Abstract
Despite tuberculosis resurgence and extensive dendritic cell (DC) research, there are no in vivo studies evaluating DC within regional lymphoid tissue during airways infection with virulent Mycobacterium tuberculosis (Mtb) H37Rv. Using DC-specific antibodies, immunocytochemistry, flow cytometry and Ziehl–Neelsen (ZN) for bacilli staining, we searched for Mtb and DC changes within mediastinal lymph nodes, after intratracheal (ITT) inoculation of virulent Mtb. ZN and immunocytochemistry in frozen and paraffin sections of mediastinal lymph nodes identified Mtb until day 14 after ITT inoculation, associated with CD11c+ and Dec205+ DC. Analysing CD11c, MHC-CII, and Dec205 combinations by flow cytometry in MLN suspensions revealed that CD11c+/MHC-CII+ and CD11c+/Dec205+ DC did not increase until day 14, peaked on day 21, and sharply declined by day 28. No changes were seen in control, saline-inoculated animals. The costimulatory molecules evaluated in CD11c+ DCs followed a similar trend; the CD80 increase was negligible, slightly surpassed by CD40. CD86 increased earlier and the three markers peaked at day 21, declining by day 28. While antigen-specific proliferation was not evident for MLN CD4+ T cells at 2 weeks postinfection, delayed-type hypersensitivity responses upon ITT inoculation revealed that, as early as day 3 and 7, both the priming and peripheral systemic immune responses were clearly established, persisting until days 14–21. While airways infection with virulent Mtb triggers an early, systemic peripheral response maintained for three weeks, this seems dissociated from regional events within mediastinal lymph nodes, such as antigen-specific T-cell reactivity and a delay in the influx and local activation of DC.
Keywords: virulent Mycobacterium tuberculosis, dendritic cells in vivo, mediastinal lymph nodes
Introduction
Tuberculosis (TB) is a major cause of death worldwide, affecting one-third of the world's population. This disease is caused by Mycobacterium tuberculosis (Mtb), an acid-fast bacillus transmitted primarily via the respiratory route. According to the World Health Organization, TB is still the world's most neglected health crisis.1–3
In mice and humans, M. tuberculosis infection is mainly controlled by macrophage activation induced through T helper 1 (Th1)-type cytokines, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) have a central role in this process by inducing macrophage activation and inducible nitric oxide synthase (iNOS) expression. The NO produced in this process is essential – at least for mice – to kill intracellular mycobacteria.4,5 This protective activity fails if there is a marked release of Th2-type cytokines.6,7 This interplay of cytokines is clearly depicted in a BALB/c model of pulmonary tuberculosis following intratracheal infection.8–11 In this model, an initial phase is dominated by high production of Th1 cytokines that, together with high levels of TNF-α and iNOS, temporarily controls the infection. Granulomas develop in this phase. Four weeks after infection, expression of Th1 cell cytokines, TNF-α and iNOS starts to decline. Gradually, pneumonic areas prevail over granulomas. Pneumonia, in coexistence with a high burden of bacteria, causes the death.
It is widely accepted that the initial phase of the Mtb infection within the lungs occurs in alveolar macrophages, where the bacteria replicates inducing cytokines that initiate the local inflammatory response in the lungs.12 However, despite the potential function that dendritic cells (DC) from lymphoid tissues might play in the initiation of immune responses, their role during pulmonary TB has not yet been established. Furthermore, most of the knowledge that has been generated to date, has not explored the participation of DC in the infection in vivo, especially DC from the mediastinal lymph nodes, the nearest draining regional lymphoid tissue of the lungs.
