Abstract
While effective for the prevention and treatment of allergic rhinitis (AR) symptoms, currently available medications do not reverse allergen specific hypersensitivities. Therefore, pharmacotherapeutics are not curative and their daily use is often required for years. These investigations were conducted to determine whether immunostimulatory sequence oligodeoxynucleotide (ISS-ODN) delivery protects previously sensitized mice from AR hypersensitivity responses and modulates their allergen specific immune profiles. Mice were first sensitized with ovalbumin (OVA) and alum, twenty-four hr before beginning a series of seven daily intranasal (i.n.) allergen challenges, subsets of mice received a single i.n. or intradermal (i.d.) dose of ISS-ODN or control oligodeoxynucleotide (C-ODN), a single intraperitoneal (i.p.) injection of dexamethasone (DXM), or no intervention. Mice receiving i.d. or i.n. ISS-ODN were found to have attenuated immediate and late phase effector cell responses to i.n. OVA challenge. Specifically, ISS-ODN treated mice had less histamine and cysteinyl leukotriene release and eosinophilic inflammation in their nasal passages than mice treated with C-ODN. In addition, splenocytes from ISS-ODN but not C-ODN treated mice displayed attenuated OVA-specific interleukin (IL)-4, IL-5, and IL-13 but increased interferon-γ responses. Finally, ISS-ODN was generally a more effective treatment than DXM, both in blunting AR hypersensitivity responses and in shifting T helper 2 Th2-biased immune parameters towards Th1 dominance. As ISS-ODN delivery rapidly attenuated effector cell responses in this AR model in an allergen independent manner, the present results suggest that therapy with ISS-ODN alone may be an effective alternative to corticosteroid medications for the clinical management of AR.
Keywords: immunostimulatory sequence DNA, CpG motif, immunotherapy, immunomodulation, allergic rhinitis
Introduction
Prevalence estimates suggest that seasonal AR occurs in 10% and perennial AR in 10–20% of individuals living in industrialized countries and for children, prevalence rates may be as high as 40%.1 Such figures suggest that AR is one of the most common chronic diseases in the industrialized world. Moreover, the economic burden and morbidity associated with AR are substantial. In 1996, AR treatment costs in America alone were estimated at 5·9 billion dollars.2 Poorly controlled AR is also a major risk factor for sinus infections and may contribute to the development of asthma and to exacerbations in those who already have the disease.1,2 Finally, symptomatic AR patients may have difficulties with learning and mentation, may need to miss days from work or school, and in a number of other ways, can have their quality of life compromised.3 Unfortunately, while there are a number of effective therapeutic options for AR patients, each has its limitations and disease associated costs and morbidities remain high.
Immunotherapy (IT) with allergen extracts was initially reported to be effective for the desensitization of atopic patients by Noon in 1911, and for many years it was a first line therapy for allergic diseases such as AR.4,5 However, in the last decade, IT has gradually fallen out of favour for a number of reasons. In particular, IT is relatively ineffective, completely replacing medication use for only about 30–50% of AR patients. In addition, IT injections have the potential for inducing allergic reactions including rare, but life threatening anaphylactic reactions.5 Furthermore, IT is a relatively impractical therapeutic modality, as it requires comprehensive allergy testing to identify allergens for the IT cocktail, its therapeutic effects take several months to develop, and IT requires frequent visits to a doctor's office and frequent injections.
In the last decade, pharmaceutical interventions such as non-sedating, long acting antihistamines and topical corticosteroids have proven both safe and highly effective for the prevention and treatment of AR symptoms. As a consequence, these medications have gradually replaced IT as first line interventions for a majority of patients.6 However, as presently available pharmacological interventions do not appreciably attenuate the aeroallergen hypersensitivities that fuel AR, they are generally needed on a daily basis and their ongoing use may be required for many years. Therefore, despite many disadvantages, IT has one distinct advantage over AR medications; it can induce aeroallergen tolerance and extinguish medication dependence in a subset of patients.
As an alternative to both IT and traditional medications, we have investigated the use of immunostimulatory sequence oligodeoxynucleotide (ISS-ODN), as an allergen-independent immunomodulatory agent. In models of allergic conjunctivitis and asthma, ISS-ODN delivery immediately or up to 6 weeks before allergen challenge, has been found to attenuate shock organ hypersensitivity responses and the T helper 2 (Th2)-biased immune profiles of previously allergen sensitized mice.7–9 To further evaluate the allergen independent immunomodulatory activities of ISS-ODN, in the present studies we determined whether a single dose of ISS-ODN, delivered systemically (intradermal; i.d.) or locally (intranasal; i.n.), might also protect sensitized mice from immediate and late phase AR hypersensitivity responses induced by repeated i.n. allergen challenges.
