Abstract
Activation of the CD4+ T-cell mediated immune response relies on the proteolytic capacity of enzymes involved in modulating major histocompatibility complex (MHC) II-associated antigen presentation in antigen-presenting cells (APC). The MHC II-associated chaperone molecule p41 isoform of invariant chain (inhibitory p41 Ii) has been suggested to regulate stability and activity of cathepsin L in these APC. In the present study the human lymph node distribution of non-inhibitory p31 Ii and inhibitory p41 Ii have been compared by differential labelling, using two specific monoclonal antibodies. The distribution of p41 Ii, but not p31 Ii, matched the distribution of cathepsins L and H in subcapsular and cortical sinuses and germinal centres. Co-localization of p41 Ii with cathepsin H was confirmed in strongly CD68+ sinus-lining macrophages, acting as APC. Furthermore, p41 Ii was determined together with cathepsins L and H in tingible body macrophages, highly phagocytic, but not antigen-presenting cells inside germinal centres. With respect to the physiological function that these two populations of macrophages have in human lymph nodes, our results support a regulatory function of p41 Ii towards cathepsins L and H in human macrophages, associated with the processes of phagocytosis rather than antigen presentation.
Keywords: cathepsin H, cathepsin L, antigen presentation, lymph node, p41 invariant chain
Introduction
Antigen-presenting cells (APC) play an important role in T-cell mediated immune response. Afferent lymph draining from different tissues is filtered as it trickles through the subcapsular and cortical sinuses of lymph nodes. The antigen-collecting function is aided by APC in sinuses (by sinus-lining macrophages and veiled dendritic cells in afferent lymph), in paracortex by interdigitating dendritic cells and by non-phagocytic follicular dendritic cells for presentation to germinal centre B cells in cortex.1
In addition to processing of endocytosed or phagocytosed antigen, the capacity of APC to activate CD4+ T-cell response relies on their ability to mature major histocompatibility complex class II molecules (MHC II), to facilitate loading of antigen peptides on MHC II and to transport these complexes to the cell surface for recognition by the T-cell receptor.2 MHC II reach the endocytic pathway by associating with the invariant chain (Ii), a transmembrane chaperone molecule with multiple physiological roles. Ii facilitates proper folding and targeting of MHC II and prevents premature peptide binding en route to endosomal compartments of APC.3 Furthermore, Ii is involved in endocytosis of MHC II–Ii complexes from the plasma membrane, in induction of multivesicular bodies, in retention of MHC II-Ii complexes in the endosomal pathway and in promoting differentiation of immature B cells.4
Another function of Ii has been suggested, i.e. Ii acting as a specific inhibitor of cysteine proteases (cathepsins), present inside endocytic pathway of APC. Ii exists in humans in alternatively spliced forms (isoforms), p31 Ii and p41 Ii,5 the latter containing an additional 64-amino acid sequence at the C-terminal luminal end (inhibitory p41 fragment), which is structurally related to inhibitors thyropins.6,7 p41 fragment is in vitro a potent inhibitor of human cathepsin L (Ki = 1·7 pm), a moderate inhibitor of human cathepsin H (Ki = 5·3 nm), but does not inhibit cathepsin S (Ki > 100 nm).7,8 The physiological role of p41 Ii toward cathepsin L has been reported9,10 upon experiments performed with mouse bone-marrow-derived APC. However, it has been shown that mouse cathepsin L in fact corresponds to human cathepsin V with regard to structure similarities.11
Cathepsins are implicated in processing of antigen and in stepwise degradation of Ii (i.e. in its removal from MHC II) inside late endosomal compartments of APC.12 Cathepsins S and L were suggested as the key enzymes involved in the processing of Ii13–15 and their regulation by endogenous protein inhibitors could modulate antigen presentation.16 On the other hand, cathepsins are necessary for the complete intracellular catabolism within lysosomes, eliminating cell residues by highly phagocytic cells, such as tingible body macrophages in germinal centres.17
This study was focused on inhibitory role of Ii towards two potential target enzymes (cathepsins L and H) in macrophages inside human lymph nodes. To distinguish between human Ii isoforms in tissue samples two different monoclonal antibodies (mAb) were prepared: first recognizing non-inhibitory portion and second recognizing inhibitory p41 sequence of human Ii. Colocalization of inhibitory p41 Ii isoform was confirmed with cathepsin H in both studied macrophage populations, but with cathepsin L only in tingible-body macrophages. Our results support regulatory function of p41 Ii towards cathepsins L and H in human macrophages, associated with phagocytosis rather than antigen presentation.
