Abstract
Enteroaggregative Escherichia coli (EAEC) is an emerging enteric pathogen that causes acute and chronic diarrhoea in a number of clinical settings. EAEC diarrhoea involves bacterial aggregation, adherence to intestinal epithelial cells and elaboration of several toxigenic bacterial mediators. Flagellin (FliC-EAEC), a major bacterial surface protein of EAEC, causes interleukin (IL)-8 release from several epithelial cell lines. The host response to flagellins from E. coli and several other bacteria is mediated by Toll-like receptor 5 (TLR5), which signals through nuclear factor kappa B (NF-κB) to induce transcription of pro-inflammatory cytokines. p38 mitogen-activating protein (MAP) kinase (MAPK) is a member of a family of stress-related kinases that influences a diverse range of cellular functions including host inflammatory responses to microbial products. We studied the role of p38 MAPK in FliC-EAEC-induced IL-8 secretion from Caco-2 human intestinal epithelial cells and THP-1 human monocytic cells. We found that IL-8 secretion from both cell types is dependent on p38 MAPK, which is phospho-activated in response to FliC-EAEC. The role of TLR5 in p38 MAPK-dependent IL-8 secretion was verified in HEp-2 cells transiently transfected with a TLR5 expression construct. Activation of interleukin-1 receptor-associated kinase (IRAK) was also observed in Caco-2 and TLR5-transfected HEp-2 cells after exposure to FliC-EAEC. Finally, we demonstrated that pharmacological inhibition of p38 MAPK reduced IL-8 transcription and mRNA levels, but did not affect NF-κB activation. Collectively, our results suggest that TLR5 mediates p38 MAPK-dependent IL-8 secretion from epithelial and monocytic cells incubated with FliC-EAEC, and that this effect requires IL-8 promoter activation independent of NF-κB nuclear migration.
Keywords: bacterial infection; chemokines, inflammation; protein kinases; signal transduction
Introduction
Enteroaggregative E. coli (EAEC) is an emerging enteric pathogen that causes diarrhoea in various clinical settings. EAEC is primarily recognized as a cause of endemic and persistent childhood diarrhoea in developing areas. EAEC diarrhoea is frequently seen in children attending day-care, in travellers, and in immunocompromised persons in developed countries.1 EAEC diarrhoea in children is associated with increased levels of faecal lactoferrin, interleukin (IL)-8 and IL-1β.2 In addition, some international travellers with EAEC diarrhoea have increased IL-8 and IL-1β concentration in their stools.3 Elevated faecal IL-8 concentration has been described as a marker of inflammation in travellers who developed EAEC diarrhoea.4
We previously reported that the 65-kDa flagellin from EAEC strain 042 (FliC-EAEC) causes IL-8 release from Caco-2 cells and other intestinal epithelial cell lines.5 Subsequent work has shown that bacterial flagellins have pro-inflammatory and immunomodulatory activity in various experimental models, including triggering acute respiratory complications in experimental gram-negative bacterial sepsis.6,7 Most if not all of the responses to bacterial flagellin are believed to be mediated by Toll-like receptor (TLR) 5.8–10 Studies suggest that activation of TLRs by microbial products involves several important signal transduction molecules, including interleukin-1 receptor-associated kinase (IRAK), nuclear factor kappa B (NF-κB) and p38 mitogen activating protein (MAP) kinase (MAPK), ultimately leading to inflammatory cytokine production.11,12
Recent studies13,14 suggest that IL-8 secretion from intestinal epithelial cells in response to bacterial pathogens involves activation of p38 MAPK by flagellin. However, the effect of this activation and its importance in human epithelial cells remain unknown for EAEC flagellin. The objective of this study was to investigate the role of p38 MAPK in IL-8 secretion from Caco-2 human intestinal epithelial cells, HEp-2 human epithelial cells transiently expressing TLR5, and THP-1 human monocytic cells exposed to FliC-EAEC, in order to better characterize the complex signalling pathways involved in the host response to flagellin.
Materials and methods
Cell culture
Caco-2 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and grown in Dulbecco's modified Eagle's minimal essential medium (DMEM) with 4·5 g/l d-glucose, 1 × nonessential amino acids, 2 mm glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml) (Sigma, St Louis, MD), and 10% fetal bovine serum (FBS) (Hyclone, Logan, UT). Cells were seeded at high density in polystyrene culture dishes and used for experiments 5–7 days after becoming confluent. The monocytic cell line THP-1 was obtained from ATCC and cultured in RPMI 1640 supplemented with 10% FBS, 2 mm l-glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml). HEp-2 cells were maintained in Ham's F12 medium with penicillin (100 U/ml), streptomycin (100 µg/ml) and 5% FBS.
TLR5 transient expression in HEp-2 cells
pEF6/V5-His containing the full-length human TLR5 gene (phTLR5) was a gift from A. Aderem (University of Washington, Seattle, WA). pEGFP-N1 vector (Clontech, Palo Alto, CA) expressing the green fluorescent protein (GFP) was used as a transfection control. Prior to transfection, HEp-2 cells were released with 0·25% trypsin/EDTA and seeded at 105/well in 12-well polystyrene dishes (VWR International, West Chester, PA). After 24–48 hr, cells were transfected with 0·5 µg each of phTLR5 and pEGFP-N1 per well using 22 mm ExGen-500 polyethylenimine transfection reagent (MBI Fermentas; Burlington, ON, Canada) per well. Expression of GFP was confirmed at 48 hr by fluorescence microscopy.
Expression and purification of EAEC flagellin
The full-length gene encoding the EAEC flagellin fliC-EAEC with an N-terminal 6XHis tag5 was maintained in Top10F′ (Invitrogen, Groningen, the Netherlands). Recombinant FliC-EAEC was prepared by cobalt affinity chromatography as in Donnelly & Steiner,15 diluted in PBS, and stored at −20° until use. For treatment of HEp-2 and THP-1 cells, flagellin was purified free of lipopolysaccharide (LPS) by polymyxin B chromatography (Detoxi-Gel; Pierce, Rockford, IL). Flagellin thus prepared did not cause IL-8 release from untransfected HEp-2 cells, in contrast to crude preparations which release IL-8 from LPS-responsive HEp-2 cells.
