which IFN-γ, given either during the primary sensitization or during the secondary immune response, decreased IgE production and airway inflammation and normalized airway function.53,54 Moreover, systemic as well as pulmonary IFN-γ gene delivery was proven to be effective not only for preventing but also for suppressing established allergen-induced airway hyperresponsiveness (AHR) in a mouse model.55,56 However, no beneficial effects were observed in clinical trials on asthma patients with subcutaneous or aerosolized IFN-γ.57,58 Moreover, transfer of ovalbumin (OA)-specific IFN-γ-secreting Th1 cells increased, instead of decreasing, Th2-induced airway inflammation.59,60 Administration of IL-12, the most powerful Th1-polarizing cytokine, also suppressed allergen-induced airway eosinophilia and AHR, but a complete inhibition of allergen-specific IgE synthesis was obtained only when IL-12 was given during sensitization, but not during a secondary challenge.61 It is also of note that local and systemic application of endotoxin modulates systemic and local Th1/Th2 immune responses in a distinct but similarly IL-12-dependent manner.62 Transfer of the IL-18 gene, another cytokine produced by cells of the innate immunity, which also exhibits a powerful Th1-polarizing effect, prevented the development of, and reversed, established allergen-induced AHR.63 However, it has recently been reported that injection of IL-18 together with antigen into mice to which Th1 cells had previously been administered induced severe airway inflammation and AHR.64
Even more clear-cut are the effects achieved by administration of ODNs containing CpG motifs found in bacterial, but not vertebrate, DNA. Experiments using allergens, such as ragweed and house dust mites, demonstrated that allergic asthma could be reduced or eliminated by the administration of CpG ODNs during or even after the sensitization phase.65 CpG-ODN treatment increased the ratio of IFN-γ- to IL-4-secreting cells by approximately 4-fold, decreased the number of allergen-specific IgE-producing cells to an equivalent degree, and diminished the influx of inflammatory cells into the lungs.65 These effects were long-lasting; when mice were challenged with allergen some weeks after CpG treatment, they continued to generate a strong Th1-cell (rather than Th2-cell) memory response, which protected them from lung inflammation.65 This was accompanied by a reduction in allergen-specific IgE and IgG1 and an increase in IgG2a-secreting cells in mice treated with CpG ODNs.65 These outcomes are consistent with the ability of CpG DNA to stimulate the production of Th1-type cytokines that induce isotype switching to IgG2a rather than IgE and IgG1.65 These antiallergic effects were observed when CpG ODNs were administered alone, mixed with allergen or physically linked to allergen, and the greatest success in generating a Th1-dominated response resulted when the CpG ODN was coupled directly to allergen.65 Presumably, cross-linking improves allergen uptake and ensures that the immune cells exposed to allergen are also exposed to CpG DNA, and are therefore stimulated to induce a Th1-cell response. At optimal ODN:allergen conjugation ratios, CpG ODNs not only prevented the development of allergic rhinitis, but also reversed established airway disease.65
As expected, the administration in vivo of the entire infectious agents has yielded different results according to the type of infection. Suppression of allergen-induced airway eosinophilia by IL-12 and IFN-γ production caused by Mycobacterium bovis–bacillus Calmette-Guèrin (BCG) has been consistently reported in different experimental models.66,67 These findings are consistent with the observations in humans showing reduced prevalence of allergy in BCG-vaccinated children.68–71 Similarly, gram-positive lactic acid bacteria inhibited the secretion of Th2-type cytokines and this effect was shown to be dependent on APCs and the involvement of IL-12 and IFN-γ.72
In contrast, results obtained with viral infections were more contradictory. Neonatal priming with respiratory synctytial virus (RSV) in newborn mice increased recruitment of inflammatory cells (including Th2 cells and eosinophils) during reinfection, whereas delayed priming led to enhanced IFN-γ production and less severe disease during reinfection.73 Infection with influenza virus inhibited the development of allergen-induced Th2 responses in the lung, which was attributable to generation of a Th1 immune response, as it correlated with a significant increase in IFN-γ levels and could not be observed in IFN-γ- and IL-12 p35-deficient animals.74 This finding is in apparent contrast with the results of a recent study showing that influenza A viral infection unexpectedly enhanced later allergen-specific asthma by promoting dual allergen-specific Th1 and Th2 responses.75 However, it is consistent with another report showing bystander suppression of allergic airway inflammation by IFN-γ produced by lung resident memory CD8+ T cells.76