Materials and methods
Production and selection of anti-invariant chain monoclonal antibodies
Recombinant p41 Ii isoform (recombinant protein, comprising only the luminal domain of human p41 Ii with the inhibitory p41 fragment at the C-terminal end) was used as an antigen for immunization of BALB/c mice. Prior to immunization, lyophilized recombinant p41 Ii was dissolved in 6 m urea with 10 mm dithiothreitol (DTT) at room temperature. Sample was dialysed against 20 mm BIS-TRIS buffer, pH 6·2, at 4° overnight. Precipitated aggregates were removed by centrifugation and refolded Ii molecules were found soluble in the supernatant. For hybridoma production splenocytes from immunized mice and mouse myeloma cells (NS1/1-Ag4-1) were fused.18 Positive clones were screened and selected by antigen-immobilized enzyme-linked immunosorbent assay (ELISA) using three different sources of the antigen: (a) inhibitory recombinant p41 Ii (described above); (b) recombinant p31 Ii (non-inhibitory recombinant protein, comprising only the luminal domain of human p31 Ii and lacking inhibitory fragment at the C-terminal end, dissolved and refolded exactly as described for recombinant inhibitory p41 Ii); and (c) non-covalent inhibitory p41 fragment/cathepsin L complex, isolated from human kidney tissue.8 Recombinant p41 Ii and p31 Ii isoforms were isolated from inclusion bodies from Escherichia coli. N-terminal His-tag allowed purification by Ni-chelate chromatography. Both lyophilized Ii isoforms were provided by Dr Klaus Dornmair from Max-Planck-Institute for Psychiatry, Martinsried, Germany. Two selected hybridomas (2C12 and 5F6), producing antibody of interest, were cloned and mAb purified by affinity chromatography on Protein A-Sepharose CL-4B (Pharmacia Biotech, Uppsala, Sweden). Monoclonality of prepared mouse anti-Ii antibodies (2C12 mAb and 5F6 mAb) was confirmed by isoelectric focusing as described.19 Specific antibodies against human cathepsins L, H and S, used in this study, were also prepared and characterized in our laboratory as previously reported: mouse anti-cathepsin S 1E3 mAb20 mouse anti-cathepsin H 1D10 mAb18 and sheep anti-cathepsin L polyclonal antibody (pAb).21
Characterization of anti-invariant chain monoclonal antibodies by ELISA
The specificity and cross-reactivity of anti-Ii (2C12 mAb and 5F6 mAb) were compared by antigen-immobilized ELISA using standard protocol at our department.18 The following antigen were included: (a) inhibitory recombinant p41 Ii; (b) non-inhibitory recombinant p31 Ii; (c) isolated non-covalent p41 fragment/cathepsin L complex from human kidney;8 (d) inhibitory p41 fragment alone, detached by high pressure liquid chromatography from the isolated complex with cathepsin L;8 and (e) recombinant cathepsin L22 all applied at the same concentration (5 µg/ml). Absorbance was measured at 405 nm.
Characterization of anti-invariant chain mAb by Western blot analysis
For Western blot analysis, samples (recombinant and isolated antigen) were separated on 8–25% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gels using PhastSystem (Pharmacia Biotech). Proteins were transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) by passive diffusion accelerated with higher temperature (70°). Non-specific binding was blocked with 0·4% Tween 20 in phosphate-buffered saline (PBS), pH 7·2. After this and all subsequent steps, the membrane was washed with PBS, containing 0·05% Tween 20. Membrane was incubated with primary anti-Ii 2C12 mAb or 5F6 mAb, followed by horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). antibody were detected using 0·05% 3,3′-diaminobenzidine (Sigma, Saint Louis, MO) and 0·01% H2O2 in 0·05 m Tris-HCl buffer, pH 7·5. Residual proteins on the polyacrylamide gel were visualized using silver staining protocols from PhastSystem (Pharmacia Biotech).
Tissue specimens
All investigations concerning human tissues were approved by the Ethics Committee of the Ministry of Health of the Republic of Slovenia. Lymph nodes, non-invaded by tumour cells, were obtained from patients undergoing mastectomy and auxiliary dissection for breast carcinoma at the Institute of Oncology in Ljubljana. Tissue sections from formalin fixed, paraffin embedded lymph nodes were prepared (3 µm) and proceed as reported.23
Immunohistochemistry
Distribution of inhibitory p41 isoform (p41 Ii) and non-inhibitory p31 isoform (p31 Ii) of invariant chain and cathepsins L, H and S was studied in human lymph nodes, together with cell type specific markers for macrophages (CD68 is a member of the LAMP family – lysosome-associated membrane glycoproteins)24,25 and for follicular dendritic cells. Tissue sections were labelled with mouse 2C12 mAb, mouse 5F6 mAb (two anti-Ii mAb, described above), mouse anti-CD68 mAb (KP1), mouse CNA.42 mAb against follicular dendritic cells (both obtained from DAKO, Glostrup, Denmark), anti-cathepsin L pAb, anti-cathepsin H 1D10 mAb and anti-cathepsin S 1E3 mAb (described above). Furthermore mouse anti-HLA-DR mAb (TÜ36, BD Pharmingen, San Diego, CA) and mouse anti-cystatin C 1A2 mAb26 were applied to label dendritic cells in paracortex. Colocalization of p41 Ii with cathepsins L, H and S was performed with the same (primary) antibody. Immunolabelling of tissue sections for light microscopy (inverted transmission microscope Olympus IMT-2) was performed as reported.23 For confocal fluorescence microscopy nonspecific binding was blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich, Steinheim, Germany) and adequate 10% normal serum (Sigma). Primary antibody (10 µg/ml) against the selected antigen were added for 45 min at 37°. Another incubation for 45 min at 37° followed with species-specific or isotype-specific (only after primary mouse CNA.42 mAb) and Alexa Fluor™-labelled secondary antibody (Molecular Probes, Eugene, OR). In case of labelling with two mouse mAb, of which one was anti-p41 Ii 2C12 mAb, tissue sections were labelled in three steps. First, non-labelled primary mAb (5F6 mAb, for example) was added, followed by anti-mouse Alexa Fluor™ 546-labelled secondary antibody, and thirdly, tissue sections were incubated with 2C12 mAb, directly labelled with fluorophore Alexa Fluor™ 488. Washing steps with PBS were performed after each incubation in order to remove the excess of the applied antibody. Controls omitting the primary and/or secondary antibody and preblocking experiments were performed. Fluorescence microscopy and optical slicing were performed using a confocal laser scanning microscope LSM 510 (Carl Zeiss). Alexa Fluor™ 488 and Alexa Fluor™ 546 were excited with an argon (488 nm) and He/Ne (543 nm) laser, respectively. The emission signal was filtered using BP 505–530 nm (green fluorescence of Alexa Fluor™ 488) and LP 560 nm (red fluorescence of Alexa Fluor™ 546) filters. Excitation lasers were used successively, avoiding the overlapping emission of Alexa Fluor™ 488 in red spectra. Carl Zeiss LSM 3·0 software was used.
