Abstract
We investigated whether inhibitory natural killer cell receptor (iNKR) expression contributes to impaired antigen-specific cytotoxicity and interferon-γ (IFN-γ) production by CD8 T cells during chronic infection. iNKR immunoglobulin-like transcript-2 (ILT2/CD85j) is expressed on 40–55% of cytomegalovirus (CMV)-, Epstein–Barr virus (EBV)- and human immunodeficiency virus (HIV)-specific CD8 T cells in both healthy and HIV-infected donors. Other iNKRs (CD158a, b1, e1/e2, k, CD94/NKG2A) are expressed on only a small minority of CD8 T cells and are not preferentially expressed on tetramer-staining virus-specific cells. In normal donors, ILT2 is expressed largely on perforin+ CD27– effector cells. However, in HIV-infected donors, only a third of ILT2+ cells are also perforin+. In both normal and HIV-infected donors, ILT2+ cells are prone to spontaneous apoptosis. Therefore, ILT2 is normally expressed during effector cytotoxic T-lymphocyte (CTL) differentiation, but can also be expressed when effector maturation is incomplete, as in HIV infection. The effect of ILT2 on CD8 cell function was assessed by preincubating effector cells with ILT2 antibody. While blocking ILT2 engagement has no appreciable effect on cytotoxicity, it increases antiviral IFN-γ production by approximately threefold in both normal and HIV-infected donors. Thus, ILT2 expression, increased on antiviral CD8 cells in chronic infection, may interfere with protective CD8 T-cell function by suppressing IFN-γ production.
Keywords: NK receptor, CD8, CTL, interferon-γ, HIV
Introduction
Human immunodeficiency virus-1 (HIV-1)-infected individuals generate an adaptive CD8 T-cell response that only partially controls infection (reviewed in refs 1–3). Although HIV-specific CD8 T cells constitute as many as 10% of peripheral blood CD8 T cells, only a fraction produce interferon-γ (IFN-γ) in response to HIV antigens.4–6 Moreover, freshly isolated cells are often unable to lyse HIV-infected target cells.4,7–9 These functional defects in HIV infection have been linked to aberrant signalling secondary to down-modulation of the T-cell signalling molecules, CD3ζ and CD28, lack of perforin expression and incomplete effector-cell differentiation in CD8 T cells.7,8,10,11 CD8 T-cell triggering can also be hindered by engagement of inhibitory cell-surface signalling molecules, including inhibitory natural killer cell receptors (iNKR), which are expressed on previously activated CD8 T cells.12,13 iNKRs are reported to be expressed at higher frequencies on CD8 T cells during HIV infection.14–16
More than 30 NKRs have been identified as proteins in humans, and genomic analysis suggests that more than 100 possible NKR genes may be expressed.17 The inhibitory subset of NKRs (iNKR) are predicted to interfere with T-cell activation by signalling through immunoreceptor tyrosine-based inhibition motifs (ITIM).12,13 NKRs can be classified into two broad groups: immunoglobulin (Ig) superfamily molecules, such as killer inhibitory receptors (KIR), recognizing specific human leucocyte antigen (HLA) alleles as their ligands, and immunoglobulin-like transcript (ILT) receptors, recognizing a broader spectrum of class Ia and Ib HLA alleles; and C-type lectin receptors of the CD94 family that bind to HLA-E. Current data suggest that inhibitory receptors may raise the threshold of T-cell activation, protect the organism against autoimmunity and participate in terminating a primary immune response.12,13,18–20
The expression of HLA alleles known to bind particular NKRs has been linked to disease prognosis in HIV infection.21–23 HIV nef-mediated down-modulation of HLA-A and HLA-B, which also serve as NKR ligands, might influence the CD8 T-cell cytotoxicity of HIV-infected targets.24,25 Furthermore, HLA-G, the preferred ligand of CD85j/ILT2,26,27 is up-regulated on monocytes and T lymphocytes in HIV infection, perhaps as a consequence of enhanced interleukin-10 (IL-10) production.28,29 These findings suggest that expression of iNKRs may play a role in HIV infection, possibly by inhibiting CD8 T-cell triggering. Previous studies have reported conflicting results regarding NKR expression on CD8 T cells in HIV infection.14–16 However, none of these studies investigated NKR expression on antigen-specific CD8 T cells. NKR-expressing cells have oligoclonal T-cell receptor (TCR) usage, suggesting that NKRs are expressed after antigen-driven T-cell expansion.30,31 Here we studied the expression and function of multiple NKRs [five KIR receptors (CD158a, CD158b1, CD158e1/e2, CD158k), CD85j/ILT2 and the C-type lectin receptors (CD94, NKG2A)] on HIV, cytomegalovirus (CMV) and Epstein–Barr virus (EBV)-specific CD8 T cells in HIV-infected and healthy donors. Only CD85j/ILT2 was found to be highly expressed on the surface of antiviral CD8 T cells, identified by tetramer staining. In HIV infection, where the majority of circulating CD8 T cells are antigen-experienced,32,33≈ 40% of circulating CD8 T cells express CD85j/ILT2. Moreover, 40–60% of tetramer-positive CD8 T cells that recognize these persistent viruses (HIV, EBV and CMV) during chronic infection are ILT2+ in both HIV-seropositive and -seronegative donors. Blocking CD85j/ILT2 engagement significantly restores diminished IFN-γ production in response to viral antigens, but has little, if any, effect on dampened antiviral cytotoxic function. Therefore, although iNKR expression may reduce the likelihood of immune-mediated pathology by diminishing immunoreactivity, our results suggest that in chronic infections increased iNKR expression may inhibit a protective immune response.
Materials and methods
Subjects
Subjects were eight healthy volunteers and 17 HIV-seropositive donors of diverse disease stages expressing HLA class I alleles A2.1 and/or B8. Of the HIV seropositive subjects, five were long-term non-progressors (LTNP), six were stage A subjects receiving highly active antiretroviral therapy (HAART) and six had stage B or stage C disease. Informed consent was obtained from each subject. The study was approved by the Institutional Review Boards. Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll gradient centrifugation and used either as fresh cells or thawed cryopreserved cells, frozen using a programmed cell freezer (Gordinier Model 9000; Gordinier, Roseville, MI). Flow cytometry results with thawed cells were comparable to those obtained with freshly isolated cells.
Tetramers
Tetramers of A2.1 or B8 class I molecules complexed with epitopic peptides encoded by HIV, EBV or CMV (Table 1), were produced in our laboratory as previously described,4 or obtained from the National Institute of Allergy and Infectious Diseases Major Histocompatibility Complex (MHC) Tetramer Core Facility (Yerkes Regional Primate Research Center, Atlanta, GA).34 The concentration of tetramers used for staining was titrated to minimize background staining. Specificity was confirmed for each tetrameric complex using peptide-specific cytotoxic T-lymphocyte (CTL) lines. The sensitivity of detection of tetramer-positive cells was 0·01%. For phenotypic analysis, streptavidin–phycoerythrin (PE) tetramer-stained populations were required to be well-separated on flow cytometric dot-plots of tightly gated lymphocytes costained for tetramers and CD8-Cy5 (mAb B9.11; Immunotech, Westbrook, ME) and to represent at least 0·05% of CD8 T cells in the sample.