DC function as the first-line sentinels in immune surveillance of epithelia and peripheral tissues such as skin, gut and airways,13 which are in direct contact with the external milieu. In the respiratory tract, under steady-state conditions DC form a contiguous network throughout the epithelial surfaces, and their resident density somehow reflects the degree and intensity of local exposure to inhaled antigens.14 In general, the primary function of DC in these tissues is to sample incoming antigenic material which may then be processed and presented to antigen specific T cells within T-cell areas in the nearest regional lymph nodes, where the interaction between DC and the specific, naive T cells takes place, presumably involving both signal 1 (immunogenic peptide on major histocompatibility complex (MHC) molecules) and signal 2 (costimulation). Costimulation is provided mainly by cell surface-associated costimulatory molecules, particularly through the interaction between CD40, and especially, the CD80 and CD86 molecules on APC and the T-cell associated CD28 molecule.15,16 Soluble cytokines and mediators that are present in the microenvironment during the antigen-presenting cell (APC)–T-cell interaction also contribute to proliferation and differentiation in the responding lymphocyte.15
The interactions between DC and pathogens are of prime importance in establishing an appropriate immune response. On the other hand, it has been reported that DCs themselves are able to internalize and react to several microbes.16,17 In vitro, for instance, DCs infected with M. tuberculosis showed a consistent up-regulation of the cell surface molecules CD80 and CD86, compared to uninfected cells.18
Other studies suggest that during mycobacterial infections, host DC located in the lung migrate to the T-cell areas of the regional draining lymph nodes. There, they present antigens to T cells and promote the expansion of IFN-γ-secreting CD4+ T cells, stimulating the interleukin (IL)-12 response of DC to mycobacterial antigens. It has also been shown that CD40-stimulation of bacillus Calmette–Guèrin (BCG)-infected DC, increases the production of inflammatory cytokines like IL-1, wich plays a critical role in antimycobacterial immunity.19,20
We attempted to address some of these issues in vivo by studying DC from mediastinal lymph nodes (MLN) during the pulmonary infection with Mtb H37Rv, using the above mentioned model in BALB/c mice. Cell suspensions from MLN from tuberculous animals and control non-infected mice were analysed by flow cytometry on days 1, 3, 7, 14, 21, 28 and 90 postinfection. By combining markers such as MHC-CII, CD11c and Dec205, we found that MHC-CII+/CD11c+ and MHCII+/Dec205+ DCs, slowly increased during the course of the disease up to the end of the first phase (day 21), declining by day 28, which corresponds to the initiation of the progressive phase of the infection. In situ, Mtb was detected by Ziehl–Neelsen in MLN as early as day 14 post-intratracheal (ITT) infection.
Materials and methods
Experimental model of pulmonary tuberculosis in mice
The experimental model of Mtb infection by ITT inoculation has been previously described in detail8,10 using male BALB/c mice from 6 to 8 weeks of age. Briefly, the virulent M. tuberculosis strain H37Rv was cultured in Proskauer and Beck medium modified by Youmans. After 1 month of culture, bacilli were harvested and adjusted to 106 cells in 100 µl of sterile, endotoxin-free sterile saline solution, aliquoted and maintained at −70° until use. Immediately before use, bacteria were counted and their viability checked. To induce Mtb infection, mice were anaesthetized with 56 mg/kg of intraperitoneal pentothal. The trachea was carefully exposed via a small midline incision, and 100 µl of PBS with 106 suspended viable bacteria was inoculated. The incision was then closed with sterile silk, and the mice were maintained in a vertical position until the effect of anaesthesia was over. The control group consisted of mice injected by the ITT route with sterile pyrogen-free saline solution, using the same technique. Experiments were performed in a P3 biosecurity facility in accordance with the institutional guidelines for animal care and experimentation. Three separate experiments were done. At least five mice were killed at each selected time point along the infection.
Immunohistochemistry
MLN were pooled from five mice from each group of mice at a given time point, embedded in Tissue/Teck and frozen sections of 5 µm thickness were obtained and mounted on silane (Sigma, St Louis, MO)-treated slides. Endogenous peroxidase activity was blocked with 6% of H2O2 in phosphate-buffered saline (PBS) for 15 min After that, MLN sections were incubated with the primary biotinylated antibody anti-CD11c (Pharmingen San Diego CA) overnight at 4°, followed by streptavidin-horseradish peroxidase conjugated. Enzyme-linked antibody was revealed by reacting with 3,3′- diaminobenzidine/H2O2 for 10–15 min at room temperature. Finally, tissue sections were stained with Ziehl–Neelsen to identify the mycobacteria, and counterstained with haematoxylin for analysis.
Regional lymph node cell suspensions
MLN from at least five mice per experimental group and time point were identified and removed under a stereomicroscope, cut into small pieces and digested for 90 min in complete medium (RPMI-1640 plus 10% fetal calf serum, 2 mm glutamine, 100 U of penicillin G per ml, and 100 µg of streptomycin per ml) containing 1 mg/ml collagenase type 2 (Worthington Biochemical Corporation, Lakewood, NJ), and 2 U/ml of DNase I (Gibco, BRL, Carlsbad, CA). Red blood cells were lysed and cells were passed through a 100-µm cell strainer (Becton Dickinson, San Jose, CA). MLN cell suspensions were thoroughly washed in culture medium to remove excess of enzymes and then resuspended in fluorescence-activated cell sorting (FACS) buffer. For cell culture, MLN were obtained under aseptic conditions and cell suspensions were prepared under sterile conditions.