Materials and methods
Reagents
Ovalbumin (OVA, Grade V) and aluminium hydroxide (alum) were purchased from Sigma Chemical Co. (St. Louis, MO). Endotoxin free phosphorothioate ISS-ODN (5′-TGACTGTGAACGTTCGAGATGA-3′) and control oligodeoxynucleotide (C-ODN; 5′-TGACTGTGAAGGTTAGAGATGA-3′) were purchased from Trilink (San Diego, CA). Dexamethasone (DXM) was purchased from Merck & Co. (West Point, PA).
Animals
Four−6-week-old female BALB/c mice obtained from Jackson Laboratories (Bar Harbor, ME) were used in all experiments. The studies reported herein followed the principles for laboratory animal research, as outlined in the Animal Welfare Act and Department of Health, Education and Welfare (National Institutes of Health) guidelines for the experimental use of animals and experimental protocols were approved by our institutions animal welfare committee.
Sensitization, ISS-ODN delivery, and allergen challenge
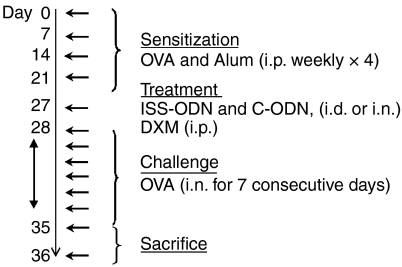
The procedure for allergen sensitization and challenge are summarized in Fig. 1. Briefly, on days 0, 5, 14, and 21, mice were sensitized by i.p. injection of 25 µg of OVA and 1 mg of alum in 300 µl of phosphate-buffered saline (PBS). Six days after the last OVA sensitization, select groups of mice received an i.d. injection of either 100 µg ISS-ODN or control oligodeoxynucleotide (C-ODN) in 50 µl of saline in the base of the tail. Alternatively, mice were lightly anaesthetized with isoflurane (Schering-Plough Animal Health Corp., Union, NJ), and 100 µg ISS-ODN or C-ODN was delivered i.n. in 30 µl of PBS (15 µl per nostril). To compare the effects of ISS-ODN with a standard AR therapy, a group of control mice received a single i.p. injection of 5 mg/kg of DXM in PBS. Beginning 1 day after receiving an immunomodulatory intervention, mice received 7 daily i.n. OVA challenges. On each challenge day, mice received i.n. OVA (500 µg) by methods just reviewed. Fifteen min after the final OVA challenge, the frequency of sneezing and nasal rubbing events (four repetitive nasal rubbing movements equalled one event) were recorded for each mouse (clinical score) by a blinded observer over a 1-min interval. Mice were then killed for further analysis 15 min or 24 hr after the last OVA challenge.
Figure 1.
Allergic rhinitis model. BALB/c mice (four per group) were s.c. sensitized with OVA and alum on three occasions spaced 1 week apart. One week after the last sensitization (Day 28), mice received a series of seven daily i.n. OVA challenges. Additional subsets of mice received ISS-ODN or C-ODN via the i.d. or i.n. routes, or i.p. DXM, one day prior to initiating i.n. OVA challenges (Day 27). Mice were killed for analyses 15 min or 24 hr after the last i.n. OVA challenge.
Nasal lavage and histology
At death, the mouse tracheas were cannulated, a catheter was guided into the nasopharynx, and the nasal passages were gently perfused with 1 ml of PBS, which was collected in a Petri dish for further analysis. Nasal lavage fluid (NLF) collected 15 min after the last i.n. OVA challenge was centrifuged to separate cells and supernatants. Histamine and total cysteinyl leukotriene (LTC4, LTD4, and LTE4) levels in NLF supernatants were determined with commercial enzyme-linked immunosorbent assay (ELISA) kits (Immunotech, Westbrook, MN and Oxford Biomedical Research, Oxford, MI, respectively), according to the manufacturer's directions. Alternatively, NLF interleukin (IL)-5 and interferon-γ (IFN-γ) levels were determined by ELISA with a commercial kit (Pharmingen, San Diego, CA), according to the manufacturer's guidelines. NLF cell pellets were resuspended in 1 ml PBS and cytospin onto glass slides, acetone fixed, and stained with Leukostat (Fisher Scientific). A blinded observer counted 200 cells per slide to determine the percentage of eosinophils in the NLF. In addition, nasal septums surgically removed from mice 24 hr after their final i.n. OVA challenges were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. Tissue inflammation and oedema for each nasal mucosal section were then scored on a four-point scale by a blinded pathologist.
OVA specific immune parameters
Serum samples were collected from mice 1 day before the first OVA challenge and at the time of death, for OVA-specific immunoglobulin G1 (IgG1), IgG2a, and IgE determinations, as previously described.10,11 For IgE analyses, sera were first mixed with a 50% slurry of protein G-sepharose beads (Pharmacia, Piscataway, NJ) to remove IgG and improve OVA-specific IgE detection sensitivity. Samples were then added to OVA-coated ELISA plates along with serial dilutions of a high titre OVA IgE standard. Subsequent ELISA techniques were routine. OVA-specific IgG1 and IgG2a levels were determined by similar methods, but serum samples were not pretreated with protein G-sepharose beads. Immunoglobulin E and IgG1 levels are presented as mean percentage increases [(post-challenge immunoglobulin/pre-challenge immunoglobulin) × 100] for four mice per group.
