Abstract
We recently showed that increased in vitro heterophil functional efficiency translates to increased in vivo resistance to a systemic Salmonella enteritidis (SE) infection utilizing a parental pair of broiler chickens (lines A and B) and the F1 reciprocal crosses (C and D). Heterophils produce cytokines and modulate acute protection against Salmonella in young poultry. Therefore, we hypothesize that heterophils from SE-resistant chickens (A and D) have the ability to produce an up-regulated pro-inflammatory cytokine response compared to that of heterophils from SE-susceptible chickens (B and C). In this study, heterophils were isolated from day-old chickens and treated with either RPMI-1640 (as the control), or phagocytic agonists (SE, or SE opsonized with either normal chicken serum or immune serum against SE) and cytokine mRNA expression assessed using real-time quantitative reverse transcription–polymerase chain reaction. Heterophils from SE-resistant chickens (A and D) had significantly higher levels of pro-inflammatory cytokine (interleukin (IL)-6, IL-8, and IL-18) mRNA expression upon treatment with all agonists compared to heterophils from SE-susceptible lines (B and C). Further, heterophils from SE-resistant chickens had significantly decreased mRNA expression levels of transforming growth factor-β4, an anti-inflammatory cytokine, when compared to heterophils from SE-susceptible chickens. These data indicate cytokine gene expression in heterophils may be a useful parameter in determining resistance to Salmonella, as indicated by our previous in vivo SE studies. Therefore, heterophil functional efficiency and cytokine production may be useful biomarkers for poultry breeders to consider when developing new immunocompetent lines of birds.
Keywords: cytokines, heterophils, salmonella, chickens
Introduction
There are two functional arms of the immune system: innate (natural) and acquired (adaptive) immunity. Historically, the innate immune response was thought of as the first-line of defence and functioned to limit infection until the acquired response was initiated.1 However, recent studies indicate the innate immune response provides instruction for the acquired immune response2–5 which begins with the recognition of self from (infectious) non-self by detecting molecules unique to invading organisms referred to as pathogen-associated molecular patterns (PAMP) that are recognized by pattern recognition receptors (PRR) on host immune cells.2,6–10 The end product of microbial recognition by cells of the innate response is the activation of intracellular signalling pathways that initiate cellular processes, such as activation of microbicidal killing mechanisms, the production of pro-inflammatory and anti-inflammatory cytokines, and the production of costimulatory molecules required for antigen presentation to the acquired immune system.3,9,11,12
As the first cells to migrate to the site of infection, polymorphonuclear leucocytes (PMN) are critical components of the innate immune response and the subsequent inflammatory response.13–16 Heterophils are the primary PMN in chickens, and are the avian counterpart to mammalian neutrophils.17,18 Functionally, heterophils modulate the acute innate host response through rapid phagocytosis of invading microbes and foreign particles, production of oxygen intermediates, and by releasing proteolytic enzymes.19–22 The phagocytic process is triggered by two distinct cell surface receptors, one involving the recognition of the carboxy-terminus of immunoglobulin molecules by the Fc receptor and the other where complement receptors recognize complement fragments.23,24 Until recently, heterophils were thought to be terminally differentiated cells, deficient of transcriptional activity, and with little protein synthesis. However, using real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR) measuring specific mRNA expression of various cytokines, we recently showed that heterophils isolated from day-old outbred chickens have a distinct pro- or anti-inflammatory cytokine response dependent on the phagocytic agonist used as a stimulant.25
Cytokines are essential effector molecules of innate and acquired immunity that initiate and coordinate cellular and humoral responses aimed at eradicating pathogens. Proper communication between pathogen and host cells via PRRs and PAMPs initiates signal transduction pathways which in turn induces the expression of cytokines and costimulatory molecules which control immune function and subsequently direct the appropriate immune response.10 The recent cloning of chicken cytokines has enabled the development of a more comprehensive array of reagents for investigating the innate and acquired immune responses at cellular and molecular levels. Chicken orthologues of the T helper 1 (Th1) cytokines interferon-γ (IFN-γ)26 and interleukin-18 (IL-18),27 the pro-inflammatory cytokines IL-1β28 and IL-6,29 the chemokine IL-8,30,31 and the anti-inflammatory cytokine transforming growth factor-β4 (TGF-β4)32 have been cloned and sequenced. This information makes it possible to design probes and primers to quantify cytokine mRNA expression using real-time quantitative RT–PCR in a specific population of cells, such as heterophils.
Recently, we have characterized the in vitro heterophil functional efficiency and in vivo resistance to Salmonella enteritidis (SE) in a parental pair of broilers (lines A and B) and the F1 reciprocal crosses (C = A hen × B rooster and D = B hen × A rooster). First, we have found differences in in vitro heterophil functional efficiency between the two parental lines (A > B) of broilers as well as the F1 reciprocal crosses (D > C).22,33 Second, we have found a relationship between the in vitro heterophil functional inefficiency and susceptibility of these chickens to Salmonella infections. Two different in vivo SE challenge models have shown differences in resistance to an extraintestinal SE infection between the parental lines. Using a mortality model, line A chickens were more resistant to an SE infection than line B chickens (1% versus 33·7% mortality, respectively; Swaggerty and Kogut, unpublished data). Further, an SE organ invasion study has confirmed the differences in susceptibility to extraintestinal SE infection between lines A and B (57% and 89% SE-positive, respectively; Ferro and Kogut, unpublished data).
