Abstract
Cells of the innate immune system express a large repertoire of germ-line encoded cell-surface glycoprotein receptors including Toll-like receptors (TLRs). TLRs recognize conserved motifs on microbes and induce inflammatory signals. Evidence suggests that individual members of the TLR family or other non-TLR surface antigens either physically or functionally interact with each other and cumulative effects of these interactions instruct the nature and outcome of the immune response to a particular pathogen.
Keywords: innate immunity, TLRs
Pattern recognition and toll-like receptor (tlr) signalling pathway: introduction and background
One of the major differences between innate and adaptive immune system is genetic, the latter relying on somatic rearrangement of genes to give rise to tailor-made highly specific antigen receptors. There is no theoretical limit to the number of possible receptors for the infinite number of antigen which the host could encounter during its lifetime. On the other hand, cells of the innate immune system do not rearrange their genes, but express a large repertoire of germ line encoded cell surface glycoprotein receptors which recognize conserved patterns unique to microbial surfaces (pathogen-associated molecular patterns; PAMPs), allowing these cells to distinguish dangerous non-self materials from self-molecules. Hence, these molecules are often referred to as pattern recognition receptors (PRR).1 Historically it is believed that the ability of the innate immune system to recognize a great variety of pathogens is genetically limited.
PRR include a family of non-phagocytic TLR, which act as pathogen sensors, orchestrate inflammatory responses and play a central role in overall recognition of PAMPs by the innate immune system. On the other hand scavenger receptors (SR), mannose receptors and β-glucan receptors recognize ligands on microbial surfaces directly and mediate the engulfment of particulates.2–4 Macrophages (Mφ) also express a range of opsonic phagocytic receptors (Fc and complement receptors) that recognize antibody and complement coated particles, respectively.5,6 Apart from PRRs, innate immune cells express a large number of surface antigens which control migration, adhesion, activation or down modulation of the cells involved in innate immunity. Integrins, immunoglobulin superfamily (IgSF), glycosylphosphatidylinositol (GPI)-anchored proteins and G-protein coupled receptors are selected examples in this category.7–9 The PRRs or other cell surface antigens are usually shared to a varying degree by cells of the innate immune system, which include polymorphonuclear phagocytes (PMN), monocytes/Mφ, dendritic cells, natural killer cells and to some extent, epithelial or endothelial cells. All of these cells have specialized functions, although they interact and co-operate to mount an effective immune response against pathogens. Therefore, the biology of receptor collaboration should be interpreted in the much wider context of cellular co-operation in immune responses. In theory, different receptors could cross-talk with one another to increase and diversify the recognition and overall handling of microbial infection by the innate immune system, otherwise limited by the central genetic bottleneck. Cells of the innate immune system also communicate with one another through soluble mediators like cytokines and chemokines, but those interactions are beyond the scope of this review.
How PRRs convert the information gleaned from recognition of a pathogen to an appropriate cellular response has been a subject of intensive investigation. Two principal classes of PRRs have been proposed: those that mediate phagocytic uptake, and those that lead to activation of pro-inflammatory pathways.10 Most PRRs do not possess the cytoplasmic motifs shown to activate pro-inflammatory responses and only with the description of TLRs did it become clearer how innate immune activation occurred in response to PAMPs.10–13 The Toll receptors are conserved from Drosophila to humans and there are nine TLRs in mice and 10 in humans. Each TLR recognizes a restricted subset or even a single molecule produced by microbes (Table 1) and it is now accepted that the TLRs are the principal membrane signalling molecules through which mammals sense infection.11
Table 1.
Toll-like receptors and their ligands
| Toll-like receptor | Identified ligands |
|---|---|
| TLR-1/TLR-2 | Tri-acyl lipopeptides (bacterial, mycoplasmal), soluble factors |
| TLR-2 | Peptidoglycan, lipopeptide, zymosan, glycosylphosphoinositols, glycolipids, LTA, LAM, porins, atypical LPS, HSP70 (host) |
| TLR-3 | ds RNA (viral) |
| TLR-4 | LPS, Taxol (plant), fusion and envelope proteins (viral), HSP60 (bacterial), multiple host proteins |
| TLR-5 | Flagellin |
| TLR-6/TLR-2 | Di-acyl lipopeptide (mycoplasma) |
| TLR-7 | Synthetic ligands: Imidazoquinoline, Loxoribine, Bropirimine |
| TLR-8 | ssRNA,73 |
| TLR-9 | Unmethylated CpG DNA |
| TLR-10 | ? |
| TLR-11 | Uropathogenic bacteria |
What is not clearly established is whether TLRs can directly recognize their ligands as some studies suggest14–16 or whether an accessory molecule such as MD-2 or an intermediary similar to Drosophila Spaetzle performs this function.13,17–19 The signalling pathways of the TLRs have now been characterized in some detail; for recent reviews see.11,20 All TLRs, interleukin (IL)-1 receptor and other TLR–IL-1R (TIR) domain-containing receptor with the exception of TLR-3, share a common signalling pathway that depends on the adaptor myeloid differentiation factor 88 (MyD88). TLR mediated pro-inflammatory cytokine production in response to microbial recognition is critically dependent on MyD88 and its downstream mediators IRAK-4 and TRAF-6 that activate JNK and nuclear factor (NF)-κB.20 The importance of this pathway to host defence against a wide range of organisms was demonstrated when it was shown that MyD88-deficient macrophages are completely unresponsive to immunostimulatory components including lipopolysaccharide (LPS), peptidoglycan, lipoproteins, CpG DNA, flagellin, and imidazoquinolines, suggesting an essential role of MyD88 in mediating multiple inflammatory TLR responses.20 More recently TLR-4 and TLR-3-mediated MyD88-independent pathways have been described20,21 but these remain poorly characterized and mostly do not induce pro-inflammatory gene expression.
