Abstract
Macrophages play important roles in the clearance of dying and dead cells. Typically, and perhaps simplistically, they are viewed as the professional phagocytes of apoptotic cells. Clearance by macrophages of cells undergoing apoptosis is a non-phlogistic phenomenon which is often associated with actively anti-inflammatory phagocyte responses. By contrast, macrophage responses to necrotic cells, including secondarily necrotic cells derived from uncleared apoptotic cells, are perceived as proinflammatory. Indeed, persistence of apoptotic cells as a result of defective apoptotic-cell clearance has been found to be associated with the pathogenesis of autoimmune disease. Here we review the mechanisms by which macrophages interact with, and respond to, apoptotic cells. We suggest that macrophages are especially important in clearing cells at sites of histologically visible, high-rate apoptosis and that, otherwise, apoptotic cells are removed largely by non-macrophage neighbours. We challenge the view that necrotic cells, including persistent apoptotic cells are, of necessity, proinflammatory and immunostimulatory and suggest that, under appropriate circumstances, persistent apoptotic cells can provide a prolonged anti-inflammatory stimulus.
Keywords: apoptosis, cell interactions, inflammation: inflammatory mediators including eicosanoids, macrophages, monocytes, phagocytosis
Introduction
When cells die they are either lost directly to the environment − as in the sloughing off of epithelial cells from body surfaces − or, more commonly, they are engulfed by their adjoining neighbours or by specialized phagocytes, the macrophages. Typically, cells dying purposefully by programmed cell death or apoptosis are thought to be phagocytosed by mechanisms that fail to incite inflammatory or immune reactions. Indeed, some of these mechanisms are actively anti-inflammatory and tolerogenic. By contrast, cells that die accidentally and catastrophically − by thermodynamically downhill processes summed up in the term ‘necrosis’ − are capable of activating proinflammatory and immunostimulatory responses. It is accepted widely that the acquisition of necrotic properties by apoptotic cells as a result of failed or inefficient clearance has proinflammatory consequences that can lead to the development of autoimmune disease. Thus, engulfment of apoptotic cells by phagocytes is not limited to safe packaging and disposal of unwanted cells and their contents: this process clearly plays an important regulatory role in the immune system. Perhaps not surprisingly, however, the immunological consequences of a particular mode of cell death or the effects of failed clearance are not as straightforward as they might be perceived.
Here we review the relationship between the apoptotic cell and its professional ‘scavenger’ the macrophage, focusing especially on recent evidence that extends and challenges the often-accepted dogma that apoptotic cells are anti-inflammatory and immunosuppressive, whereas necrotic cells − including those derived from apoptotic cells − are proinflammatory and immunostimulatory. From the background of in vitro research, we consider in detail the molecular mechanisms underlying interaction between apoptotic cells and macrophages and discuss recent in vivo studies that shed new light on the immunological implications of these interactions. Numerous excellent reviews on the subject of apoptotic-cell clearance and implications for immunity have appeared elsewhere, including several published recently.1–13 We refer the reader to these not only as a source of important historical literature, because constraints on space prevent us from including an extensive bibliography here, but also as a series of informed views on additional aspects of this increasingly complex area − including, for example, the dendritic cell and its relationship with apoptotic cells (see especially12) − that are beyond the scope of this review.
The macrophage as the professional scavenger of apoptotic cells
The macrophage is considered widely to be the professional phagocyte of the apoptotic cell. This is based upon the frequent association between macrophages and apoptotic cells in tissues, very often the apoptotic cell and its fragments being visible in phagocytic vacuoles within the macrophage cytoplasm. In general, apoptotic cells are rarely observed in situ but their presence in a histological snapshot is a sure indication that the rate of apoptosis in the tissue sample is high. One of the most famous examples is the frequent apoptosis that occurs during development, for example that of the cells of the interdigital webs deleted during limb modelling (Fig. 1). In the immune system are numerous examples of locations of high-rate apoptosis − the thymus and the follicles of secondary lymphoid tissues (where the macrophages engulfing apoptotic cells are known as ‘tingible body’ macrophages), providing classical examples. Furthermore, the resolution phase of acute inflammation is characterized by the activity of infiltrating macrophages that clear large numbers of apoptotic granulocytes at inflammatory sites (reviewed in Maderna & Godson14). However, multiple and varied lineages of cells have the power to engulf their apoptotic neighbours (Table 1) and a significant factor that confounds full understanding of the normal rates of cell death in tissues is the efficiency with which the deleted cells are engulfed and lost from histological sight. It is known that cells displaying no gross histological features of cell death can signal the need to be phagocytosed via molecular changes at their plasma membranes.15 It is reasonable to suspect, therefore, that cell death in situ may often proceed undetected.
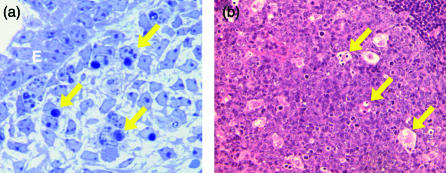
Figure 1.
Engulfment of apoptotic cells in situ. (a) Clearance of apoptotic cells in the mouse footplate during the dissolution of the interdigital web at embryonic day 14·5. Arrows show examples of macrophages that have phagocytosed apoptotic mesenchymal cells with classical morphological features of the death programme. E, epidermis. Toluidine blue stain. (b) Standard haematoxylin- and eosin-stained section of lymph node showing tingible body macrophages (arrows) of a germinal centre containing engulfed remnants of apoptotic cells.
Table 1.
Mammalian phagocytes of apoptotic cells and known receptors of intercellular interaction
| Phagocyte | Molecules |
|---|---|
| Macrophage | Lectin, αvβ3, CD36, SR-A, MER, CD14, ABCA1, PSR, CR3, CR4, CD91/ calreticulin, CD31, FcγR, SHPS-1, Oxidation-specific receptors |
| Dendritic cell | αvβ3, αvβ5, CD36 |
| Fibroblast | Lectin, αvβ3, PSR |
| Kidney mesangial cell | αvβ3 |
| Testis Sertoli cell | SR-B1 |
| Ovarian thecal cell | SR-B1 |
| Smooth muscle cell | PSR? |
| Endothelial cell | Lectin |
| Epithelial cell | αvβ5, PSR |
| Hepatocyte | Asialoglycoprotein receptor |
| Mesenchymal cell | ? |
See Fig. 1 for abbreviations.
Is the macrophage truly, then, the professional phagocyte of the apoptotic cell? In general, whenever apoptotic cells are visible by standard histological techniques − showing the classical morphological features of this mode of cell death (see Kerr et al.16) − they are colocalized with macrophages. Analysis of apoptotic epithelial cell remnants visualised by a caspase-generated epitope on cytokeratin 18 confirms this association.17 However, numerous examples exist of so-called ‘amateur’ phagocytes playing significant roles in engulfment of deleted cells (Table 1). In lowly organisms such as the nematode worm, Caenorhabditis elegans, engulfment of cells that are deleted during development is mediated by non-macrophage neighbours, for these animals possess no macrophages. Such neighbourly phagocytes in mammals include epithelial cells of the thymus,18 parenchymal cells of the liver19 and stromal cells of the bone marrow.20 Further examples are documented in Table 1. Intriguingly, mice lacking macrophages develop ostensibly normally,21 supporting the notion that ‘amateur’ phagocytes assist in the clearance of apoptotic cells when macrophages become overwhelmed.22 An alternative view that we support here, however, is that many cells destined for removal fail to show the hallmark morphological features of apoptosis and thus remain histologically unidentifiable as dying cells. It would be predicted that such cells, perhaps outnumbering the classically apoptotic cells in most developed tissues, are engulfed efficiently, and essentially invisibly (at least by standard histology) by their neighbours. In this scenario, the roles of the ‘amateur’ and ‘professional’ phagocyte are reversed, with the macrophage playing the supportive role as the back-up phagocyte to the tissue neighbour. We propose that the macrophage is the professional scavenger of apoptotic cells and, in addition to its role in the clearance of dead and dying cells of the circulation, is especially important in the clearance of cell corpses that have overwhelmed the neighbourly nonmacrophage engulfment system.