The objective of the present study was to further characterize the innate immune response in these four lines of broilers by evaluating cytokine mRNA expression levels in isolated heterophils. The specific objectives were to (1) determine the basal levels of constitutively expressed mRNA of IL-6, IL-8, IL-18, and TGF-β4 and (2) determine if isolated heterophils have a differential cytokine response following stimulation with phagocytic agonists (SE, SE opsonized with either normal chicken serum (NCS-OpSE) or immune serum prepared from SE-infected chickens (IgY-OpSE)). Based on the findings of this study and our previous reports, we propose that isolating heterophils and evaluating cytokine mRNA expression levels can be used as an indicator of overall immune competence when selecting for immunologically competent poultry.
Materials and methods
Experimental chickens
Parental and F1 reciprocal cross broiler chickens used in this study were obtained from a commercial breeder. To maintain confidentiality, the lines were designated A, B, C, and D where lines A and B are parental lines and C and D are F1 reciprocal crosses of the two parent lines (C = A hen × B rooster and D = B hen × A rooster). Fertilized eggs were set in incubators (GQF Manufacturing Company, Savannah, GA; Jamesway Incubator Company, Inc., Ontario, Canada; or Petersime Incubator Co., Gettysburg, PA) and maintained at wet and dry bulb temperatures of 32·2°C and 37·8°C, respectively. After 10 days of incubation, the eggs were candled; non-fertile and non-viable eggs were discarded. The viable eggs were returned to the incubator until day 18 when they were transferred to hatchers (Humidaire Incubator Company, New Madison, OH) and maintained under the same temperature and humidity conditions until hatch. At hatch, chickens were placed in their respective floor pens (120 × 120 cm) containing wood shavings, provided supplemental heat, water, and a balanced, un-medicated corn and soybean meal based chick starter diet ad libitum. The feed was calculated to contain 23% protein and 3200 kcal of metabolizable energy/kg of diet, and all other nutrient rations met or exceeded the standards established by the National Research Council.34
Bacteria preparation
A poultry isolate of SE (#97-11771) was obtained from the National Veterinary Services Laboratory (Ames, IA) and approved by the United States Department of Agriculture Animal and Plant Health Inspection Service for use in our facilities. SE was cultured in tryptic soy broth (Difco Laboratories, Becton Dickinson Co., Sparks, MD) overnight at 41°. The bacteria were pelleted (7700 g, 10 min), washed with cold phosphate-buffered saline (PBS), and centrifuged (7700 g, 10 min). The supernatant was discarded, and the pellet was re-suspended in 1 ml cold RPMI-1640. A stock solution of SE (1 × 109 colony forming units (cfu)/ml) was prepared in RPMI-1640. Bacterial concentration was determined spectrophotometrically (Spectronic 20D spectrophotometer, Milton Roy Co., Golden, CO) using a standard curve with a reference wavelength of 625 nm. SE were prepared fresh for each experiment and kept on ice until used.
Opsonization of SE
The SE bacteria were opsonized as previously described.19 Briefly, SE (109 cfu/ml) was suspended in normal chicken serum (NCS-OpSE) or hyper-immune serum from adult chickens immunized against SE (IgY-OpSE) (v:v 4 : 1), incubated for 30 min at 39° on a rotary shaker, and stored at 4° until used.
Isolation of peripheral blood heterophils
Heterophils were isolated from the peripheral blood of 35 chickens (per line) 24 hr posthatch as previously described.19 Briefly, blood from chickens was collected in vacutainer tubes containing disodium ethylenediaminetetraacetic acid (EDTA) (BD vacutainer, Franklin Lakes, NJ) and mixed thoroughly. The blood and EDTA for each line was pooled and diluted 1 : 1 with RPMI-1640 media containing 1% methylcellulose and centrifuged at 35 g for 15 min at 4°. The supernatant was transferred to a new conical tube and diluted with Ca2+- and Mg2+-free Hank's balanced salt solution (1 : 1), layered onto discontinuous Histopaque® gradients (specific gravity 1·077 over 1·119) and centrifuged at 190 g for 1 hr at 4°. The histopaque layers were collected, washed with RPMI-1640 (1 : 1) and pelleted at 485 g for 15 min at 4°. The cells were then re-suspended in fresh RPMI-1640, counted on a haemocytometer, and diluted to 1 × 107/ml in RPMI-1640. All tissue culture reagents and chemicals including endotoxin-free RPMI-1640 (without phenol red), Hanks balanced salt solution, methylcellulose, Histopaque® 1119, and Histopaque® 1077 were obtained from Sigma Chemical Company (St. Louis, MO).
Isolation of total RNA
Purified heterophils (1 × 107/treatment group) isolated from each line of chickens were treated with 300 µl RPMI-1640, SE, NCS-OpSE, or IgY-OpSE for 30 min at 39° on a rotary shaker. Heterophils treated with endotoxin-free RPMI-1640 served as control to establish basal cytokine levels. The treated heterophils were then pelleted (485 × g for 15 min at 4°), washed with RPMI-1640 and pelleted again (485 × g for 15 min at 4°). The supernatant was then discarded and the cells re-suspended in 350 µl lysis buffer (Qiagen RNeasy mini RNA extraction kit, Qiagen Inc., Valencia, CA), and frozen. The lysed heterophils were transferred to QIAshredder homogenizer columns (Qiagen Inc.) and centrifuged for 2 min at 8000 g. The total RNA was extracted from the homogenized lysate according to the manufacturer's instructions, eluted with 50 µl RNase-free water, and stored at −80° until TaqMan analyses were performed.