The majority of the in vivo studies in humans also support the possibility that a shift from Th2 to Th1 in the allergen-specific response may occur, and its lack may play a role in the increased prevalence of allergy. Several studies have indeed shown that allergic subjects have not only increased production of Th2-type cytokines but also reduced production of IFN-γ by peripheral blood cells77 and in exhaled breath condensate.78 However, it has been well demonstrated, both at the clonal level and using flow cytometry, that nonatopic subjects usually recognize most allergens, but the response to allergens of their CD4+ T cells is mainly characterized by IFN-γ rather than Th2-type cytokine production and they have neither allergic symptoms nor any type of inflammatory response to allergen challenge.79,80 Accordingly, it has recently been shown that, while peanut-allergic donors show Th2 polarization by peanut-specific T cells, nonallergic children and, more importantly, children who have outgrown their allergy show Th1 skewing to peanut antigens.81 Of note, CXCL10, a chemokine induced by IFN-γ, promotes dominance of environmental allergen-driven IFN-γ over IL-4 responses,82 and allergic subjects seem to be hyporesponsive to the CXCL10-mediated Th1 immunity-promoting loop in comparison with nonatopic individuals.83 Reduced neonatal IL-12 production has also been associated with stronger neonatal Th2 responses and weaker Th1 responses to allergen in the postnatal period.84 Moreover, insufficient IL-12 production by DCs from atopic subjects has been reported.85 Finally, increases in IL-12 mRNA-expressing cells have been found to accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy.86
However, in contrast to nonatopic individuals, atopic subjects exhibit not only enhanced expression of c-maf and GATA-3,87 which are involved in Th2-cell differentiation,19 but also defective expression of T-bet,88 a transcription factor essential for Th1 development.19 Very recently, it was shown that individuals lacking one functional GATA-3 allele exhibit markedly decreased Th2-cell frequencies in vivo and in vitro.89 Th2 cell-mediated effector functions were also dramatically decreased, whereas the Th1 responses were elevated.89 Recently, it has been reported that children who grew up in East Germany had a marked bias towards type 0 Th (Th0)/Th1 responsiveness, regardless of whether they were atopic, whereas the children of West Germany, particularly when they were atopic, showed Th2 polarization.90 This may be consistent with the observation of high levels of endotoxin in children with a reduced risk for atopy91 and elevated levels of CD14 and TLR2 in sera from farmers' children, who are at lower risk of allergy.92 Taken together, these findings, and particularly those related to both in vitro and in vivo experiments with CpG ODNs, provide an impressive body of experimental evidence to support the role of missing immune deviation as a possible explanation for the increased prevalence of allergy.
Experimental evidence in favour of or against the suppressive effect of allergen-specific Th2 responses
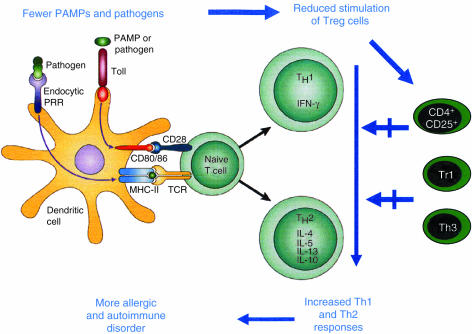
Despite repeated claims in the recent literature, the experimental evidence in favour of a role of reduced immune suppression as an explanation for the increased prevalence of allergy (a role which is summarized in Fig. 2) is currently weaker and even more contradictory than that in favour of the reduced shifting effect from Th2 to Th1 responses. As mentioned above, there are at least two main families of Treg cells: (i) natural CD4+ CD25+ Treg cells, which do not produce cytokines, act via cell-to-cell contact, and are mainly devoted to the suppression of autoimmune responses, and (ii) adaptive Treg cells, which represent a heterogeneous family of cells that mainly act via the production of IL-10 and TGF-β and are mainly induced by exogenous antigens.30 Natural Treg cells have recently been found to express the products of the Foxp3 and GITR genes, the first being essential for their differentiation and the second for their counter-regulation.27,28Foxp3 mutations in both mice (Scurfy mice) and humans have been identified. Foxp3 gene mutations in humans result in the immune dysregulation polyendocrinopathy, enteropathy, and X-linked inheritance syndrome (IPEX). Characteristic symptoms of the disorder include early-onset IDDM, severe enteropathy, often leading to the diagnosis of Crohn's disease or ulcerative colitis, skin disorders, variable autoimmune phenomena, and severe infections.93 Although in some cases IgE levels may be elevated and eosinophilia is sometimes present, both clinical and laboratory findings support the concept that Th1-mediated pathologies and deficient immune responses to pathogens are clearly prevalent over allergic manifestations. This suggests that at least natural Foxp3-expressing Treg cells are more important for suppressing Th1- than Th2-mediated disorders. In agreement with this prediction, we have recently observed that human Th2 cells are much less susceptible than Th1 cells to the suppressive activity of natural CD4+ CD25+ Foxp3-expressing autologous thymocytes, because of their responsiveness to different cytokines.94
Figure 2.
The hypothesis of reduced immune regulation as an explanation for the increased prevalence of allergy as a consequence of improved hygiene. The reduced microbial burden during childhood results in reduced stimulation of Treg cells, with consequent implementation of both Th1 and Th2 responses which are responsible for the increased prevalence of both autoimmune and allergic disorders, respectively.