An important unresolved question is how, given the common MyD88-dependent signalling pathway shared by almost all the TLRs, discriminatory signals are transmitted from the TLR that has recognized its ligand to the cell nucleus. In the first instance some TLRs show a high degree of promiscuity making discrimination between micro-organisms less precise. In the second instance all the TLRs, with the exceptions of TLR-4 and TLR-2 that additionally require the adaptor TIRAP, and TLR-3 that senses viral RNA, signal exclusively through the Myd88, IRAK-4, TRAF-6 pathway to activate NF-κB and JNK. The TLR-3 and TLR-4 signalling pathways are more complex, also having an MyD88-independent pathway that signals through the MyD88 like molecule, Trif (a protein encoded by the gene Lps2) to the transcription factor IRF-3.11,22,23 Furthermore, studies have shown or suggested that TLR-independent sensing mechanisms exist for the prototypical TLR-4 ligand, LPS, and that LPS or its contaminants can also be recognized by multiple other surface and intracellular proteins that are able to activate the transcription factor NF-κB in a TLR-independent fashion.24–26 Other mechanisms that can modify immune response have been described, for instance, transcription factors not activated in the TLR-mediated signalling pathway, such as members of the signal transducer and activator of transcription (STAT) family, can be activated by both bacterial infection and LPS stimulation of macrophages, influencing expression of interferon (IFN)-regulated genes.27,28
Despite these reservations it is presently well accepted that TLR-mediated signalling is the primary mechanism of pathogen detection. A number of mechanisms have been proposed by which TLRs might discriminate between micro-organisms and these include: (i) TLR interactions with other TLRs (homophilic or heterophilic); (ii) TLR interactions with other non-TLR innate receptors. Interactions among non-TLR innate molecules are beyond the scope of this review. Interactions could be either cis- or trans-cellular, at the cell-surface or intracellular, contact/close proximity dependent and simultaneous or contact-independent and sequential. Such interactions could augment, inhibit or synergize with the functions of either participating partner. The aim of this review is to discuss selected examples from the above categories to illustrate principles of receptor collaboration and how such interactions ultimately contribute to host defence and immune pathology.
Simultaneous heterophilic cis interactions between different tlrs
TLRs recognize a restricted subset or even a single molecule produced by microbes, overlapping with other members of the family and yet functioning as principal signalling molecules through which mammals distinguish large numbers of micro-organisms. In a study using dominant negative forms of receptors Ozinsky and colleagues proposed that TLRs might discriminate between organisms by functioning in a combinatorial repertoire.29 As shown in this study both TLR-2 and TLR-6 are recruited within the phagosome and in collaboration they recognize peptidoglycan, a Gram-positive bacterial component. By contrast, TLR2 recognizes another bacterial component, lipopeptide, independently of TLR-6. Moreover, unlike TLR-4, homodimerization of the TLR-2 cytoplasmic tail does not induce tumour necrosis factor-α (TNF-α), but TLR-2 could physically associate with TLR-6 or TLR-1 and the cytoplasmic domain of TLR-2 could form heteromeric functional pairs with TLR-6 and TLR-1, leading to cytokine induction. In a follow up study the same group has shown that among TLR-2, TLR-6 and TLR-1-transfected HEK.293 cells, only TLR-2 responds to phenol-soluble modulin (PSM), a factor secreted by Staphylococcus epidermidis. However, cotransfection of TLR-6 or TLR-1 in TLR-2-expressing cells enhances and inhibits TLR-2 mediated PSM response, respectively.30 Transfection of dominant negative forms of TLR-1, TLR-2 or TLR-6 in either TLR-2 or TLR-2 + TLR-6 expressing cells showed that both dominant negative TLR2 and TLR-6 blocked the response in both cases to a varying degree. However, dominant negative TLR-1 failed to block the TLR-2 and TLR-6 combined response confirming the specificity of this heterophilic interaction between TLR2, TLR-6 and TLR-1. Transfection of the chimaeric form of TLR-1 and TLR-6 containing the cytoplasmic tail of TLR-6 and TLR-1, respectively, confirmed that the extracellular domain of TLR-1 is sufficient to inhibit TLR-2 responses, but both domains are needed for TLR-6 to enhance TLR-2 function. In summary, TLRs recognize distinct sets of PAMPs either alone or in collaboration with other members of the family to form heterodimers. The nature of the ligand and the participating receptor partner determine the nature and magnitude of the ultimate response.
