Abstract
In view of the presence of a large number of epithelial cells in the alveoli of the lung and their ability to produce various cytokines and chemokines, the possible role of alveolar epithelial cells in the innate immune response to tuberculosis was examined. The human alveolar epithelial cell line A549 was used as a model. The ability of A549 cells to induce nitric oxide (NO) in response to Mycobacterium tuberculosis infection was taken as an in vitro correlate of innate immunity. M. tuberculosis infection induced A549 cells to produce significant levels of NO and to express inducible nitric oxide synthase mRNA at 48 hr of infection. However, the amount of NO released at this point was not mycobactericidal. Cytokine stimulation (interferon-γ, tumour necrosis factor-α, interleukin-1β, alone or in combination) of the infected A549 cells induced a higher concentration of NO. The study of colony-forming units (CFU) as a measure of the mycobactericidal capacity of A549 cells revealed a reduction in CFU of M. tuberculosis by 39·29% (from 10·62 ± 0·48 – 6·392 ± 0·54) following cytokine stimulation of the infected cells. Interestingly γ-irradiated M. tuberculosis H37Rv could also induce higher than basal level of NO. Therefore we examined mycobacterial antigenic components for their possible role in NO production. We observed that A549 cells produced significantly higher amounts of NO at 48 hr when treated with mycobacterial whole cell lysates, cell wall or cell membrane preparations. The release of NO and the resultant mycobactericidal activity could be further enhanced by simultaneously conditioning the M. tuberculosis infected A549 cells with cytokine and mycobacterial components. These results suggest that alveolar epithelial cells respond to their microenvironment, which is constituted of various cytokines and macrophage-processed antigens and may contribute to the innate immune response to tuberculosis.
Keywords: epithelial cells, A549, NO, Mycobacterium tuberculosis, innate immune response
Introduction
Tuberculosis is acquired through inhalation of droplets containing live Mycobacterium tuberculosis, and the lung is the major site of disease activity. Although most studies of the interactions between M. tuberculosis and lung cells have focused on alveolar macrophages1–4 it is possible that the interactions between M. tuberculosis and alveolar epithelial cells play an important role in the immune response during pulmonary tuberculosis. Until recently the alveolar epithelial cells were considered as ‘bystanders’ only.5 Subsequently several reports have appeared in the literature on the innate immune response against M. tuberculosis infection mediated by cells like neutrophils and dendritic cells.6–8 However, not much is known about the part played by the alveolar epithelial cells during tuberculosis infection.
Type II alveolar epithelial cells are ideally situated to play a role in local regulation of inflammatory response within the alveolar space.9,10 These cells produce inflammatory mediators such as interleukin-8 (IL-8),11,12 prostaglandins,9 nitric oxide (NO)13,14 and complement proteins15,16 capable of modulating local immune response. An in vitro experimental model using the alveolar epithelial cell line A549 has demonstrated that H37Rv, a virulent strain of M. tuberculosis can replicate within the A549 cell line at a low multiplicity of infection (1–10 per organism) and grow more rapidly in A549 cells than the avirulent H37Ra strain.17–22 These studies, however, did not focus on the ability of M. tuberculosis to induce NO production as a mediator of innate immune response.
NO is a short-lived, readily diffusible molecule of great biological importance. It plays a key role in signal transduction, neurotransmission, and host-defense mechanism. NO is produced by nitric oxide synthase (NOS), a heme-protein that catalyses the oxidation of l-arginine to NO and citrulline.23,24 The inducible NOS (iNOS or NOS2) has been identified as a protective locus against tuberculosis using a transgenic mouse model NOS2–/–, and NO is shown to be mycobactericidal in a murine system.25 Although there are conflicting reports as to whether human monocytes/macrophages are able to kill M. tuberculosis in an iNOS-dependent manner, in earlier communications from our laboratory26,27 we have reported that proinflammatory cytokine stimulation of monocytes/ex vivo matured macrophages from active tuberculosis patients leads to NO production that brings down the colony-forming units (CFU) of M. tuberculosis in these cells. The present study was designed to see whether infection of alveolar cells with M. tuberculosis results in induction of NO. The possible bactericidal activity of the induced NO on the CFU counts of M. tuberculosis in the A549 cells was also studied. In order to demonstrate that the NO release was an active process, mRNA expression for the iNOS gene was correlated for each set of experiments. IL-8 estimation and mRNA expression for IL-8 was undertaken as a measure of activation of the A549 cell line. To understand whether interaction with live mycobacteria is mandatory, γ-irradiated H37Rv and several mycobacterial subcellular components were incorporated in the test systems. We demonstrate that the alveolar epithelial cells upon infection with M. tuberculosis or stimulation with mycobacterial components induce release of NO. Additional cytokine stimulation brings about reduction in CFU that correlates significantly with the concentration of released NO.
Materials and methods
Alveolar epithelial cell line
The human pulmonary type II alveolar epithelial cell line A549 was purchased from National Center for Cell Science, Pune, India and was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, and 10 µg/ml ampicillin, an antibiotic with no significant antimycobacterial activity at this concentration, in a humidified 5% CO2 atmosphere. Epithelial cells were seeded in six-well tissue culture plates (106 cells per well) 48 hr before use. Immediately before experiment, medium was replaced with serum-free DMEM supplemented with 2 mm glutamine and 10 µg/ml ampicillin.