A kinetics study was conducted to determine the optimal time required for peak cytokine mRNA expression levels. Heterophils isolated from each line of chickens were stimulated for 0, 0·5, 1, 2, and 4 hr with SE, NCS-OpSE, and IgY-OpSE and cytokine mRNA expression levels were quantitated. Cytokine mRNA expression for heterophils from lines A and B are shown following stimulation with SE (Table 1). Peak mRNA expression levels occurred after 0·5 hr of stimulation.
Table 1.
Time course evaluation of cytokine mRNA expression levels
| Line A | Line B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine mRNA expression (40-Ct ± SEM) | Cytokine mRNA expression (40-Ct ± SEM) | |||||||||
| Hours post stimulation | 0 | 0·5 | 1 | 2 | 4 | 0 | 0·5 | 1 | 2 | 4 |
| IL-6 | 11·94 ± 0·12 | 14·3 ± 0·12 | 14·27 ± 0·11 | 14·29 ± 0·17 | 14·25 ± 0·18 | 9·54 ± 0·11 | 7·32 ± 0·09 | 7·23 ± 0·12 | 7·26 ± 0·06 | 7·25 ± 0·10 |
| IL-8 | 14·64 ± 0·25 | 15·98 ± 0·22 | 15·98 ± 0·16 | 15·96 ± 0·12 | 15·96 ± 0·18 | 11·28 ± 0·17 | 8·49 ± 0·12 | 8·44 ± 0·12 | 8·44 ± 0·07 | 8·40 ± 0·07 |
| IL-18 | 9·45 ± 0·15 | 10·75 ± 0·15 | 10·75 ± 0·15 | 10·73 ± 0·17 | 10·7 ± 0·09 | 7·77 ± 0·11 | 6·08 ± 0·04 | 6·08 ± 0·08 | 6·03 ± 0·12 | 6·02 ± 0·08 |
| TGF-β4 | 6·27 ± 0·09 | 4·45 ± 0·18 | 4·44 ± 0·14 | 4·45 ± 0·17 | 4·45 ± 0·16 | 8·48 + 0·14 | 9·58 ± 0·09 | 9·55 ± 0·10 | 9·52 ± 0·12 | 9·52 ± 0·08 |
Real-time quantitative RT–PCR
Cytokine mRNA expression in control and phagocytic agonist-treated heterophils from each line were quantitated using a method described by Kaiser35 and Moody.36 Primers and probes for cytokine and 28S RNA-specific amplification have been described previously25,35 but for clarity are provided (Table 2).
Table 2.
Real-time quantitative RT–PCR probes and primers
| RNA target | Probe/primer sequence | Exon boundary | Accession number* | |
|---|---|---|---|---|
| 28S | Probe | 5′-(FAM†)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ | X59733 | |
| F‡ | 5′-GGCGAAGCCAGAGGAAACT-3′ | |||
| R§ | 5′-GACGACCGATTGCACGTC-3′ | |||
| IL-6 | Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3′ | 3/4 | AJ250838 |
| F | 5′-GCTCGCCGGCTTCGA-3′ | |||
| R | 5′-GGTAGGTCTGAAAGGCGAACAG-3′ | |||
| IL-8 | Probe | 5′-(FAM)-CTTTACCAGCGCGTCCTACCTTGCGACA-(TAMRA)-3′ | 1/2 | AJ009800 |
| F | 5′-GCCCTCCTCCTGGTTTCAG-3′ | |||
| R | 5′-TGGCACCGCCAGCTCATT-3′ | |||
| IL-18 | Probe | 5′-(FAM)-CCGCGCCTTCAGCAGGGATG-(TAMRA)-3′ | 4/5 | AJ416937 |
| F | 5′-AGGTGAAATCTGGCAGTGGAAT-3′ | |||
| R | 5′-ACCTGGACGCTGAATGCAA-3′ | |||
| TGF-β4 | Probe | 5′-(FAM)-ACCCAAAGGTTATATGGCCAACTTCTGCAT-(TAMRA)-3′ | 5/6 | M31160 |
| F | 5′-AGGATCTGCAGTGGAAGTGGAT-3′ | |||
| R | 5′-CCCCGGGTTGTGTGTTGGT-3′ |
Genomic DNA sequence;
5-carboxyfluorescein;
forward;
reverse.
Real-time quantitative RT–PCR was performed using the Reverse Transcriptase qPCR Master Mix RT–PCR kit (Eurogentec, Seraing, Belgium). Amplification and detection of specific products were performed using the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Warrington, UK) with the following cycle profile: one cycle of 50° for 2 min, 96° for 5 min, 60° for 30 min, and 95° for 5 min, and 40 cycles of 94° for 20 s, 59° for 1 min. Quantification was based on the increased fluorescence detected by the ABI PRISM 7700 Sequence Detection System due to hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. The passive reference dye 6-carboxy-χ-rhodamine, which is not involved in amplification, was used for normalization of the reporter signal. Results are expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye passes a significance threshold (Rn) (Table 3). To correct for differences between RNA levels between samples within the experiment, the difference factor for each sample was calculated by dividing the mean Ct value for 28S rRNA-specific product from all samples. Results were expressed as 40-Ct values for basal levels or fold change in the phagocytic agonist-treated samples.