Sequential transcellular interaction between tlr family members
Functions of innate receptors are often studied in isolation of the overall function of the cell types in which they are expressed. The vascular endothelium is a multifunctional cell monolayer involved in immune and inflammatory processes and plays a critical role in PMN migration by production of proinflammatory cytokines, chemokines or adhesion molecules. In a recent study Fan et al. discussed the transcellular cross-talk between TLR-4 and TLR-2 pathways in the context of cell–cell interaction between PMN and endothelial cells, resulting in PMN migration and profound physiological consequences.31 In brief, this study showed that LPS treatment induced TLR-2 in the lung in vivo, or in cultured endothelial cells in vitro. LPS-induced TLR-2 expression depended on TLR-4, MYD-88 and the NF-κB signalling pathway. Moreover, transcellular signalling of oxidant radicals from PMN (which also depended on LPS–TLR-4 signalling) to endothelial cells significantly amplified the LPS-TLR-4 mediated TLR-2 induction. TLR-2 up-regulation was significantly reduced in neutropenic mice, and could be restored by wild-type (WT) PMN, but not by gp91phox–/– PMN. Similarly in a coculture system WT PMN, but not gp91phox–/– PMN enhanced LPS–TLR-4-dependent TLR-2 expression in endothelial cells, confirming the role of the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system of PMN in this pathway. To study the physiological consequences of TLR-2 induction in vivo, they showed that sequential challenge with LPS and peptidoglycan led to the sequential induction of TLR-2 and intracellular adhesion molecule-1 (ICAM-1) in WT mice, where ICAM-1 induction depends on TLR-2. ICAM-1-dependent adhesion of PMN is an important determinant of PMN migration. Therefore, the authors studied PMN migration in an air pouch model; sequential challenge with LPS and PGN induced a marked increase in PMN migration in WT mice, but as expected, this did not occur in gp91phox–/– or TLR-4–/– mice. This study revealed a hitherto unique example where TLR-4, TLR-2 and the NADPH oxidase system are involved in a transcellular sequential positive feed back loop, which ultimately leads to PMN migration.
Simultaneous cis interaction between tlr and other innate receptors at the cell-surface
The combinatorial mechanism still does not avoid the problem of extensive overlap between TLR signalling pathways. More recently it has been shown that the recruitment of additional adaptors such as TIRAP (downstream of MyD88) and TRIF by particular TLRs can activate additional signalling pathways.11,32,33 TRIF recruitment may help to explain the MyD88 independent pathway attributed to TLR-3 and TLR-4, but other TLRs have known ligands that are not recognized by either TLR-3 or TLR 4 yet have a role in microbial discrimination that depends on the shared MyD88 signalling pathway. Furthermore, TLR-4 has many ligands and at least two signalling pathways, so how this molecule can relay specific signals in response to a particular ligand remains unclear. An unexplored area is whether, given the multiplicity of receptors that can recognize microbes, any of the non-TLR PRRs transmit signals that assist the TLR system in discriminating between particles and so help to regulate subsequent cellular activation.
Interaction between TLR-4, LBP, MD-2 and CD-14
The best understood description of collaboration between TLR and other immune receptors derived from the identification of different members of the LPS signalling complex. TLR-4 was the first discovered mammalian homologue of Drosophila Toll.34 Positional cloning of the LPS non-responsive mouse strain, C3H/HeJ, revealed a point mutation in the signalling domain of the TLR-4 protein.35, 36 Similarly, another LPS hyporesponsive strain C57BL10/ScCr, lacked the entire genomic region. Finally, targeted deletion of TLR-4 gene confirmed that TLR-4 is indispensible for LPS signalling.37 However, requirement for other molecules was suggested by in vitro studies showing that transfection of TLR-4 cDNA did not confer LPS responsiveness, an observation that was subsequently explained by the cloning of another LPS recognition molecule MD-2, an extracellular adaptor protein.38 Physical association between MD-2 and TLR-4 is critical for LPS responses. A range of in vitro studies demonstrated that LPS hyporesponsiveness in cells expressing TLR-4 alone or TLR-4 with mutant MD-2 was rescued by transfection of MD-2 or soluble MD-2 protein.39,40 Finally, mice lacking MD-2 did not respond to LPS and were resistant to endotoxic shock confirming the nonredundant role of MD-2 in LPS signalling.41 Similarly CD-14, a GPI-anchored protein expressed by myelomonocytic cells, was implicated in LPS signalling. Targeted disruption of CD-14 gene also displayed an LPS-resistant phenotype;42 however, mice lacking CD-14 are still able to respond to high concentrations of LPS. LBP, a serum glycoprotein shown to bind LPS, has also been implicated in LPS signalling.43 However, among all these molecules only TLR-4 has a signalling domain. Taken together, the present consensus is that LBP first binds LPS which, acting as a lipid transferase, catalyses transfer of an LPS monomer from the bacterial cell wall to CD14. In turn, CD14 binds and markedly augments LPS responses. LPS has to interact with TLR-4–MD-2 complexes to transduce the signal. Crosslinking studies demonstrated that the TLR-4-MD-2 complex requires membrane CD-14 to get close to LPS, suggesting that LPS needs to be transferred from CD-14 to TLR-4–MD-2. The molecular mechanisms underlying LPS transfer from CD14 to TLR-4–MD-2, however, remain to be elucidated.