Table 1.
Major histocompatibility complex (MHC) class I tetramers used in this study
| Virus | Antigenic peptide | Amino acids | Peptide sequence | Restricting element |
|---|---|---|---|---|
| CMV | pp65 | 495–503 | NLVPMVATV | HLA-A0201 |
| EBV | BMLF1 | 280–288 | GLCTLVAML | HLA-A0201 |
| EBV | BZLF1 | 190–197 | RAKFKQLL | HLA-B8 |
| HIV | gag | 77–85 | SLYNTVATL | HLA-A0201 |
| HIV | RT | 309–317 | ILKEPVHGV | HLA-A0201 |
| HIV | RT | 294–302 | YTAFTIPSI | HLA-A0201 |
| HIV | env | 593–600 | YLKDQQLL | HLA-B8 |
Flow cytometry
PBMCs were suspended at 1 × 106 cells/50 µl in fluorescence-activated cell sorter (FACS) blocking buffer (Hanks' balanced salt solution containing 10% human AB serum, 0·5% human IgG and 5 mm EDTA) for 15 min at 4° and then incubated with streptavidin–PE-conjugated tetramer for an additional 40 min at 4°. For external staining, cells were washed and resuspended in FACS buffer [2% fetal calf serum (FCS) in phosphate-buffered saline (PBS)] and stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) to CD27, ILT2 or CD45RA, PE-conjugated ILT2 and phycoerythrin-cyanin 5·1 (Cy5)-conjugated CD8 mAb or with IgG-conjugated FITC, -PE and -Cy5 isotype-matched controls (Coulter Immunotech, Westbrook, ME or BD PharMingen, San Diego, CA). The anti-iNKR immunoglobulins EB6 (anti-p58.1/CD158a, IgG1), GL183 (anti-p58.2/CD158b1, IgG1), Z27 (anti-p70/CD158e1/e2, IgG1), Q66 (anti-p140/CD158k, IgM), XA185 (anti-CD94, IgG1), Z199 (anti-NKG2A, IgG2b) were generated in the laboratory of Lorenzo Moretta.30,35–37 The myeloma supernatant HP-F1 (anti-ILT2/CD85j, IgG1) was a gift of Dr Miguel Lopez-Botet (Barcelona, Spain).38 iNKR staining was followed by secondary antibody staining using 2 µl of FITC-conjugated goat anti-mouse immunoglobulin (DAKO, Carpinteria, CA). After incubation for 30 min at 4°, cells were washed and resuspended in FACS buffer, containing 1% formaldehyde, for analysis. For internal staining with FITC-conjugated perforin mAb δG9 (Pharmingen), cells were resuspended in 50 µl of FACS buffer and permeabilized using the Caltag Laboratories (Burlingame, CA) Fix and Perm kit, according to the manufacturer's protocol. All samples were analysed on a FACScalibur using cell quest software (Becton Dickinson) on a CD8high lymphocyte-gated population, unless stated otherwise. Because we were initially limited to three-colour staining, we were unable to simultaneously stain for CD3 and CD8 together with the other markers we were studying. In a few samples we verified that the CD8high gate included very few NK cells (< 2%) and excluded few CD8dim CD3+ CD8 T cells (< 9%), and that our results were not substantially altered by using a CD3+ CD8dim+high gate.
Cell selection
CD4+ cells were depleted using CD4 Dynabeads (Dynal MPC, Oslo, Norway). Tetramer-positive or CD8+ cells were positively selected using anti-PE (after staining with streptavidin PE-conjugated tetramer) or anti-CD8 microbeads, respectively, and MS+ MACS separation columns (Miltenyi Biotec Inc., Auburn, CA) following the manufacturer's protocol.
In vitro stimulation
PBMCs (1 × 106 cells/well in 48-well plates) in culture medium (RPMI-1640 containing 10% FCS, 2 mm HEPES, 100 µg/ml streptomycin, 100 U/ml penicillin, 2 mm l-glutamine, 50 µm 2-mercaptoethanol) were treated with 10 ng/ml anti-CD3 (Clone UCT1; Coulter Immunotech), harvested at designated time-points, and analysed by flow cytometry for ILT2, perforin and CD8 expression.
Detection of apoptosis
PBMCs (1 × 106 cells/well in 48-well plates) in culture medium were treated with 0, 10 or 100 ng/ml anti-CD3 and harvested 8 hr later for staining with annexin V–FITC (BD Pharmingen), ILT2–PE and CD8–Cy5 in 10 mm HEPES (pH 7·4), containing 140 mm NaCl and 2·5 mm CaCl2. Samples were analysed within 90 min of staining.
CD8 T-cell lines and clones
HIV, CMV or EBV-specific CD8 T-cell lines were generated by stimulating PBMCs or immunomagnetically selected HIV-specific IFN-γ-producing cells, isolated as described previously,39 with 5 µg/ml antigenic peptide (Table 1). The next day, recombinant interleukin-2 (rIL-2) (60 IU/ml; Chiron Oncology, Emeryville, CA) and/or recombinant interleukin-15 (rIL15) (25 ng/ml; R & D Systems, Minneapolis, MN) were added, and cells were cultured for an additional 10–14 days with biweekly addition of cytokines. For cloning, immunomagnetically selected HIV-specific IFN-γ-producing cells were seeded at five cells/well in microtitre plates in the presence of HIV peptide-labelled autologous adherent cells. rIL-2 (60 IU/ml) and rIL-15 (25 ng/ml) were added 1 day later and biweekly thereafter. Every 10–14 days, cells were restimulated with peptide-labelled autologous cells.
CTL assay
Log-phase autologous B lymphoblastoid cell lines (B-LCL) target cells were labelled for 1 hr at 37° with 100 µCi of 51Cr, washed and resuspended in culture medium at 105 cells/ml, as described previously.4 Labelled targets (104 cells/well) in triplicate U-bottom microtitre wells were incubated for 1 hr at 37° with peptides (5 µg/ml or the indicated concentrations). Effector cells (100-µl volumes) at indicated effector/target (E : T) ratios were added and the plates incubated at 37° over CO2 for 4 hr. Supernatants (35 µl) were counted on a Top Count microplate reader (Packard, Meriden, CT) and percentage specific cytotoxicity was calculated from the average counts per minute (c.p.m.) as follows:
The spontaneous release for all experiments was < 20%. Peptide-specific cytotoxicity was calculated as the difference between percentage specific cytotoxicity against peptide-loaded targets and targets incubated with medium. For ILT2-blocking experiments, effector cells were incubated in culture medium with an equal volume of undiluted HP-F1 myeloma supernatant, isotype-control IgG1 (Beckman Coulter) or medium for 10 min at 4° before being added to radiolabelled target cells.