At death, spleens were harvested aseptically and teased to make single cell suspensions. Splenocytes were cultured at 5 × 106 cells/ml in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 100 µg/ml streptomycin and 100 U/ml penicillin, with OVA (50 µg/ml) at 37°, 5% CO2 and culture supernatants were harvested at 72 hr for cytokine ELISA. Pharmingen reagents were used for IL-5, and IFN-γ determinations and reagents from R & D Systems (Minneapolis, MN) were used to measure IL-4 and IL-13 levels.
LTC4 synthase mRNA expression and cysteinyl leukotriene production by bone marrow-derived macrophages (BMDMs): Bone marrow derived macrophages were obtained by irrigating femurs with BMDM media (Dulbecco's modified Eagle's minimal essential medium with 30% L929-cell conditioned medium and 10% heat-inactivated FCS) and then culturing the harvested cells for one week in BMDM media. Mature BMDMs were incubated (10 X106 cells/ml) with ISS-ODN or C-ODN at 10 µg/ml or were left unstimulated for 24 hr. RNA was then isolated from the BMDMs and electrophoresed on 1% agarose/2·2 m formaldehyde gels and transferred onto nylon membranes (Hybond N; Amersham; Hercules, CA). The membranes were probed with [32P]-labelled full-length murine LTC4 synthase cDNA12 washed under high-stringency conditions, and exposed to autoradiographic film. Loading equivalency and transfer efficiency were established by comparing the 28S ribosomal bands on these membranes by methylene blue staining. Alternatively, BMDMs from the 24-hr cultures were further stimulated with calcium ionophore (A23187 at 1 µm) before culture supernatants were collected for ELISA of cysteinyl leukotriene levels.
Statistics
Statistical analyses were conducted using Statview software. Two-tailed unpaired Student's t-tests were used to analyse all data. The Bonferroni correction factor was included in the calculation of P-values, to account for the increased probability of type-I errors when multiple groups are statistically compared. Results were considered statistically significant if P-values were < 0·05.
Results
ISS-ODN rapidly protects sensitized mice from developing immediate phase AR hypersensitivity responses
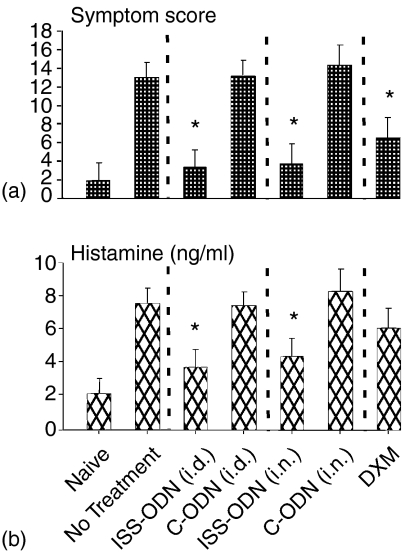
Mice were OVA sensitized and i.n. allergen challenged, as outlined in Fig. 1. Subsets of mice received a single dose of ISS-ODN or C-ODN by either the i.d. OR i.n. route 24 hr before their first of seven daily i.n. OVA challenges or were left untreated. Immediate-phase AR hypersensitivity responses were assessed 15 min after the last i.n. OVA challenge. Compared to OVA-sensitized and challenged mice receiving no intervention or C-ODN, mice treated with i.d. OR i.n. ISS-ODN prior to initiation of i.n. OVA challenges averaged 75% lower immediate phase AR clinical scores (Fig. 2a) and 50% lower NLF histamine levels (Fig. 2b). As corticosteroids are generally considered the most effective therapeutic for AR patients, an additional group of OVA-sensitized mice was treated with a single i.p. dose of DXM prior to initiating i.n. OVA challenges. However, DXM-treated mice were generally less well protected against immediate phase AR responses than mice treated with i.d. OR i.n. ISS-ODN.
Figure 2.
Immediate phase AR hypersensitivity responses. Mice (four mice/group) were sensitized and challenged as outlined in Fig. 1. (a) Fifteen min after the final i.n. OVA challenge, mice received clinical scores, as described in methods and (b) NLF samples were collected for histamine determinations. Results are reflective of three separate experiments (*P ≤ 0·05 for comparisons to OVA sensitized and challenged mice receiving no intervention).