Mycobacteria
M. tuberculosis H37Rv (American Type Culture Collection, Rockville, MD) was maintained in Lowenstein Jensen medium slants. Mycobacteria were subcultured 15 days prior to use in 8 ml Middlebrook 7H9 (DIFCO, Becton Dickinson India Ltd, Haryana, India) supplemented with albumin dextrose catalase. Prior to experiments the culture tubes were vortexed, allowed to stand for one minute. The upper 6 ml was withdrawn and centrifuged at 220 g for 10 min. The pellet was resuspended in DMEM supplemented with 10% FCS and passed through an 8-µm filter to prepare a single cell suspension. The dispersed bacterial concentration was measured by taking absorbance at 600 nm using MacFarland's nephelometric standards.27 The bacterial concentration was adjusted to a density of 108 bacteria/ml, aliquoted and kept as a single lot at 4°. For infection, the H37Rv suspension from this lot was taken out and added in the six-well plates at 1 : 10 ratio (A549 cell: H37Rv).
Infection of A549 cells by M. tuberculosis H37Rv and cytokine stimulation
Lung alveolar epithelial cells were infected with M. tuberculosis H37Rv and stimulated with three prime cytokines (interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and IL-1β), that are thought to be present in the internal milieu of the infected lung. A549 cells grown in six-well tissue culture wells were charged with warmed DMEM supplemented with 2% FCS prior to infection and cytokine exposure. Initially the monolayers were treated with 350 µmol of NG-monomethyl-arginine to suppress any constitutive NOS activity for 2 hr (data not shown).28 In these experiments (in triplicate) confluent monolayers of A549 were infected with M. tuberculosis H37Rv at a ratio of 1 : 10 (cells : M. tuberculosis) in DMEM supplemented with 10% FCS for 6 hr followed by 2 hr treatment with amikacin (20 µg/ml), an aminoglycoside antimicrobial agent, to inhibit the growth of extracellular mycobacteria. The monolayer was washed three times with DMEM to remove extra cellular mycobacteria. The final wash medium was plated on the Lowenstein Jensen (LJ) slant to confirm the absence of extracellular mycobacteria in the medium.17
Following washing, infected monolayers were either kept as such or stimulated with the cytokines TNF-α, IFN-γ and IL-1β individually or in combination of two or three and incubated at 37° in a 5% CO2 atmosphere. TNF-α, IFN-γ and IL-1β were added at a concentration of 10 ng/ml, 250 units/ml and 50 units/ml, respectively.29,30 In another set, as a control experiment, uninfected monolayers were exposed to the cytokines TNF-α, IFN-γ and IL-1β as described above. A NO assay was performed at 24 and 48 hr, respectively. All experiments were done in triplicate. The kinetics of NO secretion showed an optimal production at 48 hr after cytokine stimulation. Therefore, this time period was considered for all further observations (data not shown). In parallel experiments subsequent addition of l-arginine, which is a substrate for the enzyme iNOS, resulted in further increase in the production of NO (data not shown). A549 cells were also grown on cover slips and infected with M. tuberculosis in the same ratio (MOI 1 : 10). Monolayers were then washed as described above and the cover slips were air dried and methanol fixed and stained using a modified cold kinyoun stain. The percentage of cells infected with M. tuberculosis was determined by counting the number of epithelial cells associated with mycobacteria. Counts were performed in high power field, one slide for each time point and experiments were performed in triplicate. We also undertook an invasion assay following a previously published protocol12,17,21,22 to demonstrate that M. tuberculosis was able to invade A549 cells. Briefly, amikacin was added to the culture medium to kill extracellular organisms, followed by washing and lysing A549 cell monolayers to release the mycobacteria. The lysates were cultured on LJ slants after appropriate dilutions. CFU of mycobacteria was determined after 3–4 weeks of incubation at 37°.
An XTT assay31[sodium 3′-(1-phenylamino carbonyl)-3-4-tetrazolium-bis (4-methoxy-6-nitro) benzene sulphonic acid hydrate] (Sigma, St Louis, MO) was performed at each time point to measure the cell growth and viability of infected A549 cell line. A CFU assay was performed at 48 hr. Isolation of messenger RNA and reverse transcription–polymerase chain reaction (RT–PCR) was also performed at 48 hr.
Stimulation of A549 with γ-irradiated H37Rv or components of H37Rv
In a parallel set of experiments the A549 cell lines were treated either with killed mycobacteria (γ-irradiated H37Rv) or different subcellular components of H37Rv. γ-Irradiated M. tuberculosis, whole cell lysate (WCL), cell wall, cell membrane, cytosol and lipoarabinomannan (LAM) were all derived from M. tuberculosis H37Rv and were kindly provided by Dr J. T. Belisle, Colorado State University, Fort Collins, CO [http://www.cvmbs.colostate.edu/microbiology/tb]. The aim of the experiment was to determine whether M. tuberculosis bacterial components of the cell surface and other subcellular components released after infection could also induce NO release. Confluent monolayers of A549 were stimulated with γ-irradiated H37Rv at a ratio of 1 : 10 (cells : γ-irradiated H37Rv) or were stimulated with different components of H37Rv at the following concentrations: WCL (10 µg/ml), cell wall (5 µg/ml), cell membrane (5 µg/ml), cytosol (5 µg/ml) and LAM (5 µg/ml).32 These concentrations of mycobacterial components were found to be optimum for NO production (data not shown). An NO was performed at 24 and 48 hr. All experiments were repeated at least three times. An assay was performed at each time point. Isolation of messenger RNA and RT–CR was also performed at 48 hr.
Infection of A549 cells followed by stimulation with cytokines and mycobacterial components
To determine the response of infected A549 cells to the mycobacterial components and to the cytokine present in the milieu of infected lung, the cells were infected with H37Rv followed by simultaneous stimulation with cytokines and different components of H37Rv as were used in the earlier experiments. NO assay was performed at 24 and 48 hr. XTT assay was performed to measure the cell growth and viability in each well. A CFU assay was performed at 48 hr. Isolation of messenger RNA and RT–PCR was also performed at 48 hr.