Flow cytometry of MLN cell suspensions (phenotype)
Cells were washed in PBS containing 1% bovine serum albumin and 0·01% sodium azide (FACS buffer), and 1 × 106 cells were incubated with specific antibodies for 30 min on ice. To reduce nonspecific binding, cells were incubated with 2.4G2 blocking reagent for 15 min Monoclonal antibodies used were as follows: Anti-I-A/I-E- fluorescein isothiocyanate (MHC–CII-FITC clone 269), allophycocyanin-labelled CD11c (CD11c-APC clone HL3), phycoerythrin (PE)-labelled antibodies against CD80, CD86, anti-CD40-biotinylated all from Pharmingen, BD, San Jose, CA; and anti-Dec205 (kindly donated by R. M. Steinman). In the case of biotinylated antibodies, these were followed by streptavidin (SA)-PECy5. The analysis was performed in a FACScalibur flow cytometer using the CellQuest program (Becton Dickinson Immunocytometry Systems) recording at least 50 000 events for each assessment.
Antigen-specific T-cell proliferation in regional lymphoid tissue
MLN were aseptically removed at day 14 from five BALB/c mice infected ITT with M. tuberculosis H37Rv, and from control mice inoculated with sterile saline solution, to prepare cell suspensions. MLN cell suspensions from both groups were labelled with CFSE and cultured with medium alone, concanavalin A (5 µg/ml) or Mtb H37Rv sonicate (20 µg/ml). After 5 days of culture, cells were harvested and analysed by flow cytometry by immunolabelling with CD4-PE.
Delayed-type hypersensitivity
Delayed-type hypersensitivity (DTH) responses in the footpad were used to evaluate the systemic cellular immune response against locally (ITT) inoculated Mtb. Culture filtrate was harvested from M. tuberculosis H37Rv grown as described above for 5–6 weeks, then antigens were precipitated with 45% (w/v) ammonium sulphate, washed and re-dissolved in sterile, pyrogen-free saline solution. Mice inoculated ITT with either Mtb or isotonic sterile endotoxin-free saline solution, were all subsequently injected subcutaneously with 0·02 ml (20 µg/ml) of Mtb culture filtrate antigens, into the hind footpad at different times post-ITT infection. Before and 24 hr after the sonicated-challenge, footpad swelling was monitored with an engineer's micrometer (Mitutoyo, Japan).
Results
Histological and immunohistochemistry features of mediastinal lymph nodes along the infection
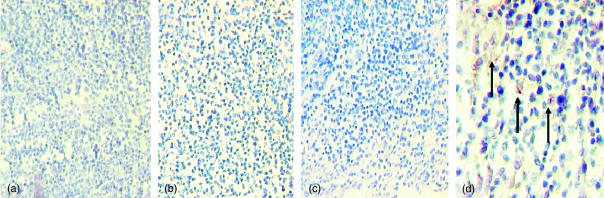
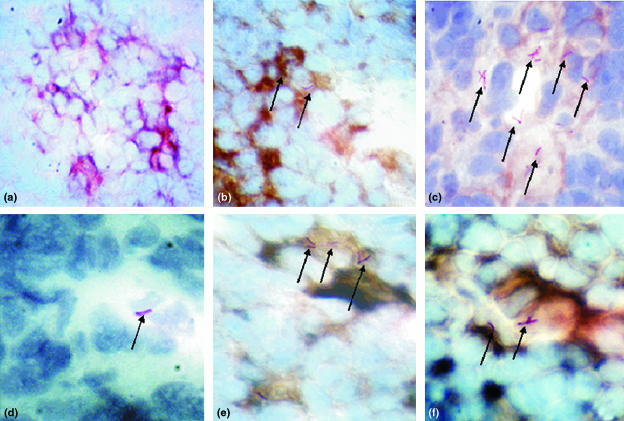
Searching first for bacilli, we analysed paraffin sections of MLN from BALB/c mice at different time points after intratracheal inoculation, we found that, at least by Ziehl–Neelsen staining, we could not detect mycobacteria during early time points (days 3–7); only on day 14 after the infection (Fig. 1d, arrows). We then looked for CD11c+ DCs within the regional lymph nodes upon ITT inoculation of Mtb (day 28). We noticed that the gross histological appearance seemed somehow altered because there was not a clear regionalization of the CD11c+ DC in the MLN of infected mice, compared to non-infected control nodes (not shown). By performing double staining with antibodies to DCs and Ziehl–Neelsen in tissue sections from these regional lymph nodes at day 28, we could identify red mycobacteria, though apparently only associated with either DEC 205+(Fig. 2f, arrows) or CD11c+ DC (Fig. 2b,c,e, arrows). Seemingly cell-free mycobacteria were very difficult to identify.