Our view is that macrophages are attracted to sites of high-rate apoptosis where dying cells reach the stage of histological visibility. We suggest that while macrophages are able to interact with early apoptotic cells23,24 (Dransfield, personal communication), they have a preference for interactions with phases of the process that are later than those preferred by the phagocytic neighbours of apoptotic cells. Recent in vitro evidence lends support to the view that macrophages often fail to engulf apoptotic cells until long after they have acquired the morphological hallmarks of apoptosis.25 Although macrophages appear also to be able to interact with cells at an early stage of apoptosis prior to chromatin condensation, it is noteworthy that this stage of ‘recognizability’ may be transient.24 Perhaps this mechanism, which involves early changes in surface sugars, also underlies the rapid recognition of apoptotic cells by their neighbours?
The association between the classically apoptotic cell and the macrophage in vivo may not therefore be representative of the true relationship between the dying cell and its professional phagocyte. Rather, this association may simply reflect our ability to recognize only restricted stages of the apoptosis programme in standard histological material. Nevertheless, the macrophage's role in apoptotic-cell clearance should not be underestimated: it is a key phagocyte of circulating leukocytes that reach the end of their lives and plays an important role in clear-up operations at sites of frequent and synchronous apoptosis. Furthermore, the macrophage's role as the professional scavenger of apoptotic cells is likely to prove to be critically important in the regulation of immune responses. Of particular note here is the ability of the macrophage to provide tolerogenic signals in the adaptive immune response to apoptotic cell-derived antigens.26,27
Close encounters between macrophages and apoptotic cells
The present molecular picture of the interaction between macrophage and apoptotic cell is bewildering (Fig. 2). Largely drawn from in vitro studies, a broad array of phagocyte receptors, apoptotic-cell-associated ligands and intermediate molecules has emerged. Included on the phagocyte are integrins (e.g. αvβ328), scavenger receptors (e.g. CD3629), immunoglobulin super-family (IgSF) molecules (e.g. CD3130), receptors for complement (e.g. CD91/calreticulin31) and for microbial components (e.g. CD1432), as well as molecules involved in engagement of sugars and phospholipids (e.g. lectins33 and PSR34, respectively). Implicated thus far on the apoptotic cell are fewer defined structures but these encompass lipid, carbohydrate and protein moieties, the most renowned being the exposed anionic phospholipid, phosphatidylserine (PS), which resides normally on the inner leaflet of the plasma membrane.35 An increasingly wide variety of soluble, intermediate factors is emerging whose role is to opsonise apoptotic cells creating molecular bridges between components of the apoptotic-cell and phagocyte surfaces. For example, the first component of complement, C1q, as well as certain of its collectin relatives, links the apoptotic-cell surface with the phagocyte receptor complex CD91/calreticulin;31 the secreted glycoprotein, milk fat globule EGF factor 8, MFG-E8 (lactadherin) bridges via discrete binding sites the αvβ3 integrin on macrophages and the exposed PS on apoptotic cells.36 Further details of molecules currently implicated in apoptotic-cell clearance can be found in Fig. 2.
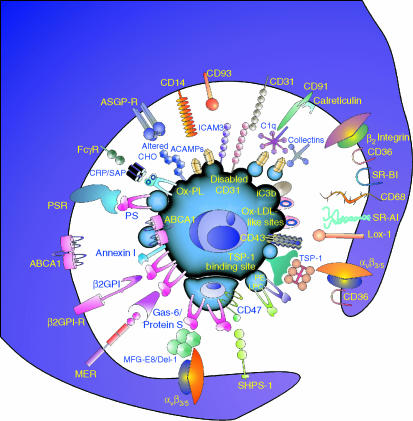
Figure 2.
Close encounters between apoptotic cells and phagocytes. Schematic view of molecules implicated in interactions mediating recognition, binding and engulfment of a theoretical, ‘consummate’ apoptotic cell with a similarly theoretical phagocyte. Molecules used by nonmacrophages as well as macrophages are included (see Table 1 for comparison). αvβ3, αvβ5 vitronectin receptor integrins; ABCA1, ATP-binding cassette transporter A1; ACAMPs, apoptotic cell-associated molecular patterns; ASGP-R, asialoglycoprotein receptor; β2GPI, β2 glycoprotein I; β2GPI-R, β2GPI-receptor; β2 integrins include CR3 and CR4; C1q, first component of complement; CHO, carbohydrate; CRP, C-reactive protein; Del-1, developmental endothelial locus-1; Gas-6, growth arrest specific gene-6; iC3b, inactivated complement fragment C3b; ICAM-3 (CD50), intercellular adhesion molecule-3; Lox-1, oxidised low density lipoprotein receptor 1; LPC, lysophosphatidylcholine; MER, myeloid epithelial reproductive tyrosine kinase; MFG-E8, milk fat globule epidermal growth factor-8; Ox-PL oxidised phospholipids; PE, phosphatidylethanolamine; PS, phosphatidylserine; PSR, PS-receptor; SAP, serum amyloid protein, SHPS-1, Src homology 2 domain-bearing protein tyrosine phosphatase substrate-1; SR-AI, scavenger receptor AI; SR-BI, scavenger receptor BI; TSP-1, thrombospondin-1.
Pattern recognition in apoptotic-cell clearance
In recent years, numerous components of the innate immune system including CD14, CD91/calreticulin, C1q, C3bi, collectins and pentraxins have been implicated in the clearance of apoptotic cells by macrophages. This has led to the proposal that apoptotic cells, like microbes, can present conserved molecular patterns to innate immune molecules to mediate or facilitate the clearance process. Such apoptotic-cell-associated molecular patterns (ACAMPs)37,38 may share structural homology with pathogen-associated molecular patterns (PAMPs), conserved structures decorating microorganisms that are recognized by innate immune receptors, pattern recognition receptors (PRRs).39,40 It is tempting to speculate that CD14, for example, a prototypic pattern recognition receptor41 that interacts with bacterial lipopolysaccharide, a prototypic PAMP, has an affinity for ACAMPs displaying lipopolysaccharide (LPS)-like structures. While every molecule in three-dimensional structure naturally displays a molecular pattern it is molecular grouping, perhaps to form repetitive structures, that is likely to underlie ACAMP formation. Thus, although currently speculative, it is immediately conceivable that the basis for ACAMP formation could be the topological association of certain patterns of molecules in distinct domains of the apoptotic plasma membrane.