Cell viability assay (XTT assay)
To check for the cell proliferation and viability, overall activity of mitochondrial dehydrogenase in each set of experiments was measured according to the method of Roehm et al.31 A549 cells were grown in a 96-well tissue culture plate in four different concentrations (102, 103, 104, 105 and 106), followed by cytokine stimulation or infection or antigen stimulation, according to the experimental design described above. 25 µl of XTT in DMEM was added to each well and incubated at 37° for 24 hr. Overall activity of mitochondrial dehydrogenase in each well was measured spectrophotometrically at 490 nm using 650 nm as the reference wavelength.
NO assay
Concentration of nitrite produced by A549 cells as a measure of the production of NO was determined by the method of Ding et al.33 Briefly, 100 µl supernatant was removed from each culture well at 24 and 48 hr, centrifuged at 400 g for 10 min to make it cell free, incubated with 100 µl of Griess reagent (1% sulphanilamide, 0·1% napthylethylenediamine dihydrochloride, 2·5% phosphoric acid) at room temperature for 10 min Absorbance was read at 540 nm in a Beckman DU-64 spectrophotometer.
The concentration of nitrite (NO2) was determined by using sodium nitrite as standard. Nitrite release was reported as µm/1 × 106 cells per well. Cell-free medium was used as blank for the assay.
Messenger RNA isolation and RT–PCR
Messenger RNA was isolated from either M. tuberculosis infected or uninfected epithelial cells cultured in a six-well plate that were treated with cytokines or mycobacterial components at 37° for 48 hr. The cells were scraped using a cell scraper and collected after centrifugation at 400 g for 15 min.34 Messenger RNA (mRNA) was isolated using mRNA capture kit (Roche Biochemicals). Briefly the cells were lysed using lysis buffer, biotinylated oligo (dt)20 was added directly to the tubes and incubated for 5 min at 37°. Fifty microlitres of the mix was transferred to streptavidin-coated PCR tubes and incubated for 5 min at 37°. The mix was then removed and tubes washed with the washing buffer. The captured mRNA was used directly for RT–PCR using specific primers and a Techne thermocycler. For each sample, one aliquot without RT, was used as negative control for PCR amplification. The housekeeping β-actin gene was used as an internal control. Reverse transcription was carried out at 42° for 60 min according to the manufacturer's protocol.
Primer sequences were as follows: iNOS: TGGAATTCACTCAGCTGTGC (sense), GATGTTGTAGCGCTGGACG (antisense);35 IL-8: ATGACTTCCAAGCTGGCCGTG (sense), TTATGAATTCTCAGCCCTTCTTCAAAAACTTCTC (antisense);36β-actin: CCAAGGCCAACCGCGAGAAGATGAC (sense), AGGGTACATGGTGGTGCCGCCAGAC (antisense); (one step RT–PCR kit; Roche Biochemicals, Mumbai, India).
Cycling conditions were as follows: initial denaturation at 94° for 2 min, 35 cycles each of denaturation at 94° for 30 s, annealing for 1 min, extension at 68° for 1 min with final extension at 68° for 7 min. The annealing temperature for iNOS was 54° and for IL-8 and β-actin it was 65° and 66°, respectively.35,36 The RT–PCR products were run and visualized on 1·5% agarose gel with ethidium bromide along with the 100 base pair marker (MBI fermentas; Genetix Biotech Asia Pvt Ltd, New Delhi, India).
CFU assay
To determine the effect of nitric oxide produced by the infected A549 cell, A CFU assay was performed for each set of experiments. Infected A549 cells were harvested at 48 hr according to the experimental design described above, and lysed with 0·5 ml of 1% Triton-X-100 for 15 min followed by repeated passage through a 21-gauge needle to release intracellular mycobacteria.4,27 Serial 10-fold dilutions of the lysate were prepared in DMEM as 10−1, 10−2,10−3,10−4 and then plated on LJ medium slants. Colonies were counted after 3 weeks of incubation at 37° with 5% CO2 and CFU were calculated taking the dilutions into consideration. The number of initially ingested mycobacteria was calculated by lysing the infected A549 cells 6 hr after amikacin treatment and inoculating onto LJ slants, after suitable dilutions. On an average three to five mycobacteria were taken up by each cell and 40% of the A549 cells were infected (data not shown).
IL-8 assay
Pulmonary epithelial cells release IL-8 in response to M. tuberculosis infection.11,12,36 As an indicator of active response of A549 cells to M. tuberculosis infection in our experimental protocol, the cytokine IL-8 was assayed in cell culture supernatants at 48 hr. A solid phase sandwich enzyme-linked immunosorbent assay method using matched antibody pairs was applied, according to the manufacturer's instructions (Diaclone Research, Sancon, France). Briefly, the culture supernatants were added to the IL-8-cytokine specific monoclonal antibody-coated wells along with biotinylated IL-8-specific monoclonal antibody. After incubation and washing, the enzyme (strepavidin peroxidase) was added. After incubation and washing to remove unbound enzyme, a substrate solution was added to induce a coloured reaction product. The intensity of the coloured reaction is a direct measure of the concentration of cytokine IL-8 present in the culture supernatant.
Statistical analysis
Each experiment was repeated thrice and results are expressed as mean with standard error of mean (mean ±SEM), Student's t-test and t-paired tests were used to assess significance using Graph Pad Prism™ software.