ISS-ODN rapidly protects sensitized mice from developing late phase AR hypersensitivity responses
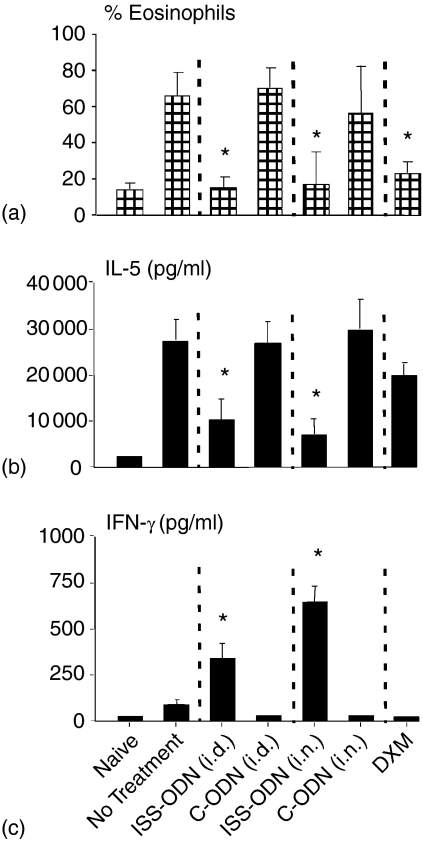
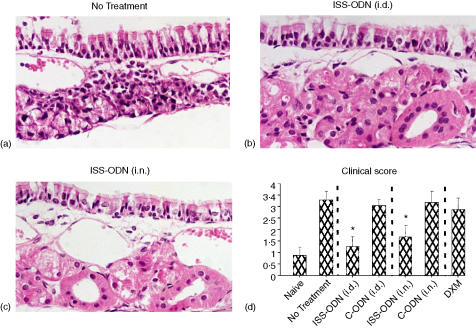
Additional groups of mice were sensitized, treated, and challenged (Fig. 1) and NLF and nasal septums were collected 24 hr after the final i.n. OVA challenge. On average, NLF from mice receiving C-ODN or no intervention had nearly 70% eosinophils, while NLF from i.d and i.n. ISS-ODN treated mice had less than 20% eosinophils (Fig. 3a). In addition, compared to untreated and C-ODN treated mice, mice receiving ISS-ODN had mean NLF IL-5 levels that were reduced by over 60% and NLF IFN-γ levels that were significantly higher (Fig. 3b). Moreover, i.d and i.n. ISS-ODN treated mice had mean mucosal tissue inflammation scores that were less than 50% of these control mice (Fig. 4). Finally, as with the immediate phase hypersensitivity response, ISS-ODN was generally more effective than DXM in protecting sensitized mice from allergen induced late phase nasal hypersensitivity responses.
Figure 3.
Late phase AR hypersensitivity response: NLF analysis. Mice (four mice/group) were sensitized and challenged as outlined in Fig. 1. Twenty-four hr after the final i.n. OVA challenge, NLF samples were collected for evaluation of (a) eosinophil and (b) IL-5 and (c) IFN-γ content. Results are reflective of three separate experiments (*P ≤ 0·05 for comparisons to OVA sensitized and challenged mice receiving no intervention).
Figure 4.
Late phase AR hypersensitivity response: tissue analysis. Mice were sensitized and challenged as outlined in Fig. 1. Twenty-four hr after the final i.n. OVA challenge, nasal septums were removed and stained by routine methods. Representative tissue sections from (a) mice receiving no intervention, (b) i.d. ISS-ODN, or (c) i.n. ISS-ODN are presented. (d) Pathology scores (tissue inflammation and edema) for nasal tissue sections (four mice/group). Results are reflective of three separate experiments (*P ≤ 0·05 for comparisons to OVA sensitized and challenged mice receiving no intervention).
ISS-ODN inhibits local leukotriene release by allergen-sensitized and i.n. challenged mice and inhibits BMDM leukotriene synthesis
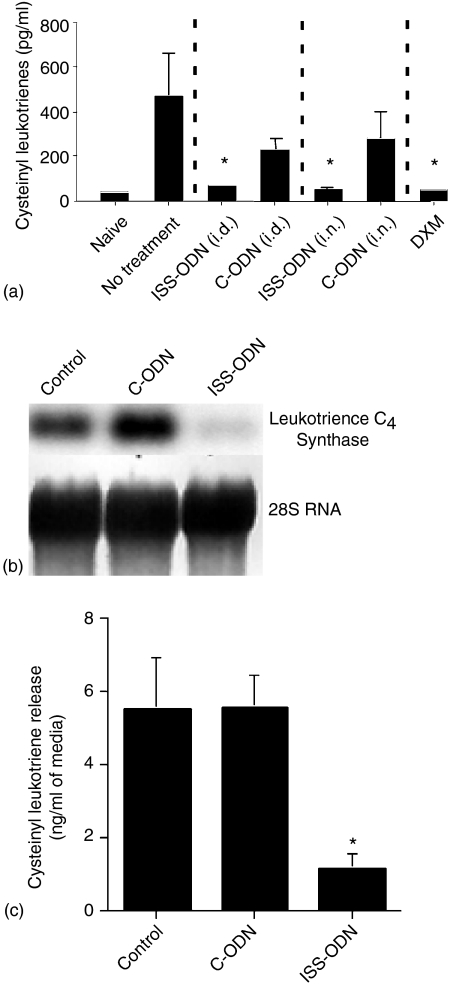
Although cysteinyl leukotrienes are known to contribute to allergic hypersensitivity responses, the effects of ISS-ODN on allergen induced cysteinyl leukotriene release have yet to be investigated. Therefore, we measured the total cysteinyl leukotriene levels of NLF samples collected from experimental mice 15 min after their final i.n. OVA challenge. Treatment with either ISS-ODN or DXM reduced NLF cysteinyl leukotriene levels by as much as 90%(Fig. 5a) compared to no treatment. Surprisingly, while C-ODN treatment did not measurably influence other AR outcome measures (Figs 2–4), NLF samples from mice receiving C-ODN did have modest reductions in cysteinyl leukotriene content compared to samples from untreated mice.