IFN-γ production
Adherent PBMCs (1 × 106 cells/well) were cultured with medium, antigenic or irrelevant peptide (5 ng/ml, Table 1) for 1 hr at 37°. Non-adherent cells were cultured in medium, anti-ILT2 blocking antibody or isotype control antibody, as described above, for 30 min at 4° before adding to peptide-loaded adherent cells. Two hours later, 20 µm Brefeldin A (Sigma, St Louis, MO) was added, and 4 hr later the non-adherent cells were stained for CD8 and intracellular IFN-γ and analysed by flow cytometry, as described previously.4
Statistical analysis
The difference between groups was analysed using the two-tailed Student's t-test. When results using the same samples were compared, a paired two-tailed t-test was used.
Results
CD85j/ILT2 is highly expressed on antigen-specific CD8 T cells in normal donors and in HIV infection
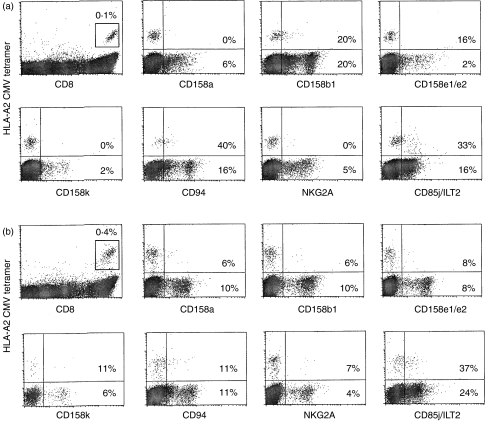
We assayed PBMCs for expression of the KIR receptors p58.1/CD158a, p58.2/CD158b1, p70/CD158e1/e2 and p140/CD158k, for the C-type lectin proteins CD94 and NKG2A, and for CD85j/ILT2, on total, as well as on EBV and CMV antigen-specific, CD8 T cells in six healthy donors and six HIV-seropositive donors (Fig. 1, Table 2). On average, less than 20% of CMV- and EBV-specific T cells of all CD8 T cells were found to stain for KIR or C-type lectin receptors in normal donors. The proportion of cells staining for these receptors was either similar or lower in HIV-infected donors. Moreover, the KIR and C-type lectin receptors were not expressed on a higher proportion of tetramer-positive cells compared with all circulating CD8 T cells.
Figure 1.
Immunoglobulin-like transcript-2 (CD85j/ILT2) is highly expressed on antigen-specific cells in healthy and human immunodeficiency virus (HIV)-seropositive individuals. Representative flow cytometry analysis is shown of inhibitory natural killer cell receptor (iNKR) expression on total and cytomegalovirus (CMV)-specific CD8 T cells in peripheral blood mononuclear cells (PBMCs) from (a) a healthy HIV-seronegative individual and (b) an asymptomatic HIV-seropositive donor. The first dot-plot for each sample displays the percentage of human leucocyte antigen (HLA)-A0201-CMV tetramer-positive cells in the lymphocyte gate. All the tetramer-positive cells are CD8high and form a well-separated population. Other dot-plots displaying NKR expression are gated on CD8high cells. The percentage of tetramer-positive and total CD8+ cells that stain above background for each iNKR are shown. The high proportion of CMV tetramer-positive cells in (a) that are CD94+ is not representative of the group of normal donor samples (Table 2).
Table 2.
Natural killer cell receptor (NKR) expression on Epstein–Barr virus (EBV) and cytomegalovirus (CMV) tetramer-positive CD8 T cells from six human immunodeficiency virus (HIV)-seropositive and six HIV-seronegative donors
| % of CD8 T cells | % of tetramer +cells | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NKR molecule | NKR type | Antibody | Ligand | HIV seronegative | HIV seropositive | P-value | HIV seronegative | HIV seropositive | P-value |
| CD158a (p58.1) | KIR | EB6 | HLA-Cw4 | 16 ± 14 | 5 ± 2 | 0·04 | 15 ± 15 | 5 ± 3 | 0·08 |
| CD158b1 (p58.2) | KIR | GL183 | HLA-C | 12 ± 7 | 8 ± 1 | 0·1 | 8 ± 5 | 3 ± 1 | 0·01 |
| CD158e1/e2 (p70) | KIR | Z27 | HLA-B | 4 ± 5 | 4 ± 3 | 0·4 | 9 ± 7 | 3 ± 1 | 0·05 |
| CD158k (p140) | KIR | Q66 | HLA-A | 3 ± 2 | 3 ± 2 | 0·4 | 6 ± 8 | 3 ± 2 | 0·1 |
| CD94 | Lectin | XA185 | HLA-E | 15 ± 14 | 20 ± 27 | 0·4 | 18 ± 17 | 13 ± 11 | 0·3 |
| NKG2A | Lectin | Z199 | HLA-E | 12 ± 12 | 4 ± 1 | 0·06 | 12 ± 21 | 2 ± 1 | 0·1 |
| CD85j (ILT2) | ILT | HPF1 | HLA-G* | 23 ± 14 | 40 ± 11 | 0·04 | 42 ± 24 | 41 ± 15 | 0·89 |
Immunoglobulin-like transcript (ILT2) binds to all major histocompatibility complex (MHC) class I molecules, including human leucocyte antigen-G (HLA-G).
Significant differences between HIV-seropositive and seronegative donors are shown in bold.
While ILT2 is expressed on just over 20% of all CD8 T cells in normal donors, > 40% of EBV- and CMV-specific cells in normal donors are ILT2+. In HIV-infected donor samples, ≈ 40% of tetramer-positive, as well as total, CD8 T cells express ILT2. These results suggest that ILT2 is expressed on a subpopulation of antigen-experienced T cells. In HIV infection, there are few naïve CD8 T cells in the circulation.32,33 Because most circulating cells are antigen-experienced in HIV infection, the frequency of ILT2 expression on all CD8 T cells is similar to that on tetramer-staining subsets. In normal donors, where ≈ 50% of circulating cells are naïve,32,33 the proportion of ILT2-expressing cells is about half of that observed in the tetramer-staining cells. However, antigen-experienced antiviral CD8 T cells in normal donors express ILT2 to the same extent as in HIV infection.
ILT2 is expressed comparably on HIV-, EBV- and CMV-specific CD8 T cells in HIV infection, irrespective of disease stage. Because ILT2 was the only iNKR we tested that was highly expressed on the surface of CD8 T cells in HIV infection, we focused on analysing ILT2 expression in HIV-specific, as well as EBV- and CMV-specific CD8 T cells. Samples from 17 HIV-infected donors were analysed for T cells recognizing four HIV, one CMV and two EBV epitopes (Table 1). Most donors recognize one or two of those peptides at a frequency of > 0·05%, which was our cut-off value for analysis to provide sufficient events to obtain reproducible phenotypic characterization. Twenty-seven tetramer-positive populations were analysed. ILT2 was found to be expressed at a significantly higher level on total and tetramer-positive CD8 T cells, irrespective of viral antigen or HIV disease stage (Table 3). Taking all samples together, 43 ± 14% of total CD8 T cells and 48 ± 19% of tetramer-positive CD8 T cells are ILT2+, in this extended analysis of HIV-infected individuals. No statistically significant difference was found between ILT2 expression on total and tetramer-positive cells between the different HIV+ groups. Moreover, the CD8 T cells specific for each of the chronic infections studied (CMV, EBV, HIV) have similar proportions of ILT2+ cells (Table 3). Therefore, ILT2 is expressed on many antiviral CD8 T cells in HIV infection across the disease spectrum, from LTNPs to patients with acquired immune-deficiency syndrome (AIDS).