Integrins and LPS
Although interactions among TLR-4, MD-2 and CD-14 are central to LPS signalling, other molecules may synergize with this pathway. Complement receptor-3 (CD11b-CD18 or CR3), a member of the β2 integrin family has been reported to bind LPS. Its role in LPS signalling, however, is doubtful. Although expression of CR3 in Chinese hamster ovary cells expressing TLR-4–MD-2, but not CD14, is sufficient to induce LPS mediated NF-κB induction, CD18 deficient humans cells respond normally. A recent study by Vogel and colleagues suggested that CD-14 and CR3 differentially synergize with TLR-4 for expression of the full repertoire of LPS/Taxol-inducible genes, where inducible protein-10 and ICSBP are totally dependent on CD-14, but maximal induction of cyclo-oxygenase-2 and of IL-12 p35 and p40 requires CR3 along with TLR-4 and CD-14.44
Dectin-1 co-operation with TLRs
We and others44,45 recently showed that dectin-1, a leucocyte-expressed PRR, may provide additional signals to enable the TLR system to generate pro-inflammatory responses to fungal pathogens.
Dectin-1 is a C-type lectin that recognizes fungal wall-derived β-glucans.46,47 In addition to recognizing β-glucans, dectin-1 also mediates the phagocytosis of live yeast and fungal-derived zymosan particles48 as well as zymosan particle-induced inflammatory cytokine production by macrophages.44,45 The latter observation is interesting because there is compelling evidence that zymosan particle-induced pro-inflammatory cytokine production depends on TLR-2 and -6 in particular,14,29,49,50 yet pure agonists of TLR-2 such as PAM3 and CSK4are poor inducers of inflammatory mediators.45 Dectin-1 and TLRs recognize different epitopes on fungal particles, and the TLR ligand can be destroyed by hot alkali treatment, or released from zymosan particles following chloroform/methanol/water extraction, without compromising zymosan phagocytosis in macrophages that express low levels of dectin-1.51
In macrophages we showed that although TLR2 and MyD88 were required for zymosan induced TNF-α production, increased dectin-1 expression markedly enhanced this response (Fig. 1 and ref. 45). Dectin-1 and TLR-2 and-6 colocalized in areas of contact between zymosan particles and macrophages, even in the presence of cytochalasin D,44 suggesting that TLR recruitment does not require particle phagocytosis.50,52 Phagocytosis of zymosan particles was also not required for pro-inflammatory cytokine production, and inhibition of zymosan particle internalization by a variety of inhibitors including cytochalasin D, wortmannin and toxin B44 led to a marked enhancement of cytokine production, suggesting that fungal particle internalization may be a mechanism to limit collaborative signalling. Furthermore, deletion of the tyrosine residues found in the ITAM-like motif of dectin-1 abrogated TNF-α production suggesting that these residues were important for fungal particle-induced cytokine production.44 The production of another zymosan-induced, TLR dependent pro-inflammatory cytokine, IL-12, was also promoted by dectin-1.45
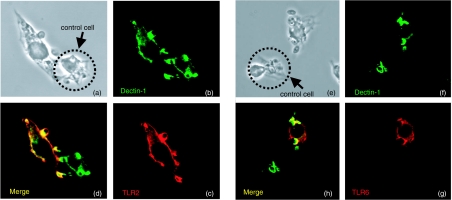
Figure 1.