The situation concerning the role of adaptive Treg cells, particularly those involved in the regulation of allergy, is less clear. Some of these CD4+ T cells have also been found to express CD25, but the fact that even effector cells can express the same molecule makes it very difficult to discriminate between them. Even the induction of Foxp3 on, and the mechanisms responsible for its expression by, adaptive Treg cells are still a matter of controversy. So, we are presently facing a heterogeneous and not easily identifiable family of cells, and this accounts for why the presently available data are contradictory. BALB/c RAG–/– mice, injected with CD4+ CD25+ T cell-depleted cells from OA-specific transgenic mice, showed a decrease in IL-4 and IL-5 production in the airways and had impaired antigen-induced Th2-cell differentiation, but increased differentiation of Th1 cells, suggesting that CD4+ CD25+ T cells modulate the Th1/Th2 balance towards Th2 cells.95 In another study, removal of CD4+ CD25+ T cells was found to reduce the expression of Th2-type cytokines, IL-4, IL-5, and IL-13.96 With regard to the few studies performed in humans, no altered numbers or functions of CD4+ CD25+ T cells were found in adult patients with IgE-mediated cow's milk allergy.97 In contrast, defective numbers of CD4+ CD25+, allergen-specific Treg cells were recently found in atopic individuals, which appeared to be particularly marked during the season of pollen exposure.98 With regard to the latter study, there are, however, at least two major points that need to be addressed. First, it is intriguing that the defect in CD4+ CD25+ T-cell numbers was mainly observed during the pollen season, when there is a striking increase in circulating allergen-specific Th2 effectors which also express CD25 because of their activation status, thus making it difficult to distinguish between the decrease in CD4+ CD25+ Treg cell numbers and the increase in CD4+ CD25+ effector T-cell numbers. Secondly, unlike adaptive Treg cells, these cells do not produce either IL-10 or TGF-β. Finally, it is also intriguing, in the light of the hygiene hypothesis, that the defect in atopic individuals appeared to be limited to allergen-specific T cells and was not observed with the polyclonal T-cell response. The suggested mechanism of reduced immune suppression as a consequence of reduced microbial burden during childhood should in fact result in a general reduction in the activity of Treg cells. Thus, the possibility that the increased prevalence of allergy attributable to reduced microbial burden during childhood results from a defective stimulation of CD4+ CD25+ CD25+ CD25+ Treg cells remains unproved.
More convincing results have been obtained by looking at Treg cells acting through the production of suppressive cytokines, such as IL-10 and TGF-β. T cells engineered to secrete TGF-β very effectively reduced airway inflammation and AHR, and this effect was clearly dependent upon TGF-β.99 Similar effects were obtained after IL-10 gene transfer in the airways100 or when CD4+ Th cells were engineered to produce IL-10.101 Even the suppressive activity of Mycobacterium vaccae on airway eosinophilia was found to be clearly related to the induction of allergen-specific Treg cells able to produce IL-10 and TGF-β,102 although in a subsequent study M. vaccae administration during allergen sensitization or challenge suppressed asthmatic features without inducing an increase in either IFN-γ or IL-10 in the lung lavage fluid.103 Accordingly, transfer of Tr1 cell clones coincident with OA immunization inhibited Th2, but not Th1, responses through IL-10 secretion.104
A suppressive role for IL-10 has also been demonstrated in both allergen-specific immunotherapy (SIT) and normal responses to allergens.105 However, T cells generated by SIT were CD4+ CD25+ and produced both IL-10 and TGF-β.106 More importantly, it seems that IL-10 produced by Treg cells acts mainly by shifting the allergen-specific antibody production from the dangerous IgE to the protective IgG4 isotype rather than by inducing a Th2-cell tolerance.107 Indeed, IL-10 is not suppressive on Th2 cells in all experimental models and sometimes favours rather than inhibits Th2 responses. For example, IL-4 has been found to be able to drive the immune response towards a Th2 phenotype in Leishmania major infection.108 More importantly, disruption of the IL-10 gene resulted in a significant increase in IFN-γ, but reduced secretion of IL-4, and prevented skin eosinophilia in a murine model of allergic dermatitis, suggesting a promoting rather than suppressive role for this cytokine in a mouse model of allergic skin inflammation.109 These discordant effects of IL-10 may obviously be caused by the genetic background, as IL-10-dependent events in mice are strongly strain-dependent. Nevertheless, it cannot be denied that the role of Treg cells, which certainly suppress Th1 responses, in regulating allergen-specific Th2 responses is still controversial.