Pharmacological inhibitors of signalling proteins
SB-203580 (SB) and Bay 11–7082 (Bay 11) (Sigma) were dissolved in dimethyl sulphoxide (DMSO) and stored in aliquots at − 20°. Caco-2 cells cultured in a 24-well plate were fed with fresh media and treated with inhibitors for 1 hr at 37°. FliC-EAEC (500 ng/ml) was then added to the media and incubated for 3 hr and IL-8 measured in the supernatant by ELISA (OptEIA IL-8; BD Biosciences, Mississauga, ON, Canada). 500 ng/ml was the smallest dose that reproducibly caused maximal IL-8 stimulation (data not shown). Undifferentiated THP-1 cells were transferred to six-well plates at a density of 2 × 106 cells/well, and 500 ng/ml of FliC-EAEC added. After 6 hr of incubation, the medium was collected and the cells pelleted by centrifugation at 500 g for 10 min, and the supernatant was used for measurement of IL-8 concentration.
Western blotting and immunoprecipitation
FliC-EAEC treatment of cells cultured in six-well plates for various times was followed by two washes, each with 2 ml of ice-cold Hank's Balanced Salt Solution (Sigma). Treated cells were then lysed in 250–500 µl of lysis buffer (50 mm Tris, pH 7·5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2·5 mm sodium pyrophosphate, 1 mmβ-glycero-phosphate, 1 mm Na3VO4, 1 µg/ml leupeptin and 1 mm PMSF) on ice for 5–10 min and then scraped into microcentrifuge tubes. The tubes were centrifuged at 15 000 g for 5 min to pellet debris, and the supernatant was transferred to another tube for Western blots and immunoprecipitation.
Caco-2 and HEp-2 proteins (50–100 µg in cleared cell lysate) were resolved by 9% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to 0·2 µm polyvinylidene fluoride (PVDF) membranes. Blots were then blocked for 1 hr with 5% nonfat milk in Tris-buffered saline (Tris 20 mm and NaCl 0·3 m, pH 7·4) with 0·05% Tween-20 (TBST). Primary antibodies used were as follows: rabbit antiphospho-p38 MAP kinase (Cell Signaling, Beverly, MA) 1 : 500, mouse anti-IRAK (BD Biosciences) 1 : 1000, and mouse antiphosphotyrosine primary antibody, clone 4G10 (gift from Dr Zakaria Hmama, Division of Infectious Diseases, University of British Columbia, Vancouver, BC, Canada) at a dilution of 1 : 10 000. Proteins were detected by enhanced chemiluminescence (ECL-Plus; Amersham, Piscataway, NJ).
For immunoprecipitation, 1 µg of rabbit anti-p38 MAPK (Cell Signaling) was added per 100 µg of protein and incubated overnight at 4° on a rotating platform. 50 µl of protein A-sepharose (Bio-Rad, Hercules, CA) was added to the sample and incubated at 4° for 1 hr on a rotating platform. Immune complexes were washed three times with cold lysis buffer, followed by three more washes with cold phosphate-buffered saline (PBS). The beads were then resuspended in 4 × gel loading buffer, boiled for 5 min and resolved by SDS-PAGE as indicated above.
Electrophoretic mobility shift assays
Caco-2 cells seeded in six-well plates were used at least 7 days after plating. Cells were fed with 1 ml fresh growth medium and incubated with signalling inhibitors for 60 min followed by FliC-EAEC or IL-1β for 30–60 min. Cells were rinsed with ice-cold PBS, scraped into PBS, pelleted by brief centrifugation, and resuspended in 400 µl lysis buffer (10 mm HEPES, 10 mm KCl, 0·1 mm EDTA, 0·1 mm EGTA, 1 mm dithiothreitol (DTT) and 0·1 mm PMSF at pH 7·9). After 15 min at 4°, 23 µl of 10% Nonidet P-40 was added and cells were placed on ice for 2 min. Nuclei were pelleted by brief centrifugation and then resuspended in 50 µl nuclear extract buffer [20 mm HEPES, 420 mm KCl, 1 mm EGTA, 1 mm EDTA, and 1 × protease inhibitor cocktail for mammalian cells (Sigma) at pH 7·9]. After 30 min incubation on ice, the debris was pelleted at 12000 g for 2 min, and the supernatant was flash-frozen in liquid nitrogen until testing. Supernatants were tested for protein concentration (Bio-Rad DC assay) and diluted in nuclear extract buffer to 1 mg/ml.
A double-stranded NF-κB-binding probe of sequence 5′-AGTTGAGGGGACTTTCCCAGGC-3′ was end-labelled with γ-32P-ATP using polynucleotide kinase (MBI Fermentas, Burlington, ON, Canada) and purified by Sephadex G-25 chromatography (Amersham) and ethanol precipitation. To measure NF-κB activity, a reaction mixture containing 5 µg of nuclear extract, 7·5 µl of binding buffer (20 mm HEPES, 50 mm KCl, 0·1 mm EDTA, 5% glycerol, 200 µg/ml bovine serum albumin, and 1 mm DTT, pH 7·9), and 10 ng of sheared salmon sperm DNA (Sigma) in 20 µl total volume was incubated for 20 min on ice, and 1 µl of labelled NF-κB probe added. Following a 15-min incubation at room temperature, reactions were separated by 4% nondenaturing PAGE, and gels were dried onto filter paper and examined by autoradiography. NF-κB activity was measured by delayed mobility, and specificity was verified by including a 100-fold excess of unlabelled NF-κB-binding oligonucleotide in some samples. Autoradiographs were scanned and densitometry performed using NIH Image software (National Institutes of Health, USA).
IL-8 promoter/reporter assays
Caco-2 cells were seeded at approximately 90% confluence in 12- or 24-well plates. After 24 hr, medium was changed to growth medium without penicillin or streptomycin, and cells were transfected with 100 ng/well of pEGFP-N1 and 400 ng/well of an IL-8 promoter/luciferase vector16 using Lipofectamine 2000 (Invitrogen) at a DNA:reagent ratio of 1 : 2–1 : 3. After 48 hr, cells were pretreated with inhibitors for 1 hr, and then stimulated with FliC-EAEC or IL-1β for 6 hr. Supernatants were removed and tested for IL-8 concentration, and cells were lysed with a 1 : 1 mixture of growth medium or PBS and Bright-Glo reagent (Promega). After 2 min of lysis, cells were read in a microplate luminometer (10-s integration). The lysates were recovered and GFP fluorescence measured in a Versa-Fluor Fluorometer (Bio-Rad). The ratio of luminescence to fluorescence in arbitrary units (to correct for cell number and transfection efficiency) was calculated for each sample, and the fold increase in this value compared to that for controls within the same experiment was defined as the fold increase in expression.