Labelling of anti-p41 invariant chain 2C12 mAb with fluorophore Alexa Fluor™ 488
Anti-p41 Ii 2C12 mAb was labelled with Alexa Fluor™ 488 using Alexa Fluor™ 488 Protein Labelling Kit as recommended by the producer (Molecular Probes). Preserved affinity of 2C12 mAb for p41 Ii after labelling with Alexa Fluor™ 488 fluorophore was determined by antigen-immobilized ELISA as described under ‘Characterization of anti-invariant chain mAb by ELISA’ and compared to nonlabelled 2C12 mAb. Anti-p41 Ii 2C12 mAb, directly labelled with Alexa Fluor™ 488, was applied to tissue sections, in order to avoid the use of antimouse Alexa Fluor™ 488-labelled secondary antibody in colocalization studies with another mouse mAb, i.e. anti-Ii 5F6 mAb, anti-CD68 mAb, antifollicular dendritic cell mAb, and anti-cathepsin H, anti-HLA-DR mAb and anti-cystatin C mAb, as described above.
Preblocking experiment with anti-invariant chain mAb
The specificity of anti-Ii mAb (2C12 and 5F6) was also tested by blocking their paratopes with the antigen prior to localization studies on tissue sections. Two different antigens (inhibitory recombinant p41 Ii and non-inhibitory recombinant p31 Ii) were added separately to anti-Ii mAb in 1 : 3 molar ratio (mAb : antigen) and incubated for 1 hr at 37°. Two parallel labellings of tissue sections were performed with 2C12 mAb and 5F6 mAb, respectively, once without preblocking and secondly, with preblocking with the antigen. Confocal micrographs were taken and the intensity of labelling of morphologically similar areas of lymph nodes was compared.
Results
Generation of two different anti-human invariant chain monoclonal antibodies
Two different mouse anti-Ii antibodies (2C12 mAb and 5F6 mAb, both immunoglobulin G1 (IgG1) were generated against two distinct Ii epitopes on the luminal portion of human Ii isoforms. Monoclonality of purified anti-Ii antibody was confirmed by isoelectric focusing, showing one set of bands for each of the characterized antibodies: at pI 6·5 for 2C12 mAb and at pI 5·6 for 5F6 mAb (data not shown).
Characterization of generated anti-invariant chain monoclonal antibodies by antigen-immobilized ELISA
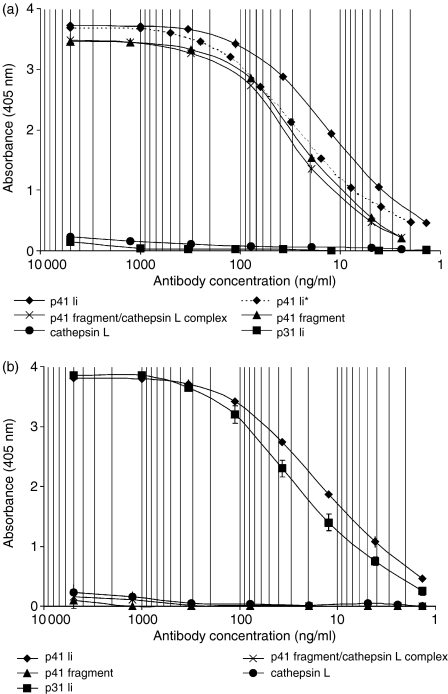
Strong reaction of 2C12 mAb was observed with recombinant inhibitory p41 Ii, as well as with the inhibitory p41 fragment detached from the complex and inhibitory p41 fragment in the isolated complex with cathepsin L (Fig. 1a). A signal of anti-Ii 2C12 mAb with recombinant non-inhibitory p31 Ii was negligible (Fig. 1a). In addition, affinity of 2C12 mAb for p41 Ii has been preserved after labelling of 2C12 mAb with Alexa Fluor™ 488 fluorophore (Fig. 1a). 5F6 mAb reacted with both recombinant (p41 and p31) Ii isoforms (Fig. 1b). However, 5F6 mAb did not react with the isolated inhibitory p41 fragment, detached from the complex or inhibitory p41 fragment in the isolated complex with cathepsin L (Fig. 1b). Cross-reactivity of anti-Ii 2C12 mAb and 5F6 mAb towards cathepsin L was not significant (Figs 1a, b).
Figure 1.