In contrast, there is compelling experimental evidence to support the induction by several microbial products of an immune deviation in allergen-specific responses from Th2 to Th1. The possibility cannot be excluded that, in addition to missing immune deviation, reduced immune suppression can play a role in explaining the increased prevalence of allergy, but additional experimental work is required to demonstrate such a role. Thus, there is no evidence that allergen-specific Th1 responses in humans are pro-inflammatory, inasmuch as allergen-specific T cells from nonatopic subjects are able to produce high amounts of IFN-γ-79,80 as well as IFN-induced Th1-recruiting chemokines,82,83 such as CXCL9 and CXCL10, without any clinical manifestation or sign of inflammatory reactions. More importantly, while the possible side-effects of increasing the suppressive activity of Treg cells are still unknown, there is not evidence to suggest, as it has been done10, that shifting allergen-specific T-cell responses from Th2 to Th0/Th1 for the prevention and/or cure of allergic diseases, and it may be dangerous.
Concluding remarks
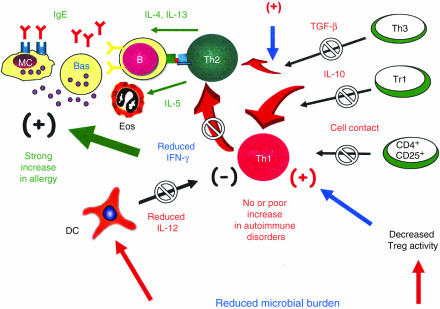
The reason for the increased prevalence of allergic disorders that has occurred during the past few decades in developed countries is still a matter of controversy. Several epidemiological studies suggest that the reduced microbial exposure of children, as a result of Westernised lifestyles, is mainly responsible for this event, and this theory is known as the ‘hygiene hypothesis’. However, the immunological changes induced by higher standards of hygiene during childhood are still being debated. Two main explanations have been suggested: (i) missing immune deviation from Th2 to Th1, caused by reduced production of Th1-polarizing cytokines by cells of the innate immunity in response to stimulation of their TLRs by microbial components, and (ii) reduced activation of Treg cells caused by reduced stimulation of the immune system. The epidemiological observations, showing increased prevalence not only of Th2-mediated allergic disorders but also of some Th1-mediated autoimmune or chronic inflammatory disorders, have been overemphasized or wrongly interpreted. So far, there is an impressive body of evidence, which tends to be neglected, in favour of the existence of immune deviation induced by pathogenic and nonpathogenic agents which can modify allergen-specific responses from Th2 to Th1. The experimental evidence in favour of an important role of Treg cells in dampening allergen-specific Th2 responses is still scarce and sometimes contradictory. This is probably a result of the heterogeneity of Treg cells and of incomplete knowledge of their origin and function. It is likely that the reduced microbial burden during childhood results in both missing immune deviation and reduced immune suppression, as summarized in Fig. 3.
Figure 3.
A less simplistic model illustrating the possible immunological events that can occur as a consequence of the reduced microbial burden during childhood. Production of IL-12 by DCs is diminished, thus resulting in a reduction of Th1 polarization with a lack of IFN-γ production. However, the reduced Th1 polarization caused by lower IL-12 production may be balanced by the enhanced activation of Th1 cells, as a result of reduced Treg stimulation. This may explain why there is no increase, or only a small increase, in the prevalence of Th1-mediated autoimmune disorders in rich countries, but increased prevalence of some inflammatory disorders, such as proliferative glomerulonephritis, in poor countries. In contrast, both the reduced production of IL-12 (and therefore IFN-γ) and the reduced stimulation of Treg cells may favour Th2 responses towards allergens. Thus, under conditions of reduced microbial burden, Th2 responses are much more likely to occur than Th1 responses, and this may explain the ‘allergy epidemics’.
Because allergen-specific Th2 responses are probably subjected to the double negative control of Th1-polarizing cytokines (IFNs and IL-12) and immunosuppressive cytokines (IL-10 and TGF-β), which are both produced in response to chronic and repeated stimulation of the immune system, the reduced microbial burden during childhood may well explain the ‘allergy epidemics’ that have occurred in the past few decades in Westernized countries. However, it must not be forgotten that the role of IL-10 in the regulation of Th1 immunity in humans is much less important than that of IL-4, compared with the mouse, as recently definitively demonstrated in a model of human tissue engraftment in SCID animals.110 Thus, taking all these considerations into account, it is reasonable to conclude that in humans the mutual antagonism between Th1 and Th2 cytokines plays the major role in the counter-regulation of T-cell mediated effector responses.
Acknowledgments
The experiments reported in this review have been supported by grants from the Ministry of Education and Ministry of Health.