Results
NO production by A549 cell line following cytokine stimulation
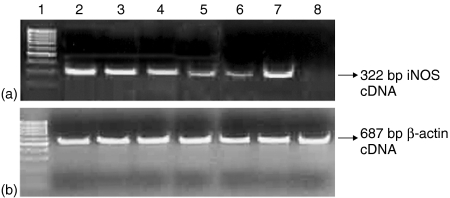
The A549 cell line, which is functionally similar to the human lung alveolar epithelial cells, was used as a model to study the innate immune response to tuberculosis. Induction of NO release was studied as an in vitro correlate. Because alveolar macrophages along with CD4 T cells release IFN-γ, TNF-α and IL-1β in the internal milieu of the infected lung1,2,37 the A549 cells were stimulated with cytokines IFN-γ, TNF-α and IL-1β individually or in combination. Significant concentrations of NO were released after stimulation with IFN-γ alone (35·26 ± 5·1 µm/l, P <0·0001), which was comparable to that produced by the mixture of all the three cytokines (38·76 ± 5·2 µm/l, P < 0·0001) at 24 hr (Table 1). The kinetics of secretion showed an optimal production at 48 hr after cytokine stimulation, therefore this time point was considered for all further observations. Enhanced production of NO after addition of arginine confirmed that the iNOS pathway of NO production is functional in A549 cells, as arginine is the substrate for iNOS enzyme.23,24,33 Any constitutive activity of cNOS was inhibited by addition of NG-monomethyl-arginine at the beginning of the experiments.28 IFN-γ was found to be significant inducer of iNOS alone or in combination with other cytokines (P < 0·001) (Table 1). To specifically examine whether the NO production is due to up regulation of iNOS mRNA expression in the alveolar epithelial cell line A549, iNOS specific cDNA synthesis following RT–PCR was performed (Fig. 1). When mRNA was isolated from these cells at 48 hr and RT–PCR performed for iNOS gene a good amount of RT–PCR product of 322 base pairs was obtained, which was in accordance with the published literature.37β-actin was used as an internal control. The cell viability was not affected by any of the cytokine used as checked by XTT assay (data not shown).
Table 1.
Production of nitrite by human alveolar epithelial cells A549 (µmol/1 × 106 cells)
| A549 cells | A549 cells infected with M.tuber culosis | |||
|---|---|---|---|---|
| Treatment | 24 hr | 48 hr | 24 hr | 48 hr |
| No cytokine | 12·44±3·3 | 13·30±3·4 | 20·44±5·5 | 29·53±5·7* |
| IFN-γ | 35·26±5·07* | 40·24±6·68* | 41·16±5·1* | 61·27±4·9* |
| TNF-α | 15·56±6·1 | 19·46±2·9 | 23·75±4·7* | 31·56±3·6* |
| IL-1β | 9·750±1·8 | 21·08±5·9 | 18·51±2·6 | 38·03±6·0* |
| IFN-γ+TNF-α | 29·43±4·5* | 50·30±4·1* | 37·86±4·9 | 55·46±6·2* |
| Cytokine mix | 38·76±5·2* | 55·05±7·8* | 48·86±4·9* | 56·75±7·7* |
P<0·0001 when paired t-tests were performed between A549 cells alone and cells infected with M. tuberculosis; A549 cells alone and cells stimulated with cytokines; A549 cells alone and cells infected with M. tuberculosis followed by cytokine stimulation.
Infected cells were simultaneously stimulated with various cytokines individually or as a mixture. ‘Cytokine mix’ has all three cytokines. IFN-γ TNF-αand IL-β were added at a concentration of 250 unit/ml, 10 ng/ml and 50 units/ml, respectively. All the experiments were performed in triplicate and results presented as mean ± SEM.
Figure 1.
RT–PCR result for iNOS gene (322 bp product) mRNA expression at 48 hr. (a) Lane 1: 100 bp marker. Lane 2: A549 cells treated with IFN-γ. Lane 3: A549 cells infected M. tuberculosis H37Rv and treated with IFN-γ. Lane 4: A549 cells treated with cytokine mixture. Lane 5: A549 cells treated with TNF-α. Lane 6: A549 cells infected with M. tuberculosis H37Rv and treated with TNF-α. Lane 7: A549 cells infected with M. tuberculosis H37Rv and treated with cytokine mixture. Lane 8: Untreated A549 cells. (b) β-actin gene mRNA (control, 687 bp product) demonstrating equal loading in all the corresponding lanes as in (a).
M. tuberculosis stimulated NO production by A549 cell line
The percentage of A549 cells infected with M. tuberculosis H37Rv assessed by microscopy of kinyoun-stained cell preparations showed that 40% of A549 cells were infected after 6 hr. This observation is consistent with studies that demonstrated by invasion assay that 42 ± 5% of A549 cells were invaded by M. tuberculosis after 4 hr incubation.17,21,22,35
Production of NO by M. tuberculosis-infected A549 cells indicated the active participation of alveolar epithelium in the innate immune response to tuberculosis. The kinetics of secretion showed an optimal production at 48 hr post-infection. Therefore this time point was considered for all further observations. When A549 cells were infected with M. tuberculosis the level of NO produced were negligible initially but rose significantly at 48 hr (29·53 ± 5·7 µm/l, P < 0·0001) and continued to increase up to 100 hr (66·09 ± 4·8 µm/l) (data not shown). When these infected cells were subsequently stimulated with cytokines the amount of NO produced was much higher than that produced by uninfected cells stimulated with cytokines. Again IFN-γ was the most potent cytokine to induce this effect (Table 1). An up-regulation of iNOS mRNA was observed at 48 hr when RT–PCR was performed on these samples (Fig. 1). The viability of the cells was not affected by M. tuberculosis infection as the overall mitrochondrial dehydrogenase activity continued to increase in all experimental wells, as determined by XTT assay (data not shown).