Figure 1.
In situ assessment of the presence of Mtb H37Rv in mediastinal lymph nodes at different time points after intratracheal inoculation. Days 1 (a), 3 (b), 7 (c), and 14 (d).Arrows in d indicate the Ziehl–Neelsen+ (red colour) bacilli. Magnification: (a–c) 20×, (d) 40×.
Figure 2.
Association of mediastinal lymph node DCs and M. tuberculosis H37Rv in situ. Ziehl–Neelsen staining and immunocytochemistry of MLN from control (a) and H37Rv-infected (b–f) mice were performed to ascertain potential colocalization of MtbH37Rv (arrows) with DCs (brown colour; a, b, c, e = CD11c; d = isotype control antibody, f = Dec205). All pictures were taken at 100× magnification. (c–f) Each is a zoom-magnified area to show the red bacilli. In (c), only a slight brown colour development was allowed to better visualize the red mycobacteria.
Cytofluorometry analysis of CD11c+ subsets (MHC-CII+ and DEC205+) and costimulatory molecules in MLN DC during H37Rv infection
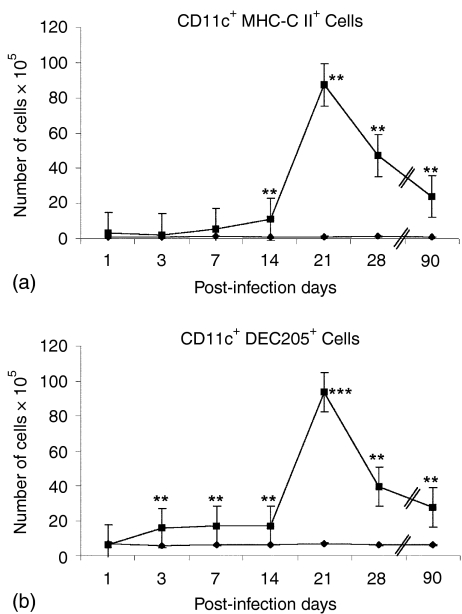
MLN cell suspensions from both groups (control and infected) were analysed by flow cytometry using double staining on days 1, 3, 7, 14, 21, 28 and 90 postinfection, combining specific markers for DC (Fig. 3): CD11c+ plus MHC-CII+ DC and CD11c+ plus Dec205+ DC (Fig. 3a and b, respectively). Because the size, and with it the cellularity, of mediastinal nodes of infected animals was definitively bigger than that of uninfected mice, and sometimes the percentages obtained from dot-plots were not much different between the two groups of animals, we decided therefore to express all these percentages as actual, absolute DC numbers. We observed that these two subsets of double positive DC slightly increased since the first week postinoculation in mice infected with Mtb H37Rv, reaching a peak at day 21 and declining after day 28. In contrast, no changes were observed for these DC populations in the control group of mice.
Figure 3.
CD11c+/MHC-CII+ and CD11c+/CD205+ DC increased in the MLN during the first phase of the pulmonary disease. BALB/c mice were infected ITT with M. tuberculosis H37Rv (▪), while control mice were inoculated with sterile saline solution (⋄). MLN cell suspensions from both groups were analysed by flow cytometry on days 1, 3, 7, 14, 21, 28 and 90 postinfection, combining (a) CD11c plus MHC-CII and (b) CD11c plus Dec205. Each group (control and infected) consisted of at least five mice per time point. Results are presented as the mean ± standard error of three different experiments. Statistically significant differences are indicated by asterisks (*P < 0·05; **P < 0·01; ***P < 0·001).