Abbreviations
- AHR
airway hyperresponsiveness
- APC
antigen-presenting cell
- BCG
bacillus Calmette-Guerin
- CTLA
cytotoxic T lymphocyte-associated
- DC
dendritic cell
- ds
double-stranded
- GITR
glucocorticoid-induced TNFR-related
- TNFR
related
- IBD
inflammatory bowel diseases
- IDDM
insulin-dependent diabetes mellitus
- IFN
interferon
- IL
interleukin
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked inheritance syndrome
- LPS
lipopolysaccharide
- MARE
maf response elements
- MS
multiple sclerosis
- NK
natural kiler
- OA
ovalbumin
- ODN
oligodeoxynucleotide
- PAMP
pathogen-associated molecular pattern
- R
receptor
- STAT
signal transducer and activator of transcription
- T-bet
T-box expressed in T cells
- TGF
transforming growth factor
- Th0
type 0 T helper
- Th1
type 1 T helper
- Th2
type 2 T helper
- Th3
type 3 T helper
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
- Treg
T regulatory
References
- 1.Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 2.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–64. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 3.Sperger JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:s118–27. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Strachan DP. Hay fever, hygiene and household size. Br Med J. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: an epidemiological study. Br Med J. 2000;320:412–7. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alm JS, Swartz J, Lilja G, et al. Atopy in children with an anthroposophic style of life. Lancet. 1999;353:1484–8. doi: 10.1016/S0140-6736(98)09344-1. [DOI] [PubMed] [Google Scholar]
- 7.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. New Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani S. Regulation of Th2 development in allergy. Curr Opin Immunol. 1994;6:838–46. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FD. The coming-of-age of the hygiene hypothesis. Respir Res. 2001;2:129–32. doi: 10.1186/rr48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanbakhah M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 11.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. New Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Santeliz J, Karp CL. The germless allergic disease: revisiting the hygiene hypothesis. Nature Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 13.Sabroe I, Read RC, Whyte MKB, et al. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003;74:1630–5. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 14.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin-12) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan MH, Sun YL, Hoey T, et al. Impaired IL-12 responses and enhanced development of Th2 cells in STAT-4-deficient mice. Science. 1996;382:174–7. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 16.Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 17.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory role of IFN-γ and IL-4 on the in vitro development of human Th1 and Th2 cells. J Immunol. 1992;148:2142–7. [PubMed] [Google Scholar]
- 18.Parronchi P, De Carli M, Manetti R, et al. IL-4 and IFN (s) (alpha and gamma) exhibit opposite regulatory effects on the development of cytolytic potential by TH1 or TH2 human T cell clones. J Immunol. 1992;149:2977–82. [PubMed] [Google Scholar]
- 19.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–83. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Agrawal A, Doughty B, et al. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 21.Redecke V, Hacker H, Datta SK, et al. Activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2729–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 22.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick C, Bottomly K. Lipolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 24.Abbas AK, Murphy K, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 25.Suri-Payer E, Amar AZ, Thornton AM, et al. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 26.Weiner HL. Induction and mechanism of action of transforming growth factor beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 27.Roncarolo MG, Bacchetta R, Bordignon C, et al. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;6:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 30.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 32.Platts-Mills T, Vaughan J, Squillace S, et al. Sensitization, asthma and a modified Th2 response in children exposed to cat allergen: a population based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 33.Holt PG, Macaubas C, Strumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402 (6760 Suppl):12–7. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 34.Lynch NR, Lopez RI, DiPrisco-Fuenmayor MC, Hagel I, Medouze L, Viana G, Ortega C, Prato G. Allergic reactivity and socioeconomic level in a tropical environment. Clin Allergy. 1987;17:199–207. doi: 10.1111/j.1365-2222.1987.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 35.Pugliatti M, Sotgiu S, Solinas G, et al. Multiple sclerosis epidemiology in Sardinia: evidence for a true increasing risk. Acta Neurol Scand. 2001;103:20–6. doi: 10.1034/j.1600-0404.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 36.Stefano S, Maura P, Alessandra S, et al. Does the ‘hygiene hypothesis’ provide an explanation for the high prevelence of multiple sclerosis in Sardinia? Autoimmunity. 2003;36:257–60. doi: 10.1080/0891693031000151607. [DOI] [PubMed] [Google Scholar]