Translocation of molecules from intracellular to extracellular sites is a proven mechanism whereby cells that have engaged their apoptosis programme can become rapidly ‘visible’ to phagocytes. Exposure of PS on the apoptotic cell42 and, at least in certain circumstances, its subsequent oxidation43 appears to be a necessary prerequisite for clearance. Therefore it might be predicted that ACAMP-containing domains include regions of exposed PS. While necessary, PS exposure is not sufficient to ensure clearance25,44 and additional critical changes in the apoptotic-cell surface might comprise the colocalization of further molecules with these PS-rich domains, including other plasma membrane molecules, soluble factors and translocated intracellular components. An example of the latter is Annexin I which, following caspase activation, is recruited from its usual intracellular location to domains of the apoptotic-cell surface that are rich in PS.45 Significantly, this mechanism is conserved in the nematode, C. elegans. Additional, as yet ill-defined, intracellular components may be recruited to the apoptotic-cell surface according to the phase of the process.46 Topological changes in plasma membrane structures include the caspase-dependent capping of the major surface sialoglycoprotein CD43 early after induction of apoptosis in lymphocytes.24 Membrane components of apoptotic cells bound by the globular heads of C1q and its cousin, mannose-binding lectin (MBL) are also localized in distinct plasma-membrane patterns.31,47
Determining the nature of ACAMPs presents a significant challenge to our understanding of the apoptotic-cell surface. Regardless of structural features, profoundly different mechanisms must underlie the functional effects of PRR engagement by ACAMPs versus PAMPs, as the macrophage response to the latter is proinflammatory and to the former normally anti-inflammatory. One key difference is due conceivably to differential signalling by toll-like receptors (TLRs), PAMP responsiveness being TLR-dependent, whereas ACAMP responsiveness may be TLR-independent. In support of this, it has been shown that necrotic, but not apoptotic, cells induce expression of proinflammatory and tissue repair genes in macrophages via TLR2-dependent activation of NF-κB.48 A recent study indicates further that TLR4 plays no role in the engulfment of apoptotic cells.49
We have proposed previously two distinct scenarios for the role of CD14 as a PRR in the clearance of apoptotic cells compared to its role in the clearance of LPS38,50 which are detailed and extended in (Fig. 3). Broadly, these can be divided into (a) altered receptor complex formation and (b) dominant anti-inflammatory signalling. The first scenario envisages ACAMP-dependent assembly of a receptor complex involving the GPI-anchored CD14 in cooperation with molecules other than TLR2 and TLR4 that fail to transduce proinflammatory signals (as they do in response to LPS) and/or activate anti-inflammatory signalling pathways. In the second scenario, CD14 may interact with ACAMPs and PAMPs via common mechanisms. Here activation of the default proinflammatory signalling pathways would be prevented by dominant effects of anti-inflammatory responses produced by additional receptor–ligand interactions between the phagocyte and the apoptotic cell. One clear example of such an interaction that dominantly suppresses the proinflammatory response of macrophages to LPS and that promotes resolution of inflammation through stimulation of anti-inflammatory cytokine secretion is that of the macrophage PSR engaging with PS on the apoptotic cell.34,51 Additional mechanisms are also likely to militate against proinflammatory innate immune responses to apoptotic cells. Thus, surface binding of the pentraxin, C-reactive protein not only opsonizes apoptotic cells to be engulfed effectively along with production of the anti-inflammatory cytokine transforming growth factor (TGF)-β1, but also helps prevent cell lysis and possible proinflammatory consequences through inhibiting assembly of the terminal components of complement in the plasma membrane of the apoptotic cell.52 The bridging molecule MFG-E8 can link PS on the apoptotic cell with the αvβ3 integrin of the phagocyte and functions clearly as a trigger of engulfment.36,53 It will be of great interest to know whether this pathway also engages dominant anti-inflammatory responses in phagocytes.
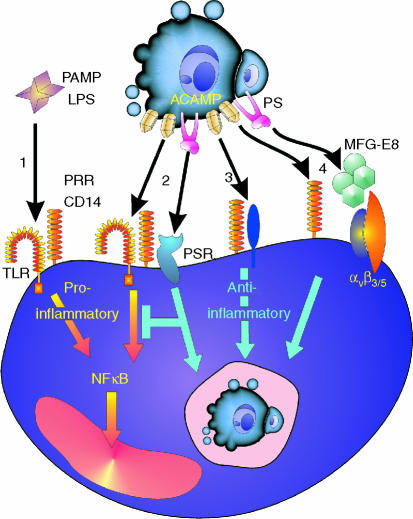
Figure 3.
Outline of possible scenarios for common mechanisms of pattern recognition of PAMPs and ACAMPs with differential macrophage responses illustrated by the pattern recognition receptor (PRR), CD14. 1: Proinflammatory response to a prototypic PAMP, LPS, in which the GPI-anchored CD14 cooperates with TLRs to activate NFκB and elicit proinflammatory responses. 2: engagement of CD14 by ACAMPs may engage a similar default inflammatory signalling pathway but here additional receptor–ligand interactions, e.g. the PS/PSR interaction shown, dominantly suppress the proinflammatory response and may also engage anti-inflammatory signalling pathway(s). 3: Alternatively, in a ligand-dependent manner, CD14 may associate with different signal-transduction partners that activate anti-inflammatory rather than proinflammatory responses in the phagocyte. 4: A further scenario is that CD14–ACAMP interactions merely tether the apoptotic cell to the phagocyte and that additional receptor/ligand interactions such as PSR/PS or, as illustrated, vitronectin receptor/MFG-E8/PS, activate anti-inflammatory responses in the macrophage.
Discrete or redundant mechanisms?
Why does the process of apoptotic-cell clearance involve so many molecules? It is assumed commonly that the multiplicity of molecules reflects redundancy of mechanisms, stressing the biological importance of the clearance process. Undoubtedly, it can be argued that the clearance process itself effectively provides apoptosis with biological importance and a case can be made for the existence of redundant, failsafe mechanisms; but there are likely to be further explanations for the apparent multiplicity of pathways. For example, multiple molecules could reflect multiple phases in the interaction between phagocyte and apoptotic cells. As implied above, there may be distinct mechanisms that support the clearance of early versus late stages of apoptosis. Furthermore, mounting evidence indicates that the significance of the molecules involved in the clearance process lies in their functional activity in mediating defined steps in the underlying intercellular interactions (Fig. 4). These steps have been termed previously ‘tethering’ and ‘tickling’54 and in chronological order are categorized here as (1) recognition (the beginnings of the intercellular interaction); (2) binding (tethering); and (3) signalling (for initiation of engulfment and other responses of the phagocyte including production of anti-inflammatory mediators). Molecular interactions in these three categories culminate in (4) phagocytosis (confinement of the apoptotic cell or body within the phagocyte) (Fig. 4). Examples of molecular partners falling into the first three of these categories include: (1) phagocyte CD31 interacting with disabled CD31 on the apoptotic cell, which leads to productive intercellular interaction;30 (2) SHPS-1 on the phagocyte interacting with CD47 on the apoptotic cell to mediate intercellular adhesion (tethering);55 and (3) phagocyte PSR interacting with phosphatidylserine on the apoptotic cell to initiate phagocytic signalling and anti-inflammatory mediator release.34 Clearly, some molecules may have multiple functions within this scheme: a tethering receptor on the phagocyte may function additionally as a signal transduction molecule, for example. Multiple, low-affinity receptor–ligand interactions may serve to form a high-avidity complex − a phagocytic synapse2,8,10 akin to the immunological synapse formed between T cells and antigen-presenting cells.
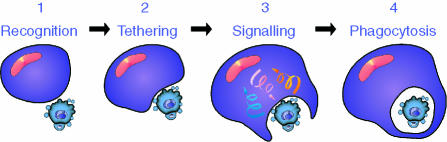
Figure 4.
Phases of interaction between apoptotic cell and macrophage. (1) Initial contact events (e.g. CD31/CD31) precede (2), the tethering stage (involving, e.g. CD14, CD47) which facilitates the initiation of (3) ‘outside-in’ and ‘inside-out’ signal transduction events (involving, e.g. PS/PSR, integrins) that activate the phagocyte. The phagocyte responses include reinforcement of intercellular adhesion, production of anti-inflammatory cytokines and (4) phagocytosis.
Further apparent molecular diversity may be rooted in the tissue context of the clearance event (and it should be noted that macrophages from different locations have different constitutive capacities to clear apoptotic cells,56) or the stage of apoptosis. Thus, the preferential usage of particular molecular interactions between macrophages and apoptotic cells may be dependent not only on the state of activation of the macrophage but also on the lineage of the apoptotic cell and the point it has reached in the apoptosis programme when encountered by the phagocyte. Supportive evidence includes recent observations indicating that MFG-E8 and its closely related structural and functional homologue, Del-1 (developmental endothelial locus-1) are produced by distinct populations of macrophages;57 C1q appears to be important for the clearance of apoptotic cells in the kidney but plays no part in apoptotic keratinocyte clearance;58 CD43 appears to be involved at an early stage in the apoptosis programme, whereas the pentraxins and collectins may preferentially facilitate uptake of late apoptotic cells.59,60 Therefore the interaction of an individual macrophage with a specific lineage of apoptotic cell at a particular stage in its apoptosis programme may be far simpler than might be apparent from Fig. 2. Characterization of additional molecules associated with distinct stages of apoptosis that are implicated in the clearance process (see, for example Fujii et al.46) will, it is hoped, simplify rather than complicate this picture further.