Table 3.
Immunoglobulin-like transcript-2 (ILT2) is expressed on antiviral tetramer-positive CD8 cells responding to cytomegalovirus (CMV), Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV) in all stages of disease in HIV infection
| Donors | No. of tetramer- positive samples analysed | Tetramer-positive cells expressing ILT2 (%) | |
|---|---|---|---|
| Tetramer | |||
| CMV | 6 | 6 | 51 ± 27 |
| EBV | 13 | 13 | 46 ± 19 |
| HIV | 5 | 8 | 44 ± 18 |
| Disease stage | |||
| LTNP* | 5 | 8 | 45 ± 21 |
| Stage A | 6 | 11 | 55 ± 20 |
| Stage B or C | 6 | 8 | 43 ± 13 |
No significant differences between tetramers or disease stages were identified.
LTNP (long-term non-progressors) are defined as individuals who have been infected with HIV for at least 5 years and who had maintained CD4 counts of > 450/mm3 in the absence of antiviral therapy. Disease staging was based on the lowest recorded CD4 count according to the staging system of the Centers for Disease Control. All stage A donors were receiving highly active antiretroviral therapy (HAART).
In normal donors ILT2 is expressed on effector CD8 T cells
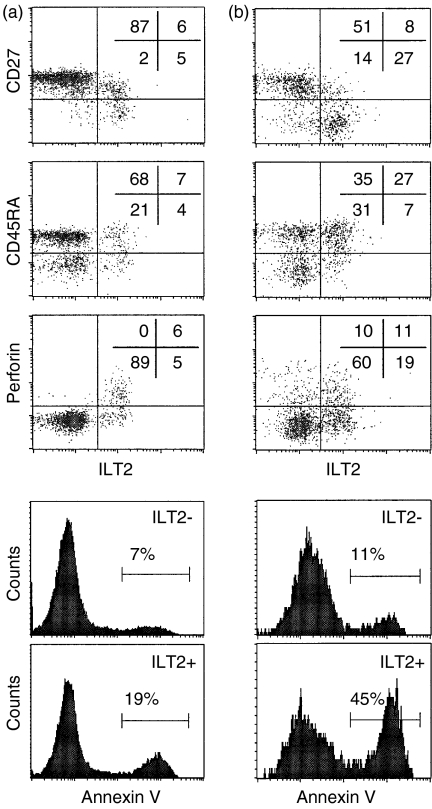
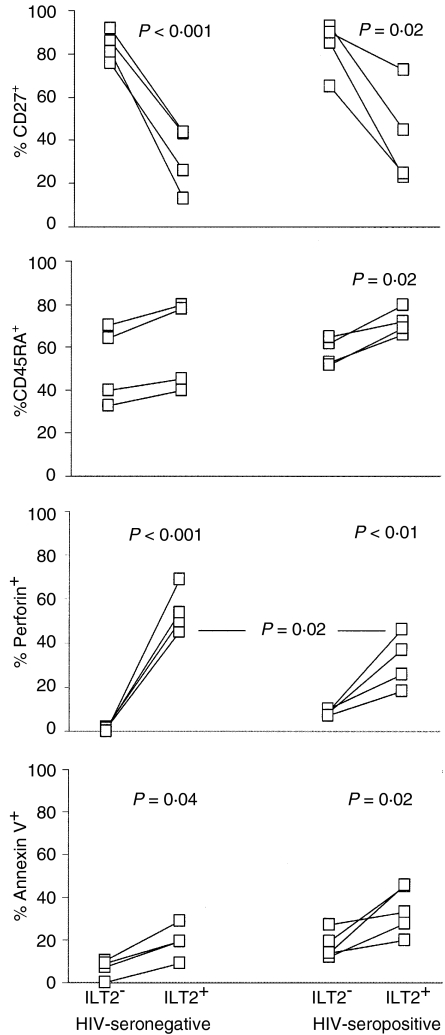
As ILT2 is expressed on activated CD56+ CD8 T cells and increased in HIV infection, we next investigated whether ILT2+ CD8 T cells are effector cells, because effector maturation in activated CD8 T cells is impaired in HIV infection. PBMCs were co-stained for ILT2 and T-cell markers that change during CD8 T-cell differentiation. Two of the best markers for CTL differentiation are perforin up-regulation and CD27 down-modulation.40 In four healthy donors, 32 ± 15% of ILT2+ and 84 ± 7% of ILT2– CD8 T cells were demonstrated to express CD27 (P < 0·001) (Figs 2 and 3). Moreover, 55 ± 10% of ILT2+ CD8 T cells stain for perforin, while only 1 ± 1% of ILT2– CD8 T cells are perforin-positive (P < 0·0001) (Figs 2 and 3). However, there is no significant correlation between ILT2 and CD45RA expression, because 61 ± 21% of ILT2+ and 52 ± 18% of ILT2– CD8 T cells are CD45RA+ (P = 0·5) (Figs 2 and 3). This lack of correlation is probable because CD45RA is expressed on both naïve CD8 T cells and effector CTLs.41 The high level of perforin staining and lack of CD27 staining on ILT2+ versus ILT2– CD8 T cells suggests that ILT2 is expressed on differentiated effector CD8 T cells in normal donors.
Figure 2.
Immunoglobulin-like transcript-2 (CD85j/ILT2) expression correlates with CD8 T-cell differentiation into effector cells. Representative flow cytometry analysis is shown of gated CD8high cells in peripheral blood mononuclear cells (PBMCs) from (a) an human immunodeficiency virus (HIV)-seronegative donor and (b) an HIV-seropositive donor. Cells were co-stained for ILT2 and CD27, CD45RA, or perforin. ILT2+ cells are preferentially CD27– and perforin-positive. For annexin V staining, cells co-stained for CD8, ILT2 and annexin V were analysed on gated subpopulations of ILT2+ or ILT2– CD8high cells. Spontaneous apoptosis was substantially higher amongst ILT2+ cells.
Figure 3.
Immunoglobulin-like transcript-2 (CD85j/ILT2+) CD8 T cells are more often CD27– and perforin+ and have a higher rate of spontaneous apoptosis. Comparison of phenotypic properties of ILT2+ and ILT2– CD8 T cells in four healthy donors and four asymptomatic human immunodeficiency virus (HIV)-infected donors. The analysis was performed as described in the legend of Fig. 2.