- 37.Cook SD, Cromarty JI, Tapp W, et al. Declining incidence of multiple sclerosis in the Orkney Islands. Neurology. 1985;35:545–51. doi: 10.1212/wnl.35.4.545. [DOI] [PubMed] [Google Scholar]
- 38.Farrokyar F, Swarbrick ET, Levine EJ. A critical review of epidemiological studies in inflammatory bowel disease. Scan J Gastroenterol. 2001;36:2–15. doi: 10.1080/00365520150218002. [DOI] [PubMed] [Google Scholar]
- 39.Sewell DL, Reinke EK, Logan LH, et al. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol Lett. 2002;82:101–10. doi: 10.1016/s0165-2478(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 40.Hilliquin P, Allanore Y, Coste J, et al. Reduced incidence and prevalence of atopy in rheumatoid arthritis. Results of a case-control study. Rheumatology. 2000;39:1020–6. doi: 10.1093/rheumatology/39.9.1020. [DOI] [PubMed] [Google Scholar]
- 41.van Roon JAG, Bijlsma JWJ. Th2 mediated regulation in RA and spondyloarthropathies. Ann Rheum Dis. 2002;61:951–4. doi: 10.1136/ard.61.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oro AM, Guarino TJ, Driver R, et al. Regulation of disease susceptibility: decreased prevalence of IgE-mediated disease in patients with multiple sclerosis. J Allergy Clin Immunol. 1996;97:1402–8. doi: 10.1016/s0091-6749(96)70210-5. [DOI] [PubMed] [Google Scholar]
- 43.Tremlett HL, Evans J, Wiles CM, et al. Asthma and multiple sclerosis: an inverse association in a case-control general practice population. QJM. 2002;95:753–6. doi: 10.1093/qjmed/95.11.753. [DOI] [PubMed] [Google Scholar]
- 44.Tang L, Benjaponpitak S, DeKruyff RH, et al. Reduced prevalence of allergic disease with multiple sclerosis is associated with enhanced IL-12 production. J Allergy Clin Immunol. 1998;102:428–35. doi: 10.1016/s0091-6749(98)70131-9. [DOI] [PubMed] [Google Scholar]
- 45.Johnson RJ, Hurtado A, Merszei J, et al. Hypothesis: dysregulation of immunologic balance resulting from hygiene and socioeconomic factors may influence the epidemiology and cause of glomerulonephritis worldwide. Am J Kidney Dis. 2003;42:575–81. doi: 10.1016/s0272-6386(03)00801-1. [DOI] [PubMed] [Google Scholar]
- 46.Manetti R, Annunziato F, Tomasevic L, et al. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-alpha and interleukin-12. Eur J Immunol. 1999;25:2656–60. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 47.Koski GK, Kariko K, Xu S, et al. Innate immunity system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J Immunol. 2004;172:3989–93. doi: 10.4049/jimmunol.172.7.3989. [DOI] [PubMed] [Google Scholar]
- 48.Parronchi P, Brugnolo F, Annunziato F, et al. Phosphorothioate oligodeoxynucletides promote the in vitro development of human allergen-specific CD4+ T cells into Th1 effectors. J Immunol. 1999;163:5946–53. [PubMed] [Google Scholar]
- 49.Tighe H, Takabayashi K, Schwartw D, et al. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J Allergy Clin Immunol. 2000;106:124–34. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 50.Annunziato F, Cosmi L, Manetti R, et al. Reversal of human allergen-specific CRTH2+ Th2 cells by IL-12 or the PS-DSP30 oligodeoxynucleotide. J Allergy Clin Immunol. 2001;108:815–21. doi: 10.1067/mai.2001.119156. [DOI] [PubMed] [Google Scholar]
- 51.Brugnolo F, Sampognaro S, Fintoni T, et al. The novel synthetic immune response modifier R-848 (Resiquimod) has a powerful effect on the in vitro switching of human allergen-specific CD4+ Th2 lymphocytes into IFN-γ-producing cells. J Allergy Clin Immunol. 2003;111:380–8. doi: 10.1067/mai.2003.102. [DOI] [PubMed] [Google Scholar]
- 52.Tournoy KG, Kips JC, Pauwels RA. Is Th1 the solution for Th2 in asthma? Clin Exp Allergy. 2002;31:17–29. doi: 10.1046/j.0022-0477.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 53.Lack G, Bradley KL, Hamelmann E, et al. Nebulized but not parenteral IFN-gamma decreases IgE production and normalizes airway function in a murine model of allergen sensitization. J Immunol. 1994;152:2546–54. [PubMed] [Google Scholar]
- 54.Lack G, Bradley KL, Hamelmann E, et al. Nebulized IFN-gamma inhibits the development of secondary allergen responses in mice. J Immunol. 1996;157:1432–9. [PubMed] [Google Scholar]
- 55.Dow SW, Schwartze J, Heath TD, et al. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice. Hum Gene Ther. 1999;10:1905–14. doi: 10.1089/10430349950017266. [DOI] [PubMed] [Google Scholar]
- 56.Li XM, Chopra RK, Chou TY, et al. Mucosal IFN-gamma gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216–9. [PubMed] [Google Scholar]
- 57.Martin RJ, Boguniewicz M, Henson JE, et al. The effects of inhaled interferon-gamma in normal human airways. Am Rev Respir Dis. 1993;148:1677–82. doi: 10.1164/ajrccm/148.6_Pt_1.1677. [DOI] [PubMed] [Google Scholar]