Multitasking: secondment of molecules to apoptotic-cell clearance
As discussed above, there is clear evidence that molecules of the innate immune system better known for their activities in clearing microbial pathogens and their products are also functional in enabling macrophages to clear apoptotic cells. It remains doubtful from the available evidence that any of the molecules implicated so far in the clearance process are dedicated to that process: active molecules associated with the apoptotic cell appear to be modified, disabled or translocated, whereas macrophage receptors and intermediate factors are ‘seconded’ (or vice versa) to clearance of apoptotic cells from other processes including angiogenesis, immunity and repair. Even the novel PSR34 which is found on the surface of all cells known to be capable of engulfing apoptotic cells,2 also has a ‘day-job’ in the nucleus where it is probably functional in chromatin remodelling.61,62 Whether the future holds any molecules specific for the apoptotic-cell clearance process remains to be seen.
Consequences of Macrophage/Apoptotic-Cell interactions
Anti-inflammatory effects of apoptotic cells
Macrophages do not simply provide a neutral means to package, isolate and degrade apoptotic cells, although this may be one of their functions (see Kurosaka et al.63). Rather, their active responses to the apoptotic cells they engulf have inflammatory and immunological consequences. There is no doubt that apoptotic cells can not only fail to stimulate,64,65 but can actively suppress66 proinflammatory mediator release from engulfing macrophages in vitro. A convincing body of evidence shows that mononuclear phagocytes responding to apoptotic cells release anti-inflammatory cytokines, including interleukin (IL)-10 and TGF-β1, which may act as key local autocrine or paracrine anti-inflammatory factors and immunosuppressants66–68 (Ogden, Gregory et al., submitted). While the mechanisms that determine anti-inflammatory mediator release have yet to be detailed, the process appears to be PS-dependent and it has been suggested that the PSR may play an important signalling role in this pathway, particularly with respect to TGF-β1.2,51,69 Notionally, as annexin I can stimulate IL-10 production, this could provide an additional mechanism whereby IL-10 is secreted by mononuclear phagocytes engaging with apoptotic cells via annexin I-dependent mechanisms.70
Direct suppression of proinflammatory responses may follow apoptotic–cell interaction via mechanisms that do not require soluble intermediates. One such signalling pathway may emanate from SHPS-1 following its ligation by CD47,55 because it is known that proinflammatory cytokine production by immature dendritic cells is inhibited by SHPS-1 signalling.71 However, SHPS-1 activity is also known to cause inhibition of phagocytosis in macrophages.72 Like SHPS-1, CD31 possesses intracellular immunoreceptor tyrosine-based inhibitory motifs suggesting that it too could inhibit proinflammatory macrophage responses. Furthermore, the kinase domain of Mer has the potential to generate direct anti-inflammatory macrophage responses to apoptotic cells possibly through its ability to engage PS on apoptotic cells via the product of the growth-arrest-specific gene, gas6 see8,73 (Fig. 2).
Additional mechanisms contribute to the anti-inflammatory relationship between the phagocyte and the apoptotic cell. First, apoptotic cells can themselves produce immunomodulatory factors such as IL-1074 and TGF-β1.75 Each of these anti-inflammatory mediators is capable of up-regulating the capacity of macrophages to clear apoptotic cells77 (Ogden, Gregory et al., submitted). Furthermore, by making contact with apoptotic neutrophils, activated monocytes can become switched from a proinflammatory to an anti-inflammatory state.77 Thus the interplay between apoptotic cells and mononuclear phagocytes appears to create a microenvironment that not only suppresses immune and inflammatory responses, but also facilitates efficient apoptotic-cell clearance.
Proinflammatory effects of apoptotic cells
The anti-inflammatory and immunosuppressive engulfment of apoptotic cells by macrophages is often simply contrasted to the effects of necrotic cells, which are taken to be proinflammatory and immunostimulatory. As we have discussed, there is strong evidence supporting this view. However, a body of in vitro investigations challenges its simplicity. Let us consider first the ‘accepted wisdom’ that macrophages are able to discriminate between apoptotic cells and necrotic cells and respond accordingly. Furthermore, let us not forget the related accepted dogma that apoptotic cells must be cleared while their membranes are intact, for if they become permeabilized (often referred to as ‘secondary necrosis’), they can switch macrophages' responses from anti-inflammatory to proinflammatory. When comparing the relationship between an apoptotic versus a necrotic cell and the macrophage, it is important to recognize the lineage of the dying cell. In the case of granulocytic leukocytes, lysed, necrotic cells stimulate proinflammatory responses in macrophages including release of the inflammatory eicosanoid, thromboxane, granulocyte-macrophage colony stimulating factor (GM-CSF), macrophage inflammatory protein 2, IL-8 and tumour necrosis factor (TNF)-α.65,78 Recent work suggests that among the macromolecular contents of these cells, liberated proteases provide a proinflammatory stimulus endowing necrotic neutrophils, but not lymphocytes, with proinflammatory properties towards macrophages.78 Intriguingly, this work demonstrated that the membranes of either necrotic or apoptotic cells provided macrophages with an anti-inflammatory stimulus (leading to TGF-β1 release) which may well be related to phagocyte receptor or bridging molecule accessibility to PS in the membranes of both necrotic and apoptotic cells. Liberated neutrophil elastase may also promote inflammation by cleaving the PSR and/or other anti-inflammatory signalling receptors.79 Support for the concept that necrotic cells are not necessarily proinflammatory has been provided by studies of necrotic fibroblastic cells which, like their apoptotic counterparts, fail to induce proinflammatory cytokine production.80 Other work has demonstrated that necrotic cells are unable to activate macrophages themselves but can enhance their activation by LPS, whereas apoptotic cells inhibit proinflammatory macrophage responses.81
An additional mechanism underlying the differential abilities of apoptotic and necrotic cells to stimulate inflammatory responses has been proposed based on the extracellular activity of the DNA-binding protein HMGB-1, which can leak out of necrotic cells inciting inflammatory responses from macrophages but remains sequestered in apoptotic cells, bound irreversibly to chromatin.82,83 Macrophage activation by HMGB-1 is LPS-like, being dependent upon TLR2 and TLR4.84 Absence of available extracellular HMGB-1 may provide a molecular basis for the lack of proinflammatory effects of late/leaky apoptotic cells such as lymphocytes, which lack the stimulatory protease mechanism described above. Therefore, cells undergoing secondary necrosis may possess two distinct mechanisms that prevent proinflammatory stimulation: (1) in common with necrotic cells, PS accessibility (perhaps together with other molecules involved in the phagocytic synapse) at the plasma membrane and (2) HMGB-1 sequestration. Because in most, although not all, studies undertaken thus far, the anti-inflammatory effects of apoptotic cells can inhibit the proinflammatory stimuli dominantly, it would be of interest to know the circumstances (if any) under which the anti-inflammatory effect of the necrotic cell membrane could prevent the proinflammatory effects of released factors such HMGB-1.
It is reasonable to conclude that the onset of secondary necrosis does not necessarily carry the dire inflammatory consequences we are perhaps led to expect. Again in contrast to expectations, numerous studies indicate that clearance of apoptotic cells can have inflammatory consequences. It is well documented that autoantibodies, including those against PS or nuclear autoantigens displayed in blebs at the apoptotic-cell surface, can opsonize apoptotic cells triggering proinflammatory responses in macrophages that engulf via Fc receptors (reviewed in Savill et al. and Hart et al.8,13). Less well known is the role of IgG immune complexes that can bridge apoptotic neutrophils to macrophages via Fcγ receptors that trigger proinflammatory responses in the phagocyte.85 Other instances of apoptotic cells stimulating proinflammatory responses abound86–90 and, while in some cases this may be a feature of the lateness of the phase of target-cell apoptosis encountered by the phagocyte, in other cases it appears to be a feature of the kinetics of the interaction itself. For example, a recent study found that macrophages responding to apoptotic cells in combination with TLR ligands exhibited an early secretion of proinflammatory cytokines including TNF-α that was followed by TGF-β1 release.91 The significance of this early proinflammatory response is as yet unknown, although it could be important when apoptotic cells are being generated during an infection with pathogenic parasitic organisms such Trypanosoma cruzi and Leishmania major that are able to subvert the anti-inflammatory effects of apoptotic cells to their advantage.92–94
Balancing acts
The macrophage's response to the apoptotic cell may therefore be (a) neutral, (b) anti-inflammatory or (c) proinflammatory and so an important issue in our understanding of the macrophage's responses to apoptosis is the balance between these responses at sites of cell death. There are common mechanisms through which necrotic and apoptotic cells interact with macrophages and it is conceivable that mononuclear phagocytes respond to common chemotactic signals95–97 released from both apoptotic and necrotic cells. The differential responsiveness of the macrophage in an apoptotic versus necrotic milieu is therefore likely to depend largely on the balance of pro- versus anti-inflammatory signals in the phagocyte's immediate vicinity.