Quantitative PCR
Caco-2 cells were seeded in six-well plates and allowed to differentiate for at least 7 days. Cells were fed with fresh growth medium and inhibitors added for 1 hr, followed by FliC-EAEC or IL-1β for 1 hr. Total or cytoplasmic RNA was isolated (RNeasy mini kit; Qiagen, Mississauga, ON, Canada) and quantified by absorbance at 260 nm. Equal µg quantities of RNA were reverse-transcribed using oligo-dT as primer with Superscript II (Invitrogen). Quantitative real-time PCR was performed using Sybr Green PCR Master Mix (ABI Prism, Applied Biosystems, Foster City, CA) according to the manufacturer's instructions, with cDNA corresponding to 100 ng total RNA as template. Parallel reactions were set up using primer pairs specific for IL-8 (forward: 5′-ATGACTTCCAAGCTGGCCGTGGCT; reverse: 5′-TCTCAGCCCTCTTCAAAAACTTCTC) and β-actin (forward: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA; reverse: 5′-CTAGAAGCATTGCGGTGGACGATGGAGGG). Samples were analysed on an Opticon real-time PCR system (MJ Research, Waltham, MA). Generation of the appropriate PCR product was verified by melting curve analysis and electrophoresis. The cycle number to reach the standard threshold fluorescence was measured for each sample, and the difference between IL-8 cycle number and actin cycle number (dCT) was determined. The fold increase in IL-8 mRNA was then calculated as 1/2 raised to the power of the difference between the dCT for each sample and the dCT of untreated Caco-2 cells in each experiment (ddCT).
Statistical analysis
All results were expressed as mean ± standard error of mean (SEM) from at least three or more independent experiments. Statistical significance was calculated using Student's t-tests or one-way anova with Tukey HSD test. Densitometry for comparison of bands was performed using the Un-Scan-it software (Silk Scientific Inc., Orem, UT) or NIH Image. Background reading was subtracted before reading each band three times. Average values of each band were then used to compute differences.
Results
FliC-EAEC-induced IL-8 secretion from Caco-2 intestinal epithelial and THP-1 monocytic cells is dependent on p38 MAP kinase
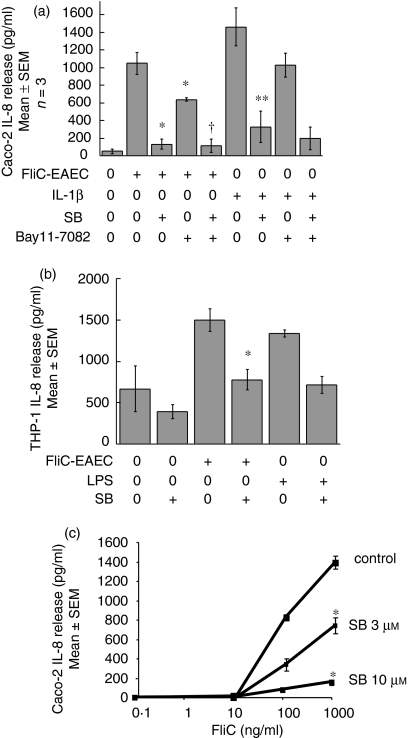
To determine whether p38 MAP kinase is involved in IL-8 secretion, Caco-2 cells were pretreated with the specific p38 MAP kinase inhibitor SB-203580 (SB), and then exposed to FliC-EAEC. As shown in Fig. 1(a), preincubation of Caco-2 cells with SB (10 µm) for 1 hr resulted in 90% inhibition of FliC-EAEC-induced IL-8 secretion (P < 0·05). Pre-treatment with an NF-κB inhibitor Bay 11-7082 (20 µm) resulted in approximately 50% inhibition of IL-8 secretion. Similarly, pretreatment of Caco-2 cells with either SB or Bay 11 caused inhibition of IL-1β-induced IL-8 secretion. The concentrations of Bay 11 and SB used above were not found to be toxic, as confirmed by trypan blue staining of Caco-2 cells after 6 hr of treatment with the inhibitors (data not shown). Moreover, SB and Bay 11 alone did not cause IL-8 release. Similar results were observed in THP-1 cells, where LPS was used as a positive control for IL-8 release (Fig. 1b).
Figure 1.
(a) Effect of p38 MAP kinase and NF-κB inhibition on FliC-EAEC-induced IL-8 secretion from Caco-2 cells. Cells pretreated for 1 hr with SB-203 580 (10 µm) and/or Bay 11-7082 (20 µm) were exposed to FliC-EAEC (500 ng/ml) or IL-1β (10 ng/ml) for 3 hr. Supernatants were analysed for IL-8 by ELISA. Overall P < 0·001 by anova; *P < 0·01 vs. FliC alone (Tukey HSD); †P = not significant vs. FliC + SB; **P < 0·01 vs. IL-1β alone. (b) Effect of p38 inhibition on FliC-induced IL-8 release from THP-1 cells. Doses used: FliC-EAEC (500 ng/ml); LPS (10 µg/ml); SB (10 µm). Overall P < 0·005 by anova; *P < 0·05 vs. FliC alone. (c) Dose–response of the effect of p38 inhibition on FliC-induced IL-8 release from Caco-2 cells. *P < 0·05 vs. control (Student's t-test).
As shown in Fig. 1(c), 3 µm SB caused approximately 50% inhibition (P < 0·05) of 100 ng/ml and 1000 ng/ml FliC-EAEC-induced IL-8 secretion, while 90% inhibition was seen with 10 µm SB (P < 0·05). The magnitude of inhibition seen in this experiment is consistent with the data in Fig. 1(a).