- 58.Boguniewicz M, Martin RJ, Martin D, et al. The effects of nebulized interferon-gamma in asthmatic airways. J Allergy Clin Immunol. 1995;95:133–5. doi: 10.1016/s0091-6749(95)70162-1. [DOI] [PubMed] [Google Scholar]
- 59.Randolph DA, Carruthers CJL, Szabo SJ, et al. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol. 1999;162:1375–83. [PubMed] [Google Scholar]
- 60.Hansen G, Berry G, DeKruyff RH, et al. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kips JC, Brusselle GJ, Joos JF, et al. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 1996;153:535–9. doi: 10.1164/ajrccm.153.2.8564093. [DOI] [PubMed] [Google Scholar]
- 62.Gerhold K, Blumchen K, Bock A, et al. Endotoxins prevent murine IgE production, Th2 immune responses and development of airway eosinophilia but not airway hyperreactivity. J Allergy Clin Immunol. 2002;110:110–6. doi: 10.1067/mai.2002.125831. [DOI] [PubMed] [Google Scholar]
- 63.Walter DM, Womg CP, DeKruyff RH, et al. IL-18 gene transfer by adenovius prevents the development of and reverses established allergen-induced airway hyperreactivity. J Immunol. 2001;166:6392–8. doi: 10.4049/jimmunol.166.10.6392. [DOI] [PubMed] [Google Scholar]
- 64.Sugimoto T, Ishikawa Y, Yoshimoto T, et al. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naïve host mouse. J Exp Med. 2004;199:535–45. doi: 10.1084/jem.20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Rev Immunol. 2004;4:1–10. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 66.Erb KJ, Holloway JW, Sobeck A, et al. Infection of mice with Mycobacterium bovis-Bacillus Calmette Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–9. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Major T, Wohlleben G, Reibetanz B, et al. Application of heat killed Mycobacterium bovis-BCG into the lungs inhibits the development of allergen-induced Th2 response. Vaccine. 2002;20:1532–40. doi: 10.1016/s0264-410x(01)00496-0. [DOI] [PubMed] [Google Scholar]
- 68.Tsai JJ, Liu YH, Shen HD, et al. Prevention of Der p2-induced airway inflammation by Mycobacterium-bacillus Calmette Guerin. J Microbiol Immunol Infect. 2002;35:152–8. [PubMed] [Google Scholar]
- 69.Shirikawa T, Enomoto T, Shimazu S, et al. The inverse association between tuberculin responses and atopic disorders. Science. 1997;275:77–9. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 70.Marks GB, Ng K, Zhou J, et al. The effect of neonatal BCG vaccination on atopy and asthma at age 7–14 years: an historical cohort study in a community with a very low prevalence of tuberculosis infection and a high prevalence of atopic disease. J Allergy Clin Immunol. 2003;111:541–9. doi: 10.1067/mai.2003.171. [DOI] [PubMed] [Google Scholar]
- 71.Townley RG, Barna IB, Patino C, et al. The effect of BCG vaccine at birth on the development of atopy or allergic disease in young children. Ann Allergy Asthma Immunol. 2004;92:350–5. doi: 10.1016/S1081-1206(10)61574-8. [DOI] [PubMed] [Google Scholar]
- 72.Pochard P, Gosset P, Grangette C, et al. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002;110:617–23. doi: 10.1067/mai.2002.128528. [DOI] [PubMed] [Google Scholar]
- 73.Culley FJ, Pollot J, Openshaw PJM. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196:1381–6. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wollheben G, Muller J, Tatsch U, et al. Influenza A virus infection inhibits the efficient recruitment of Th2 cells into the airways and the development of airway eosinophilia. J Immunol. 2003;170:4601–11. doi: 10.4049/jimmunol.170.9.4601. [DOI] [PubMed] [Google Scholar]
- 75.Dahl ME, Dabbagh K, Liggitt D, et al. Viral induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–43. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 76.Mardsland BJ, Harris NL, Camberis M, et al. Bystander suppression of allergic airway inflammation by lung resident memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:6116–21. doi: 10.1073/pnas.0401582101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smart JM, Horak E, Kemp AS, et al. Polyclonal and allergen-induced cytokine responses in adults with asthma: resolution of asthma is associated with normalization of IFN-γ responses. J Allergy Clin Immunol. 2002;110:450–6. doi: 10.1067/mai.2002.127283. [DOI] [PubMed] [Google Scholar]
- 78.Shahid SK, Kharltonov SA, Wilson NM, et al. Increased interleukin-4 and decreased IFN-γ in exhaled breath condensate of children with asthma. Am J Respir Crit Care Med. 2002;165:1290–3. doi: 10.1164/rccm.2108082. [DOI] [PubMed] [Google Scholar]
- 79.Wierenga EA, Snoek M, Jansen HM, et al. Functional subsets of allergen-reactive human CD4+ T cells. Immunol Today. 1991;12:392–5. doi: 10.1016/0167-5699(91)90137-I. [DOI] [PubMed] [Google Scholar]
- 80.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 81.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gangur V, Simons ER, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-γ over IL-4 responses. FASEB J. 1998;12:705–13. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 83.Campbell JD, Gangur V, Simons FER, et al. Allergic humans are hyporesponsive to a CXCR3 ligand-mediated Th1 immunity-promoting loop. FASEB J. 2004;18:329–31. doi: 10.1096/fj.02-0908fje. [DOI] [PubMed] [Google Scholar]