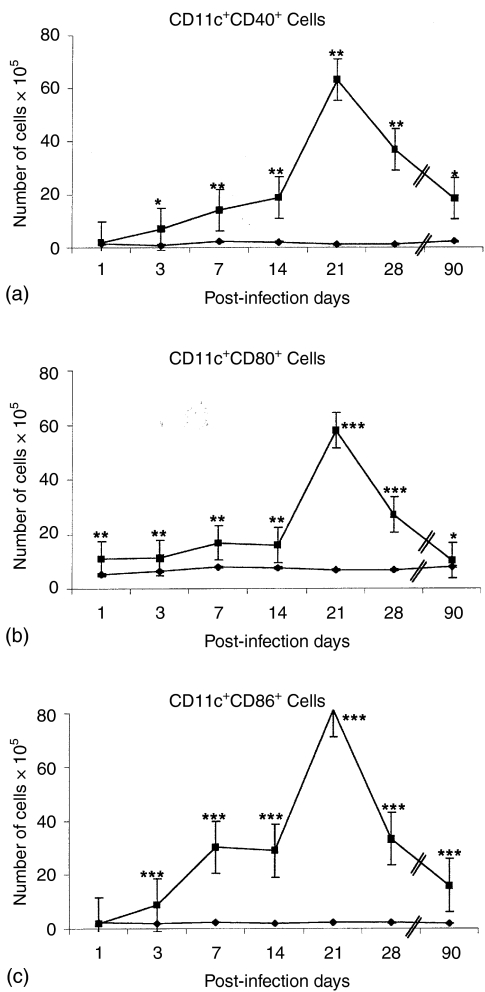
Expression of costimulatory molecules (CD40, CD80, CD86) in MLN CD11c+ DC during the course of the pulmonary infection with Mtb H37Rv. In order to ascertain which was the activation status of these CD11c+ DC in situ, we analysed the classical costimulatory molecules by double staining, pairing the labelling of either CD40, CD80 or CD86 to antibodies to CD11c+. All double-labelled DC subsets: CD11c+ CD40+, CD11c+ CD80+ and CD11c+ CD86+ DCs increased after day 14 postinfection (Fig. 4a,b,c). However, the CD11c+ CD86+ subset increased earlier, from day 3 (Fig. 4c) and the percentage of this subpopulation was higher than the other two populations at day 21 (Fig. 4a,b). Interestingly, the three DC subsets decreased by day 28 and 90 postinfection.
Figure 4.
Expression of costimulatory molecules (CD40, CD80 and CD86) in CD11c+ DC during the course of pulmonary disease. BALB/c mice were infected ITT with M. tuberculosis H37Rv (▪) while control mice were inoculated with sterile saline solution (⋄). MLN cell suspensions from both groups were analysed by flow cytometry on days 1, 3, 7, 14, 21, 28 and 90 postinfection, combining (a) CD11c plus CD40 (b) CD11c plus CD80, and (c) CD11c plus CD86. The mean result ± standard error of three separate experiments is shown, significant differences are indicated by asterisks (*P < 0·05; **P < 0·01; ***P < 0·001).
Assessment of systemic cellular immune response
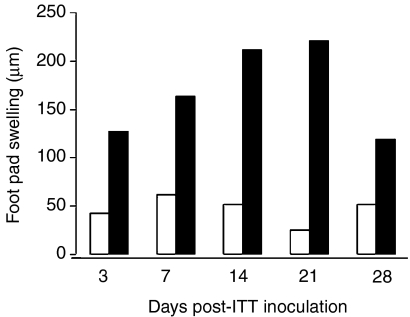
The systemic, cellular immune response was determined by DTH, which reflects the presence of antigen specific, effector T cells in the periphery. Figure 5 shows that in the group infected with Mtb, the increase of DTH was already detected at day 3, clearly seen at day 7, reaching a peak between days 14 and 21, and declining by day 28. No DTH responses were detected in the control group during the experiments.
Figure 5.
Systemic cellular immune response after ITT inoculation of Mtb H37Rv. The systemic, cellular immune response was analysed by DTH on days 3, 7, 14, 21 and 28 after mice were inoculated intratracheally with Mtb H37Rv (black bars) or with saline solution (white bars). The data are the mean of five mice assessed per time point for each group (control and infected), and the results are representative of three experiments.