Specificity of anti-human invariant chain 2C12 (a) and 5F6 (b) mouse monoclonal antibodies, determined by different antigen-immobilized ELISAs. 2C12 and 5F6 mAb were added in the concentration range of 1 ng/ml (9·4 × 10−12 m) to 5 µg/ml (3·4 × 10−8 m). Curve marked with (p41*) represents the signal of Alexa Fluor™ 488-labelled 2C12 mAb with recombinant inhibitory p41 Ii.
Characterization of generated anti-invariant chain mAb by Western blot analysis
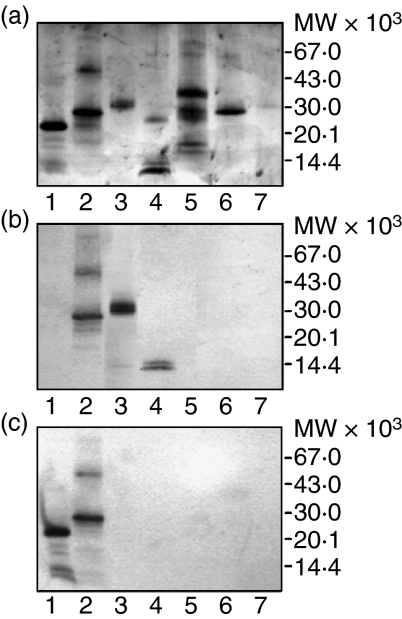
2C12 mAb specifically recognized recombinant inhibitory p41 Ii (Fig. 2b, lane 2), as well as inhibitory p41 fragment alone, i.e. detached from the isolated complex with cathepsin L (Fig. 2b, lane 4, two bands at 14 000 MW), and inhibitory p41 fragment in the isolated complex with cathepsin L (Fig. 2b, lane 3, above 30 000 MW). No cross-reactivity of 2C12 mAb was observed with shorter non-inhibitory recombinant p31 Ii without p41 fragment (Fig. 2b, lane 1). 5F6 mAb recognized both recombinant Ii isoforms (Fig. 2c, lane 1 and 2) but not the isolated inhibitory p41 fragment, detached from the complex (Fig. 2c, lane 4) or inhibitory p41 fragment in the isolated complex with cathepsin L (Fig. 2c, lane 3). No cross-reactivity of 2C12 mAb (Fig. 2b) or 5F6 mAb (Fig. 2c) was observed with cathepsin L (lane 3 and 7), cathepsin H (lane 6) or cathepsin S (lane 5). Taking together, 2C12 mAb (Fig. 2b) reacted with all tested antigen, which contain inhibitory p41 fragment and 5F6 mAb (Fig. 2c) reacted with both Ii isoforms.
Figure 2.
Specificity of anti-human invariant chain 2C12 and 5F6 mAbs determined by Western blot analysis. Antigens were subjected to SDS–PAGE (a) and transferred onto PVDF membrane (b, c) for the reaction with 2C12 mAb(b) and 5F6 mAb(c). All samples but one (lane 3), were reduced with 0·01 m DTT and exposed to 100° in the presence of SDS for 5 min prior to SDS–PAGE. Samples: (lane 1) recombinant p31 Ii (lane 2) recombinant p41 Ii (lane 3) isolated non-covalent p41 fragment/cathepsin L complex (non-reduced) (lane 4) isolated non-covalent p41 fragment/cathepsin L complex (reduced) (lane 5) recombinant procathepsin S (lane 6) cathepsin H (lane 7) recombinant cathepsin L. Bands at higher molecular masses (lane 2) represent oligomers of recombinant p41 Ii. Cathepsin L from the complex was under reducing conditions (lane 4) separated to heavy chain (below 30 000 MW) and light chain (band below inhibitory p41 fragment, i.e. below 14 000 MW) compared to single-chain recombinant cathepsin L (lane 7). Pro-cathepsin S, its mature form and pro-region are also visible under reducing conditions (lane 5).
Blocking of 2C12 and 5F6 monoclonal antibody paratopes with inhibitory p41 and non-inhibitory p31 invariant chain isoforms
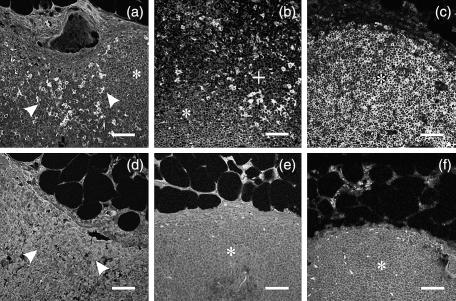
Pre-blocking experiment with inhibitory p41 Ii (Fig. 3d) but not non-inhibitory p31 Ii (not shown) showed abolished reaction of 2C12 mAb towards lymph node tissue antigen, whereas reaction of 5F6 mAb was blocked by adding non-inhibitory p31 Ii (Fig. 3f) or inhibitory p41 Ii (not shown).
Figure 3.
Lymph node distribution of p41 and p31 invariant chain isoforms (immunofluorescence). Human lymph node tissue sections were labelled with anti-Ii primary mAb (2C12 mAb and 5F6 mAb) and anti-mouse Alexa Fluor™ 488-labelled secondary antibody in single labelling experiment. (a, b) 2C12 mAb(c) 5F6 mAb(d) 2C12 mAb, preblocked with inhibitory recombinant p41 Ii(e) control, without primary anti-Ii mAb and (F) 5F6 mAb, preblocked with non-inhibitory recombinant p31 Ii. The positions of sinus (arrows), paracortex (+) and cortex (*) are denoted. Bars: 50 µm.