Antigenic components of H37Rv are good inducers of NO
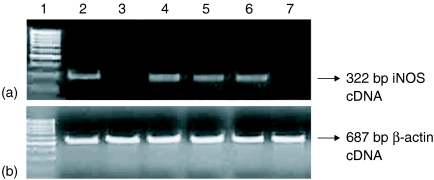
To determine whether induction of NO by M. tuberculosis could be due to bacterial components released after infection of cells or is caused by a constitutively expressed bacterial component, we substituted killed (γ-irradiated) H37Rv for live bacteria. A549 cell line (106 cells/ml) when stimulated with γ-irradiated H37Rv (1 × 107 cells/ml) did not produce significant levels of NO at 24 and 48 hr. But when incubated further until 72 hr, the production rose to a significant level (24·87 ± 4·8 µm/l, P < 0·0001). Though the concentration of NO was much less than that produced by live M. tuberculosis-infected A549 cells, the observation that killed M. tuberculosis is capable of causing induction of iNOS indicates that one or more constitutive components of the bacteria are competent to initiate a pathway that results in NO induction. In a subsequent experiment (Fig. 2) we observed that the WCL of M. tuberculosis induced up-regulation of NO indicating that some bacterial components too were able to exert their effects even when not presented in the form of whole particulate bacteria. We therefore surveyed several subcellular fractions of M. tuberculosis H37Rv. Among the various components of M. tuberculosis, WCL stimulated higher production of NO (57·11 ± 6·2 µm/l; P < 0·001) followed by cell wall (51·46 ± 5·0 µm/l; P < 0·001) and cell membrane fraction (41·89 ± 5·5 µm/l;P < 0·001) even at 24 hr of incubation when compared with basal value (12·44 ± 3·3 µm/l). In contrast, the cytosolic preparation did not produce significant amounts of NO at that timepoint (Fig. 2). The NO release was sustained till 48 hr of incubation by mycobacterial components. Because LAM is a major component of M. tuberculosis cell wall, we examined the ability of purified LAM to induce NO production. The cells stimulated with LAM produced insignificant amount of NO at 24 hr. At 48 hr, however, production rose to significant level (Fig. 2) when compared to basal level production, but not significant as compared to NO concentration at 48 hr after infection with live H37Rv (Table 1). Synthesis of iNOS was observed in cells stimulated with WCL, cell wall and cell membrane fractions of M. tuberculosis H37Rv as shown by synthesis of iNOS cDNA by RT–PCR (Fig. 3). The cell viability was not affected by any of the mycobacterial component used, as determined by XTT assay (data not shown).
Figure 2.
M. tuberculosis H37Rv cell components induce NO production by A549 cells. A549 cells were stimulated with different mycobacterial components. Cell culture supernatant was harvested at 24 and 48 hr following stimulation. Results were the mean ± SEM of three independent experiments (n = 3). *P < 0·001 when paired t-test were performed between A549 cells alone and cells stimulated with mycobacterial components at 24 hr. φP < 0·001 when paired t-tests were performed between A549 cell alone and cells stimulated with mycobacterial components at 48 hr. WCL, whole cell lysate of H37Rv; CW, cell wall of H37Rv; MEM, membrane fraction of H37Rv; CYT, cytosol fraction of H37Rv; LAM, lipoarabinomannan of H37Rv.
Figure 3.
RT–PCR result for iNOS gene (322 bp product) mRNA expression at 48 hr. (a) Lane 1: 100 bp marker. Lane 2: A549 cells stimulated with whole cell lysate of H37Rv. Lane 3: A549 cells stimulated with cytosol of H37Rv. Lane 4: A549 cells stimulated with LAM of H37Rv. Lane 5: A549 cells stimulated with cell wall fraction of H37Rv. Lane 6: A549 cells stimulated with membrane fraction of H37Rv. Lane 7: A549 cells only. (b) β-actin gene mRNA (control, 687 bp product) demonstrating equal loading in all the corresponding lanes as in (a).
Booster effect of mycobacterial components and cytokine mixture on NO production by infected cells
To determine whether the mycobacterial antigens processed and presented by alveolar macrophages in the lung has any role to play in the mycobacteria–alveolar epithelial cell interaction, infected A549 cells were stimulated with cytokine mixture and with various mycobacterial components. When the infected cells were stimulated with mycobacterial components alone the production of NO lasted only 48 hr but when infected cells were stimulated with cytokine-mixture along with mycobacterial antigens the induction of NO continued until 100 hr (Fig. 4). When the level of NO released from these cells were compared with NO released by cells infected and cytokine mixture treated, the enhanced production was statistically significant in case of WCL and cell wall (P < 0·05). This increased production was observed after 48 hr of incubation and beyond. The viability of the infected cells was not affected by simultaneous stimulation with cytokine mixture together with various antigenic components of M. tuberculosis as determined by XTT assay (data not shown).
Figure 4.
M. tuberculosis components induced NO production from A549 cells infected with M. tuberculosis followed by stimulation with a cytokine mix along with different mycobacterial components. For estimation of NO, cell culture supernatants were harvested at 24, 48, 72 and 100 hr following stimulation. Results were the mean ± SEM of three independent experiments (n = 3). When paired t-tests were performed between A549 cells alone and infected A549 cells stimulated with the cytokine mixture and mycobacterial components, the P-value was <0·001 in the case of all components. Paired t-tests were also performed between infected A549 cells stimulated with cytokine mixture only and infected A549 cells simultaneously treated with the cytokine mixture and different antigenic components. The P-value (ϕ) was < 0·05 in experiments using WCL and CW only. WCL, whole cell lysate of H37Rv; CW, cell wall of H37Rv; MEM, membrane fraction of H37Rv; CYT, cytosol fraction of H37Rv; LAM, lipoarabinomannan of H37Rv. (Infection with H37Rv and cytokine stimulation was common in all experiments shown here as a bar diagram.)