ILT2 is expressed on CD8 T cells that are not terminally differentiated CTLs in HIV infection
In HIV infection, many antiviral CD8 T cells have compromised function in response to antigen and do not have the phenotype of effector CTLs.1,2 They express some markers associated with activation, but mostly are not directly cytotoxic and do not express perforin. We therefore studied the phenotype of CD8 T cells expressing ILT2 in four HIV-seropositive donors. In HIV-infected donors, as in normal donors, ILT2 expression correlates with CD27 down-modulation. While 42 ± 23% of ILT2+ CD8 T cells are CD27+, 83 ± 13% of ILT2– CD8 T cells are CD27+ (P=0·02) (Figs 2 and 3). In HIV-seropositive samples, unlike HIV-seronegative samples, the expression of ILT2 and CD45RA correlate somewhat, as 72 ± 6% of ILT2+ CD8 T cells are CD45RA+ compared with 58 ± 6% of ILT2– CD8 T cells (P=0·02) (Figs 2 and 3). This association is probably evident in HIV-seropositive samples as the proportion of naïve CD8 cells is substantially reduced during HIV infection.32,33 ILT2 also correlates with perforin expression in HIV-infected samples, as only 9 ± 1% of ILT2– CD8 T cells stain for perforin, while 32 ± 12% of ILT2+ cells are perforin-positive (P = 0·009). Nonetheless, a significantly lower percentage of ILT2+ CD8 T cells stain for perforin in HIV-seropositive compared with HIV-seronegative samples (32 ± 12% vs. 55 ± 10%; P = 0·02). Therefore, many CD8 T cells that up-regulate ILT2 in HIV infection are not effector CTLs.
ILT2+ CD8 T cells are prone to spontaneous apoptosis
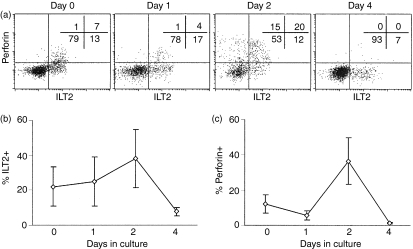
A significant fraction of CD4 and CD8 T cells in HIV infection spontaneously undergo apoptosis in short-term culture,42 although only a minor proportion (much less than 1%) are HIV infected. As most effector T cells are terminally differentiated and do not survive, if ILT2 is expressed on terminally differentiated effector CD8 T cells, we would expect a higher proportion of ILT2+ cells, compared with ILT2– cells, to stain for annexin V, either spontaneously or after in vitro T-cell activation. In four normal donor samples, spontaneous apoptosis of ILT2+ CD8 T cells is more than twice as great in ILT2+ compared with ILT2– CD8 T cells (19 ± 8% vs. 6·5 ± 4·5%, P = 0·04). (Figs 2 and 3) As expected, spontaneous apoptosis is much higher in samples from five asymptomatic HIV-infected donors and in ILT2+ cells in these samples compared with ILT2– samples: 32 ± 10% of ILT2+ CD8 cells from HIV-infected donors are annexin V+ compared with 18 ± 7% of ILT2– cells (P=0·02). We did not find any significant increase in annexin V staining in cultures 8 h after activation with CD3 antibodies compared with spontaneous apoptosis in mock-treated cultures (data not shown). This is probably because annexin V staining associated with activation-induced cell death only occurs later. However, because ILT2 expression is induced within 2 days of T-cell activation (Fig. 4), it is not feasible to compare later time-points.
Figure 4.
Immunoglobulin-like transcript-2 (ILT2) and perforin expression change in parallel during the in vitro activation of CD8 T cells with anti-CD3. Peripheral blood mononuclear cells (PBMCs) from three normal donors were activated with a suboptimal concentration of anti-CD3 and analysed for expression of perforin and ILT2 on CD8+ lymphocytes on the indicated days. (a) Data from a representative donor; (b) and (c), the proportion of CD8 T cells staining for ILT2 and perforin, respectively, in all the samples.
ILT2 and perforin expression change in parallel after short-term T-cell activation in vitro
To confirm the association of ILT2 expression with T-cell differentiation, PBMCs from three normal donors were activated in vitro with a suboptimal concentration of anti-CD3, and co-stained for perforin and ILT2 on CD8 T cells over 4 days (Fig. 4). Before culture, ≈ 20% of CD8 T cells are ILT2+, and about half of those are also perforin-positive. Although there was little change in ILT2 or perforin expression 1 day later, within 2 days a larger proportion of cells expressed both perforin and ILT2. By day 4, fewer cells expressed either marker. We interpret these data to show activation of effector function in antigen-experienced cells in the culture, as naïve T cells do not differentiate into effector cells within 2 days. Both perforin and ILT2 are up-regulated in tandem, although some perforin-positive cells are ILT2– and some ILT2+ cells are perforin-negative. This heterogeneity probably reflects the heterogeneous nature of antigen-experienced cells. Moreover, the loss of ILT2 and perforin expression by day 4 probably represents apoptosis after activation of the antigen-experienced CD8 T cells. Few, if any, naïve T cells are activated to express perforin by day 4 in these cultures, because of the low concentration of anti-CD3 used (data not shown).
ILT2 expression has little effect on the cytotoxic function of CD8 T cells
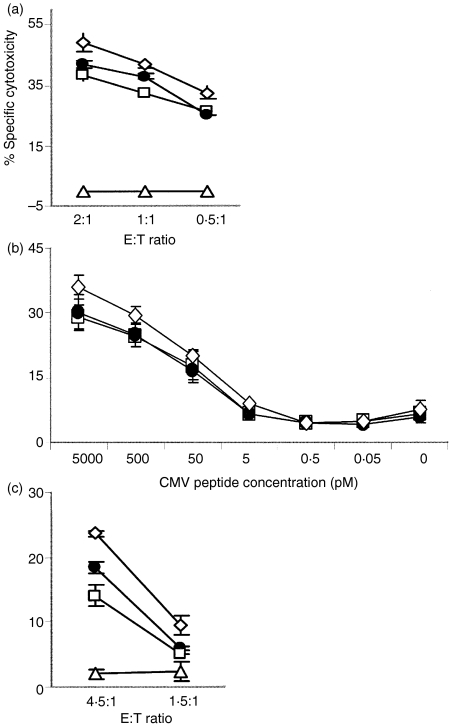
To study the functional relevance of ILT2 up-regulation in HIV infection, we tested the impact of ILT2 expression on cellular cytotoxicity and IFN-γ generation using antibodies that block ILT2 receptor engagement. An HIV-specific CD8 T-cell clone and three viral peptide-stimulated CD8 T-cell lines with a high proportion (50–100%) of ILT2+ cells were preincubated with medium, the ILT2 blocking antibody, HP-F1, or isotype-control antibody, before being used as effector cells in a 4-h chromium-release assay against autologous peptide-coated target cells. The cytotoxicity of the clone was slightly improved by blocking ILT2 engagement (Fig. 5a), but blocking ILT2 has no discernible effect on the cytotoxicity of the three cell lines (data not shown). Because ILT2 might have an effect only at limiting concentrations of antigen, we also tested the effect of ILT2 antibody in cytotoxicity assays using a range of doses of CMV antigenic peptide to arm target cells. No substantial increase in specific cytotoxicity in the presence of the anti-ILT2 blocking antibody was seen at any peptide concentration (Fig. 5b). T cells maintained in culture up-regulate adhesion and cytotoxic molecules, and may therefore have a reduced threshold for cytotoxic function and be less susceptible to the inhibitory effect of ILT2 than cells in vivo. We therefore tested the effect of blocking ILT2 engagement using freshly isolated, CMV tetramer-enriched CD8 T-effector cells from two HIV-seropositive donors. In one sample, but not the other (data not shown), cytotoxicity was found to be enhanced somewhat by blocking ILT2 signalling (Fig. 5c). Thus, ILT2 expression may inhibit the cytotoxicity of some (but not all) CD8 T cells, but only to a limited extent.