In cases of overwhelming, synchronous apoptosis − activated, for example, by exposure to ionising radiation (see Lorimore et al.90) or by an infectious agent, or simply during development − release of chemotactic factors from apoptotic cells (including secondarily necrotic cells) represents a call for recruitment of further mononuclear phagocytes to assist in clear-up operations. Specialized approaches to visualizing apoptotic cells in situ indicate that their remnants are more common than was appreciated previously.17 When the overwhelming dying cells are neutrophils at an inflammatory site, protease release from dead cells may well provide a mechanism for sustained neutrophil recruitment until such time as sufficient macrophages are present at the site to ensure efficient engulfment of apoptotic neutrophils at an early stage in their apoptosis programme.
Macrophage/Apoptotic–Cell interactions in vivo: Consequences of Defective clearance
Much of the current knowledge of the apoptotic-cell clearance process stems from molecular cell biological studies of macrophages interacting with apoptotic cells in vitro. Whole-animal studies lag behind but, with the increasing availability of appropriate genetically modified models, are set to move apace. Already, the immunological implications of defective apoptotic-cell clearance have been found to be associated with the pathogenesis of autoimmune disease. This association may be logical, but is it over-simplified?
Simplifying the molecular picture: lessons from knock-outs
Genetic studies in the worm C. elegans have identified a number of genes required for the efficient phagocytosis of cells that are deleted predictably during worm development.3,98 These genes are conserved evolutionarily (Fig. 5) and signify mechanisms underlying the neighbourly engulfment processes of non-specialized phagocytes because C. elegans does not contain macrophages. Although the studies are much less advanced, evolutionary conservation has also been noted in Drosophila, which does possess an ancient macrophage, the haemocyte.37,99 The functional attributes of these conserved molecules encompass surface receptor–ligand interactions and intracellular signalling, notably in relation to the extension of cell surfaces that occurs in migration as well as phagocytosis.10,100–103 The worm also possesses a functional PSR.104 In representing ‘minimal’ requirements for the engulfment of dying cells, these molecules provide a simplifying background to the mammalian macrophage picture described above and depicted in Fig. 2. However, in order to perform its function as professional scavenger of apoptotic cells effectively in vivo, which of the multitude of molecules thus far implicated in apoptotic-cell clearance does the macrophage require?
Figure 5.
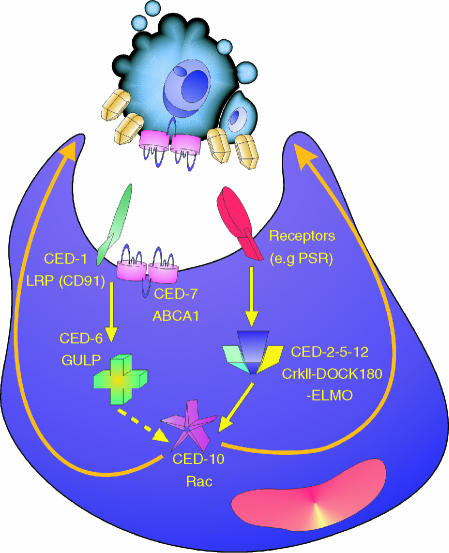
Apoptotic-cell clearance pathways conserved between worms and mammals. Mutagenesis studies in Caenorhabditis elegans have identified seven genes involved in clearance that comprise two partially redundant pathways, one involving ced-1, -6, and -7, the other involving ced-2, -5, -10 and -12. Mammalian homologues of the CED-1, -6 and -7 proteins are LDL-related receptor protein (LRP, also known as CD91), engulfment adaptor protein (GULP), and the ATP-binding cassette transporter, ABCA1, respectively. The homologous proteins in the ced-2, -5, -10, -12 pathway are the adapter proteins CrkII and Dock180, the Rho family GTP-binding protein Rac and ELMO (engulfment and motility), respectively. The latter, in complex with Dock180. acts as a guanine nucleotide exchange factor for Rac. The ced-2, -5, -10, -12 pathway is functional not only in mobilizing the actin cytoskeleton in extending the cytoplasm to surround the apoptotic cell during engulfment but also in cell migration.
Use of ‘knock-out’ mice has begun to help answer this question. As detailed in Table 2, several lines of mice possessing engineered defects in individual genes implicated in apoptotic-cell clearance have been reported including C1q, ABCA1, SR-A, Mer, PSR, MFG-E8 and CD1453,105–110 (Devitt, Gregory et al., submitted). In the context of redundancy of molecules mediating apoptotic-cell clearance, it would be surprising if absence or non-functionality of individual molecules were to produce phenotypic defects. Indeed, in the case of the SR-A knock-out mouse, no persistence of apoptotic cells was apparent in the thymus in vivo, even following induction of massive apoptosis by irradiation, despite the fact that SR-A-deficient macrophages are compromised in their capacity to engulf apoptotic thymocytes in vitro.107 In unchallenged merkd mice, which lack Mer kinase activity, clearance of apoptotic cells in the thymus also appears to be normal,108 again suggestive of redundant clearance mechanisms in this tissue. However, upon induction of synchronous apoptosis by dexamethasone, defective apoptotic-cell clearance in the thymus can be revealed in these animals.108
Table 2.
Genetically modified mice with altered phenotypes of apoptotic-cell clearance
| Defect | |||||
|---|---|---|---|---|---|
| Genetic modification | in vivo | in vitro | Tissue / cell affected | Autoimmunity | Refs |
| C1qa–/– | Yes | Yes | Kidney, peritoneal macrophage | Increased antihistone,ANA antibodies,glomerulonephritis | 105,110 |
| C4–/– | Yes | NT | Elicited peritoneal macrophage | NT | 110 |
| CD93–/– | Yes | No | Elicited peritoneal macrophage | Normal | 122 |
| SR-A–/– | No | Yes | ND | NT | 107 |
| ABCA1–/– | Embryonic footplate, elicitedperitoneal macrophage | Glomerulonephritis | 106,123 | ||
| PU.1–/– | Yes | NT | Embryonic footplate, macrophage | NT | 21 |
| merkd | Yes | Thymus (induced apoptosis),elicited peritoneal macrophage | Increased antichromatin,anti-DNA, anti-IgGantibodies, mild renalpathology | 108,111 | |
| TGase2–/– | Yes | Yes | Thymus (induced apoptosis),liver (induced apoptosis),peritoneal macrophage, Kupffercell | Increased autoantibodies,glomerulonephritis | 76 |
| Gal3–/– | Yes | Yes | Elicited peritoneal macrophage.Phagocytic defect not restrictedto apoptotic cells | NT | 124 |
| PSR–/– | Yes | Yes | Developing lung and brain, fetalliver, elicited peritonealmacrophage | NT | 109,125 |
| MFG-E8–/– | Yes | Yes | Lymphoid, tingible bodymacrophage, elicited peritoneal macrophage | Late-onset increased anti-dsDNA, antinuclearantibodies,glomerulonephritis | 53 |
| CD14–/– | Yes | Yes | Primary and secondary lymphoid,lung, liver, gut, peritonealmacrophage, bone marrowmacrophage | Normal | Devitt, Gregory et al., submitted |
Gal-3–/–, galectin-3 deficient; merkd, kinase-defective mutant of mer; ND, none detected; NT, not tested; SR-A, scavenger receptor-A; TGase2, tissue transglutaminase. See Fig. 1 for other abbreviations.