- 84.Prescott SL, Taylor A, King B, et al. Neonatal interleukin-12 capacity is associated with variations in allergen-specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy. 2003;33:566–72. doi: 10.1046/j.1365-2222.2003.01659.x. [DOI] [PubMed] [Google Scholar]
- 85.Reider N, Reider D, Ebner S. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J Allergy Clin Immunol. 2002;109:89–95. doi: 10.1067/mai.2002.120556. [DOI] [PubMed] [Google Scholar]
- 86.Hamid QA, Shotman E, Jacobson MR, et al. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254–60. doi: 10.1016/s0091-6749(97)70106-4. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura Y, Ghaffar O, Olivenstein R, et al. Gene expression of the GATA-3 transcription factor is increased in atopic asthma. J Allergy Clin Immunol. 1999;103:215–22. doi: 10.1016/s0091-6749(99)70493-8. [DOI] [PubMed] [Google Scholar]
- 88.Finotto S, Neurath MF, Glickman JM, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 89.Shapenko A, Leipe J, Niesner U, et al. GATA-3 in human T cell helper type 2 development. J Exp Med. 2004;199:423–8. doi: 10.1084/jem.20031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Renz H, von Mutius E, Illi S, Wolkers F, Hirsch T, Welland SK. Th1/Th2 immune responses profiles differ between atopic children in eastern and western Germany. J Allergy Clin Immunol. 2002;109:338–42. doi: 10.1067/mai.2002.121459. [DOI] [PubMed] [Google Scholar]
- 91.Gehring U, Bischoff W, Fahlbusch B, et al. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002;166:939–44. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]
- 92.Lauener RP, Birchler T, Adamaski J, et al. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet. 2002;360:465–6. doi: 10.1016/S0140-6736(02)09641-1. [DOI] [PubMed] [Google Scholar]
- 93.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, poliendocrinopathy, enteropathy, and X-linked inheritance (IPEX) a syndrome of systemic autoimmunity caused by mutations of Foxp3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–8. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Cosmi L, Lotta F, Lazzeri E, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103:3117–21. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- 95.Suto A, Nakajima H, Kagami S-I, et al. Role of CD4+CD25+ regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. Am J Respir Care Med. 2001;164:680–7. doi: 10.1164/ajrccm.164.4.2010170. [DOI] [PubMed] [Google Scholar]
- 96.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–9. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- 97.Tiemessen MM, Van Hoffen E, Knulst AC, et al. CD4+CD25+ regulatory T cells are not functionally impaired in adult patients with IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2002;110:934–6. doi: 10.1067/mai.2002.128856. [DOI] [PubMed] [Google Scholar]
- 98.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 99.Hansen G, McIntire JJ, Yeung VP, et al. CD4+ T helper cells engineered to produce latent TGF-beta reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stampfli MR, Cwiartka M, Gajewska BU, et al. Interleukin-10 gene transfer to the airways regulates allergic mucosal sensitization in mice. Am J Respir Cell Mol Biol. 1999;21:586–96. doi: 10.1165/ajrcmb.21.5.3755. [DOI] [PubMed] [Google Scholar]
- 101.Oh W-J, Seroogy CM, Meyer EH, et al. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol. 2002;110:460–8. doi: 10.1067/mai.2002.127512. [DOI] [PubMed] [Google Scholar]
- 102.Zuani-Amorim C, Sawicka E, Manlius C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nature Med. 2002;8:625–9. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 103.Smitt JJ, Van Loveren H, Hoekstra MO, et al. Mycobacterium vaccae administration during allergen sensitization or challenge suppresses asthmatic features. Clin Exp Allergy. 2003;33:1083–9. doi: 10.1046/j.1365-2222.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 104.Cottrez F, Hurst SD, Coffman RL, et al. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol. 2000;165:4848–53. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- 105.Blaser K, Akdis CA. Interleukin-10 T regulatory cells and specific allergy treatment. Clin Exp Allergy. 2004;34:328–31. doi: 10.1111/j.1365-2222.2004.01909.x. [DOI] [PubMed] [Google Scholar]
- 106.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 107.Nouri-Aria KT, Wackholz PA, Francis JM, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 108.Chatelain R, Mauze S, Coffman RL. Experimental Leishmania major infection in mice: role of IL-10. Parasite Immunol. 1999;21:211–8. doi: 10.1046/j.1365-3024.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 109.Laouini D, Alenius H, Tsitsikov E, Geha RS. IL-10 skews the immune response to Th2 and is critical for skin eosinophilia in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058–66. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shapenko A, Niedobitek GU, Kalden JR, et al. Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. J Immunol. 2004;172:6427–34. doi: 10.4049/jimmunol.172.10.6427. [DOI] [PubMed] [Google Scholar]