Figure 5.
ISS-ODN effects on cysteinyl-leukotriene release in vivo and in vitro. (a) Mice (four mice/group) were sensitized and challenged as outlined in Fig. 1. Fifteen min after the final i.n. OVA challenge, NLF samples were collected to measure total cysteinyl leukotriene levels. (b) BMDMs were incubated with ISS-ODN, C-ODN, or were left unstimulated for 24 hr. RNA was then harvested for Northern blot analysis of LTC4 synthase mRNA expression. Equivalence in RNA loading was established by comparing methylene blue staining of 28S RNA on the membranes. (c) BMDMs described in (b) were activated with calcium ionophore for an additional 15 min before supernatants were harvested for cysteinyl leukotriene determinations. All results are reflective of three separate experiments (*P ≤ 0·05 for comparisons to OVA sensitized and challenged mice receiving no intervention).
We recently reported that LPS conditioning inhibits cysteinyl leukotriene production at least in part by reducing steady state leukotriene C4 synthase mRNA levels. Therefore, we determined whether BMDM incubation with ISS-ODN attenuated leukotriene C4 synthase mRNA expression, by Northern blot analysis. As can be seen in Fig. 5(b), BMDM leukotriene C4 synthase mRNA expression was inhibited dramatically by 24 hr of culture with ISS-ODN but not C-ODN. Finally, BMDMs cultured with ISS-ODN or C-ODN were further activated with calcium ionophore for 15 min and cysteinyl leukotriene release was assessed. Compared to unstimulated BMDMs and BMDMs cultured with C-ODN, cysteinyl leukotriene levels were about 75% lower in supernatants from BMDM cultures in which ISS-ODN was added (Fig. 5c).
ISS-ODN attenuates Th2-biased immune profiles in allergen sensitized and i.n. challenged mice
Sera were obtained before the first and after the last i.n. OVA challenge for allergen-specific IgG1, IgG2a, and IgE determinations. In addition, splenocytes were harvested from mice 24 hr after their last i.n. OVA challenge to analyse allergen-specific cytokine responses. Mice receiving i.d and i.n. ISS-ODN had mean post-challenge increases in OVA specific serum IgE and IgG1 that were significantly blunted compared to those of mice receiving no intervention or C-ODN (Table 1). However, under the experimental conditions used for these studies, ISS-ODN treatment did not influence IgG2a levels, which were very low in all experimental groups (data not shown).
Table 1.
ISS-ODN modulation of Th2-biased adaptive immune profiles
| Intervention | %IgE increase | %IgG1 increase | IL-4 (pg/ml) | IL-5 (pg/ml) | IL-13 (pg/ml) | IFN-γ (pg/ml) |
|---|---|---|---|---|---|---|
| None | 478 ± 83 | 432 ± 119 | 2379 ± 840 | 2468 ± 783 | 146 140 ± 32 680 | 159 ± 23 |
| ISS-ODN (i.d.) | 187 ± 34* | 166 ± 44* | 472 ± 221* | 154 ± 60* | n.d.* | 772 ± 163* |
| C-ODN (i.d.) | 549 ± 186 | 798 ± 27 | 6086 ± 3039 | 2452 ± 1170 | 112 112 ± 21 493 | 173 ± 71 |
| ISS-ODN (i.n.) | 197 ± 47* | 134 ± 67* | 459 ± 137* | 186 ± 35* | 856 ± 856* | 617 ± 131* |
| C-ODN (i.n.) | 425 ± 101 | 413 ± 57 | 2517 ± 704 | 2459 ± 619 | 105 151 ± 5704 | 133 ± 37 |
| DXM | 200 ± 37* | 286 ± 175 | 2782 ± 1196 | 902 ± 86 | 197 433 ± 81 995 | 164 ± 41 |
Mice (four mice/group) were sensitized and challenged as outlined in Fig. 1. Sera were collected for OVA-specific IgE and IgG1 determinations 1 day before the first and 1 day after the last i.n. OVA challenges. (Post-challenge immunoglobulin/pre-challenge immunoglobulin) × 100=% immunoglobulin increase. Splenocytes were harvested to assess OVA specific cytokine production at the time of the second bleed. Results are reflective of three separate experiments.
n.d.=none detected.
P ≤ 0.05 for comparisons to OVA sensitized and challenged mice receiving no intervention.