Further examples of single-gene defects causing apoptotic-cell-persistence phenotypes are ABCA1, PSR, C1q, MFG-E8 and CD14. Animals lacking ABCA1 display defective apoptotic-cell clearance during footplate development but fail, ultimately, to show any developmental abnormalities, although many of the animals tend to die soon after birth as a result of perivisceral haemorrhage.106 Like ABCA1, PSR-deficiency compromises engulfment of apoptotic cells during development not only of worms but also of mice.104,109 PSR deficiency in mice also leads to neonatal lethality caused apparently by the accumulation of dead cells in the developing lung and brain.109 Single-gene defects can also cause persistence of apoptotic cells in unchallenged adult tissues. In seminal work, it was demonstrated that C1q deficiency caused persistence of apoptotic cells in kidney glomeruli of genetically susceptible mice.105 By deliberate instillation of apoptotic cells, the clearance defect was also demonstrable in the peritoneal cavity.110 The tingible-body macrophages of germinal centres appear to be affected especially in MFG-E8-deficient mice, resulting in defective engulfment of apoptotic B cells in secondary lymphoid tissues.53 Our recent work reports that CD14-deficiency leads to persistence of apoptotic cells in multiple tissues in adult mice including thymus, spleen, lung, liver and gut (Devitt, Gregory et al., submitted). Taken together, these results suggest that certain of the molecules implicated in apoptotic-cell clearance are functionally redundant (e.g. SR-A), others are essential for proper development (e.g. PSR), others are important when rates of apoptosis in relevant tissues are normal (e.g. C1q, MFG-E8, CD14) and others are required for efficient clearance when rates of apoptosis are accelerated (e.g. Mer). The tissue context of the various phenotypes emphasizes the concept raised above that individual molecules may assume particular importance in specific tissues.
The study of further knock-out animals, including multiple mutants, is likely to clarify further the issue of redundancy in the mechanisms underlying clearance of apoptotic cells. In addition to the effects of molecules involved directly in the interaction between apoptotic cells and phagocytes, indirect effects of molecules in modulating apoptotic-cell clearance − such as TGase2 in promoting clearance via production of TGF-β176 – are also likely to emerge.
Defective clearance of apoptotic cells: association with autoimmune disease
The finding that the C1q-deficient mouse develops glomerulonephritis with immune deposits105 provided the first in vivo evidence supporting the long-standing notion that apoptotic cells provide an important source of autoantigens and that inefficient clearance of apoptotic cells could lead to autoimmune disease. Some mutant mice in which apoptotic cells persist in adult tissues as a result of defective clearance follow this pattern, but others do not. Thus animals defective in the Mer tyrosine kinase also produce increased titres of lupus-like autoantibodies.108,111 Recent work has shown that MFG-E8-deficient mice similarly develop high titres of autoantibodies and consequent glomerulonephritis.53 TGase2-deficient animals produce high titres of autoantibodies and develop immune complex glomerulonephritis, which may be due in part to the defect in macrophage clearance of apoptotic cells displayed by these animals and in part by the absence of active TGF-β production. By contrast, mice lacking CD14 fail to generate increased levels of autoantibodies and fail to develop autoimmune disease, despite the extensive persistence of apoptotic cells in multiple tissues (Devitt, Gregory et al., submitted). Therefore, while defective apoptotic-cell clearance is often associated with autoimmune disease, there is not a rigorous causative link.
What then, are the mechanisms that link the defective clearance of apoptotic cells with increased autoimmune responses? Undoubtedly, apoptotic cells are rich sources of autoantigens, including those of nucleosomes that are sequestered in surface blebs.112,113 A simplistic view is that, because apoptotic cells engulfed by macrophages tend to engender immunosuppressive or tolerogenic phagocyte responses, then in cases of such responses being inhibited increased autoimmunity might be logically expected. Given our discussion above, secondarily necrotic cells may not only fail to incite proinflammatory responses in macrophages but also continue to promote anti-inflammatory responses. Therefore, defective apoptotic-cell clearance would only be expected to promote autoimmunity if the anti-inflammatory effects of apoptotic cells became switched to proinflammatory effects. In the specific case of genetically modified animals, loss of molecules promoting anti-inflammatory responses − the Mer tyrosine kinase, for example − might be expected to result in a switched response of phagocytes to apoptotic cells. Interestingly, the tethering of apoptotic cells to macrophages is normal in merkd mice.108 Similar arguments could be made for MFG-E8-deficient macrophages, which also tether apoptotic cells normally but fail to engulf them efficiently.53 Again it might be predicted that in such macrophages, anti-inflammatory responses to apoptotic cells are inhibited. In stark contrast, loss of molecules such as CD14 that promote tethering of apoptotic cells without affecting anti-inflammatory responsiveness (Devitt, Gregory et al., submitted) would not be expected to result in inflammation or autoimmunity. Indeed, it is conceivable that in such situations the persistence of apoptotic cells might promote longevity of anti-inflammatory signals.
The significance of tissue-specific effects and genetic background in linking autoimmunity with apoptotic-cell clearance are as yet poorly understood. For example, the germinal centre phenotype of the MFG-E8-deficient mouse53 may be critical in driving the autoimmune disease in these animals. The autoimmune phenotype of the C1q-deficient mouse requires a mixed genetic background (129 X C57BL/6) and the basal apoptotic-cell clearance defect appears to be tissue-restricted.105 Of critical importance is whether defective clearance of apoptotic cells represents a primary pathogenic component in autoimmune diseases such as systemic lupus erythematosus (SLE) or whether, alternatively, acquired defects in the clearance of apoptotic cells emerge secondarily to autoimmunity. For example, inhibition of clearance by nucleosomes or autoantibodies may serve to amplify, rather than cause, autoimmune responses. This issue is contentious, studies of apoptotic-cell clearance in SLE-prone mutant mice reporting conflicting results. Thus it has been suggested that such animals have an intrinsic defect in apoptotic-cell clearance that may be important in the initiation of autoimmunity.114 Others have concluded that macrophages from lupus-prone animals do not display a constitutive defect in their capacity to clear apoptotic cells.115,116 Rather, in the premorbid phase, macrophages were found to clear apoptotic cells normally, defective clearance being manifest during disease progression and mediated by serum factor(s).116
We support the notion that defective apoptotic-cell clearance only plays a primary role in autoimmune disease pathogenesis only under special circumstances: when the clearance defect is accompanied by a modified response of the phagocyte to the (inefficiently cleared) apoptotic cell. Thus, the autoimmune disease-inducing effects of C1q, Mer and MFG-E8 would be predicted to require defective anti-inflammatory signalling via CD91/calreticulin, Mer tyrosine kinase and αvβ3, respectively. In other words, persistence of apoptotic cells and autoimmune disease are by no means linked inextricably. Indeed, we speculate that persistence of apoptotic cells in the context of no defect in anti-inflammatory/immunosuppressive signalling could militate against autoimmunity.
Conclusions
In recent years, considerable progress has been made in defining some of the molecular details of the final clearance phase of the apoptosis programme. The macrophage remains the most frequently studied and consequently best understood phagocyte of apoptotic cells. We should keep in mind, however, that much of cell death in situ is probably proceeding unnoticed − simply because the phagocytic clearance (by neighbouring cells as well as macrophages) of the earliest dying cells is not readily tractable. Undoubtedly, more detailed approaches to the study of apoptosis in situ are warranted to fill this notable gap in current knowledge. While it may not be the most frequent phagocyte of apoptotic cells, the macrophage's contribution to immune homeostasis is likely to be immense. Its anti-inflammatory and immunosuppressive tendencies in responding to apoptotic cells makes the macrophage a potentially powerful regulator of adaptive immune responses, including autoimmune and antitumour responses.