Dectin-1 and TLR-2 and -6 colocalize in nascent zymosan phagosomes. V5-tagged TLR-2 and -6 (shown in red) and HA-tagged dectin-1 (green) colocalize within 5 min of initiating zymosan uptake. (a) Transmission image of two adjacent cells binding zymosan particles. (b) Dectin-1 HA staining. (c) TLR-2 V5 staining. (d) Staining showing colocalization. The cell indicated with an arrow was not transfected with TLR-2 and is presented asa staining control. (e) Transmission image of two adjacent RAW-D1 macrophages. (f) Dectin-1 is present in nascent phagosomes. (g) TLR-6 is also present in nascent phagosomes. (h) Merge shows that TLR-6 and dectin-1 colocalize. (Reproduced from 45)
In addition to mediating phagocytosis of zymosan particles, dectin-1 mediates the production of reactive oxygen intermediates (ROIs) independently of TLRs. As was the case for zymosan induced TNF-α production, however, these responses depend on the ITAM-like motif of dectin-1.45 These observations demonstrate that dectin-1 activates signalling pathways distinct from those of the TLRs, and our observations suggest that these signalling pathways share few, if any, mediators.48 Dectin-1, by some yet to be described mechanism, delegates zymosan induced pro-inflammatory responses to the TLRs. The implications of these findings are that phagocytosis of microbes and subsequent inflammatory responses are differentially regulated depending on the nature of ligand and the receptors involved.
FcγR interactions with TLRs
Receptors for the Fc portion of immunoglobulin G (IgG), the Fcγ receptors, allow innate immune cells to recognize and bind immune IgG complexes rapidly and efficiently. The sequelae of FcγR cross-linking include internalization by phagocytosis or endocytosis, antigen presentation, antibody-dependent cellular cytotoxicity and the release of mediators of inflammation.5
IL-12 produced by antigen-presenting cells is a pro-inflammatory cytokine essential for both cell-mediated immune responses and to bias T-helper cells to a Th1 phenotype.53–55 A range of stimuli including intact Gram-positive and Gram-negative microbes, intracellular protozoa and fungi as well as bacterial products such as LPS and LTA, induce the production of IL-12 by macrophages and other innate immune cells.56 IL-12 production by these cells in response to microbes and microbial products depends on TLRs57,58 and is mediated by MyD88.59 Down-regulation of IL-12 secretion by macrophages, which is necessary to protect the host, has been attributed to the cytokines IL-4, IL-10, IL-13 and TGF-β.60,61
Co-ligation of phagocytic receptors and TLRs resulted in modulation of IL-12 induction in response to multiple inflammatory stimuli, including LPS and LTA, independently of other cytokines.56,62 In macrophages these stimuli, on their own, induced high levels of IL-12 production and modest amounts of IL-10 (an antagonist of cellular immunity and septic shock), but coligation of FcγR, using either antibody-opsonized erythrocytes or soluble antibodies, along with TLR ligands, resulted in the simultaneous abrogation of IL-12 production and high levels of IL-10 induction.62,63 The abrogation of IL-12 production was not specific to FcγRs and coligation of complement and scavenger receptors, using either complement opsonized or maleylated-bovine serum albumin-coated erythrocytes, had the same effect. Treatment of macrophages with various pharmacological inhibitors showed that the abrogation of IL-12 production resulting from phagocytic receptor ligation did not depend on phagocytosis of erythrocytes, tyrosine phosphorylation or protein synthesis, but on changes in intracellular calcium levels56 whilst the IL-10 induction was specific for FcγR ligation.62 Ligation of FcγR on macrophages thus seems to activate an anti-inflammatory cell programme that has a damping effect on TLR-mediated pro-inflammatory signals, perhaps to limit excessive inflammation.62 Further evidence of interactions between FcγRs and TLRs can be found in B cells, where the induction of rheumatoid factor depends on both an IgG2a–chromatin immune complex and ligation of the MyD88-dependent TLR, TLR-9.64
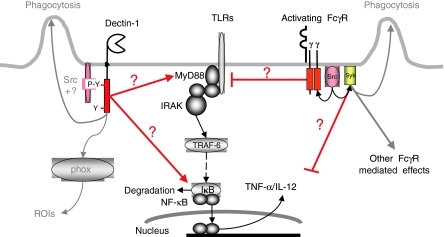
It is thus likely that interactions, either direct or through mediators, occur between FcγRs and TLRs whereby FcγRs modulate TLR-derived signals. Whether these events occur at the cell surface, as appears to be the case for dectin-144 or within phagosomes as appears to be the case for DCs65 remains to be established. Figure 2 diagrammatically illustrates our current understanding of the interactions between the TLRs and dectin-1/FcγR.
Figure 2.