In addition to modulating IgE and IgG1 responses, treatment with ISS-ODN also inhibited OVA-specific splenic IL-4, IL-5, and IL-13 production by over 80%, compared to treatment with C-ODN or no treatment (Table 1). Moreover, in response to culture with OVA, splenocytes from i.d and i.n. ISS-ODN treated mice produced threefold higher amounts of IFN-γ than splenocytes from untreated and C-ODN treated mice. In contrast to ISS-ODN therapy, DXM treatment only attenuated OVA-specific serum IgE levels and splenic IL-5 responses, while splenic IL-4 and IL-13 responses were similar to those of sensitized mice receiving C-ODN or no intervention. Furthermore, unlike ISS-ODN treated mice, DXM treated mice did not develop an OVA specific IFN-γ response.
Discussion
AR is one of the most common chronic diseases in the industrialized world.1 Unfortunately, while the medications presently used to treat AR are highly effective in controlling symptoms, they are ineffective as curative measures, as they do not reverse the allergen-specific hypersensitivities that perpetuate the disease.5,13 In contrast, traditional IT can induce long-lasting aeroallergen tolerance and obviate the need for medications but only for a relatively small subset of AR patients.5,14 Recently, we found that allergen/ISS-ODN covaccination was more effective than IT with allergen alone for the induction of airway allergen tolerance in previously Th2-sensitized mice11 and Hussain and colleagues have reported that mice receiving ISS-ODN at the time of OVA/alum sensitization were protected from the development of AR.15 As an alternative to medications and allergen-specific IT with or without ISS-ODN, the present studies assessed whether ISS-ODN delivery attenuates AR hypersensitivity responses and Th2-biased immune profiles of previously sensitized mice in an allergen-independent manner.
Compared to sensitized mice receiving no intervention or treatment with C-ODN, sensitized mice treated with ISS-ODN by either the i.d. or i.n. route, were protected from allergen-induced immediate and late phase AR hypersensitivity responses. Moreover, although corticosteroids are the most effective AR medications presently available, mice treated with DXM prior to initiating i.n. OVA challenges were generally less well protected from hypersensitivity responses than mice receiving ISS-ODN. In experimental models of allergic conjunctivitis and asthma, we have previously observed that allergen-independent ISS-ODN delivery protected against one and two topical allergen challenges, respectively.7,9 The present results further highlight the rapid and potent antiallergic activities of ISS-ODN, demonstrating that a single dose of ISS-ODN also protects Th2-sensitized mice from AR hypersensitivity responses induced by seven daily i.n. allergen challenges.
Originally known as the slow reacting substance of anaphylaxis16 cysteinyl leukotrienes are known to play important roles in both immediate and late phase hypersensitivity responses.17 However, while the antiallergic activities of ISS-ODN have been fairly well characterized, effects on cysteinyl leukotriene production and release have not been previously investigated. In the present studies, ISS-ODN treatment was found to reduce cysteinyl leukotriene release into the NLF of OVA-sensitized and i.n. challenged mice and to inhibit leukotriene C4 synthase mRNA expression and cysteinyl leukotriene release by in vitro cultured BMDMs.
In addition to inhibiting AR hypersensitivity responses, allergen-independent ISS-ODN delivery modified the immune profiles of the OVA-sensitized mice used in these studies, attenuating parameters of Th2-biased immunity, while inducing an antigen-specific IFN-γ response from cultured splenocytes. These observations are consistent with other published studies that demonstrate a rapid but temporary (4–6 weeks) attenuation of Th2-biased immune profiles with allergen-independent ISS-ODN therapy.8 In previous investigations, allergen/ISS-ODN immunizations were found to induce a persistent and Th1-biased shift in the immune profiles of previously Th2-sensitized mice (8 weeks), while treatment with ISS-ODN alone did not.11,18 Taken together, these observations suggest that immune memory is not irreversibly impacted by allergen-independent ISS-ODN delivery but that repeated environmental allergen exposures in the context of innate immune activation by ISS-ODN would likely imprint a persistent antiallergic Th bias on allergen specific immune memory by committing increasing numbers of B and T cells with each successive allergen encounter.
The mechanisms by which ISS-ODN treatment inhibited local histamine and cysteinyl leukotriene release during the immediate phase AR hypersensitivity response, remain to be determined. Nonetheless, given that basophilic cells are a major source of histamine and cysteinyl leukotriene release in the allergic nose17 it appears that ISS-ODN treatment may have compromised their ability to contribute to the immediate phase hypersensitivity response. It should be noted that while ISS-ODN treatment inhibited allergen-challenge induced IgE and IgG1 production modestly, only trace amounts of IgE are required to fully arm basophilic cells for allergen induced degranulation.10,19,20 Therefore, we consider it likely that ISS-ODN inhibited the activities of basophilic cells in the nasal mucosa by additional mechanisms. We and others have previously shown that murine mast cells express Toll-like receptor-9 and produce cytokines in response to ISS-ODN stimulation but that ISS-ODN activation does not directly inhibit mast cell degranulation.21,22 However, as ISS-ODN stimulation was found to inhibit cysteinyl leukotriene production by BMDMs, it is conceivable that ISS-ODN treatment might also inhibit cysteinyl leukotriene synthesis and possibly even histamine synthesis by basophilic cells. Alternatively, IL-4 and IL-13 have recently been shown to amplify immediate hypersensitivity responses.23 Therefore, ISS-ODN-mediated inhibition of allergen-specific Th2 cytokine production could also contribute to its attenuation of the immediate phase AR hypersensitivity response. Finally, we have recently found that ISS-ODN inhibits mast cell recruitment to the lungs of allergen sensitized and airway challenged mice21 suggesting that ISS-ODN treatment could also have inhibited local mast cell recruitment, in this AR model.