Lymph node distribution of inhibitory p41 and non-inhibitory p31 invariant chain isoforms
p41 Ii+ cells were found in subcapsular, cortical (Fig. 3a) and medullary sinuses (not shown), paracortex (Fig. 3b, top) and germinal centres (Fig. 4f, green), compared with other less labelled parts of cortex, i.e. primary follicles. The difference between the number of p41 Ii+ cells labelled in paracortex (Fig. 3b, top) and those in primary follicles (Fig. 3b, bottom) was clearly observed. In comparison to p41 Ii, more uniform distribution of p31 Ii (Fig. 3c) was observed throughout the lymph node, i.e. in cortex, paracortex and sinuses. Goat anti-mouse IgG Alexa Fluor™ 488-labelled secondary antibody did not bind to lymph node tissue (control, Fig. 3e). These results were also confirmed in double labelling experiment with 5F6 mAb and 2C12 mAb, where both antibodies showed positive reaction in sinuses, paracortex and secondary follicles, but not in primary follicles, where cells were labelled only with 5F6 mAb but not with 2C12 mAb (figure not shown). p41+ cells in paracortex (Fig. 3b, top) were also positive for MHC II and for cystatin C (figure not shown).
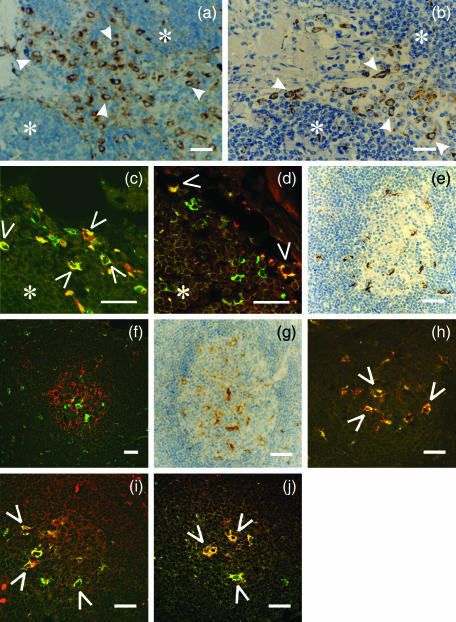
Figure 4.
Co-localization study of inhibitory p41 invariant chain with cathepsins H, L and S in sinuses with afferent lymph (a–d) and in germinal centres (e–j). Human lymph node tissue sections were labelled with different (primary) antibodies: (a) anti-cathepsin S 1E3 mAb (b) anti-cathepsin L pAb (c) anti-p41 Ii 2C12 mAb (green) and anti-CD68 mAb (red) (d) anti-p41 Ii 2C12 mAb (green) and anti-cathepsin H 1D10 mAb (red) (e) anti-CD 68 mAb (f) anti-p41 Ii 2C12 mAb (green) and anti-follicular dendritic cell mAb (red)(g) anti-cathepsin S 1E3 mAb (h) anti-p41 Ii 2C12 mAb (green) and anti-CD 68 mAb (red)(i) anti-p41 Ii 2C12 mAb (red) and anti-cathepsin L pAb (green) (j) anti-p41 Ii 2C12 mAb (green) and anti-cathepsin H 1D10 mAb (red). HRP-labelled secondary antibody was applied in a, b, e and g. Specific signal from primary Alexa Fluor™ 488-labelled 2C12 mAb (green) is shown in c, d, f, h, j, whereas Alexa Fluor™-labelled secondary antibodies were applied with all other primary antibodies (listed above). Before merging the confocal images (c, d, f, h, i and j) the signals for red fluorescence (from Alexa Fluor™ 546) and green fluorescence (from Alexa Fluor™ 488) were adjusted to comparable levels. Yellow colour (open arrows) indicates colocalization of two labelled antigens. (*) cortex. Bars: 25 µm.
Co-localization study of cathepsins with inhibitory p41 invariant chain inside sinuses with afferent lymph
Cathepsin S (Fig. 4a, arrows), cathepsin L (Fig. 4b, arrows), cathepsin H (Fig. 4d, open arrows) and p41 Ii (Fig. 4c) exhibited a similar staining pattern inside sinuses with afferent lymph, which corresponded predominantly to strongly CD68+ cells (Fig. 4c, open arrows), i.e. sinus-lining macrophages. No significant colocalization of p41 Ii with cathepsin L was detected in macrophages in sinuses (not shown), although other macrophage population (tingible body macrophages in germinal centres, see results below) was strongly positive for both tested antigen, i.e. for p41 Ii and cathepsin L, on the very same tissue sections. On the contrary, colocalization of p41 Ii with cathepsin H (Fig. 4d, open arrows) was observed in CD68+ sinus-lining macrophages. In sinuses small-sized CD68– cells were also labelled positively for p41 Ii (Fig. 4c, green), but no colocalization with cathepsin H was observed there (Fig. 4d, green).