Table 3.
Standard curve data from real-time quantitative RT–PCR on total RNA extracted from stimulated heterophils
| ΔRn* | Log dilutions | Ct† | R2‡ | Slope | |
|---|---|---|---|---|---|
| 28S | 0·05 | 10−1−10−5 | 8–22 | 0·9952 | 3·1657 |
| IL-6 | 0·02 | 10−1−10−5 | 21–36 | 0·9911 | 3·7263 |
| IL-8 | 0·02 | 10−1−10−5 | 14–28 | 0·9885 | 3·1645 |
| IL-18 | 0·02 | 10−1−10−5 | 20–32 | 0·9914 | 4·0451 |
| TGF-β4 | 0·02 | 10−1−10−5 | 20–36 | 0·9881 | 3·1595 |
ΔRn, change in the reporter dye.
Ct, threshold cycle level: the cycle at which the change in the reporter dye levels detected passes the ΔRn.
R2, coefficient of regression.
Statistical analyses
Statistical analyses (Student's t-test) were performed using Microsoft® Excel 2000 with P ≤ 0·05. All statistical analyses are based on comparisons between the parental pair (A and B) or between the F1 reciprocal crosses (C and D). No statistical analyses were done between the parental lines and the F1 reciprocal crosses.
Results
Real-time quantitative RT–PCR
For the TaqMan experiments, replicate experiments on different days were highly repeatable, with a coefficient of variation for two replicate RT–PCR assays of log10 serially diluted RNA for the different reactions (Table 3). There was a linear relationship between the amount of input RNA and the Ct values for the various reactions (Table 3). Regression analysis of the Ct values generated by the log10 dilution series gave R2 values for all reactions in excess of 0·98 (Table 3). The increase in cycles per log10 decrease in input RNA for each specific reaction, as calculated from the slope of the respective regression line, is given in Table 3.
To account for the variation in sampling and RNA preparations, the Ct values for cytokine-specific product for each sample were standardized using the Ct value for 28S rRNA product for the same sample from the reaction run simultaneously. To normalize RNA levels between samples within an experiment, the mean Ct value for 28S rRNA-specific product was calculated by pooling values from all samples in that experiment. Tube-to-tube variations in 28S rRNA Ct values about the experimental mean were calculated. Using slopes of the respective cytokine and 28S rRNA log10 dilution series regression lines, the difference in input total RNA, as represented by the 28S rRNA, was used to adjust cytokine-specific Ct values. Standardization does not dramatically alter the distribution of the results as a whole.
Basal cytokine mRNA expression
Heterophils were isolated from non-infected healthy day-old chickens and the basal levels of IL-6, IL-8, IL-18, and TGF-β4 mRNA expression were determined (Table 4). Heterophils from the SE-resistant parental line A chickens had a significantly (P ≤ 0·05) higher expression level of IL-6 (12·32) and IL-8 (19·77) compared to heterophils isolated from the SE-susceptible parental line B chickens (8·54 and 13·93, respectively). Even though the level of expression of IL-18 was not significantly higher in heterophils from line A chickens (14·93), the trend was similar in that the values were numerically higher than observed in heterophils from line B chickens (13·87). Based on the elevated pro-inflammatory response of heterophils from line A chickens, a lower anti-inflammatory TGF-β4 response would be expected. There was not a statistical difference but the TGF-β4 values were lower in line A heterophils (13·89) compared to line B heterophils (14·13).
Table 4.
40-Ct basal levels of cytokine mRNA expression in heterophils isolated from day-old chickens
| Line | SE status | IL-6 | IL-8 | IL-18 | TGF-β4 |
|---|---|---|---|---|---|
| A | Resistant | 12·32 ± 0·59* | 19·77 ± 1·56* | 14·93 ± 0·68 | 13·89 ± 1·07 |
| B | Susceptible | 8·54 ± 0·41 | 13·93 ± 1·07 | 13·87 ± 0·81 | 14·13 ± 0·26 |
| C | Susceptible | 13·76 ± 0·93 | 17·0 ± 1·23 | 13·64 ± 0·24 | 14·89 ± 0·63* |
| D | Resistant | 15·81 ± 0·77* | 18·64 ± 0·61 | 15·12 ± 1·11* | 12·91 ± 1·05 |
Values are the average ± standard error mean of replicate experiments and are given as Ct, the threshold cycle level at which the change in reporter dye levels detected passes the ΔRn.
Statistical analyses were determined by Student's t-test. Comparisons are between parental lines A and B or between the F1 crosses C and D. No statistical analyses were conducted between the parental lines and the F1 crosses
=statistically significant at P≤0·05).