Figure 5.
Immunoglobulin-like transcript-2 (CD85j/ILT2) expression exerts little inhibitory effect on the cytotoxic function of CD8 T cells. (a) A human immunodeficiency virus (HIV) gag-specific CD8 T-cell clone showed, at most, a minimal inhibitory effect of ILT2 engagement on specific cytotoxicity. Cytotoxicity may be minimally enhanced in the presence of anti-ILT2 blocking antibody (◊) compared with isotype-control antibody (•), or no antibody (□), against gag peptide-armed targets. In the absence of peptide there is no killing (Δ). ILT2 blocking has no effect at all in specific lysis by three other antigen-specific T-cell lines (data not shown). (b) Blocking ILT2 also has no effect on specific lysis at limiting concentrations of peptide antigen. Effector cells, pretreated with blocking antibody (◊), control antibody (•), or no antibody (□), were tested for lysis of autologous B-LCLs treated with the indicated concentrations of cytomegalovirus (CMV) A0201-resticted peptide. (c) Freshly isolated peripheral blood mononuclear cells (PBMCs), stained for CMV A0201 peptide and selected for tetramer-positive CD8 T cells, were incubated with ILT2 or control antibody and used as effector cells. Blocking ILT2 increased the killing somewhat by one cell line, but not by another (data not shown). Preincunbation was performed in the presence of: (◊), blocking antibody; (•), control antibody; (□), no antibody.
ILT2 expression inhibits IFN-γ production by CD8 T cells
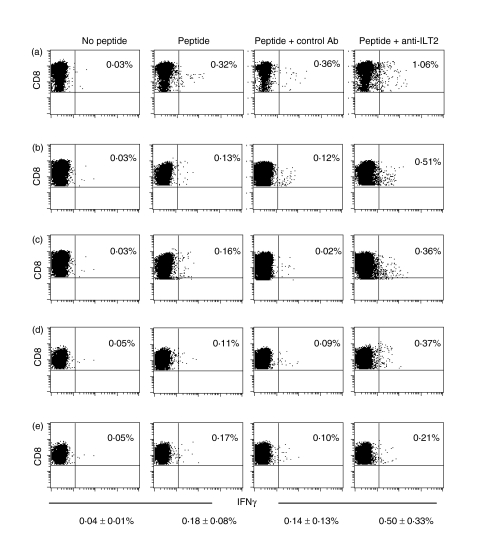
We next tested the effect of blocking ILT2 engagement on IFN-γ production in response to EBV and CMV antigenic peptides in five separate experiments. In these tests, two normal samples with a high frequency (> 0·1%) of CD8 T cells staining for EBV and/or CMV tetramers, and one HIV-seropositive sample with a high frequency of CMV tetramer-positive cells were used. Freshly isolated non-adherent PBMCs were preincubated with medium, ILT2 blocking antibody or control antibody before being added to antigenic peptide-coated autologous stimulator cells (Fig. 6). Blocking ILT2 significantly increased IFN-γ production. In the five experiments, 0·18 ± 0·08% of CD8 T cells produced IFN-γ in response to antigenic peptide, compared with a background of 0·04 ± 0·01% in the absence of peptide (Fig. 6). The proportion of IFN-γ-producing cells increased approximately threefold to 0·50 ± 0·33% after preincubation with anti-ILT2 blocking antibody (P = 0·002), but did not change significantly after incubation with control antibody (0·14 ± 0·13%). Incubation with ILT2 antibody in the absence of peptide or with irrelevant peptide did not change the background (not shown).
Figure 6.
Immunoglobulin-like transcript-2 (CD85j/ILT2) inhibits interferon-γ (IFN-γ) production by CD8 T cells. Non-adherent peripheral blood mononuclear cells (PBMCs) from one human immunodeficiency virus (HIV)-seropositive (a) and two HIV-seronegative (b–e) donors, preincubated with ILT2 blocking antibody, control antibody or no antibody, were mixed with Epstein–Barr virus (EBV) (b, c, e) or cytomegalovirus (CMV) (a, d) antigenic peptide-armed autologous adherent cells and analyzed for IFN-γ production. Percentages of CD8 T cells staining for intracellular IFN-γ are shown. The mean value ± standard deviation (SD) of the five experiments is also shown for each condition. The percentage of IFN-γ+ cells increases by approximately threefold when ILT2 engagement is blocked.
Discussion
In this study we found that ILT2, but not seven other iNKRs, is highly expressed on CMV, EBV and HIV antigen-specific CD8 T cells in HIV infection and in normal donors. Two previous studies also reported similarly enhanced ILT2 expression on EBV and/or CMV tetramer-positive CD8 T cells in a few normal donor samples.43,44 In normal donors, ILT2 expression on the surface of CD8 T cells occurs principally on effector cells that have down-modulated CD27, express perforin and have an increased level of spontaneous apoptosis. In HIV and other chronic infections, many antiviral CD8 T cells are defective in effector functions and do not have the phenotypic properties of effector CTLs (reviewed in ref. 3). Although ILT2– CD8 T cells in HIV infection are more likely than ILT2– cells to be CD27– and perforin-positive, many ILT2– cells in HIV infection are perforin-negative and CD27–. Therefore, antiviral CD8 T cells express ILT2, even though they fail to undergo terminal maturation to effector CTLs. Using an ILT2 blocking antibody, we found that ILT2 receptor engagement has a modest and variable inhibitory effect on cytotoxicity, but substantially inhibits IFN-γ production in response to antigen. Therefore, ILT2 expression raises the threshold for triggering CD8 T-cell functional responses and probably contributes to the immune regulation of CD8 T cells to prevent immunopathogenicity. However, in settings of chronic infection, such as HIV infection, ILT2 up-regulation interferes with the protective antiviral CD8 T-cell response. Not only do a high proportion of antiviral CD8 T cells (≈ 40%) express ILT2, but ILT2 engagement inhibits IFN-γ production in response to viral antigens.