FliC-EAEC activates p38 MAP kinase in Caco-2 and THP-1 cells
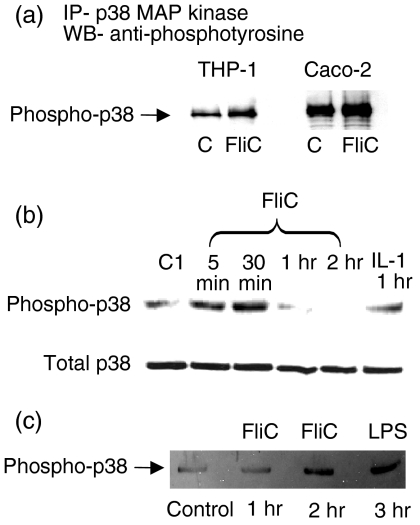
To determine if p38 MAP kinase was activated by FliC-EAEC, we immunoprecipitated lysates from FliC-EAEC-treated THP-1 and Caco-2 cells with p38 MAP kinase antibody overnight and then subjected immunoprecipitates to Western blots using an antiphosphotyrosine antibody. As shown in Fig. 2(a), exposure of THP-1 and Caco-2 cells to FliC-EAEC (500 ng/ml) for 30 min induced tyrosine phosphorylation of p38 MAP kinase when compared to untreated control samples. Densitometry confirmed that these results were statistically significant (THP-1 cells: control = 27·14 ± 5·63 density units vs. FliC = 54·95 ±10·10, P < 0·05; Caco-2 cells: control = 34·75 ± 6·75 vs. FliC =71·05±12·07, P<0·05). Subsequently we studied the time dependence of activation of p38 MAP kinase in Caco-2 cells using an antibody that recognizes the dual phosphorylation of p38 MAP kinase at Thr-180 and Tyr-182 (recognized as a marker of p38 enzyme activation). Figure 2(b) shows that maximal activation of p38 MAP kinase occurred after 30 min of incubation with 500 ng/ml of FliC-EAEC (25·67 ± 8·22 density units vs. 82·56 ±6·44, P < 0·05, for control vs. FliC-EAEC, respectively). All samples in this experiment contained equal protein concentrations in cell lysates. Similar results were obtained in THP-1 cells with LPS used as a positive control for p38 activation (Fig. 2c) (25·05 ± 7·36 density units vs. 80·75 ± 9·56, P < 0·05, for control vs. FliC-EAEC, respectively).
Figure 2.
(a) Activation of p38 MAP kinase in Caco-2 and THP-1 cells incubated with FliC-EAEC. Cells were treated with FliC-EAEC (500 ng/ml) for 30 min. Cell lysates (500 µg protein/sample) were immunoprecipitated with rabbit phospho-p38 MAP kinase primary antibody, resolved by SDS-PAGE and probed with monoclonal antiphosphotyrosine antibody (4G10) in Western blots. (b)Time course of p38 MAP kinase activation in Caco-2 cells treated with FliC-EAEC. Caco-2 cells were incubated with FliC-EAEC (500 ng/ml) or IL-β (10 ng/ml) for the times indicated. Equal amounts of protein (50 µg/sample) were analysed by Western blots using a rabbit anti-phospho-p38 MAP kinase primary antibody. Blots were then stripped and re-probed with rabbit p38 MAP kinase primary antibody.(c)FliC-EAEC activates p38 MAP kinase in THP-1 cells. Undifferentiated THP-1 cells (2 × 106 cells/sample) were incubated with FliC-EAEC (500 ng/ml) or LPS (1 µg/ml) for the times indicated. Equal amounts of protein (50 µg) from cell lysates were analysed by Western blots using rabbit anti-phospho-p38 MAP kinase primary antibody. Equal loading of proteins was confirmed by India ink staining of membranes.
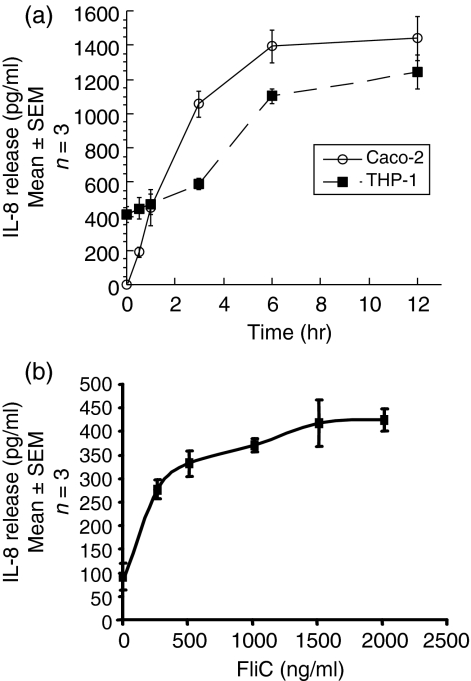
The time course of IL-8 secretion in response to flagellin was studied in Caco-2 and THP-1 cells. As shown in Fig. 3(a), IL-8 secretion from THP-1 cells increased rapidly between 3 and 6 hr, reaching an apparent plateau at 12 hr. Caco-2 cells followed a similar time pattern, although their kinetics were somewhat faster and basal IL-8 production was negligible. Near-maximal IL-8 secretion was reached in 6 hr for THP-1 cells and 3 hr for Caco-2 cells; these time points were therefore selected for measuring IL-8 secretion. At 6 hr of incubation, 250–1500 ng/ml of FliC-EAEC caused a dose-dependent increase in IL-8 secretion from THP-1 cells (Fig. 3b; P < 0·001, anova). The differences in basal IL-8 expression between THP-1 cells in the experiments in Figs 3(a) and (b) may be attributable to differences in passage number of the cells; however, similar passage numbers were used for replicates within each experiment individually. The reason for the difference in the time dependence of FliC-EAEC-induced p38 MAP kinase activation and maximal IL-8 secretion between THP-1 and and Caco-2 cells is not known.
Figure 3.
(a) Time course of FliC-EAEC-induced IL-8 secretion from Caco-2 and THP-1 cells. Undifferentiated THP-1 cells (2 × 106 cells/well) in six-well plates and differentiated Caco-2 cells in 24-well plates were incubated with FliC-EAEC (500 ng/ml) for the times indicated. IL-8 was measured by ELISA.(b)Dose–response of FliC-EAEC-induced IL-8 secretion from THP-1 cells. Undifferentiated THP-1 cells (2 × 106 cells/well) in six-well plates were incubated for 6 hr with increasing doses FliC-EAEC as indicated. IL-8 was measured by ELISA. IL-8 was significantly higher than control for all measured doses of flagellin (P < 0·01, anova).