Ligation of phagocytic receptors has differential effects on TLR-mediated signalling. Ligation of dectin-1 by particulate ligands results in phosphorylation of the membrane proximal tyrosine residue of the ITAM-like motif by tyrosine kinases including hthe Src family.48 Signals emanating from the ITAM-like motif are transmitted, by an unknown mechanism, to TLR-2 and perhaps TLR-6, promoting MYD88-dependent cytokine secretion.46,49 The zymosan-induced, dectin-1-mediated, production of ROIs is independent of the TLR system. The ITAMs of FcγRs become tyrosine phosphorylated, when ligated by either soluble antibody or antibody-coated erythrocytes, and then, by an unknown mechanism, exert an inhibitory effect on TLR-mediated IL-12 production.63,64
Scavenger receptors
SR are a large family of structurally unrelated distinct gene products implicated in modified low-density lipoprotein (mLDL) uptake and atherosclerosis. However, several members of this family are reported to be involved in phagocytic recognition of microbes and innate immunity.2 Generally, SR mediated uptake of bacteria is thought to be dissociated from TLR-4 mediated stimulation of proinflammatory cytokine release.66,67 Recently Doyle et al. showed that treatment of bone marrow culture derived murine Mφ with TLR-3, TLR-4 and TLR-9 agonists induced SR-A, MARCO and LOX-1, all members of the SR family that have been shown to bind bacteria. However, the contribution of individual TLR and expression kinetics of each SR was variable.68 Dissection of TLR signalling pathways using knock-out mice or pharmacological antagonists revealed that induction of SR was mediated by MyD-88, IRAK4 and p38. Furthermore, TLR induced SRs contributed to phagocytosis of Escherichia coli, blocked by general SR inhibitors or a specific blocking antibody. However, significant increases in SR-A, MARCO, or LOX-1 mRNA levels were not reflected in cell surface expression of respective proteins. Similarly individual contribution of induced SRs in bacteria binding was not shown explicitly.
Nonetheless, this work showed that TLRs sense PAMPs and induce a phagocytic gene programme in the same cell, which in turn promotes the ingestion of bacteria. This is the first example that although not involved directly in phagocytosis, TLR can contribute and control uptake of particulates.
Liver X receptor (LXR)
Mφ induce foam cell formation through SR mediated uptake of mLDL. However, this process is counterbalanced by degradation and efflux of cholesterol. Induction by oxysterol and synthetic agonists of LXR, transcriptional regulators in liver as well as Mφ, promotes synthesis of ABCA1 and other transporters involved in cholesterol efflux.69 Although an infectious contribution to atherosclerosis has long been suspected little direct evidence has been presented. In a recent study Castrillo et al. presented evidence that TLR-mediated microbial recognition may interfere with LXR-dependent cholesterol efflux from Mφ leading to increased susceptibility to atherosclerosis.70 The authors showed that agonists of TLR-3, TLR-4, but not TNF-α, inhibit the transcription of LXR responsive cholesterol-efflux genes including ABCA-1, both in vitro and in vivo. As expected this decrease in cholesterol-efflux genes translated functionally in reduced cholesterol efflux from Mφ. Dissection of TLR signalling pathways using knock-out mice, inhibitors or a dominant negative approach, showed that TLR-3/4-mediated inhibition of LXR responsive genes was independent of MYD-88 or NF-κB, but depended on another transcription factor IRF-3, implicated in interferon responses. In a separate study the same group has shown that agonists of LXR are also able to block LPS-induced NF-κB dependent inflammatory genes. Therefore, it is intriguing to determine the balance in mutually negative interaction between TLR and LXR, which otherwise might leave the host susceptible to either infection or atherosclerosis.
G-protein coupled receptors
Apart from transcellular cross-talk between TLR-4, TLR-2 and the NADPH oxidase system, PMN migration is also controlled at another level by cis interactions between TLR-4 and G-protein coupled chemokine receptors. Chemokine receptors are activated by specific chemokines and direct PMN migration along a concentration gradient. However, this process is negatively regulated by a family of GPCR specific kinases (GRKs), which phosphorylate and desensitize GPCR. Fan and Malik showed that LPS-TLR-4 signalling downmodulates this GRK-dependent negative pathway and thereby decreases GPCR desensitization, in turn augmenting PMN migration.71 Using a controlled PMN–Mφ coincubation assay system, the authors showed that MIP-2, a chemokine released by LPS pretreated Mφ induced GRK2 and GRK5 (two members of the GRK family) in PMN. Furthermore, MIP-2-mediated GRK2 and GRK5 induction depended on an intact phosphatidylinositol-3K (PI3K)-γ signalling pathway. However, LPS pretreatment of WT PMN, but not PMN from C3H/HeJ mice, failed to induce GRK2 and GRK5 in response to MIP-2. They also reported that LPS pretreatment decreased the internalization of MIP-2 receptors upon ligand binding. Similarly, use of antisense technology to block GRK2 and GRK5 protein expression showed a decrease in MIP-2 receptor internalization, suggesting that LPS–TLR-4 signalling blocked GRK2 and GRK5 expression, which in turn decreased receptor internalization. To show whether increased availability of receptors had any physiological relevance, data from an in vitro chemotaxis assay for PMN migration showed that LPS-pretreated PMN from WT, but not C3H/HeJ mice, displayed increased migration in response to MIP-2. This result was validated in vivo using an air pouch model. Blocking of GRK2 and GRK5 using antisense oligonucleotides confirmed their role in PMN migration. Finally, levels of GRK2 and GRK5 were measured in LPS-pretreated PMN in the presence of various inhibitors and only MEK inhibitors blocked the LPS–TLR-4 mediated down-regulation of GRK2 and GRK5. In summary, the authors suggested that MIP-2 binds to its receptor, which induces PI3K-γ mediated GRK2 and GRK5 expression, and in turn augments receptor internalization. However, simultaneous interaction between LPS and TLR-4 reduces GRK2 and GRK5 expression in a MEKK-dependent mechanism and thereby reduces receptor internalization. Increased availability of MIP-2 receptors finally translated as increased PMN migration.