Intracellular mycobactericidal activity of A549 cells
In order to relate the nitrite production by A549 cells with intracellular mycobacterial killing, the percentage viable count of intracellular M. tuberculosis was determined. The amount of NO produced from the A549 cells infected with M. tuberculosis was not sufficient to kill the mycobacteria. It was only after cytokine stimulation leading to enhanced production of NO that these cells could bring down the CFU. The reduction was 34·87% when only IFN-γ was used, 36·37% when IFN-γ + TNF-α were used, and 39·29% when all the cytokines are used to stimulate infected A549 cells. Lowered mycobacterial colony counts correlated with the log phase of NO release at 48 hr (Table 2).
Table 2.
Correlation of nitric oxide production and mycobactericidal activity of A549 cells, infected with M. tuberculosis H37Rv and stimulated with various cytokines
| Treatment | 24 hr | 48 hr | |
|---|---|---|---|
| A549+H37Rv+IFN-γ | Nitric oxide(µmol/l) | 41·16±5·1 | 61·27±4·9 |
| CFU/ml/well (×104) | 9·285±0·29 | 6·858±0·37 | |
| % Reductionin CFU | 12·07% | 34·87% | |
| A549+H37Rv+(IFN-γ+TNF-α) | Nitric oxide(µmol/l) | 37·86±4·9 | 55·46±6·2 |
| CFU/ml/well(×104) | 9·083±0·64 | 6·7±0·54 | |
| % Reduction CFU | 13·74% | 36·37% | |
| A549+H37Rv +(IFN-γ+TNF-α+IL-1β) | Nitric oxide(µmol/l) | 48·86±4·9 | 56·75±7·7 |
| CFU/ml/well(×104) | 9·283±0·61 | 6·392±0·54 | |
| % Reductionin CFU | 11·84% | 39·29% | |
| A549+H37Rv | Nitric oxide(µmol/l) | 20·02±5·5 | 29·53±5·7 |
| CFU/ml/well(×104) | 10·26±0·2 | 10·62±0·48 |
Percent reduction was calculated from CFU/ml at 6 hr after amikacin treatment and washing in each set of experiments. CFU/ml at 6 hr was 10·53 ± 0·71 which was the basal value. Basal value of nitrite at 6 hr was 5· 68 ±3·1 µm/l. IFN-γ, TNF-αand IL-1β were added at a concentration of 250 units/ml, 10 ng/ml and 50 units/ml, respectively.
IL-8 production by infected A549 cell line
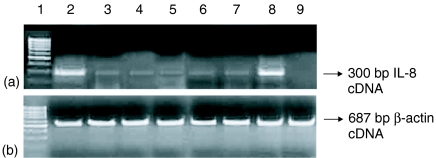
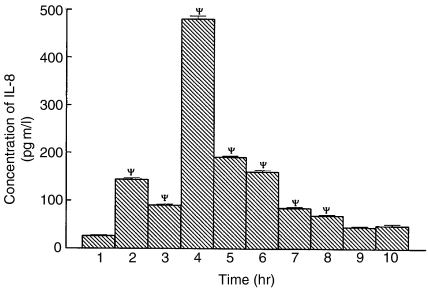
As a measure of activation of the A549 cells we undertook IL-8 estimation in the culture supernatant of A549 cells at various experimental timepoints as described earlier.11,12,36 We checked for mRNA expression for the IL-8 gene in the same experimental sets. The basal value for production of IL-8 by the A549 cells was below the detection level. Stimulation of IL-8 production and IL-8 mRNA expression (Fig. 5) following M. tuberculosis infection, indicates activation of the A549 cells. Cells infected with M. tuberculosis produced 190·5 pg/ml of IL-8 at 48 hr. The level was further increased after cytokine stimulation of the cells. The cytokine mixture stimulation further enhanced IL-8 production by the A549 cells (480·9 pg/ml at 48 hr). The level of IL-8 was also increased after stimulation of A549 cells with WCL (159·8 pg/ml), cell wall (84·51 pg/ml), membrane (69·25 pg/ml), LAM (48·65 pg/ml) and cytosol (45·35 pg/ml) fraction of M. tuberculosis H37Rv at 48 h (Fig. 6).
Figure 5.
RT–PCR result for IL-8 gene (300 bp product) mRNA expression at 48 hr. (a) Lane 1: 100 bp pair marker. Lane 2: Cytokine mixture stimulated A549 cells. Lane 3: IFN-γ stimulated A549 cells. Lane 4: IFN-γ + TNF-α stimulated A549 cells. Lane 5: TNF-α stimulated A549 cells. Lane 6: A549 cells infected with H37Rv. Lane 7: A549 cells infected with H37Rv and stimulated with IFN-γ. Lane 8: A549 cells infected with H37Rv and stimulated with cytokine mixture. Lane 9: A549 cells only. (b) β-actin gene mRNA (control, 687 bp product) demonstrating equal loading in all the corresponding lanes as in (a).
Figure 6.
Concentration of IL-8 produced by A549 cells stimulated with cytokines, infected with live M. tuberculosis (H37Rv) and different mycobacterial components. Cell culture supernatant was harvested at 48 hr following stimulation. Results are mean ± SEM of three independent experiments (n = 3). Paired t-tests were performed between A549 cells alone and A549 cells after various treatment. The P-value (ψ) was < 0·001. Lane 1: A549 cells only. A549 treated with IFN-γ (lane 2), TNF-α (lane 3), cytokine mixture (lane 4), Live H37Rv (lane 5), mycobacterial WCL (lane 6), mycobacterial cell wall component (lane 7), mycobacterial membrane preparation (lane 8), mycobacterial cytosol (lane 9), LAM (lane 10).