Transient expression of TLR5 in HEp-2 cells enables p38 MAP kinase-dependent IL-8 secretion in response to FliC-EAEC
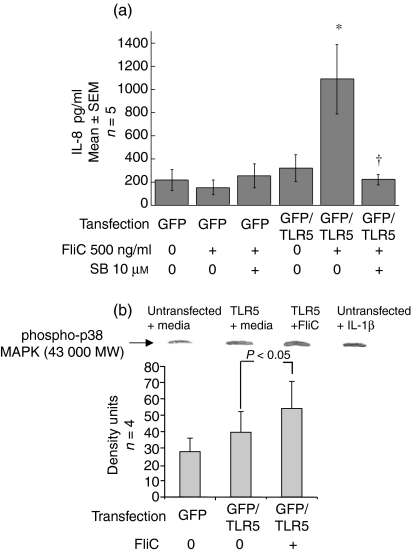
To determine if TLR5 is necessary for p38 MAP kinase activation by FliC-EAEC, HEp-2 cells, which normally express little or no TLR5 and do not secrete IL-8 in response to flagellin, were transiently transfected to express TLR5. Exposure to FliC-EAEC (500 ng/ml) for 3 hr did not increase IL-8 secretion over control levels in untransfected cells. However, TLR5-transfected HEp-2 cells secreted significant amounts of IL-8 following FliC-EAEC treatment (500 ng/ml), as shown in Fig. 4(a). Pre-incubation with SB (10 µm) completely abrogated this IL-8 response to FliC-EAEC, as indicated. The concentration of IL-8 measured in supernatants from HEp-2 cells incubated with SB alone was similar to that of untransfected, control samples. Moreover, as shown in Fig. 4(b), incubation of TLR5-transfected HEp-2 cells with FliC-EAEC (500 ng/ml) for 1 hr resulted in a significant increase in phospho-activated p38 MAP kinase (P < 0·05). In comparison, IL-1β, but not FliC-EAEC, activated p38 MAPK in untransfected HEp-2 cells (densitometry not shown).
Figure 4.
(a) Inhibition of FliC-EAEC-induced IL-8 secretion from TLR5-transfected HEp-(a) 2 cells. Untransfected and TLR5-transfected HEp-2 cells were preincubated with DMSO or SB-203 580 (10 µm) for 1 hr and then treated with FliC-EAEC (500 ng/ml) for 3 hr. Transfection efficiency was 70–80% as verified by fluorescent microscopy to detect GFP. IL-8 was measured by ELISA. Overall P < 0·005 by anova. *P < 0·05 vs. untransfected HEp-2 cells treated with FliC and vs. TLR5-transfected cells with media alone. †P < 0·05 vs. TLR5-transfected cells treated with FliC alone. (b) Activation of p38 MAP kinase in TLR5-transfected HEp-2 cells exposed to FliC-EAEC. Transiently transfected HEp-2 cells were incubated with media alone or FliC-EAEC (500 ng/ml) for 1 hr and were analysed by Western blots with rabbit anti-phospho-p38 MAP kinase antibody. Controls included untransfected HEp-2 cells treated with media alone or with IL-1β (10 ng/ml). Equal protein loading was verified by India ink staining. Bands were compared by densitometry and analysed by t-test.
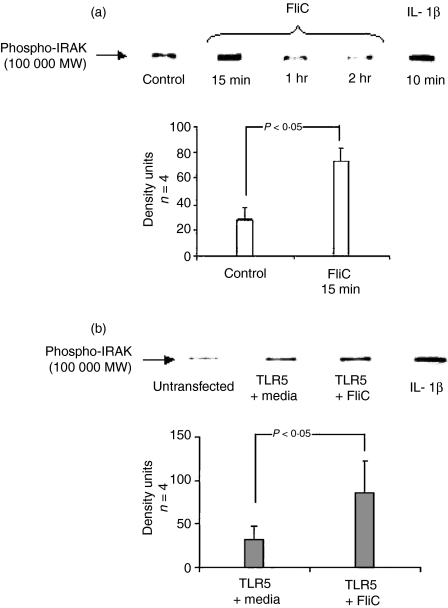
FliC-EAEC activates IRAK in Caco-2 cells and TLR5-transfected HEp-2 cells
Phosphorylation and subsequent degradation of IRAK-1 are an early signal response to TLR ligation. As part of the confirmation that TLR5-dependent pathways are required for the flagellin responses we have observed, we elected to measure activation of IRAK in Caco-2 cells and TLR5-transfected HEp-2 cells by Western blot using a primary rabbit anti-phosphoIRAK antibody. Incubation of Caco-2 cells for 15 min with FliC-EAEC (500 ng/ml) or IL-1β (10 ng/ml) for 10 min resulted in activation of IRAK, as shown in Fig. 5(a), where an intense band of 100 kDa was detected. After 2 hr of stimulation with FliC-EAEC, there was approximately a 50% reduction of the IRAK signal relative to control, reflecting degradation, a recognized consequence of IRAK activation. Similarly, IRAK activation was seen in TLR5-transfected HEp-2 cells following FliC-EAEC (500 ng/ml) exposure for 15 min (Fig. 5b). Compared to untreated TLR5-transfected cells, an approximately 3-fold increase of IRAK activation was noted in TLR5-transfected HEp-2 cells treated with FliC-EAEC. IRAK activation was also seen in untransfected HEp-2 cells exposed to IL-1β (10 ng/ml) for 15 min. IRAK phosphoactivation was not inhibited by SB (data not shown).
Figure 5.
(a) Activation of IRAK by FliC-EAEC in Caco-2 cells. Cells were treated with FliC-EAEC (500 ng/ml) or IL-1β (20 ng/ml) for the times indicated. Equal amounts of protein (50 µg/sample) were separated by SDS-PAGE and probed in Western blots using mouse anti-phospho-IRAK antibody. Equal loading of proteins was confirmed by India ink staining of membranes. Bands were compared by densitometry.(b) IRAK activation in TLR5-transfected HEp-2 cells treated with FliC-EAEC. Wild-type or TLR5-transfected HEp-2 cells were exposed to FliC-EAEC (500 ng/ml) or IL-1β (10 ng/ml) for 15 min. Cell lysates (50 µg protein/sample) were probed by Western blot using mouse anti-phospho-IRAK primary antibody. Equal loading of proteins was confirmed by India ink staining of membranes. Bands were compared by densitometry.
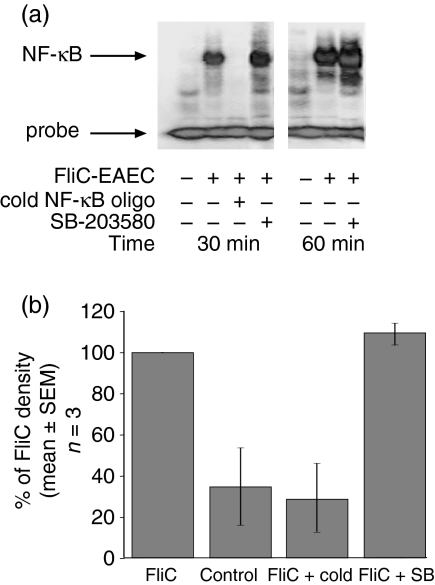
FliC-EAEC activation of NF-κB is not dependent on p38 MAPK activity
Caco-2 cells treated with FliC-EAEC for 30–60 min demonstrated a large increase in NF-κB activation as measured by electrophoretic mobility shift assay (EMSA)(Fig. 6a). In order to determine whether p38 activity is required upstream of NF-κB activation, we pretreated Caco-2 cells with SB for 1 hr prior to FliC stimulation. As shown in Fig. 6, pretreatment of cells with 10 µm SB did not reduce NF-κB activation, suggesting that the effect of this inhibitor on IL-8 release is occurring downstream of NF-κB.