Inhibition of tlr function by other receptors
Members of the TLR–IL-1R superfamily contain an intracellular TLR domain and either an immunoglobulin domain or a leucine repeat domain in their extracellular portion. IL-1R and IL-18R are prototypic example of the immunoglobulin subgroup whereas TLRs are members of the leucine repeat subgroup. However, as a general rule both subgroups have similar signalling pathways, activate NF-κB and contribute positively to inflammation. Almost certainly there is negative regulation to protect the host from uncontrolled inflammation or immune pathology, but so far our knowledge is limited in this particularly important area. Recently a receptor has been identified which has a single immunoglobulin domain and a TIR domain (SIGIRR). However, unlike other members of the TLR–IL-1R superfamily no constitutive signalling was measured by overexpression or structural modification of SIGIRR. Although the TIR domain of SIGIRR is highly conserved, it does not retain two amino acids from IL-1R that have been shown to be essential for signalling. In a recent study Wald et al. showed that epithelial cells from kidney, liver, lung and colon have a high to moderate degree of SIGIRR expression, which is down-regulated in different tissues after LPS challenge.71 Furthermore, overexpression of SIGIRR in Jurkat or HepG2 cells significantly reduces the IL-1 or IL-18 mediated NF-κB activation in these cells, but IFN-γ dependent STAT-1 activation remains unaltered. Moreover, SIGIRR-deficient mice show a more potent inflammatory response and increased susceptibility to endotoxic shock compared with WT animals. Similarly injection of IL-1, but not TNF-α, increases the induction of inflammatory mediators like KC, MIP-2, and C-reactive protein, indicating that SIGIRR can function as a negative regulator of IL-1 and LPS–TLR-4 signalling. Consistent with the in vivo data, different primary cells from SIGIRR-deficient mice show increased activation of NF-κB or JNK in response to IL-1, LPS or CPG indicating a similar role in the CPG–TLR-9 pathway. Finally, SIGIRR can be coimmunoprecipitated with TLR-4, TLR-5, TLR-9, IL-1R, or the adaptor molecules TRAF-6 and IRAK indicating possible physical contact between SGIRR and these molecules. Use of different deletion mutants of SIGIRR confirmed that amino acid 248–298 of the TIR domain was essential for its physical association with TLR-4 and TRAF-6.
Synergism between tlr and adenosine receptor
A unique example of receptor synergism between several members of the TLR family and adenosine A2A receptor (A2AR) has been reported.72 In this study murine peritoneal Mφ was treated with different TLR agonists in the presence or absence of adenosine or A2AR agonists. As expected, different TLR agonists induced TNF-α secretion. However, in the presence of A2AR agonists, TLR-2, -4, -7, -9, but not TLR-3 and -5, failed to induce TNF-α, instead inducing significant levels of vascular endothelial growth factor (VEGF), a potent stimulus for angiogenesis. Simultaneous downmodulation and up-regulation of TNF-α and VEGF, respectively, operated as an angiogenic switch shifting Mφ from an inflammatory to an angiogenic phenotype. In most cases infected or inflamed tissues suffer from ischaemia, therefore synergism between TLR and A2AR agonists may initiate a repair mechanism. In contrast, angiogenesis is a prerequisite for successful tumorigenesis, therefore it would be of great interest to know whether a potential hijacking of an angiogenic switch by infectious agents could contribute to the tumour pathology.
Conclusion
Investigators have only recently recognized the potential of receptor collaboration. However, very little is known about the biochemical nature of signal transduction pathways or physiological and pathological implications of such interactions. Genetic dissection of these pathways will shed light on the nature of these interactions and reveal how evolutionary pressures have shaped the immune system to its present day form. The expanded knowledge of receptor biology ultimately could be utilized for immune-manipulation or rational drug design.
Table 2.