Localization of inhibitory p41 invariant chain together with cathepsins S, L and H in germinal centres
Germinal centres (Fig. 4e–j) presented a different picture from the rest of the cortex (Fig. 4a–d), with regard to the localization of cathepsins and inhibitory p41 Ii. A population of CD68+ tingible body macrophages (Figs 4e, h) was strongly labelled for inhibitory p41 Ii (Fig. 4h) and for all three cathepsins S, L and H (Figs 4g, i, j). In these cells, residing in germinal centres, p41 Ii was colocalized with cathepsin L (Fig. 4i), cathepsin H (Fig. 4j) and cathepsin S (not shown). As also evident, the presence of p41 Ii (Fig. 4f, green) or cathepsins S, H and L (not shown) can not be observed in follicular dendritic cells (Fig. 4f, red).
Discussion
Regional lymph nodes are one of the first components of the immune system to come into contact with antigen from normal, inflamed or tumour tissues. In a recent study we reported the levels and localization of cathepsin S in tumours and regional lymph nodes of patients with lung cancer. Its high expression in macrophages and lymphocytes, decreased levels in lymph nodes, infiltrated with tumour cells, and a correlation of low levels of cathepsin S in tumours and surrounding lung tissue with poor patient survival, were related to the modulation of antigen presentation and consequently to antitumour immune response.20
MHC II-associated p41 isoform of invariant chain (p41 Ii) has been suggested to act as an inhibitor of cysteine protease cathepsin L. The p41 Ii contains an additional inhibitory p41 fragment, that binds non-covalently to the active site of cathepsin L.7,8 The p41 fragment/cathepsin L complex was first isolated from human kidney.27 Further studies of in vivo significance of p41 Ii were performed on mutant mice, that express either p31 or p41 Ii isoform28,29 confirming described interaction between p41 Ii and cathepsin L.9,10 It was demonstrated that the level of active cathepsin L was reduced in bone marrow derived APC (macrophages) that lack p41 Ii.9 On the other hand, MHC II expression and function were unaffected in p31 or p41 mutant mice, implying that other functional activities of p31 and p41 isoforms, as MHC II-specific chaperones, are largely shared in these animals.28,29 We compared p41 Ii content and localization in generated human monocyte-derived immature and mature dendritic cells, also applied as APC in antigen-presentation assays to T cells. No changes in p41 protein content or its localization were observed during dendritic cell maturation with tumour necrosis factor-αin vitro (Zavašnik-Bergant, unpublished data). Furthermore, it has been reported that in dendritic cells surface MHC II expression is regulated independently of Ii degradation.30
Present colocalization study has been performed on another population of APC (i.e. on macrophages) in order to confirm the proposed interaction between cathepsin L, as well as cathepsin H, with inhibitory p41 Ii in human lymph node tissue. Two populations of macrophages, both highly immunolabelled for p41 Ii in previous single labelling experiment, but with different physiological function inside lymph nodes, were taken into consideration. First, sinus-lining macrophages in afferent lymph as potential APC and second, highly phagocytic tingible body macrophages in germinal centres, the latter responsible for elimination of non-selected apoptotic B cells, but not acting as accessory APC cells for antigen presentation in germinal centres.31
Two different mouse monoclonal antibodies have been prepared and used to show a difference in expression of p41 and p31 Ii in human lymph node tissue. First epitope was located on the inhibitory p41 fragment (2C12 mAb) and the second one on the non-inhibitory luminal portion of Ii (5F6 mAb). 2C12 mAb detected less widely distributed p41 Ii and its proteolytically cleaved shorter luminal parts including inhibitory p41 fragment, whereas the signal obtained from 5F6 mAb showed the contribution of both Ii isoforms. From the titration curves for anti-Ii mAb (Fig. 1) it can be seen that 2C12 mAb affinity for p41 Ii isoform is comparable to the affinity of 5F6 mAb for p31 and p41 Ii. Therefore, we assumed that where only 5F6 mAb, but not 2C12 mAb, showed positive reaction on tissue sections in double labelling experiment, only p31 Ii was present (in primary follicles, for example). Furthermore, given that p31 Ii constitutes the majority of Ii content inside most cells32 the signal from 5F6 mAb was taken as representative for p31 Ii distribution in lymph nodes.
Non-inhibitory p31 Ii was found to be uniformly distributed and independent of morphological and physiological region of lymph node tissue. This is consistent with the described multiple physiological roles that invariant chain possesses in B cells, macrophages and dendritic cells.4 p31 Ii distribution profile did not match the profiles of cathepsins S, L and H. On the contrary, the distribution profile of inhibitory p41 Ii matched the profiles of cathepsins S, L and H. The most abundant staining was observed in sinuses with afferent lymph. We have previously reported strong colocalization of p41 Ii with cathepsin S in CD68+ sinus-lining macrophages.19 This is to be expected, since cathepsin S is a key enzyme capable to degrade Ii, releasing MHC II binding site.15 Therefore, in sinus-lining macrophages p41 Ii represents cathepsin's S substrate. On the other hand, no colocalization of p41 Ii with cathepsin L was confirmed in these cells, although cathepsin L would be the most expected target enzyme regarding binding properties, determined in vitro with isolated human proteins8 and mouse bone marrow-derived macrophages.9 However, mouse cathepsin L is more closely related to human cathepsin V than to human cathepsin L and human cathepsin V, but not human cathepsin L, has been suggested to play an important role in thymic MHC class II antigen presentation.11 Therefore, the results on mouse experimental models not necessary reflect the situation in humans. In this study confocal microscopy confirmed colocalization of p41 Ii with cathepsin H in the same macrophage population. Therefore, cathepsin H could be a physiological target for inhibition with inhibitory p41 fragment, although the binding capacity is lower compared to cathepsin L.8 Our results do not directly prove the complex formation between p41 Ii and cathepsin H in human lymph node tissue, but strongly indicate that the proposed interaction between target enzyme and p41 Ii could take place in lysosomes and late endosomes inside CD68+ sinus-lining macrophages. These results are in agreement with the detected active cathepsin H in cell lysates and phagosomes from mouse bone marrow-derived macrophages.33
We also found high content of inhibitory p41 Ii in tingible body macrophages inside germinal centres, which are responsible for the elimination of apoptotic B cells during clonal selection17 but their function in germinal centres is not antigen presentation. Besides p41 Ii, tingible body macrophages were strongly labelled for cathepsin L, cathepsin H and cathepsin S. p41 Ii was colocalized with all three cathepsins in lysosomes of these CD68+ macrophages. The possibility that high content of p41 Ii inside tingible body macrophages originated from phagocytosed apoptotic B cells can not be completely excluded. However, in this case the contribution of exogenous p41 Ii to the fluorescence signal inside tingible body macrophages would be minor, since it is evident that B cells in germinal centres were not positively labelled for p41 Ii.