Figure 6.
(a) Electrophoretic mobility shift assay to measure NF-κB nuclear migration in Caco-2 cells. Cells were pretreated with SB-203580 or DMSO vehicle for 1 hr prior to addition of 1 µg/ml FliC-EAEC for 30 or 60 min. Nuclear extracts were purified, bound to radiolabelled NF-κB-binding oligonucleotide, and separated by PAGE. Bands corresponding to unbound and NF-κB-bound probe are shown. Results are representative of three experiments. (b) Densitometric measurement on autoradiographs of NF-κB-shifted bands from three separate experiments.
SB inhibits FliC-EAEC-induced IL-8 promoter activation
The effects of p38 MAPK on IL-8 transcription may occur through several different mechanisms, including NF-κB nuclear migration, unmasking of NF-κB DNA-binding sites by histone modification, and activation of the AP-1 transcription factor. In order to determine whether the inhibitory effect of SB on FliC-EAEC-induced IL-8 release is caused by blockade of transcriptional activation, we measured the effects of SB on expression of luciferase driven by a native IL-8 promoter in transiently transfected Caco-2 cells. These cells are poorly transfectable once they have reached the confluent, differentiated state at which IL-8 release in response to FliC is maximal. However, we were able to achieve a transfection efficiency of approximately 5% in cells when they were barely subconfluent (as determined by green fluorescence), and, by waiting 48 hr after transfection, we obtained cells that remained transiently transfected but had reached a state of increased (albeit submaximal) FliC-EAEC responsiveness as measured by IL-8 release. Using this transient transfection system, we found that FliC-EAEC causes about a 3-fold increase in luciferase expression compared to media alone, vs. a 6-fold increase in response to IL-1β(Fig. 7). Pretreatment of Caco-2 cells with SB reduced this activation by about half, and pretreatment with SB and Bay 11 together completely abrogated the transcriptional activation. These results suggest that at least part of the inhibitory effect of SB on IL-8 release is occurring at the transcriptional level. Interestingly, Bay 11 alone had only a modest inhibitory effect on IL-8 release, which, together with the observations in Fig. 1(a), suggests that NF-κB activation is of relatively minor importance in flagellin signalling.
Figure 7.
IL-8 transcriptional activation in Caco-2 cells transiently transfected with GFP and IL-8 promoter/luciferase reporter vectors. Cells were pretreated with the inhibitors shown for 1 hr (SB: 10 µm; Bay 11-7082: 20 µm) followed by 6 hr of incubation with FliC-EAEC (500 ng/ml) or IL-1β (10 ng/ml). Luciferase activity (RLU) and green fluorescence (RFU) were measured sequentially in cell lysates and the ratios calculated as a measure of promoter activity per number of transfected cells. The fold increase in RLU/RFU vs. mean control values in each individual experiment is shown. Results were compiled from three separate experiments. A significant difference among the FliC-treated samples was verified by anova (P < 0·001), and individual samples were compared by t-test. *P < 0·001 vs. control. **P < 0·05 vs. FliC-EAEC.
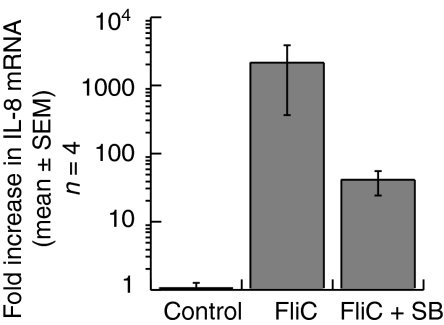
IL-8 mRNA in FliC-EAEC-treated Caco-2 cells is reduced by SB
We next used quantitative, real-time RT-PCR to determine whether the transcriptional activation of the IL-8 promoter leads to a measurable increase in IL-8 mRNA concentrations in Caco-2 cells. Differentiated Caco-2 cells were pretreated with SB 10 µm or vehicle followed by FliC-EAEC for 1 hr, and cytoplasmic RNA was isolated. IL-8 mRNA was then measured by real-time RT-PCR to quantify the effects of SB on IL-8 mRNA compared to untreated cells. Reverse-transcribed samples were amplified in parallel using IL-8- and actin-specific primers, and the difference in threshold cycle number between samples and control (untreated Caco-2 cells) was calculated to determine the fold increase in IL-8 mRNA relative to the control. As shown in Fig. 8, FliC-EAEC dramatically increased IL-8 mRNA after 1 hr in Caco-2 cells (fold increase of 2100 ± 1744). In contrast, Caco-2 cells pretreated with SB prior to FliC-EAEC had a fold increase of IL-8 mRNA of 40 ± 15·6. Within individual experiments, the reduction in IL-8 mRNA with SB treatment compared to FliC-EAEC alone was 41·5 ± 21·6% (P < 0·05; paired t-test). This finding is consistent with the amount of transcriptional inhibition described above.
Figure 8.
Quantitative real-time RT-PCR of Caco-2 cells treated for 1 hr with FliC-EAEC (500–2000 ng/ml) with or without 1 hr of pretreatment with SB-203 580 (10 µm). The fold increase in IL-8 mRNA relative to untreated Caco-2 cells was calculated for each experiment based on internal actin controls as discussed in ‘Materials and methods’.