Although more peripheral to the immediate phase hypersensitivity response, Th2 cells are known to play a central role in mediating the late phase hypersensitivity response.24,25 We and others have previously shown that ISS-ODN elicits innate cytokine responses from several cell types including macrophages, dendritic cells, natural killer cells, and B cells, but not T cells.26–28 However, several of these cytokines, including IL-10, IL-12, type 1 IFNs, and IFN-γ, are known to inhibit the functionality of Th2 cells. In our published studies we found that naïve mice injected with ISS-ODN have elevated serum IL-12 and IFN-γ levels for a week or more.28 Furthermore, we recently observed that compared to its antiallergic effect in wild type mice, ISS-ODN is less effective in protecting sensitized IFNα/β receptor, IL-12, and IFN-γ knockout mice from developing late phase pulmonary hypersensitivity responses (unpublished data). These results support the view that ISS-ODN induced cytokine production contributes to its long lasting inhibitory effects on the late phase AR hypersensitivity response. Finally, we have published that ISS-ODN exposure down-regulates IL-4 receptor and up-regulates IFN-γ receptor expression on B cells.27 Therefore, ISS-ODN may also inhibit the functions of effector cells of the late phase AR hypersensitivity response by rendering them resistant to the effects of Th2 cytokines, including IL-4, while increasing their responsiveness to IFN-γ.
As with previously published studies7,9,29 the present investigations demonstrated that ISS-ODN is a potent and rapid antiallergic therapeutic agent, equally effective by systemic and topical routes of delivery. Therefore, in consideration of future clinical applications, ISS-ODN could be delivered to AR patients in nasal sprays in a manner analogous to how nasal steroids are currently used. Moreover, consistent with previous results in models of allergic asthma and conjunctivitis7,9,29 the present investigations suggest that allergen-independent ISS-ODN therapy could prove to be an AR intervention with curative potential, as it both attenuated AR hypersensitivity responses and at least temporarily inhibited allergen-specific IgE and Th2 production by allergen-sensitized and i.n. challenged mice. Despite their reported effectiveness in this and other murine models of allergic disease, one lingering safety concern remains with ISS-ODN-based interventions; As a potent Th1 adjuvant, ISS-ODN has the theoretical potential to convert Th2-biased allergen hypersensitivities into Th1-biased allergic hypersensitivities. Nonetheless, in our own experiments, allergen/ISS-ODN covaccinations failed to prime naïve or Th2-sensitized mice for the development of Th1-biased airway hypersensitivity responses.11,30 At present, our understanding of the underlying mechanisms by which ISS-ODN immunomodulation inhibits early and late phase AR hypersensitivity responses and allergen specific immunity is far from complete. Nonetheless, if allergen independent ISS-ODN therapy can safely treat AR pathology and attenuate Th2-biased hypersensitivities in patients, it would be a major advance for the treatment of this disease, obviating the need for allergen identification and IT injections to achieve aeroallergen tolerance.
Acknowledgments
This work was supported by grants AI40682 (A.A.H., K.T., E.R.) and AR47360 (A.A.H.) from the National Institutes of Health, Korean Research Foundation grant KRF-2001-042-F00075 (C.S.R., C.H.L.), and a grant from Dynavax Technologies.
Abbreviations
- AR
allergic rhinitis
- IT
immunotherapy
- C-ODN
control oligodeoxynucleotide
- ELISA
enzyme-linked immunosorbent assay
- ISS-ODN
immunostimulatory sequence oligodeoxynucleotide
- OVA
ovalbumin
- i.d.
intradermal
- i.n.
intranasal
- i.p.