The macrophage's unified ability to phagocytose host cells as well as microorganisms has been known since the days of Metchnikoff. While the mechanisms that control responsiveness of this professional phagocyte to pathogenic microorganisms versus dying host cells are only just beginning to be defined, it is clear that there is much common ground between the macrophage's relationship with apoptotic cells and with microorganisms. Finally, let us not forget that the macrophage not only responds to dead and dying cells, it can influence cell life and death markedly through provision of survival factors or by promoting apoptosis.117–121 The macrophage and the apoptotic cell is far from a simple relationship, but continued improvement in our understanding of its underlying mechanisms is likely to yield new therapeutic targets for inflammatory, autoimmune and malignant diseases.
Acknowledgments
Work in the authors' laboratory is supported by the Medical Research Council, UK and the Leukaemia Research Fund. We acknowledge the contributions made by many colleagues in the field whose work we have not cited because of space constraints. We are particularly indebted to our Edinburgh colleagues for fruitful discussions and especially acknowledge Simon Brown, Ian Dransfield and Adriano Rossi for critical comments on the manuscript.
References
- 1.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–62. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengartner MO. Apoptosis: corralling the corpses. Cell. 2001;104:325–8. doi: 10.1016/s0092-8674(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 4.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–33. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 5.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 6.Hamon Y, Chambenoit O, Chimini G. ABCA1 and the engulfment of apoptotic cells. Biochim Biophys Acta. 2002;1585:64–71. doi: 10.1016/s1388-1981(02)00325-6. [DOI] [PubMed] [Google Scholar]
- 7.Manfredi AA, Iannacone M, D'Auria F, Rovere-Querini P. The disposal of dying cells in living tissues. Apoptosis. 2002;7:153–61. doi: 10.1023/a:1014366531885. [DOI] [PubMed] [Google Scholar]
- 8.Savill J, Dransfield I, Gregory CD, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. Opsonization of apoptotic cells and its effect on macrophage and T cell immune responses. Ann NY Acad Sci. 2003;987:68–78. doi: 10.1111/j.1749-6632.2003.tb06034.x. [DOI] [PubMed] [Google Scholar]
- 10.Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don't eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–56. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Rovere-Querini P, Dumitriu IE. Corpse disposal after apoptosis. Apoptosis. 2003;8:469–79. doi: 10.1023/a:1025538324077. [DOI] [PubMed] [Google Scholar]
- 12.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–31. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 13.Hart SP, Smith JR, Dransfield I. Phagocytosis of opsonized apoptotic cells: roles for ‘old-fashioned’ receptors for antibody and complement. Clin Exp Immunol. 2004;135:181–5. doi: 10.1111/j.1365-2249.2003.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maderna P, Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta. 2003;1639:141–51. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Van den Eijnde SM, Boshart L, Reutelingsperger CPM, De Zeeuw CI, Vermeij-Keers C. Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Diff. 1997;4:311–6. doi: 10.1038/sj.cdd.4400241. [DOI] [PubMed] [Google Scholar]
- 16.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leers MP, Bjorklund V, Bjorklund B, Jornvall H, Nap M. An immunohistochemical study of the clearance of apoptotic cellular fragments. Cell Mol Life Sci. 2002;59:1358–65. doi: 10.1007/s00018-002-8513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao WM, Murao K, Imachi H, et al. Phosphatidylserine receptor cooperates with high-density lipoprotein receptor in recognition of apoptotic cells by thymic nurse cells. J Mol Endocrinol. 2004;32:497–505. doi: 10.1677/jme.0.0320497. [DOI] [PubMed] [Google Scholar]
- 19.Bursch W, Taper HS, Lauer B, Schulte-Hermann R. Quantitative histological and histochemical studies on the occurrence and stages of controlled cell death (apoptosis) during regression of rat liver hyperplasia. Virchows Arch B Cell Pathol Mol Pathol. 1985;50:153–66. doi: 10.1007/BF02889898. [DOI] [PubMed] [Google Scholar]
- 20.Dogusan Z, Montecino-Rodriguez E, Dorshkind K. Macrophages and stromal cells phagocytose apoptotic bone marrow-derived B lineage cells. J Immunol. 2004;172:4717–23. doi: 10.4049/jimmunol.172.8.4717. [DOI] [PubMed] [Google Scholar]
- 21.Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–52. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 22.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–60. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 23.Knepper-Nicolai B, Savill J, Brown SB. Constitutive apoptosis in human neutrophils requires synergy between calpains and the proteasome downstream of caspases. J Biol Chem. 1998;273:30530–6. doi: 10.1074/jbc.273.46.30530. [DOI] [PubMed] [Google Scholar]
- 24.Eda S, Yamanaka M, Beppu M. Carbohydrate-mediated phagocytic recognition of early apoptotic cells undergoing transient capping of CD43 glycoprotein. J Biol Chem. 2004;279:5967–74. doi: 10.1074/jbc.M310805200. [DOI] [PubMed] [Google Scholar]
- 25.Devitt A, Pierce S, Oldreive C, Shingler WH, Gregory CD. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidyiserine. Cell Death Diff. 2003;10:371–82. doi: 10.1038/sj.cdd.4401168. [DOI] [PubMed] [Google Scholar]
- 26.Albert ML, Pearce SFA, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alpha (v) beta (5) and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronchetti A, Rovere P, Iezzi G, et al. Immunogenicity of apoptotic cells in vivo. Role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–6. [PubMed] [Google Scholar]
- 28.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–3. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 29.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–22. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–3. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CA, de Cathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–9. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 33.Dini L. Recognizing death: liver phagocytosis of apoptotic cells. Eur J Histochem. 2000;44:217–27. [PubMed] [Google Scholar]
- 34.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekowitz RAB, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 35.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 36.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 37.Franc NC, White K, Ezekowitz RAB. Phagocytosis and development: back to the future. Curr Opin Immunol. 1999;11:47–52. doi: 10.1016/s0952-7915(99)80009-0. [DOI] [PubMed] [Google Scholar]
- 38.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 39.Janeway CA. Approaching the asymptote − evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Medzhitov R, Janeway CA. Innate immunity. The virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 41.Pugin J, Heumann D, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 42.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–7. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 43.Kagan VE, Gleiss B, Tyurina YY, et al. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–99. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- 44.Anderson HA, Englert R, Gursel I, Shacter E. Oxidative stress inhibits the phagocytosis of apoptotic cells that have externalized phosphatidylserine. Cell Death Diff. 2002;9:616–25. doi: 10.1038/sj.cdd.4401013. [DOI] [PubMed] [Google Scholar]
- 45.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Devel Cell. 2003;4:587–98. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 46.Fujii C, Shiratsuchi A, Manaka J, Yonehara S, Nakanishi Y. Difference in the way of macrophage recognition of target cells depending on their apoptotic states. Cell Death Diff. 2001;8:1113–22. doi: 10.1038/sj.cdd.4400920. [DOI] [PubMed] [Google Scholar]
- 47.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–9. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–35. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 49.Shiratsuchi A, Watanabe I, Takeuchi O, Akira S, Nakanishi Y. Inhibitory effect of toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. J Immunol. 2004;172:2039–47. doi: 10.4049/jimmunol.172.4.2039. [DOI] [PubMed] [Google Scholar]
- 50.Gregory CD, Devitt A. CD14 and apoptosis. Apoptosis. 1999;4:11–20. doi: 10.1023/a:1009673914340. [DOI] [PubMed] [Google Scholar]
- 51.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gershov D, Kim S, Brot N, Elkon KB. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–63. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–59. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tada K, Tanaka M, Hanayama R, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Tethering of apoptotic cells to phagocytes through binding of CD47 to Src homology 2 domain-bearing protein tyrosine phosphatase substrate-1. J Immunol. 2003;171:5718–26. doi: 10.4049/jimmunol.171.11.5718. [DOI] [PubMed] [Google Scholar]
- 56.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165:2124–33. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172:3876–82. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- 58.Pickering MC, Fischer S, Lewis MR, Walport MJ, Botto M, Cook HT. Ultraviolet-radiation-induced keratinocyte apoptosis in C1q-deficient mice. J Invest Dermatol. 2001;117:52–8. doi: 10.1046/j.0022-202x.2001.01381.x. [DOI] [PubMed] [Google Scholar]