The effect of ILT2 expression on CMV-specific cells may be especially relevant as ILT2 binds to the CMV gene product, UL18.45 In this study, we used CMV peptide-armed stimulator/target cells. It would be of interest to determine whether the inhibitory effect of ILT2 cell-surface expression on CD8 T-cell functions is greater in response to CMV-infected cells than to the peptide-loaded targets used here, particularly as the frequency of ILT2+ CD8 T cells increases in immunosuppressed transplant recipients who develop CMV disease.43
The NKRs are a rapidly evolving family with little similarity between humans and rodents, and substantial divergence even between humans and closely related non-human primates.46 However, the ILT family of receptors, unlike the KIRs, is relatively conserved between ethnically diverse human populations. Unlike the other NKRs, the ILT receptors are also broadly expressed on diverse cells in the leucocyte lineage, including B cells, neutrophils and monocytes, in addition to T lymphocytes and natural killer cells.12,13 There is some evidence that ILT2 may be broadly expressed in the cytoplasm of all T cells, even naïve T cells, but is only present at high concentrations on the cell surface of previously activated cells.47 Therefore, as we stained for ILT2 without permeabilization, this study only relates to cell-surface expression. The phenotypic properties of ILT2+ cells (mostly CD27+ perforin+ in normal donors, but less so in HIV-infected donors) in our study suggest that ILT2 is expressed on the surface of activated CD8 T cells before they have completed differentiation into effector CTLs and is sustained on effector CTLs. The high rate of spontaneous apoptosis we observed is also consistent with expression on terminally differentiated effector cells and in agreement with the findings of Young et al.,44 who also found low levels of bcl-2 expression on ILT2+ cells. Because the proportion of central memory CD8 cells is normally so small, it is difficult to draw any conclusions from this study about whether ILT2 is expressed on different subsets of antigen-experienced cells without effector function (so-called central memory and effector memory cells).41 After suboptimal in vitro activation of normal donor PBMCs, cell-surface ILT2 expression is up-regulated on some CD8 T cells within 48 hr of anti-CD3 engagement, in parallel with perforin. This early response probably occurs on antigen-experienced cells, as naïve T cells require more than 3 days to differentiate into effector cells. Our findings are consistent with the results of other studies which found that ILT2+ T cells are mostly CD28+, CD27+, CD56+ and CD57+.44,48 The high level of ILT2 expression in HIV infection then reflects the massive expansion of ‘effector’ and especially ‘effector memory’ CD8 T cells compared with naïve and central memory CD8 T cells in HIV-infected individuals.32,33 Further studies are required to pinpoint the cell-surface expression of ILT2 during activation of naïve T cells and to define precisely the conditions required for continued ILT2 expression after activation.
A few earlier studies looked at NKR expression on CD8 T cells in HIV infection.14–16,49 Although one group found increased expression of several iNKRs on CD8 T cells in HIV-infected individuals, including some that we did not find to be increased in this study, others failed to reproduce these data. The apparent discrepancy could be related to the treatment status of HIV-infected donors, as enhanced expression of NKRs other than ILT2 declines after 2 years of HAART,49 which most of our HIV-infected donors were receiving. None of these studies looked at antigen-specific cells. We now find that ILT2 is also highly expressed on tetramer-positive antiviral cells in HIV infection and in normal donors. However, only about 50% of tetramer-positive cells were ILT2+. This probably reflects the fact that during these chronic infections where the frequency of infected cells actively replicating virus is extremely low, the antiviral CD8 T cells that stain with tetramers are a heterogeneous mix of cells that have a diverse history of exposure to antigen, including memory cells, recently activated cells and cells that have been only partly activated by suboptimal stimulatory signals.4,50 In fact, most HIV-specific CD8 T cells have some characteristics of effector cells and some characteristics of memory cells.2,33
iNKR signalling on CD8 T cells is thought to raise the activation threshold for antigenic stimulation to prevent immunopathogenesis and autoreactivity and participate in terminating the primary immune response.12,13 In fact, during T-cell activation, ILT2 polarizes towards the immunological synapse and ILT2 triggering recruits the Src homology protein, SHP, reduces the phosphorylation of the key proximal signalling molecules, CD3ζ and linker for activation of T cells (LAT), and more distal signalling molecules such as the extracellular signal-related kinases (ERK), and inhibits the activation of cytoskeletal changes associated with activation.51 It is therefore not surprising that blocking ILT2 triggering enhances the function of ILT2– CD8 T cells, especially IFN-γ production in response to antigen. However, blocking ILT2 has a variable effect on enhancing cytotoxicity. Our finding, of differential effects of ILT2 on IFN-γ production and cytotoxicity, supports work which suggests that the threshold for activating cytotoxicity is much higher than that for activating cytokine production.1,11,52,53 Moreover, cytotoxic function is more compromised than cytokine production during chronic infection in both humans and mouse models.4,8,54 The variable effect of ILT2 engagement on cytotoxicity might be related to variability in TCR affinity or in the co-expression of costimulatory and adhesion molecules and other inhibitory receptors, which can affect the strength and quality of T-cell activation. In fact, previous studies found more pronounced inhibition of cytotoxicity by ILT2 blocking antibody than we observed using HIV donor samples in this study.47,49
A recent study found that the iNKR gp49B1 in mice is expressed upon CD8 T-cell activation and similarly interferes with antigen-specific IFN-γ production, but not with cytotoxicity.55 Because there is little homology between human and rodent NKRs, it is possible that gp49B in mice and ILT2 in humans, although they have little homology (20% amino acid identity), play similar dominant roles in regulating CD8 T-cell function on activated CD8 T cells. As the human genome encodes ≈ 100 NKRs that might be expressed on CD8 T lymphocytes,17 and the expression of these receptors (most of which have not even been identified as proteins) are unknown, other iNKRs might also contribute to regulating CD8 T-cell function.
ILT2 is highly expressed on antiviral CD8 T cells in normal donors and HIV-infected donors, where about half of viral tetramer-staining CD8 T cells are ILT2+. Moreover, ILT2 engagement significantly stifles the IFN-γ response to antigen. Given the importance of the IFN-γ response in controlling viral infection,4,56–58 inhibition of T-cell activation by ILT2 may play an important role in impeding a protective immune response to HIV and other chronic viral infections, especially CMV. During an acute infection, ILT2 expression may help the host to maintain the delicate balance between effective immunity and immune pathogenesis by raising the threshold for triggering CTL effector function. In the setting of chronic infection, however, inhibiting CD8 T-cell function may allow the virus to gain the upper hand.
Acknowledgments
This work was supported by NIH grant AI-42519 (J.L.), amfAR fellowships 70540–30-RF (M.N.I.), 70589–32-RF (SKL) and by the Case Western Reserve University Center for AIDS Research (AI-36219). We thank Miguel Lopez-Botet (Barcelona, Spain) for ILT2 blocking antibody (HP-F1), Chiron Oncology for rIL-2, Sandra Lee for suggestions in statistical analysis and Premlata Shankar, N. Manjunath, Xiaogang Gu and Ann Schlesinger for helpful suggestions.