As observed in the parental lines, a similar trend was observed in heterophils isolated from the SE-resistant F1 cross (line D) when compared to heterophils from the SE-susceptible F1 cross (line C). IL-6 and IL-18 mRNA expression were significantly (P ≤0·05) higher in line D heterophils (15·81 and 15·12, respectively) compared to the basal levels in line C heterophils (13·76 and 13·64, respectively). The mRNA expression of IL-8 was also elevated in heterophils from line D (18·64) chickens over the levels in heterophils from line C (17·0) chickens. As seen with the parental lines, TGF-β4 mRNA expression in heterophils from the SE resistant F1 cross chickens (line D, 12·91) was significantly (P ≤ 0·05) lower compared to expression levels in heterophils isolated from the SE-susceptible cross chickens (line C, 14·89). In general, these data show that heterophils isolated from SE-resistant chickens have higher constitutive levels of pro-inflammatory cytokine mRNA with concommitant lower constitutive levels of anti-inflammatory cytokine mRNA.
IL-6 mRNA expression following stimulation
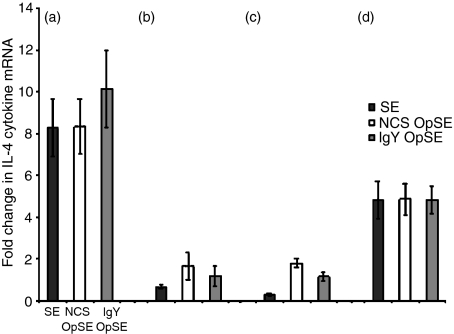
There was a significant difference in IL-6 mRNA expression between heterophils isolated from parental lines A and B (Fig. 1a, b). In both lines, there was an increase when heterophils were treated with SE, NCS-OpSE, or IgY-OpSE compared to non-stimulated control levels (Fig. 1a, b). Upon stimulation, IL-6 mRNA expression increased eight- to 10-fold in heterophils isolated from SE-resistant line A chickens (Fig. 1a) whereas expression in heterophils from SE-susceptible line B chickens increased less than twofold above basal levels (Fig. 1b). There was also a differential response between heterophils isolated from the F1 reciprocal crosses (lines C and D). Heterophils isolated from SE-resistant line D chickens (Fig. 1d) had a nearly fivefold up-regulation of IL-6 mRNA compared to a less than twofold increase in heterophils from SE-susceptible line C chickens (Fig. 1c). These data indicate that the previously observed Salmonella-resistance is influenced, in part, by the presence of a strong pro-inflammatory cytokine response, including IL-6, elicited by heterophils.
Figure 1.
Quantitation of IL-6 mRNA expression in heterophils isolated from day-old chickens in (a) line A (b) line B (c) line C, and (d) line D. Data are expressed as fold-change in IL-6 mRNA levels when treated samples (SE, NCS-OpSE, and IgY-OpSE) were compared to control heterophils treated with RPMI-1640. Error bars show SEM of three separate experiments including 35 chickens per experiment.
IL-8 mRNA expression following stimulation
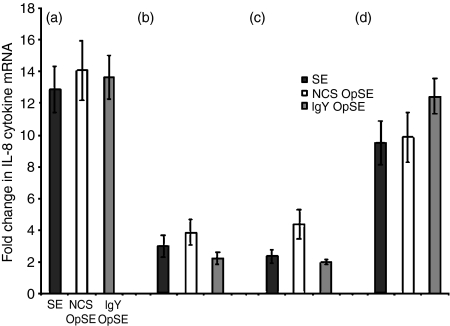
There was a significant difference in the level of IL-8, a chemotactic cytokine, mRNA expression between heterophils isolated from parent lines A and B (Fig. 2a, b). In both lines, an increase was observed when heterophils were treated with SE, NCS-OpSE, and IgY-OpSE compared to non-stimulated controls (Fig. 2a, b). IL-8 mRNA expression increased approximately 13- to 14-fold in heterophils isolated from SE-resistant line A chickens (Fig. 2a) whereas expression in heterophils from SE-susceptible line B chickens increased less than four-fold (Fig. 2b). As observed with IL-6, there was also a differential IL-8 response between heterophils isolated from the two F1 crosses (lines C and D). Heterophils isolated from SE-resistant line D chickens (Fig. 2d) had a 9·5–12·5 fold up-regulation of IL-8 mRNA compared to a less than fivefold increase in heterophils from SE-susceptible line C chickens (Fig. 2c). In addition to a strong pro-inflammatory cytokine response influencing Salmonella resistance in poultry, an elevated chemotactic response produced by heterophils is also likely involved in determining resistance.
Figure 2.
Quantitation of IL-8 mRNA expression in heterophils isolated from day-old chickens in (a) line A (b) line B (c) line C, and (d) line D. Data are expressed as fold-change in IL-8 mRNA levels when treated samples (SE, NCS-OpSE, and IgY-OpSE) were compared to control heterophils treated with RPMI-1640. Error bars show SEM of three separate experiments including 35 chickens per experiment.