T-cell proliferation in MLN of Mtb H37Rv infected mice
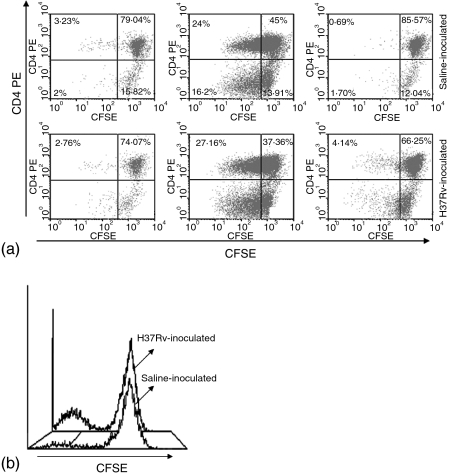
Because the peak of the DTH responses was observed between days 14 and 21, MLN were removed from mice at day 14 postinoculation with either Mtb H37Rv or sterile saline solution (control group). MLN cell suspensions from both groups were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured with medium alone, concanavalin A or sonicated Mtb. After 5 days of culture, cells were harvested and analysed by flow cytometry by immunolabelling with CD4-PE. Figure 6 shows that T cells from the control uninfected group did not proliferate when cultured with sonicated Mtb or culture medium alone, however, they responded efficiently to polyclonal stimulation with concanavalin-A. At a first glance, MLN cell suspension from Mtb infected mice appeared to show some specific T cell proliferation to the Mtb sonicate (Fig. 6a,b).However, this might not be antigen specific when compared to cultured control cells.
Figure 6.
T-cell proliferation in (MLN) regional lymphoid tissue. (a) MLN cell suspensions from control, saline inoculated (top panel) and H37Rv-infected (bottom panel) mice, were labelled with CFSE and cultured with medium alone (left histograms); Concanavalin A (middle histograms), or an Mtb H37Rv sonicate (right histograms) for 5 days. Cells were then labelled with CD4-PE antibodies and analysed by flow cytometry to determine proliferation of CD4+-specific T cells. (b) Histograms depict MLN cell suspensions from both groups (control and infected); labelled with CFSE and cultured with a sonicate of Mtb H37Rv. The MLN of five mice from each group (control and infected) were pooled to prepare cell suspensions for FACS analysis, histogram profiles representative of two different evaluations are shown.
Discussion
With the Mtb genome sequence accessible,21 state of the art research regarding the molecular biology of Mtb has been performed. Likewise, the pathogenesis of pulmonary tuberculosis has been examined in great detail. However, the ultimate mechanisms determining either the complete elimination of the bacilli leading to the total resolution of the infection; the development of chronic pulmonary disease; or even the enigmatic latent state, are still unclear. The very early interactions between Mtb and cells of the immune system are not well understood either, though it is established that monocytes/macrophages are the most likely candidates. Interestingly, the immunopathogenesis of TB using experimental animal models has been focused mainly on the examination of the lung tissue, without paying much attention to potentially important events occuring in the nearest regional lymphoid tissues, the MLN in this case. Our mouse model of progressive pulmonary tuberculosis is suitable for the exploration of these immunological events in the MLN, because it is based on aerogenic infection, the usual route of infection in humans. Besides, the rate of bacterial multiplication in the lungs correlates with the extent of tissue damage (pneumonia) and mortality, and the infection is successfully controlled as long as a strong Th1 cell response is sustained.8–10 Thus, we took advantage of this experimental model to study the DC changes from mediastinal lymph nodes along the infection.
DC are situated along the respiratory tract,22–27 and as immature DC in peripheral tissues are both ideally positioned and endowed with good capabilities for antigen uptake in the airways. However, the potential involvement of these APC has not been assessed in vivo during infection with virulent Mtb.
Upon inoculation of virulent Mtb through the airways, by staining tissue sections of mediastinal lymph nodes with Ziehl–Neelsen we were unable to detect its presence in the early phase of the infection (days 1, 3, 7 after ITT deposition), only until day 14. Though soluble and particulate model antigens deposited in the trachea have been documented to arrive into the MLN at early times (first 24–48 hr),28 to the best of our knowledge, this has not been examined with live micro-organisms such as these virulent mycobacteria. It was somehow unexpected to find out in situ that the bacilli within MLN, seemed confined to the DC markers used, CD11c+ and Dec205+. We believe this association between the DC and the virulent Mtb in situ, is an important finding. However, it would be difficult for us to assert that these DC bearing the Mtb H37Rv bacilli within the MLN, are the same ones derived from DC positioned in the airways, supposedly ferrying bacilli into the regional lymphoid tissue. We also noticed that the CD11c+ DC within the MLN of infected animals did not exhibit a clear paracortical disposition like those in control MLN, rather, they seemed dispersed throughout the tissue. Another intriguing observation was the lack of typical, well-organized granulomas within the MLN, even at 14, 21 or 28 days postinoculation, the timing when these structures are well formed within lung tissue.