References
- 1.Lieberman J, Shankar P, Manjunath N, Andersson J. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 2001;98:1667–77. doi: 10.1182/blood.v98.6.1667. [DOI] [PubMed] [Google Scholar]
- 2.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman J, Manjunath N, Shankar P. Avoiding the kiss of death: how HIV and other chronic viruses survive. Curr Opin Immunol. 2002;14:478–86. doi: 10.1016/s0952-7915(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 4.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–101. [PubMed] [Google Scholar]
- 5.Gea-Banacloche JC, Migueles SA, Martino L, et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–92. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 6.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–55. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trimble LA, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–94. [PubMed] [Google Scholar]
- 8.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray CM, Lawrence J, Schapiro JM, et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–8. [PubMed] [Google Scholar]
- 10.Stefanova I, Saville MW, Peters C, et al. HIV infection – induced posttranslational modification of T cell signaling molecules associated with disease progression. J Clin Invest. 1996;98:1290–7. doi: 10.1172/JCI118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trimble LA, Shankar P, Patterson M, Daily JP, Lieberman J. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-modulated CD3zeta and CD28, key signaling molecules for T-cell activation. J Virol. 2000;74:7320–30. doi: 10.1128/jvi.74.16.7320-7330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 13.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 14.De Maria A, Ferraris A, Guastella M, et al. Expression of HLA class I-specific inhibitory natural killer cell receptors in HIV-specific cytolytic T lymphocytes: impairment of specific cytolytic functions. Proc Natl Acad Sci USA. 1997;94:10285–8. doi: 10.1073/pnas.94.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiani MD, Aguado E, Tarazona R, Romero P, Molina I, Santamaria M, Solana R, Pena J. Expression of killer inhibitory receptors on cytotoxic cells from HIV-1-infected individuals. Clin Exp Immunol. 1999;115:472–6. doi: 10.1046/j.1365-2249.1999.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andre P, Brunet C, Guia S, Gallais H, Sampol J, Vivier E, Dignat-George F. Differential regulation of killer cell Ig-like receptors and CD94 lectin-like dimers on NK and T lymphocytes from HIV-1-infected individuals. Eur J Immunol. 1999;29:1076–85. doi: 10.1002/(SICI)1521-4141(199904)29:04<1076::AID-IMMU1076>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MJ, Torkar M, Haude A, Milne S, Jones T, Sheer D, Beck S, Trowsdale J. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA. 2000;97:4778–83. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huard B, Karlsson L. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement. Nature. 2000;403:325–8. doi: 10.1038/35002105. [DOI] [PubMed] [Google Scholar]
- 19.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–95. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 20.Halary F, Peyrat MA, Champagne E, et al. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–21. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 21.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 22.Brettle RP, McNeil AJ, Burns S, et al. Progression of HIV: follow-up of Edinburgh injecting drug users with narrow seroconversion intervals in 1983–85. AIDS. 1996;10:419–30. [PubMed] [Google Scholar]
- 23.Flores-Villanueva PO, Yunis EJ, Delgado JC, et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc Natl Acad Sci U S A. 2001;98:5140–5. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 25.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 26.Navarro F, Llano M, Bellon T, Colonna M, Geraghty DE, Lopez-Botet M. The ILT2 (LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells. Eur J Immunol. 1999;29:277–83. doi: 10.1002/(SICI)1521-4141(199901)29:01<277::AID-IMMU277>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozano JM, Gonzalez R, Kindelan JM, Rouas-Freiss N, Caballos R, Dausset J, Carosella ED, Pena J. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS. 2002;16:347–51. doi: 10.1097/00002030-200202150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Akridge RE, Oyafuso LK, Reed SG. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–9. [PubMed] [Google Scholar]
- 30.Mingari MC, Schiavetti F, Ponte M, et al. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc Natl Acad Sci USA. 1996;93:12433–8. doi: 10.1073/pnas.93.22.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabri B, Selby JM, Negulescu H, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–99. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 32.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–6. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein–Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–64. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 34.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [Google Scholar]
- 35.Sivori S, Vitale M, Sanseverino L, Parolini S, Barbaresi M, Bottino C, Moretta A. Inhibitory CD94 molecules identified by the Z199 monoclonal antibody recognize different HLA-class I molecules. Transplant Proc. 1996;28:3199–203. [PubMed] [Google Scholar]
- 36.Moretta A, Vitale M, Sivori S, et al. Human natural killer cell receptors for HLA-class I molecules. Evidence that the Kp43 (CD94) molecule functions as receptor for HLA-B alleles. J Exp Med. 1994;180:545–55. doi: 10.1084/jem.180.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pende D, Biassoni R, Cantoni C, et al. The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J Exp Med. 1996;184:505–18. doi: 10.1084/jem.184.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colonna M, Navarro F, Bellon T, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–18. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SK, Xu Z, Lieberman J, Shankar P. The functional CD8 T cell response to HIV becomes type-specific in progressive disease. J Clin Invest. 2002;110:1339–47. doi: 10.1172/JCI16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 42.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 43.Berg L, Riise GC, Cosman D, Bergstrom T, Olofsson S, Karre K, Carbone E. LIR-1 expression on lymphocytes, and cytomegalovirus disease in lung-transplant recipients. Lancet. 2003;361:1099–101. doi: 10.1016/S0140-6736(03)12855-3. [DOI] [PubMed] [Google Scholar]
- 44.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–41. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 45.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu ML. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–82. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 46.Young NT, Canavez F, Uhrberg M, Shum BP, Parham P. Conserved organization of the ILT/LIR gene family within the polymorphic human leukocyte receptor complex. Immunogenetics. 2001;53:270–8. doi: 10.1007/s002510100332. [DOI] [PubMed] [Google Scholar]
- 47.Saverino D, Fabbi M, Ghiotto F, et al. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol. 2000;165:3742–55. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- 48.Speiser DE, Valmori D, Rimoldi D, et al. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990–9. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Costa P, Rusconi S, Mavilio D, et al. Differential disappearance of inhibitory natural killer cell receptors during HAART and possible impairment of HIV-1-specific CD8 cytotoxic T lymphocytes. AIDS. 2001;15:965–74. doi: 10.1097/00002030-200105250-00004. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D, Shankar P, Xu Z, et al. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 2003;101:226–35. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514–21. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 52.Fuller CL, Braciale VL. Selective induction of CD8+ cytotoxic T lymphocyte effector function by staphylococcus enterotoxin B. J Immunol. 1998;161:5179–86. [PubMed] [Google Scholar]
- 53.Auphan-Anezin N, Verdeil G, Schmitt-Verhulst AM. Distinct thresholds for CD8 T cell activation lead to functional heterogeneity: CD8 T cell priming can occur independently of cell division. J Immunol. 2003;170:2442–8. doi: 10.4049/jimmunol.170.5.2442. [DOI] [PubMed] [Google Scholar]
- 54.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu X, Laouar A, Wan J, Daheshia M, Lieberman J, Yokoyama WM, Katz HR, Manjunath N. The gp49B1 inhibitory receptor regulates the IFN-gamma responses of T cells and NK cells. J Immunol. 2003;170:4095–101. doi: 10.4049/jimmunol.170.8.4095. [DOI] [PubMed] [Google Scholar]
- 56.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 57.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–9. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 58.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]