IL-18 mRNA expression following stimulation
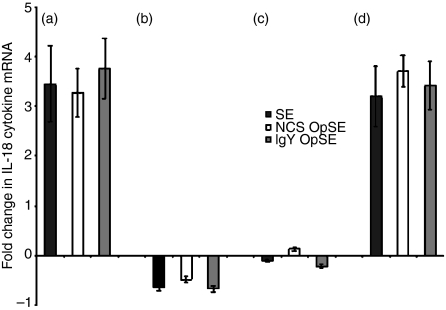
There was a significant difference in IL-18 mRNA expression between heterophils isolated from the parent lines (A and B) (Fig. 3a, b). IL-18 mRNA expression in heterophils isolated from SE-resistant line A chickens increased at least threefold above control levels when treated with each of the agonists (Fig. 3a). However, stimulation of heterophils from SE-susceptible line B chickens resulted in a slight down-regulation of IL-18 mRNA expression (Fig. 3b). The response in heterophils from line C chickens was virtually unchanged from the basal levels when stimulated with each of the agonists (Fig. 3c) whereas expression was up-regulated at least threefold in heterophils isolated from SE-resistant line D chickens upon stimulation (Fig. 3d). These data further support a role for an effective pro-inflammatory and/or Th1 cytokine response elicited by avian heterophils in contributing to resistance against Salmonella infections.
Figure 3.
Quantitation of IL-18 mRNA expression in heterophils isolated from day-old chickens in (a) line A (b) line B (c) line C, and (d) line D. Data are expressed as fold-change in IL-18 mRNA levels when treated samples (SE, NCS-OpSE, and IgY-OpSE) were compared to control heterophils treated with RPMI-1640. Error bars show SEM of three separate experiments including 35 chickens per experiment.
TGF-β4 mRNA expression following stimulation
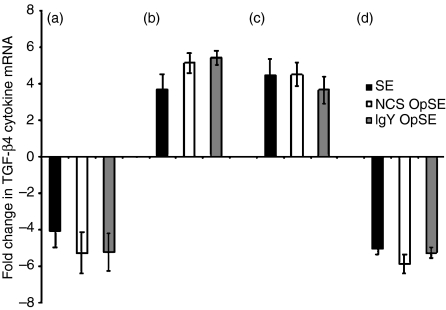
Heterophils from parental SE-resistant line A chickens had a four- to fivefold decrease in TGF-β4 cytokine mRNA expression (Fig. 4a). However, expression in heterophils from SE-susceptible parental line B chickens was up-regulated three- to fivefold when stimulated by each of the agonists (Fig. 4b). A similar pattern was observed in the F1 reciprocal crosses. The TGF-β4 mRNA expression was up-regulated (three- to fourfold) in heterophils from SE-susceptible F1 cross C chickens (Fig. 4c) while down-regulated (fivefold) in heterophils from SE-resistant F1 cross D chickens when stimulated with each of the agonists (Fig. 4d). As would be expected, levels of the anti-inflammatory cytokine TGF-β4 are the opposite of those observed for the pro-inflammatory cytokines. These data indicate that a suppressed anti-inflammatory cytokine response is a component influencing SE resistance in neonatal poultry.
Figure 4.
Quantitation of TGF-β4 mRNA expression in heterophils isolated from day-old chickens in (a) line A (b) line B (c) line C, and (d) line D. Data are expressed as fold-change in TGF-β4 mRNA levels when treated samples (SE, NCS-OpSE, and IgY-OpSE) were compared to control heterophils treated with RPMI-1640. Error bars show SEM of three separate experiments including 35 chickens per experiment.
Discussion
In the present study we isolated heterophils from SE-resistant and -susceptible day-old chickens from parental lines (A and B) and F1 reciprocal crosses (C and D) and quantitated the basal and stimulated levels of mRNA expression for various cytokines. The data indicate that avian heterophils from genetically distinct chickens have a differential cytokine profile and response to stimuli. Specifically, heterophils from SE-resistant chickens (lines A and D) have up-regulated mRNA expression levels of pro-inflammatory (IL-6), Th1 (IL-18), and chemotactic (IL-8) cytokines compared to heterophils from SE-susceptible chickens (lines B and C) upon stimulation while down-regulating TGF-β4 mRNA expression, an anti-inflammatory cytokine. Even though there are differences in cytokine mRNA expression between heterophils isolated from the distinct lines, it should be noted that cytokine mRNA levels as measured by real-time quantitative RT–PCR do not necessarily equate to the production of bioactive protein. However, real-time quantitative RT–PCR is the most highly sensitive method available to reliably quantify a broad spectrum of avian cytokines, particularly in the absence of effective bioassays.35,37,38
We recently showed, for the first time, that heterophils express increased levels of cytokine mRNA upon stimulation that is initiated by receptor-mediated phagocytosis.25 In that study, IL-6 and IL-8 mRNA expression were up-regulated, similar to what we observed in the current study. However, unlike the present study, IL-18 was down-regulated and TGF-β4 was elevated. It is likely that these differences are attributed to genetic variations between the outbred Rhode Island Red chickens used in the original study and the genetically inbred broiler lines evaluated in the present study. Nevertheless, our earlier studies utilizing the parental pair (lines A and B) and F1 reciprocal crosses (lines C and D) indicate that the differences are, in part, at the level of heterophil functional efficiency.22,33 The heterophil functional advantages appear to correlate with biological differences including the ability to mount an effective pro-inflammatory and Th1 cytokine response. The ability to initiate effective inflammatory and Th1 responses results in efficient stimulation and ultimately the direction of the acquired immune response.2,3 The differences in the cytokine response between the outbred and our inbred broiler chickens is likely to be attributed to a vast number of genetic differences associated with creating an inbred line of chickens compared to the genetics of an outbred population of birds.