It was suggested that p41 Ii may act as a chaperone molecule and by binding cathepsin L active site prevents its degradation by other present proteases.9,10 Following this explanation, in strongly acidic compartments (lysosomes) inside tingible body macrophages, the equilibrium between preserved cathepsin L in complex with p41 Ii and free active cathepsin L27 would be in favour of the free and active enzyme. The same regulation would go also for cathepsin H. Therefore, the ability of p41 Ii to preserve the active forms of cathepsins L and H should be significant for the complete degradation of apoptotic B cells after phagocytosis and their innocuous elimination by tingible body macrophages, which is crucial in order to avoid presentation of autoantigens by present follicular dendritic cells.34
No p41 Ii or cathepsins S, L and H were detected in non-phagocytic follicular dendritic cells, involved in tuning of germinal centre reaction.35 Absence of these proteolytic enzymes supports their antigen-collecting function and presentation to germinal centre B cells without intracellular processing of the presented antigen.35 In comparison to follicular dendritic cells both, in vitro generated immature and mature human dendritic cells and MHC II+ cystatin C+ interdigitating dendritic cells inside lymph node paracortex, were positively labelled for p41 Ii. If p41 Ii has an effect on cathepsins L and H, both present inside endocytic pathway of human dendritic cells (Zavašnik-Bergant, unpublished data), this interaction most probably does not directly affects MHC II-associated antigen presentation of dendritic cells.
In conclusion, we showed different distribution of p41 and p31 Ii isoforms inside human lymph nodes. Distribution of inhibitory p41 Ii corresponded to the one of cathepsins S, L and H. Colocalization of p41 Ii with cathepsin L was confirmed in tingible body macrophages but not in sinus-lining macrophages, suggesting inhibitory role of p41 Ii towards cathepsin L could be relevant, but not in all macrophage populations in human lymph nodes. Confirmed colocalization of p41 Ii with cathepsin H in both studied macrophage population supports also cathepsin H as a target enzyme for p41 Ii inhibition in lysosomes of human macrophages.
Acknowledgments
The authors thank Dr Klaus Dornmair (Max Planck Institute, Martinsried) for providing human recombinant p41 invariant chain and p31 invariant chain isoforms, Andreja Sekirnik for providing anti-cathepsin S 1E3 mAb, Tomaž Langerholc for purifying anti-invariant chain 5F6 mAb and Ms. Alenka Kljun for technical assistance. The authors acknowledge Professor Roger H. Pain for critical reading of the manuscript. This work was supported by the Ministry of Education, Science and Sport of the Republic of Slovenia.