Regarding the two subpopulations of CD11c+ DCs (MHC-CII+ and Dec205+), it was clear that though both subsets did not change much during the first 2 weeks postinoculation, they dramatically increased (about tenfold) by day 14, reaching a peak by day 21, declining afterwards. When assessing the three main costimulatory molecules (CD40, CD80 and CD86) in the the CD11c+ DCs, a very similar pattern was also noticed, perhaps with the exception of a small early increase (on day 7) for CD11c+ CD86+ DCs. At a first glance, these observations, that DC and costimulatory molecules increase after 2 weeks post-ITT inoculation, would appear as fairly normal responses. This might not be the case, however, when considering several reports that in any other regional lymph nodes so far examined upon peripheral antigen application (model or microbial antigens); both the proportions and the expression of costimulatory molecules of DCs are consistently increased in the first 24–48 hr.28–30 This also seems to apply when other antigens, model28,29or microbial ones,31–34 have been inoculated through the airways. It is thus very intriguing that in this experimental model, when inoculating virulent Mtb into the trachea, a relatively prolonged delay of about three weeks (compared to 24–48 hr in other models) occurs regarding both the influx of DCs into the regional (mediastinal) lymph nodes, as well as the up-regulation of the main costimulatory molecules required for an efficient T-cell stimulation (CD80, CD86 and CD40).
However, by means of DTH responses, we observed that T-cell priming was clearly seen already by day 3 post ITT inoculation. This systemic peripheral response was well established by day 14 and continued to increase until day 21, declining by day 28. At least regarding the timing of both the peak (day 21) and the decline (day 28) of this systemic DTH response, there was a correlation with the events observed for DC and costimulatory molecules within MLN. When assessing the proliferative capacity of CD4+ T cells obtained at day 14 from the same MLN (from either control uninfected, or H37Rv-infected animals), against mycobacterial antigens, we were somewhat surprised to observe a lack of a clear antigen-specific T-cell proliferation. However, this might also be related to the relatively low proportion of DCs and to the low levels of expression of the three costimulatory molecules within MLN at this same time-point. In fact it has long been shown that when DCs are decreased (either naturally because of the anatomical location, or induced by UV or chemicals), a decay follows in the T-cell responses.35,36
From our results, it would thus appear that early upon ITT inoculation with Mtb H37Rv, a peripheral systemic (DTH) response is clearly mounted, but this seems uncoupled from the local (regional draining lymph nodes) T cell responses. We do not know the precise mechanisms that could explain this seeming dissociation of these two antigen-specific T-cell responses. However, since mycobacteria are well known to utilize a variety of immune evasion mechanisms, this might be another one in the list. Perhaps the lack of T-cell reactivity in the draining lymphoid tissue might be related to certain mycobacterial compounds, especially lipids, some of which (lipoarabinomannans) are now appropriately referred to as ‘tuberculosis toxins’.37 We are currently exploring this last proposition.
Our results thus open several questions to be addressed in vivo, for instance the mechanisms responsible for this evident long delay in the recruitment of DC into the regional lymph nodes, and in the expression of costimulatory molecules. The other is the apparent dissociation between a well established, early peripheral T-cell reactivity, and the lack of antigen-specific CD4 responses within the regional lymphoid tissue. Altogether, our data emphasize the need to perform more research in vivo with the virulent Mtb to understand better the mechanisms of evasion of this microbe, and the potential ways to overcome them.
Acknowledgments
This work was partially supported by Grants from Conacyt; 30757-M (L. F.-R.), G36923 (R. H.-P.). L. Flores-Romo, R. Hernandez-Pando and I. Estrada-Garcia are SNI members. G. S. Garcia-Romo and A. Pedroza-Gonzalez are fellow-holders from CONACYT. Authors wish to thank J. Calderon-Amador and V. H. Rosales-Garcia (Cytometry Unit) for excellent technical support and G. Salmeron for help in the animal facilities.