We have shown that an increase in IL-1β, IL-6, and IL-8 mRNA expression by heterophils is associated with increased resistance to extraintestinal SE infections in neonatal chickens (Ferro and Kogut, unpublished data). The current in vitro study found similar results in that IL-6 and IL-8 were up-regulated in heterophils isolated from the SE-resistant lines when stimulated with SE, NCS-OpSE, or IgY-OpSE. IL-6 is a multifunctional cytokine that is produced and released by diverse populations of cells and has important roles in regulation of the immune response, differentiation and proliferation of various cells, and signal transduction pathways.39,40 In mammals, IL-6 stimulates neutrophils and enhances their degranulation.41 It is possible that the increased degranulation observed between lines A and B or lines C and D22 is attributed to an increased ability to express IL-6. Therefore, increased expression of IL-6 production may create a population of heterophils more able to respond to and eliminate pathogens.
IL-18 is an IL-1-related pro-inflammatory cytokine critical in initiating an inflammatory response.42–44 It is highly pleotropic, primarily associated with a type-1 cytokine response and involved in determining resistance or susceptibility to bacterial infections.45,46 An increased type-1 cytokine response, including an up-regulation of IL-18 is associated with increased resistance to Mycobacterium leprae in leprosy patients.45 Also, in the mouse model, a sublethal dose of Salmonella typhimurium results in a rapid pro-inflammatory cytokine response.46 An effective type-1 cytokine response dramatically enhances the innate and acquired immune response and enhances host resistance to infections.43,44 In the present study, we showed that heterophils isolated from chickens more resistant to SE infections (Ferro and Kogut, unpublished data) are capable of producing significantly higher mRNA expression levels of IL-18. Taken together, an elevated pro-inflammatory type-1 cytokine response correlates with an increased resistance against SE and may be an effective selection parameter when developing new lines of poultry with increased resistance to bacterial infections.
Additionally, IL-18 acts directly on key cells involved in innate immunity, including mammalian neutrophils.47,48 In mammals, IL-18 activates neutrophils by promoting cell migration, cytokine production, generation of a respiratory burst, and degranulation47 mediated by the p38 mitogen-associated protein (MAP) kinase signalling cascade.48 Because PMN are activated by IL-18 it is possible that increased levels of IL-18 creates a population of cells more able to respond to a pathogen(s). Avian heterophils also undergo degranulation mediated by p38 MAP kinase;49 however, the oxidative burst response is not mediated by this signalling pathway.50 The increased heterophil functional efficiency22,33 and increased resistance to SE (Ferro and Kogut, unpublished data) observed in these genetically distinct chickens may be influenced by the increased IL-18 expression by heterophils.
In addition to an effective pro-inflammatory cytokine response, a strong chemotactic response may also influence whether a chicken is resistant or susceptible to SE. In mammals, IL-8 is a chemokine involved in the recruitment of PMN to the site of infection.51,52 Similarly, in neonatal chickens, an IL-8-like chemokine is involved in heterophil recruitment to the site of infection in neonatal chickens following an intraperitoneal challenge with SE.53 We have shown a differential IL-8 response in heterophils isolated from SE-challenged chickens and observed a significant up-regulation in the SE-resistant chickens (lines A and D) compared to the SE-susceptible chickens (lines B and C) (Ferro and Kogut, unpublished data). The increased production of IL-8 would likely result in the recruitment of additional numbers of heterophils to the site of infection. Since heterophils from line A and D chickens are functionally more efficient in vitro22,33 it is likely these cells would be more efficient at eliminating an infection in vivo. These data are further supported by the in vitro study presented herein, in that heterophils isolated from SE-resistant chickens (lines A and D) demonstrated an up-regulated IL-8 response compared to heterophils from SE-susceptible chickens (lines B and C) upon stimulation with SE, NCS-OpSE, or IgY-OpSE.
In the current study, heterophils from SE-resistant lines A and D had up-regulated mRNA expression levels of pro-inflammatory (IL-6), Th1 (IL-18), and chemotactic (IL-8) cytokines, while the anti-inflammatory cytokine (TGF-β4) mRNA expression level was down-regulated. In contrast, the opposite cytokine profile was observed in heterophils from the SE-susceptible lines (B and C). These data provide evidence that the down-regulation of TGF-β4 in heterophils from line A and D chickens enables these two lines of chickens to quickly and efficiently initiate an acute pro-inflammatory response resulting in the ability to control and limit an infection more effectively. The earlier in vitro heterophil functional assays using these same four lines of chickens also support this as a possible mechanism of increased resistance against Salmonella infections.22,33 High levels of TGF-β are also associated with increased mortality in Plasmodium infections in mice.54,55 Taken together, we believe there is a direct relationship between the up-regulation of TGF-β4, decreased heterophil functional efficiency, and increased susceptibility to extraintestinal SE infections in neonatal poultry. The study presented herein provides preliminary data and indicates that additional studies are required to dissect the complex nature of the innate response to Salmonella in poultry.