Abbreviations
- APC
antigen-presenting cell
- Ii
invariant chain
- Ki
inhibition constant
- mAb
monoclonal antibody
- pAb
polyclonal antibody
References
- 1.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rew. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 2.Wubbolts RW, Neefjes J. Intracellular transport and peptide loading of MHC class II molecules: regulation of chaperones and motors. Immunol Rev. 1999;172:189–208. doi: 10.1111/j.1600-065x.1999.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 3.Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–8. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 4.Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta. 2002;1542:1–13. doi: 10.1016/s0167-4889(01)00166-5. [DOI] [PubMed] [Google Scholar]
- 5.Strubin M, Berte C, Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J. 1986;5:3483–8. doi: 10.1002/j.1460-2075.1986.tb04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenarčič B, Bevec T. Thyropins – new structurally related proteinase inhibitors. Biol Chem. 1998;379:105–11. [PubMed] [Google Scholar]
- 7.Gunčar G, Pungerčič G, Klemenčič I, Turk V, Turk D. 1999;18:793–803. doi: 10.1093/emboj/18.4.793. Crystal structure of MHC class II-associated p41, Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevec T, Stoka V, Pungerčič G, Dolenc I, Turk V. Major histocompatibility complex class II-associated p41 invariant chain fragment is a strong inhibitor of lysosomal cathepsin L. J Exp Med. 1996;183:1331–8. doi: 10.1084/jem.183.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennon-Duménil A-M, Roberts RA, Valentijn K, et al. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J. 2001;20:4055–64. doi: 10.1093/emboj/20.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiebiger E, Maehr R, Villadangos J, Weber E, Erickson A, Bikoff E, Ploegh HL, Lennon-Duménil A-M. Invariant chain controls the activity of extracellular cathepsin L. J Exp Med. 2002;196:1263–9. doi: 10.1084/jem.20020762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brömme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, elecrostatic surface potential, enzymatic characterization and chromosomal localization. Biochemistry. 1999;38:2377–85. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 12.Riese RJ, Chapman HA. Cathepsins and compartmentalization in antigen presentation. Curr Opin Immunol. 2000;12:107–13. doi: 10.1016/s0952-7915(99)00058-8. [DOI] [PubMed] [Google Scholar]
- 13.Shi GP, Villadangos JA, Dranoff G, et al. Cathepsin S required for normal MHC Class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 14.Driessen C, Bryant RAR, Lennon-Duménil A-M, Villadangos JA, Wolf Bryant P, Shi G-P, Chapman HA, Ploegh HL. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol. 1999;147:775–90. doi: 10.1083/jcb.147.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa TY, Brissette WH, Lira PD, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–17. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa TY, Rudensky AY. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol Rev. 1999;172:121–9. doi: 10.1111/j.1600-065x.1999.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 17.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rew. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 18.Schweiger A, Štabuc B, Popovič T, Turk V, Kos J. Enzyme-linked immunosorbent assay for the detection of total cathepsin H in human tissue cytosols and sera. J Immunol Meth. 1997;201:165–72. doi: 10.1016/s0022-1759(96)00218-9. [DOI] [PubMed] [Google Scholar]
- 19.Zavašnik-Bergant V, Sekirnik A, Golouh R, Turk V, Kos J. Immunochemical localisation of cathepsin S, cathepsin L and MHC class II-associated p41 isoform of invariant chain in human lymph node tissue. Biol Chem. 2001;382:799–804. doi: 10.1515/BC.2001.096. [DOI] [PubMed] [Google Scholar]
- 20.Kos J, Sekirnik A, Kopitar G, Cimerman N, Kayser K, Stremmer A, Fiehn W, Werle B. Cathepsin S in tumours, regional lymph nodes and sera of patients with lung cancer: relation to prognosis. Br J Cancer. 2001;85:1193–200. doi: 10.1054/bjoc.2001.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kos J, Šmit A, Krašovec M, Svetic B, Lenarčič B, Vrhovec I, Škrk J, Turk V. Lysosomal proteases cathepsins D, B, H, L and their inhibitors stefins A and B in head and neck cancer. Biol Chem. 1995;376:401–5. doi: 10.1515/bchm3.1995.376.7.401. [DOI] [PubMed] [Google Scholar]
- 22.Dolinar M, Barlič Magajna D, Turk V. Expression of full-length human procathepsin L cDNA in Escherichia coli and refolding of the expression product. Biol Chem. 1995;376:385–8. doi: 10.1515/bchm3.1995.376.6.385. [DOI] [PubMed] [Google Scholar]
- 23.Strojnik T, Kos J, żidanik B, Golouh R, Lah T. Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clin Cancer Res. 1999;5:559–67. [PubMed] [Google Scholar]
- 24.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysomal glycoproteins. Blood. 1993;81:1607–13. [PubMed] [Google Scholar]
- 25.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 26.Kos J, Krašovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brünner N. Cysteine proteinase inhibitors stefin A, stefin B and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–11. [PubMed] [Google Scholar]
- 27.Ogrinc T, Dolenc I, Ritonja A, Turk V. Purification of the complex of the cathepsin L and the MHC class II-associated invariant chain fragment from human kidney. FEBS Lett. 1993;336:555–9. doi: 10.1016/0014-5793(93)80875-u. [DOI] [PubMed] [Google Scholar]
- 28.Takaesu NT, Lower JA, Robertson EJ, Bikoff EK. Major histocompatibility class II peptide occupancy, antigen presentation, and CD4+ T cell function in mice lacking the p41 isoform of invariant chain. Immunity. 1995;3:385–96. doi: 10.1016/1074-7613(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 29.Takaesu NT, Lower JA, Yelon D, Robertson EJ, Bikoff EK. In vivo functions mediated by the p41 isoform of the MHC class II-associated invariant chain. J Immunol. 1997;158:187–99. [PubMed] [Google Scholar]
- 30.Villadangos JA, Cardoso M, Steptoe RJ, van Berkel D, Pooley J, Carbone FR, Shortman K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity. 2001;14:739–49. doi: 10.1016/s1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 31.Smith JP, Burton GF, Tew JG, Szakal AK. Tingible body macrophages in regulation of germinal center reactions. Dev Immunol. 1998;6:285–94. doi: 10.1155/1998/38923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kämpgen E, Koch N, Koch F, Stoger P, Heufler C, Schuler G, Romani N. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain and antigen processing capacity are down-regulated upon culture. Proc Natl Acad Sci U S A. 1991;88:3014–8. doi: 10.1073/pnas.88.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennon-Duménil A-M, Bakker AH, Maehr R, Fiebiger E, Overkleeft HS, Rosemblatt M, Ploegh HL, Lagaudriére-Gesbert C. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic content during dendritic cell activation. J Exp Med. 2002;196:529–39. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann I, Kolowos W, Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centres of patients with systemic Lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–65. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]