References
- WHO. 2000. Fact Sheet no.104. http://www.who.int/inf-fs/en/fact104.html.
- Flynn J, Chan J. Tuberculosis. latency and reactivation. Infect Immun. 2001;69:4195–201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger N, Rom W. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- Chang X, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Seah GT, Scott GM, Rook GAW. Type 2 cytokine gene activation and its relationship to extent of the disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous disease. J Immunol. 2001;166:3432–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Orozco H, Sampieri A, Pavon L, Velasquillo C, Larriva-Sahd J, Alcocer JM, Madrid MV. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pando R, Orozco H, Arriaga AK, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin 1α, tumor necrosis factor α and transforming growth factor β during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pando R, Orozco H, Honour J, Silva P, Leyva R, Rook GA. Adrenal changes in murine pulmonary tuberculosis; a clue to pathogenesis? FEMS Immunol Med Microbiol. 1995;12(1):63–72. doi: 10.1111/j.1574-695X.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pando R, Schön T, Orozco H, Serafín J, Estrada-Garcia I. Expresión of nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp Toxicol Pathol. 2001;53:257–65. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- Bodnar K, Serbina N, Flynn J. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800–9. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- McWilliam AS, Nelson DJ, Holt PG. The biology of airway dendritic cells. Immunol Cell Biol. 1995;73:405–13. doi: 10.1038/icb.1995.63. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Demangel C, Palendira U, Feng CG, Heath AW, Bean AG, Britton WJ. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect Immun. 2001;69:2456–61. doi: 10.1128/IAI.69.4.2456-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Sun Shim T, Kipnis A, Junqueira-Kipnis AP, Orme I. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–35. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- Henderson R, Watkins S, Flynn J. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–43. [PubMed] [Google Scholar]
- Nagabhushanam V, Cheers C. Non-major histocompatibility complex control of antibody isotype and Th1 versus Th2 cytokines during experimental infection of mice with Mycobacterium avium. Infect Immun. 2001;69:1708–13. doi: 10.1128/IAI.69.3.1708-1713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JP, Orme IM. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacerium tuberculosis. Infect Immun. 1994;62:1683–90. doi: 10.1128/iai.62.5.1683-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Schon-Hegrad M, Oliver J, McMenamin C, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991;173:1345–6. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B, Salomon B, Klatzmann D, Pauwels R. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–7. [PubMed] [Google Scholar]
- Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-steate turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–61. [PubMed] [Google Scholar]
- McWilliam A, Napoli S, Marsh A, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–32. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong GL, McCarthy KM, Telford J, Tamatani T, Miyasaka M, Schneeberger EE. Intraepithelial airway dendritic cells: a distintic subset of pulmonary dendritic cells obtained by microdissection. J Exp Med. 1992;175:797–807. doi: 10.1084/jem.175.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma and visceral pleura. J Exp Med. 1986;163:436–51. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaelen K, Carro-Muino I, Lambrecht B, Pauwels R. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmelli C, Demotz S, Acha-Orbea H, De Grandi P, Nardelli-Haefliger D. Trachea, lung, and tracheobronchial lymph nodes are the major sites where antigen-presenting cells are detected after nasal vaccination of mice with human papillomavirus type 16 virus-like particles. J Virol. 2002;76:12596–602. doi: 10.1128/JVI.76.24.12596-12602.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueirard P, Ave P, Balazuc A, Thiberge S, Huerre M, Milon G, Guiso N. Bordetella bronchiseptica persist in the nasal cavities of mice and triggers early delivery of dendritic cells in the lymph nodes draining the lower and upper respiratory tract. Infect Immun. 2003;71:4137–43. doi: 10.1128/IAI.71.7.4137-4143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Pinto CE, Kradin RL. The antigen-presenting activities of Ia dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–83. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Easton A, Eichlberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–66. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–6. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza S, Gaziano R, Spreca A, Bacci A, Montagnoli C, Di Francesco P, Romani L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002;168:1362–71. doi: 10.4049/jimmunol.168.3.1362. [DOI] [PubMed] [Google Scholar]
- Toews G, Bergstresser P, Streilein W. Epidermal langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- Alcay J, Kripke M. Antigen-presenting activity of draining lymph node cells from mice painted with a contact allergen during ultraviolet carcinogenesis. J Immunol. 1991;146:1717–21. [PubMed] [Google Scholar]
- Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/Calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198:653–9. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]