Discussion
Intestinal epithelial cells respond to many inflammatory stimuli by secretion of pro-inflammatory cytokines and chemokines such as IL-8.5,17 The resulting inflammation may play an important role both in protecting against enteric infections and in producing many of the symptoms. EAEC infection, in particular, is associated with elevations in faecal inflammatory markers, even in the absence of diarrhoea.2 Because EAEC are noninvasive, this inflammation is probably caused by stimulation of gut immune responses by soluble bacterial factors. We previously demonstrated that the EAEC flagellin is a very potent trigger of IL-8 release from intestinal epithelial cells in vitro, and that nonflagellated EAEC do not cause IL-8 release.2 Thus, flagellin may play a crucial role in the clinical syndrome of EAEC infection. The subsequent identification of flagellin as the activator of TLR59 has led to studies of the immune response to flagellin in the pathogenesis of many other bacterial diseases. In particular, flagellin-induced TLR5 activation has been implicated in salmonellosis18 and legionellosis.19
MAP kinase pathways are known to regulate IL-8 secretion during the host immune response to bacterial products in certain experimental systems.20,21 Some of these effects are mediated through TLRs, which can signal through NF-κB and p38 MAP kinase to induce pro-inflammatory cytokine secretion.11,12 In this study we hypothesized that FliC-EAEC-induced IL-8 secretion is mediated by TLR5 and depends on p38 MAP kinase activation. Initially, we studied the effect of pharmacological inhibition of p38 MAP kinase and NF-κB on FliC-EAEC-induced IL-8 secretion from Caco-2 cells using their specific inhibitors SB-203580 and Bay 11-7082, respectively.22 Next, we measured p38 MAPK activation, which involves dual phosphorylation of threonine and tyrosine residues at a critical site,23 by immunoprecipitation and Western blotting. We also demonstrated IRAK phosphorylation and degradation following FliC-EAEC treatment of Caco-2 cells and TLR5-transfected HEp-2 cells, as an early signalling response to TLR5 activation. Finally, we studied p38 MAPK regulation of IL-8 gene expression to demonstrate that p38 MAPK is involved in transcriptional activation of the IL-8 gene, leading to increases in IL-8 mRNA.
Caco-2 cells are a useful model for flagellin responses because of their low basal IL-8 expression and their lack of responsiveness to LPS. These and other epithelial cells do respond to flagellin.24 We found that, in Caco-2 cells, pretreatment with SB prevented IL-8 release caused by FliC-EAEC, whereas pretreatment with Bay 11-7082 produced only a 50% reduction of IL-8 release. This is an interesting finding given that NF-κB has been proposed as a central mediator in TLR signalling pathways and as a critical element in the development of innate and adaptive immune responses in the host.9,25 In fact, the potent inhibition of IL-8 release by SB was accompanied by a slight increase in NF-κB activation as shown by EMSA. This effect, which could be attributable to nonspecific ERK activation by SB,26 again points to a limited role for NF-κB in flagellin-induced IL-8 expression.
There are several possible explanations for this. First, the IL-8 promoter contains binding sites for several other transcriptional activators, including nuclear factor (NF)-IL-6, AP-1, and CAAT-enhancer-binding protein (c/EBP).27 Thus, it is not surprising that IL-8 expression in response to flagellin is partly NF-κB-independent. Moreover, p38 MAPK has been shown to regulate IL-8 protein secretion from mammalian cells through several different mechanisms, including AP-1 activation, phosphorylation of histone proteins leading to unmasking of NF-κB response elements in the IL-8 promoter28 and stabilization of IL-8 mRNA, facilitating protein expression.20
Our results suggest that more than one of these mechanisms may be involved in flagellin-induced IL-8 release. This is consistent with a recent study by Parhar et al. that found that IL-1β-induced IL-8 secretion and expression are regulated by p38 MAP kinase activation via an effect on the IL-8 promoter in Caco-2 cells.16 Of note, Parhar et al. observed no effect of SB on IL-1β activation of an NF-κB-luciferase reporter construct, again suggesting that, like flagellin, IL-1β-induced IL-8 transcriptional regulation by p38 is largely NF-κB independent. However, our observation that the effect of p38 inhibition on IL-8 protein secretion was larger than its effect on IL-8 mRNA suggests that additional pathways might be involved in flagellin-induced IL-8 production.
Yu et al.14 recently showed that Salmonella flagellin causes p38 MAPK activation in polarized T84 cell monolayers and in HeLa cells transiently transfected with a TLR5 expression vector. They also reported that p38 MAPK inhibition with SB blocked flagellin-induced IL-8 expression from epithelial monolayers with no appreciable effect on NF-κB or IRAK activation. In contrast to our results, however, they found a much more modest reduction in IL-8 mRNA following SB treatment, and therefore concluded that p38 MAPK has a cryptic post-transcriptional effect on flagellin-induced IL-8 release. There are several possible explanations for the discrepancies between these results and those reported here. First, we measured IL-8 mRNA at 60 min; in contrast, Yu et al. measured IL-8 mRNA at 90 and 120 min (and found less mRNA at the latter time point). Their measurements may therefore have been taken beyond the time of maximal SB inhibition. Secondly, Yu et al. did not address IL-8 promoter activation, so that our results provide significant new information about how p38 regulates IL-8 expression. Finally, they performed experiments in epithelial cells grown on semipermeable filters, which induces more enterocytic differentiation than cells attain when grown in culture dishes. This produces physiology in many ways resembling that of the intact small intestinal epithelium, and might therefore alter the cellular inflammatory responses in comparison to nonpolarized cells. However, the regulation of IL-8 expression in polarized vs. nonpolarized Caco-2 cells has not been thoroughly studied.
In addition to Caco-2 cells, we elected to study flagellin effects on THP-1 cells. This model is relevant to EAEC and other intestinal pathogens, because the gut-associated lymphoid tissue contains resident macrophages, which recognize microbial products that interact with the intestinal epithelial barrier.29 Phorbol 12-myristate 13-acetate (PMA)- or vitamin D3-differentiated THP-1 cells are useful models for studying these host–microbial interactions.30 However, differentiation with either PMA or vitamin D3 induces IL-8 release from THP-1 and proximal tubular epithelial cells28,31 and activates MAP kinases.32 Undifferentiated THP-1 cells are also known to express TLR5.33 We therefore decided to use undifferentiated THP-1 cells to study FliC-EAEC responses. IL-8 release from THP-1 cells in response to flagellin has not previously been shown, although IRAK activation by Salmonella enteridis flagellin has been previously described in this cell line,34 consistent with the involvement of TLR signalling pathways.
In this study, we report that undifferentiated THP-1 cells release IL-8 in response to FliC-EAEC in a time- and dose-dependent manner. As in Caco-2 cells, IL-8 secretion by FliC-EAEC in THP-1 cells was attenuated by pretreatment with SB. It is tempting to speculate that flagellin-induced, p38 MAP kinase-dependent cytokine production in monocytes may contribute to the pathogenesis of bacterial infections in humans. Moreover, undifferentiated THP-1 cells express low amounts of CD14 in comparison to PMA or D3 differentiated THP-1 cells.30 Therefore, our findings in undifferentiated THP-1 cells incubated with FliC-EAEC suggest that, unlike LPS, FliC-EAEC-induced IL-8 secretion is possible with low CD14 expression.