Discussion
Once inhaled into the alveoli of the lung, M. tuberculosis associates itself with macrophages in the alveolar spaces that play a significant role in the survival and multiplication of tubercle bacilli in the body of the infected host.1–4,23,24 Most of these earlier studies were primarily limited to the role of alveolar macrophages in immunopathogenesis of tuberculosis. Recently, however, a number of studies have described that M. tuberculosis can invade and replicate within type II alveolar epithelial cells.17,18,20 Whether M. tuberculosis enters epithelial cells either to replicate or to avoid the mechanism of host defence is not clear. Earlier it was thought that a mode of infection via non-professional phagocytic cells like epithelial cells might enable the bacteria to escape potentially hostile environment of macrophages and would create a niche for the bacteria to replicate and establish the infection.5 However, an increasing body of evidence suggests that the epithelial cells may play an important role in initiation of the acute inflammatory response to microbial pathogens by producing chemokines like IL-8 and MCP-1.11,12,21 In this study, we demonstrate that alveolar epithelial cells play an active role in the innate immunity to M. tuberculosis by elaboration of NO in response to infection with mycobacteria as also to the presence of mycobacterial components in the milieu.
Earlier reports have also demonstrated NO production by A549 cells in response to proinflammatory cytokines.13,14,28–30 For our experiments we selected three of the proinflammatory cytokines that are released when M. tuberculosis infects the alveolar macrophages and are thought to be present in the internal milieu of the infected lung.1–3,26 Alveolar macrophages and monocytes recruited early in the course of pulmonary infection with M. tuberculosis have previously been shown to be the major cellular source of immune regulatory and proinflammatory mediators including NO in the lung.4,23,24,27 Our data presented here suggest that not only monocytes/macrophages but also pulmonary epithelial cells could also have a major role to play in the pathogenesis/host defence against pulmonary tuberculosis. Here, we observed that direct infection of A549 cells with M. tuberculosis causes low-level NO release. Further, cytokine stimulation of these infected A549 cells caused substantial induction of NO production. A recent study reported that M. tuberculosis was able to directly stimulate low-level secretion of both IL-8 and monocyte chemoattractant protein-1 in pulmonary epithelial cells over a period of 1–6 days.11,12,36 When we investigated IL-8 secretion from pulmonary epithelial cell line A549 directly infected with M. tuberculosis, we also found release of this cytokine up to 48 hr. Therefore direct exposure of alveolar epithelium to virulent mycobacteria does appear to activate the epithelium to secrete chemokines as well as NO.
IFN-γ was found to be the critical mediator of NO production among the three proinflammatory cytokines used. The cytokine mixture stimulation greatly enhanced the production of NO from the A549 cells infected with mycobacteria. This could mean that the elaboration of these cytokines by the alveolar macrophages in response to mycobacterial infection are able to stimulate alveolar epithelial cells to produce NO in the actual in vivo situation. We demonstrated that de novo synthesis of NO was occurring as the iNOS mRNA expression from A549 cells was demonstrated by RT–PCR in these cells. Inducible NOS expression is also reported to be increased in macrophages during inflammation, possibly indicating that the iNOS may be readily induced in the context of priming events such as during inflammation, which is further complemented by M. tuberculosis ingestion to provide a fully activating signal for iNOS induction.4,27 Because iNOS is a highly conserved single gene14,29,30,38 the same mechanism applies for the A549 cells in all probability.
Investigations to study the mycobactericidal capacity of the alveolar epithelial cells revealed some interesting observations. The A549 cells, when infected with M. tuberculosis without cytokine stimulation, released NO was insufficient to reduce the CFU of mycobacteria. It was necessary to stimulate the alveolar epithelial cells by cytokines to bring down the CFU (Table 2). Our results confirm earlier reports4,27 that cytokine mixture leads to release of very high concentration of NO. We further demonstrated that the reduction in CFU occurred in the same wells. This observation suggested a clear relationship between the amount of NO released and the control of growth of intracellular mycobacteria within the alveolar epithelial cells.
Another interesting observation was the γ-irradiated M. tuberculosis induced production of NO by alveolar epithelial cells, although this effect was produced only after prolonged incubation for up to 72 hr. That the killed mycobacteria could induce production of NO suggested that some constitutive component of M. tuberculosis has the potential to induce NO release. Mycobacterial cell surface has unusual physicochemical properties attributable to peptidoglycan, arabinogalactan and mycolic acids present in it.2,23,24 Various subcellular components of M. tuberculosis, namely, WCL, cell wall, cell membrane, cytosol preperation and LAM were used in our experimental protocols. The results demonstrated that WCL, cell wall and cell membrane components induced significant production of NO and iNOS mRNA expression whereas cytosol and LAM could not. Our results suggested that while live M. tuberculosis has the potential to directly infect alveolar cells, NO production could be brought about by either M. tuberculosis or its antigenic components in the milieu of the alveolus. Although LAM, a highly immunogenic mycobacterial cell wall component, is associated with NO production from rodent macrophages1,2 in our experimental set up we could not find any significant inducible effect of LAM on NO production or iNOS mRNA expression from the human alveolar cell A549. Such an observation may be because of the fact that LAM has been reported to be responsible for scavenging mycobactericidal molecules like superoxide and NO from the local environment. Exposure of macrophages to high concentration of purified M. tuberculosis LAM is shown to result in defective responses to IFN-γ, including transcriptional activation, intracellular microbicidal activity, expression of major histocompatibility complex class II molecules and cytotoxicity for tumour cells.32,39 Same mechanism may be responsible for less production of NO by LAM in our experimental setup because the iNOS gene is reportedly IFN-γ responsive.1,2
We may summarize that alveolar epithelial cells play an active role in innate immunity by de novo production of NO although the amount of NO produced by direct infection of A549 cells is not sufficient for mycobacterial killing. However, our results indicate that cytokine stimulation of the infected cells lead to definite mycobacterial killing and reduction of mycobacterial load, at least in an in vitro situation. These results along with our observation of enhanced NO release induced by cell wall and cell membrane component opens a new area of investigation for immunotherapeutic interventions in pulmonary tuberculosis. Further analysis of the precise components of M. tuberculosis will be necessary to definitely identify the molecule(s) responsible for initiating NO production. The identified mycobacterial components may find clinical application in future as subunit vaccine or immune modulators to enhance the anti mycobacterial defence mechanism especially in drug resistant mycobacterial infection.