- 59.Bijl M, Horst G, Bijzet J, Bootsma H, Limburg PC, Kallenberg CG. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003;48:248–54. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- 60.Nauta AJ, Raaschou-Jensen N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–63. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 61.Clissold PM, Ponting CP. JmjC: cupin metalloenzyme-like domains in jumonji, hairless and phospholipase A2beta. Trends Biochem Sci. 2001;26:7–9. doi: 10.1016/s0968-0004(00)01700-x. [DOI] [PubMed] [Google Scholar]
- 62.Cui P, Qin B, Liu N, Pan G, Pei D. Nuclear localization of the phosphatidylserine receptor protein via multiple nuclear localization signals. Exp Cell Res. 2004;293:154–63. doi: 10.1016/j.yexcr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–9. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 64.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane-b2. J Leuk Biol. 1992;52:269–73. [PubMed] [Google Scholar]
- 65.Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis − mediation by alpha (v) beta (3)/cd36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–21. [PMC free article] [PubMed] [Google Scholar]
- 66.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 68.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–72. [PubMed] [Google Scholar]
- 69.Fadok VA, Bratton DL, Henson PM. Identification of a novel gene that mediates phosphatidylserine recognition and anti-inflammatory activity in macrophages ingesting apoptotic cells. Cold Spring Harbor Laboratory Abstracts of Programmed Cell Death Meeting, 1999. 2004:64. [Google Scholar]
- 70.Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm Res. 2004;53:125–32. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 71.Latour S, Tanaka H, Demeure C, et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–54. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- 72.Oshima K, Ruhul Amin AR, Suzuki A, Hamaguchi M, Matsuda S. SHPS-1, a multifunctional transmembrane glycoprotein. FEBS Lett. 2002;519:1–7. doi: 10.1016/s0014-5793(02)02703-5. [DOI] [PubMed] [Google Scholar]
- 73.Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003;15:31–6. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 74.Gao YK, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–96. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen WJ, Frank ME, Jin WW, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 76.Szondy Z, Sarang Z, Molnar P, et al. Transglutaminase 2-/- mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA. 2003;100:7812–7. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–77. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 78.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–54. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 79.Vandivier RW, Fadok VA, Hoffmann PR, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–70. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouckaert G, Kalai M, Krysko DV, et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15:1089–100. doi: 10.1091/mbc.E03-09-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12:919–30. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 83.Bianchi ME, Manfredi A. Chromatin and cell death. Biochim Biophys Acta. 2004;1677:181–6. doi: 10.1016/j.bbaexp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 84.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 85.Hart SP, Alexander KM, Dransfield I. Immune complexes bind preferentially to FcgammaRIIA (CD32) on apoptotic neutrophils, leading to augmented phagocytosis by macrophages and release of proinflammatory cytokines. J Immunol. 2004;172:1882–7. doi: 10.4049/jimmunol.172.3.1882. [DOI] [PubMed] [Google Scholar]
- 86.Muhl H, Nold M, Chang JH, Frank S, Eberhardt W, Pfeilschifter J. Expression and release of chemokines associated with apoptotic cell death in human promonocytic U937 cells and peripheral blood mononuclear cells. Eur J Immunol. 1999;29:3225–35. doi: 10.1002/(SICI)1521-4141(199910)29:10<3225::AID-IMMU3225>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 87.Uchimura E, Watanabe N, Niwa O, Muto M, Kobayashi Y. Transient infiltration of neutrophils into the thymus in association with apoptosis induced by whole-body X-irradiation. J Leukoc Biol. 2000;67:780–4. doi: 10.1002/jlb.67.6.780. [DOI] [PubMed] [Google Scholar]
- 88.Kawagishi C, Kurosaka K, Watanabe N, Kobayashi Y. Cytokine production by macrophages in association with phagocytosis of etoposide-treated P388 cells in vitro and in vivo. Biochim Biophys Acta Mol Cell Res. 2001;1541:221–30. doi: 10.1016/s0167-4889(01)00158-6. [DOI] [PubMed] [Google Scholar]
- 89.Kurosaka K, Takahashi M, Kobayashi Y. Activation of extracellular signal-regulated kinase 1/2 is involved in production of CXC-chemokine by macrophages during phagocytosis of late apoptotic cells. Biochem Biophys Res Commun. 2003;306:1070–4. doi: 10.1016/s0006-291x(03)01105-7. [DOI] [PubMed] [Google Scholar]
- 90.Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–69. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- 91.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–5. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 92.Freire-d, e-Lima CG, Nascimento DO, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 93.Balanco JMD, Moreira MEC, Bonomo A, Bozza PT, Amarante-Mendes G, Pirmez C, Barcinski MA. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–3. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 94.Ribeiro-Gomes FL, Otero AC, Gomes NA, et al. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172:4454–62. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 95.Horino K, Nishiura H, Ohsako T, Shibuya Y, Hiraoka T, Kitamura N, Yamamoto T. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab Invest. 1998;78:603–77. [PubMed] [Google Scholar]
- 96.Segundo C, Medina F, Rodriguez C, Martinez-Palencia R, Leyva-Cobian F, Brieva JA. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94:1012–20. [PubMed] [Google Scholar]
- 97.Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3- mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 98.Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gumienny TL, Hengartner MO. How the worm removes corpses: the nematode C-elegans as a model system to study engulfment. Cell Death Diff. 2001;8:564–8. doi: 10.1038/sj.cdd.4400850. [DOI] [PubMed] [Google Scholar]
- 100.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–4. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 101.Tosello-Trampont AC, Brugnera E, Ravichandran KS. Evidence for a conserved role for CrkII and Rac in engulfment of apoptotic cells. J Biol Chem. 2001;276:13797–802. doi: 10.1074/jbc.M011238200. [DOI] [PubMed] [Google Scholar]
- 102.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277:11772–9. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 103.Grimsley CM, Kinchen JM, Tosello-Trampont AC, et al. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–97. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Wu YC, Fadok VA, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–6. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- 105.Botto MI, Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 106.Hamon Y, Broccardo C, Chambenoit O, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 107.Platt N, Suzuki H, Kodama T, Gordon S. Apoptotic thymocyte clearance in scavenger receptor class A- deficient mice is apparently normal. J Immunol. 2000;164:4861–7. doi: 10.4049/jimmunol.164.9.4861. [DOI] [PubMed] [Google Scholar]
- 108.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 109.Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–3. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 110.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen PL, Caricchio R, Abraham V, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–66. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 114.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–32. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 115.Licht R, Jacobs CW, Tax WJ, Berden JH. No constitutive defect in phagocytosis of apoptotic cells by resident peritoneal macrophages from pre-morbid lupus mice. Lupus. 2001;10:102–7. doi: 10.1191/096120301672276558. [DOI] [PubMed] [Google Scholar]
- 116.Licht R, Dieker JW, Jacobs CW, Tax WJ, Berden JH. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun. 2004;22:139–45. doi: 10.1016/j.jaut.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Brown SB, Savill J. Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J Immunol. 1999;162:480–5. [PubMed] [Google Scholar]
- 118.Duffield JS, Erwig LP, Wei XQ, Liew FY, Rees AJ, Savill JS. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 2000;164:2110–9. doi: 10.4049/jimmunol.164.4.2110. [DOI] [PubMed] [Google Scholar]
- 119.Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in C. elegans. Nature. 2001;412:198–202. doi: 10.1038/35084096. [DOI] [PubMed] [Google Scholar]
- 120.Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature. 2001;412:202–6. doi: 10.1038/35084103. [DOI] [PubMed] [Google Scholar]
- 121.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 122.Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004;172:3406–14. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 123.Christiansen-Weber TA, Voland JR, Wu Y, Ngo K, Roland BL, Nguyen S, Peterson PA, Fung-Leung WP. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol. 2000;157:1017–29. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–97. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kunisaki Y, Masuko S, Noda M, Inayoshi A, Sanui T, Harada M, Sasazuki T, Fukui Y. Defective fetal liver erythropoiesis and T lymphopoiesis in mice lacking the phosphatidylserine receptor. Blood. 2004;103:3362–4. doi: 10.1182/blood-2003-09-3245. [DOI] [PubMed] [Google Scholar]