intraperitoneal
- BMDM
bone marrow derived macrophage
- NLF
nasal lavage fluid
References
- 1.Skoner DP. Allergic rhinitis. definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2–8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 2.Ray NF, Baraniuk JN, Thamer M, et al. Direct expenditures for the treatment of allergic rhinoconjunctivitis in 1996, including the contributions of related airway illnesses. J Allergy Clin Immunol. 1999;103:401–7. doi: 10.1016/s0091-6749(99)70463-x. [DOI] [PubMed] [Google Scholar]
- 3.Dykewicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1998;81:478–518. doi: 10.1016/s1081-1206(10)63155-9. [DOI] [PubMed] [Google Scholar]
- 4.Noon L. Prophylactic inoculation against hayfever. Lancet. 1911;1:1572–3. [Google Scholar]
- 5.Creticos PS. Immunotherapy. In: Kaplan AP, editor. Allergy. 2. Philadelphia: Saunders; 1997. pp. 26–39. [Google Scholar]
- 6.Donahue JG, Fuhlbrigge AL, Finkelstein JA, et al. Asthma pharmacotherapy and utilization by children in 3 managed care organizations. The Pediatric Asthma Care Patient Outcomes Research Team. J Allergy Clin Immunol. 2000;106:1108–14. doi: 10.1067/mai.2000.111432. [DOI] [PubMed] [Google Scholar]
- 7.Broide D, Schwarze J, Tighe H, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–62. [PubMed] [Google Scholar]
- 8.Broide DH, Stachnick G, Castaneda D, et al. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol. 2001;21:175–82. doi: 10.1023/a:1011078930363. [DOI] [PubMed] [Google Scholar]
- 9.Magone MT, Chan CC, Beck L, et al. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur J Immunol. 2000;30:1841–50. doi: 10.1002/1521-4141(200007)30:7<1841::AID-IMMU1841>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Horner AA, Nguyen MD, Ronaghy A, et al. DNA-based vaccination reduces the risk of lethal anaphylactic hypersensitivity in mice. J Allergy Clin Immunol. 2000;106:349–56. doi: 10.1067/mai.2000.107933. [DOI] [PubMed] [Google Scholar]
- 11.Takabayashi K, Libet L, Chisholm D, et al. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in th2-sensitized mice. J Immunol. 2003;170:3898–905. doi: 10.4049/jimmunol.170.7.3898. [DOI] [PubMed] [Google Scholar]
- 12.Serio KJ, Johns SC, Luo L, et al. Lipopolysaccharide down-regulates the leukotriene C4 synthase gene in the monocyte-like cell line, THP-1. J Immunol. 2003;170:2121–8. doi: 10.4049/jimmunol.170.4.2121. [DOI] [PubMed] [Google Scholar]
- 13.Pullerits T, Praks L, Ristioja V, et al. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:949–55. doi: 10.1067/mai.2002.124467. [DOI] [PubMed] [Google Scholar]
- 14.Winther L, Malling HJ, Moseholm L, et al. Allergen-specific immunotherapy in birch- and grass-pollen-allergic rhinitis. I. Efficacy estimated by a model reducing the bias of annual differences in pollen counts. Allergy. 2000;55:818–26. doi: 10.1034/j.1398-9995.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 15.Hussain I, Jain VV, Kitagaki K, et al. Modulation of murine allergic rhinosinusitis by CpG oligodeoxynucleotides. Laryngoscope. 2002;112:1819–26. doi: 10.1097/00005537-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Austen KF. The Paul Kallos Memorial Lecture. From slow-reacting substance of anaphylaxis to leukotriene C4 synthase. Int Arch Allergy Immunol. 1995;107:19–24. doi: 10.1159/000236919. [DOI] [PubMed] [Google Scholar]
- 17.Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55:7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 18.Horner AA, Raz E. Immunostimulatory sequence oligodeoxynucleotide-based vaccination and immunomodulation: two unique but complementary strategies for the treatment of allergic diseases. J Allergy Clin Immunol. 2002;110:706–12. doi: 10.1067/mai.2002.129122. [DOI] [PubMed] [Google Scholar]
- 19.Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–8. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 20.Oettgen HC, Martin TR, Wynshaw-Boris A, et al. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda RK, Miller M, Nayar J, et al. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171:4860–7. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 22.Zhu FG, Marshall JS. CpG-containing oligodeoxynucleotides induce TNF-alpha and IL-6 production but not degranulation from murine bone marrow-derived mast cells. J Leukoc Biol. 2001;69:253–62. [PubMed] [Google Scholar]
- 23.Strait RT, Morris SC, Smiley K, et al. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–42. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 24.Li XM, Schofield BH, Wang QF, et al. Induction of pulmonary allergic responses by antigen-specific Th2 cells. J Immunol. 1998;160:1378–84. [PubMed] [Google Scholar]
- 25.Hansen G, Berry G, DeKruyff RH, et al. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinman DM, Yi AK, Beaucage SL, et al. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horner AA, Widhopf GF, Burger JA, et al. Immunostimulatory DNA inhibits IL-4-dependent IgE synthesis by human B cells. J Allergy Clin Immunol. 2001;108:417–23. doi: 10.1067/mai.2001.117795. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Horner AA, Takabayashi K, et al. Immunostimulatory DNA pre-priming: a novel approach for prolonged Th1-biased immunity. Cell Immunol. 1999;198:69–75. doi: 10.1006/cimm.1999.1572. [DOI] [PubMed] [Google Scholar]
- 29.Sur S, Wild JS, Choudhury BK, et al. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999;162:6284–93. [PubMed] [Google Scholar]
- 30.Chisholm D, Libet L, Hayashi T, et al. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–54. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]