Acknowledgments
The gift of γ-irradiated H37Rv, whole cell lysate, cell wall, membrane, cytosol, LAM of M. tuberculosis H37Rv from J. T. Belisle (Colorado State University) is gratefully acknowledged. We are thankful to the Indian Council of Medical Research, India and the Department of Science and Technology, Government of India for providing financial support.
References
- 1.Ellner JJ. Review: The immune response in tuberculosis-implication for tuberculosis control. J Infect Dis. 1997;176:1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 2.Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: Roles of macrophages and monocytes. Infect Immun. 1996;64:683–90. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulton SA, Cross JV, Toossi ZT, Boom WH. Regulation of interleukin-12 by interleukin 10, transforming growth factor-β, tumour necrosis factor-α and interferon-γ in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–14. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 4.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tubercle Lung Dis. 1997;78:247–55. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Pando R, Jeyanathan M, Mengistu G, Rook GAW. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–8. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 6.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-Talk in the innate immune system. Neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–8. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 7.Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2002;70:5322–7. doi: 10.1128/IAI.70.9.5322-5327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70:4591–9. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeberger EE. Alveolar type II cells. In: Crystal RJ, West JB, editors. The Lung Scientific Foundations. New York: Raven Press; 1991. pp. 736–58. [Google Scholar]
- 10.Simon RH, Paine R. 3rd. Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med. 1995;126:108–18. [PubMed] [Google Scholar]
- 11.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–53. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickremasinghe MI, Thomas LH, Friedland JS. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB dependent network. J Immunol. 1999;163:3936–47. [PubMed] [Google Scholar]
- 13.Asano K, Chee CBE, Gaston B, Lilly CM, Gerard C, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994;91:10089–93. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins RA, Barnes PJ, Springall DR. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun. 1994;203:209–18. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson JS, Schlesinger LS. Pulmonary surfactant in innate immunity and pathogenesis of tuberculosis. Tubercle Lung Dis. 2000;80:173–84. doi: 10.1054/tuld.2000.0242. [DOI] [PubMed] [Google Scholar]
- 16.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–51. [PubMed] [Google Scholar]
- 17.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–6. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Garza J, King CH, Swords WE, Quinn FD. Demonstration of spread by Mycobacterium tuberculosis bacilli in A549 epithelial cell monolayers. FEMS Microbiol Lett. 2002;212:145–9. doi: 10.1111/j.1574-6968.2002.tb11258.x. [DOI] [PubMed] [Google Scholar]
- 19.Dobos KM, Spotts EA, Quinn FD, King CH. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect Immun. 2000;68:6300–10. doi: 10.1128/iai.68.11.6300-6310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Perez BE, Mondragon-Flores R, Luna-Herrera J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non phagocytic cells. Microb Pathog. 2003;35:49–55. doi: 10.1016/s0882-4010(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 21.McDonough KA, Kress Y. Cytotoxicity for lung epithelial cell is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–11. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta PK, King CH, White EH, Murtagh JRJJ, Quinn FD. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect Immun. 1996;64:2673–9. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celada A, Nathan C. Macrophage activation revisited. Immunol Today. 1994;15:100–2. doi: 10.1016/0167-5699(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 24.Nathan C, Hibbs J. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 25.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bose M, Farnia P. Proinflammatory cytokines can significantly induce human mononuclear phagocytes to produce nitric oxide by a cell maturation dependent process. Immunol Lett. 1995;48:59–64. doi: 10.1016/0165-2478(95)02444-1. [DOI] [PubMed] [Google Scholar]
- 27.Bose M, Farnia P, Sharma S, Chattopadhya D, Saha K. Nitric oxide dependent killing of Mycobacterium tuberculosis by human mononuclear phagocytes from patients with active tuberculosis. Int J Immunopathol Pharmacol. 1999;12:69–79. [PubMed] [Google Scholar]
- 28.Kwon S, George SC. Synergistic cytokine-induced nitric oxide production in human alveolar epithelial cells. Nitric Oxide Biol Chem. 1999;3:348–57. doi: 10.1006/niox.1999.0242. [DOI] [PubMed] [Google Scholar]
- 29.Chu SC, Marks-Konczalik J, Wu HP, Banks TC Moss J. Analysis of the cytokine stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters. Biochem Biophys Res Commun. 1998;248:871–8. doi: 10.1006/bbrc.1998.9062. [DOI] [PubMed] [Google Scholar]
- 30.Marks-Konczalik J, Chu SC, Moss J. Cytokine mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor κB-binding sites. J Biol Chem. 1998;273:22201–8. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 31.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–65. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 32.Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–906. [PubMed] [Google Scholar]
- 33.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 34.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-choroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 35.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacreium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–7. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Zhang M, Barnes PF. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998;66:1121–6. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flesch IE, Kaufmann SH. Role of cytokines in tuberculosis. Immunobiology. 1993;189:316–39. doi: 10.1016/S0171-2985(11)80364-5. [DOI] [PubMed] [Google Scholar]
- 38.Spitsin SV, Koprowski H, Michaels FH. Characterization and functional analysis of the human inducible nitric oxide synthase gene promoter. Mol Med. 1996;2:226–35. [PMC free article] [PubMed] [Google Scholar]
- 39.Sibley LD, Hunter SW, Brennan PJ, Krahenbuhl JL. Mycobacterial lipoarabinomannan inhibits γ interferon-mediated activation of macrophages. Infect Immun. 1988;56:1232–6. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]