Receptors known to collaborate with Toll-like receptors
| Toll-like receptor | Collaborating innate molecules | Class of the collaborating molecule | Reference |
|---|---|---|---|
| TLR-4 | CD14 | GPI-anchored | 42 |
| TLR-4 | CR3 | Integrins | 74 |
| TLR-2 | Dectin-1 | C-type lectin | 44 |
| TLR-3, -4, -9 | SR-A,MARCO,LOX-1 | SR | 66 |
| TLR-3, -4 | LXR | Nuclear receptor | 68 |
| TLR-4 | MIP-2 receptor | GPCR | 69 |
| TLR-4, -5, -9 | SIGIRR | IgSF | 70 |
| TLR-2, -4, -7, -9 | A2R | 71 |
Abbreviations
- ICSBP
interferon consensus sequence binding protein
- IRAK-4
interleukin receptor-associated kinase 4
- IRF-3
interferon regulatory factor 3
- JNK
c-Jun NH(2)-terminal kinase
- LBP
lipopolysaccaride binding protein
- LOX-1
lectin-like oxidized LDL receptor-1
- LTA
lipoteichoic acid
- MARCO
macrophage receptor with collagenous structure
- MEKK
mitogen-activated protein kinase kinase
- SR-A
scavenger receptor A
- TIRAP
Toll-IL-1 receptor domain-containing adapter protein
- TRAF-6
tumour necrosis factor receptor-associated factor 6
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–28. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Pomares L, Linehan SA, Taylor PR, Gordon S. Binding properties of the mannose receptor. Immunobiology. 2001;204:527–35. doi: 10.1078/0171-2985-00089. [DOI] [PubMed] [Google Scholar]
- 4.Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40:869–76. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 6.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–68. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 7.Barclay AN. Membrane proteins with immunoglobulin-like domains – a master superfamily of interaction molecules. Semin Immunol. 2003;15:215–23. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 8.Loertscher R, Lavery P. The role of glycosyl phosphatidyl inositol (GPI)-anchored cell surface proteins in T-cell activation. Transpl Immunol. 2002;9:93–6. doi: 10.1016/s0966-3274(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 10.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B, Du Hoebe KX, Ulevitch RJ. How we detect microbes and respond to them. the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–85. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 12.Underhill DM. Toll-like receptors: networking for success. Eur J Immunol. 2003;33:1767–75. doi: 10.1002/eji.200324037. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, Sano H, Iwaki D, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–25. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 15.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–7. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akashi S, Nagai Y, Ogata H, et al. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int Immunol. 2001;13:1595–9. doi: 10.1093/intimm/13.12.1595. [DOI] [PubMed] [Google Scholar]
- 18.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–51. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–6. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 20.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–8. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans. 2003;31:637–42. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 23.Doyle S, Vaidya S, O'Connell R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–63. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 24.Girardin SE, Tournebize R, Mavris M, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–42. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–4. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 26.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 27.Tsukada J, Waterman WR, Koyama Y, Webb AC, Auron PE. A novel STAT-like factor mediates lipopolysaccharide, interleukin 1 (IL-1), and IL-6 signaling and recognizes a gamma interferon activation site-like element in the IL1B gene. Mol Cell Biol. 1996;16:2183–94. doi: 10.1128/mcb.16.5.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69:598–604. [PubMed] [Google Scholar]
- 29.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar AM, O'Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge. Functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–9. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112:1234–43. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 35.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 38.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor-4. J Exp Med. 1999;189::1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohnishi T, Muroi M, Tanamoto K. N-linked glycosylations at Asn (26) and Asn (114) of human MD-2 are required for toll-like receptor 4-mediated activation of NF-kappaB by lipopolysaccharide. J Immunol. 2001;167:3354–9. doi: 10.4049/jimmunol.167.6.3354. [DOI] [PubMed] [Google Scholar]
- 40.Schromm AB, Lien E, Henneke P, et al. Molecular genetic analysis of an endotoxin nonresponder mutant cell line. a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 42.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. 1996 Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 2002;4:407–14. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 43.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–8. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 44.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–81. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 45.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown GD, Gordon S. Immune recognition: a new receptor for beta-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 48.Herre J, Marshall AJ, Caron E, et al. Dectin-1 utilizes novel mechanisms for yeast phagocytosis in macrophages. Blood 2004. doi: 10.1182/blood-2004-03-1140. (In press) [DOI] [PubMed] [Google Scholar]
- 49.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169:3876–82. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 50.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 51.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97::13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 53.Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003;9:176–80. doi: 10.1179/096805103125001586. [DOI] [PubMed] [Google Scholar]
- 54.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 56.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 57.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–85. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 59.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 60.Seki E, Tsutsui H, Nakano H, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–7. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 61.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J Exp Med. 1995;181:537–46. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–8. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 64.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217–22. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 66.Uronen-Hansson H, Allen J, Osman M, Squires G, Klein N, Callard RE. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology. 2004;111:173–8. doi: 10.1111/j.0019-2805.2003.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peiser L, De Winther MP, Makepeace K, et al. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect Immun. 2002;70:5346–54. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle SE, O'Connell RM, Miranda GA, et al. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–93. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 70.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 71.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–21. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 72.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 73.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A (2A) receptors. Am J Pathol. 2003;163:711–21. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]