Next, we asked whether FliC-EAEC-induced p38 MAP kinase activation is dependent on TLR5 in epithelial cells. To answer this, we performed transient transfection of HEp-2 cells with TLR5 and examined the responses to FliC-EAEC exposure after 48 hr of transfection. We previously reported that transient expression of TLR5 in HEp-2 cells confers IL-8 secretion upon exposure to FliC-EAEC.15 In this study we expand on those results to show that activation of p38 MAP kinase by FliC-EAEC is mediated by TLR5 in HEp-2 cells. The requirement for TLR5 in FliC-EAEC responses is further supported by the observation that FliC-EAEC treatment of Caco-2 cells and TLR5-transfected HEp-2 cells led to IRAK activation, followed by its degradation.
In summary, these results and those of others demonstrate that flagellin, through its activation of TLR5, leads to a signalling cascade that activates both p38 MAPK and NF-κB, leading to IL-8 production. Furthermore, we demonstrate that flagellin-induced activation of p38, but not NF-κB, is required for maximal IL-8 transcription and mRNA production in intestinal epithelial cells. These findings raise significant questions about the paradigm of NF-κB as the final common mediator of Toll-like receptor activation, and provide an additional mechanism whereby cells can differentially recognize and respond to various microbial products.
Acknowledgments
This work was supported by a Burroughs-Wellcome Fund Career Award in the Biomedical Sciences grant #992840 and a Canada Foundation for Innovation New Opportunities Award #4350 to T.S.S.
References
- 1.Okeke IN, Nataro JP. Enteroaggregative Escherichia coli. Lancet Infect Dis. 2001;1:304–13. doi: 10.1016/S1473-3099(01)00144-X. [DOI] [PubMed] [Google Scholar]
- 2.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Z, Okhuysen P, Gho D, Milewicz D, Dupont H. 40th Annual Meeting of the Infectious Diseases Society of America. Chicago: Infectious Diseases Society of America; 2002. A single nucleotide polymorphism in the interleukin-8 (IL-8) promoter region is associated with diarrhea due to enteroaggregative Escherichia coli (EAEC) p. 44. [Google Scholar]
- 4.Greenberg DE, Jiang ZD, Steffen R, Verenker MP, DuPont HL. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J Infect Dis. 2002;185:944–9. doi: 10.1086/339617. [DOI] [PubMed] [Google Scholar]
- 5.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest. 2000;105:1769–77. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liaudet L, Szabo C, Evgenov OV, et al. Flagellin from gram-negative bacteria is a potent mediator of acute pulmonary inflammation in sepsis. Shock. 2003;19:131–7. doi: 10.1097/00024382-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C. Role of flagellin in the pathogenesis of shock and acute respiratory distress syndrome: therapeutic opportunities. Crit Care Med. 2003;31:S39–45. doi: 10.1097/00003246-200301001-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed tlr5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 10.Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by gram-negative flagellin involves signaling via heteromeric toll-like receptor 5/toll-like receptor 4 complexes. J Immunol. 2003;170:6217–23. doi: 10.4049/jimmunol.170.12.6217. [DOI] [PubMed] [Google Scholar]
- 11.Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–40. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 12.Imler JL, Hoffmann JA. Toll and Toll-like proteins: an ancient family of receptors signaling infection. Rev Immunogenet. 2000;2:294–304. [PubMed] [Google Scholar]
- 13.Berin MC, Darfeuille-Michaud A, Egan LJ, Miyamoto Y, Kagnoff MF. Role of EHEC O157: H7 virulence factors in the activation of intestinal epithelial cell NF-kappaB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 2002;4:635–48. doi: 10.1046/j.1462-5822.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Zeng H, Lyons S, Carlson A, Merlin D, Neish AS, Gewirtz AT. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via a post-transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;17:17. doi: 10.1152/ajpgi.00503.2002. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly MA, Steiner TS. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J Biol Chem. 2002;277:40456–61. doi: 10.1074/jbc.M206851200. [DOI] [PubMed] [Google Scholar]
- 16.Parhar K, Ray A, Steinbrecher U, Nelson C, Salh B. The p38 mitogen-activated protein kinase regulates interleukin-1beta-induced IL-8 expression via an effect on the IL-8 promoter in intestinal epithelial cells. Immunology. 2003;108:502–12. doi: 10.1046/j.1365-2567.2003.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahan S, Busuttil V, Imbert V, Peyron JF, Rampal P, Czerucka D. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-kappaB and AP-1 in T84 cells. Infect Immun. 2002;70:2304–10. doi: 10.1128/IAI.70.5.2304-2310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–74. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 19.Hawn TR, Verbon A, Lettinga KD, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198:1563–72. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 21.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 22.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 24.Smith KD, Ozinsky A. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr Top Microbiol Immunol. 2002;270:93–108. doi: 10.1007/978-3-642-59430-4_6. [DOI] [PubMed] [Google Scholar]
- 25.Ruland J, Mak TW. Transducing signals from antigen receptors to nuclear factor kappaB. Immunol Rev. 2003;193:93–100. doi: 10.1034/j.1600-065x.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 26.Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol. 2000;131:99–107. doi: 10.1038/sj.bjp.0703534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–64. [PubMed] [Google Scholar]
- 28.Wilson L, Butcher CJ, Kellie S. Calcium ionophore A23187 induces interleukin-8 gene expression and protein secretion in human monocytic cells. FEBS Lett. 1993;325:295–8. doi: 10.1016/0014-5793(93)81092-e. [DOI] [PubMed] [Google Scholar]
- 29.Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 30.Hmama Z, Nandan D, Sly L, Knutson KL, Herrera-Velit P, Reiner NE. 1alpha,25-dihydroxyvitamin D (3)-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J Exp Med. 1999;190:1583–94. doi: 10.1084/jem.190.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger S, Kreft B. 1,25-dihydroxyvitamin D3 differentially regulates IL-1alpha-stimulated IL-8 and MCP-1 mRNA expression and chemokine secretion by human primary proximal tubular epithelial cells. Exp Nephrol. 2001;9:223–8. doi: 10.1159/000052615. [DOI] [PubMed] [Google Scholar]
- 32.Monick MM, Mallampalli RK, Carter AB, Flaherty DM, McCoy D, Robeff PK, Peterson MW, Hunninghake GW. Ceramide regulates lipopolysaccharide-induced phosphatidylinositol 3-kinase and Akt activity in human alveolar macrophages. J Immunol. 2001;167:5977–85. doi: 10.4049/jimmunol.167.10.5977. [DOI] [PubMed] [Google Scholar]
- 33.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 34.Moors MA, Li L, Mizel SB. Activation of interleukin-1 receptor-associated kinase by gram-negative flagellin. Infect Immun. 2001;69:4424–9. doi: 10.1128/IAI.69.7.4424-4429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]