In summary, the results of this study indicate that resistance to SE in poultry is influenced, in part, by heterophils and their ability to produce an effective pro-inflammatory cytokine and chemokine response while suppressing the anti-inflammatory response (TGF-β4). Based on the data from the present study accompanied by our previous findings utilizing these same parental and F1 reciprocal cross chickens, we propose that isolating heterophils and evaluating cytokine mRNA expression can be used as an indicator of overall immune competence when selecting for immunologically competent poultry (Ferro and Kogut unpublished data; Swaggerty and Kogut, unpublished data).22,33
Acknowledgments
The authors thank Ms Gena Lowry for outstanding technical assistance and Mr Zane Brandenberger for animal care support. All experiments were conducted according to the rules and regulations established by the United States Department of Agriculture animal care use committee and overseen by Dr J. A. Byrd, attending veterinarian. This research was funded, in part, by the UK Biotechnology and Biological Sciences Research Council (L.R. and P.K.).
Abbreviations
- Ct
threshold cycle
- EDTA
disodium ethylenediaminetetraacetic acid
- IFN
interferon
- IgY-OpSE
immune serum opsonized Salmonella enteritidis
- IL
interleukin
- MAP
mitogen-associated protein
- NCS-OpSE
normal chicken serum opsonized Salmonella enteritidis
- PAMP
pathogen-associated molecular patterns
- PBS
phosphate-buffered saline
- PMN
polymorphonuclear leucocytes
- PRR
pattern recognition receptor
- SE
Salmonella enteritidis
- TAMRA
N,N,N,Ν′-tetramethyl-6-carboxyrhodamine
- TGF
transforming growth factor
References
- 1.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. 4. New York: WH Freeman; 1992. [Google Scholar]
- 2.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Fearon DT. Innate pathways that control acquired immunity. Curr Opin Immunol. 1997;9:1–3. doi: 10.1016/s0952-7915(97)80151-3. [DOI] [PubMed] [Google Scholar]
- 5.Parish CR, O'Neill ER. Dependence of the adaptive immune response on innate immunity: some questions answered but new paradoxes emerge. Immunol Cell Biol. 1997;75:523–7. doi: 10.1038/icb.1997.83. [DOI] [PubMed] [Google Scholar]
- 6.Kogut MH, McGruder ED, Hargis BM, Corrier DE, DeLoach JR. In vivo activation of heterophil function in chickens following injection with Salmonella enteritidis-immune lymphokines. J Leukocyte Biol. 1995;57:56–62. doi: 10.1002/jlb.57.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13–9. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 8.Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 9.Romagnani S. Induction of Th1 and Th2 responses: a key role for the ‘natural’ immune response? Immunol Today. 1992;13:379–81. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Imler J-L, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–11. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 13.Yamashiro S, Kamohara H, Wang J-M, Yang D, Gong W-H, Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukocyte Biol. 2001;69:698–704. [PubMed] [Google Scholar]
- 14.Hachicha M, Rathanaswami P, Naccache PH, McColl SR. Regulation of chemokine gene expression in human peripheralblood neutrophils phagocytosing microbial pathogens. J Immunol. 1998;160:449–54. [PubMed] [Google Scholar]
- 15.Nau GJ, Richmond JFL, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci USA. 2002;99:1503–8. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci USA. 2002;99:6901–6. doi: 10.1073/pnas.092148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maxwell MH, Robertson GW. The avian heterophil leucocyte: a review. World's Poultry Sci J. 1998;54:155. [Google Scholar]
- 18.Burton RR, Harrison JS. The relative differential leukocyte count of the newly hatched chick. Poultry Sci. 1969;48:451–3. doi: 10.3382/ps.0480451. [DOI] [PubMed] [Google Scholar]
- 19.Kogut MH, Genovese KJ, Lowry VK. Differential activation of signal transduction pathways mediating phagocytosis, oxidative burst, and degranulation by chicken heterophils in response to stimulation with opsonized Salmonella enteritidis. Inflammation. 2001;25:7–15. doi: 10.1023/a:1007067426499. [DOI] [PubMed] [Google Scholar]
- 20.Desmidt M, van Nerom A, Haesebrouck F, Ducatelle R, Ysebaert MT. Oxygenation activity of chicken blood phagocytes as measured by luminol- and lucigenin-dependent chemiluminescence. Vet Immunol Immunopathol. 1996;53:303–11. doi: 10.1016/S0165-2427(96)05620-6. [DOI] [PubMed] [Google Scholar]
- 21.Genovese LL, Lowry VK, Genovese KJ, Kogut MH. Longevity of augmented phagocytic activity of heterophils in neonatal chickens following administration of Salmonella enteritidis-immune lymphokines to chickens. Avian Pathol. 2000;29:117. doi: 10.1080/03079450094144. [DOI] [PubMed] [Google Scholar]
- 22.Swaggerty CL, Pevzner IY, Lowry VK, Farnell MB, Kogut MH. Functional comparison of heterophils isolated from commercial broiler chickens. Avian Pathol. 2003;32:95–102. doi: 10.1080/0307945021000070769. [DOI] [PubMed] [Google Scholar]
- 23.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 24.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 25.Kogut MH, Rothwell L, Kaiser P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and non-opsonized Salmonella enteritidis. J Interferon Cytokine Res. 2003;23:319–27. doi: 10.1089/107999003766628160. [DOI] [PubMed] [Google Scholar]
- 26.Digby MR, Lowenthal JW. Cloning and expression of the chicken IFN-γ gene. J Interferon Cytokine Res. 1995;15:939–45. doi: 10.1089/jir.1995.15.939. [DOI] [PubMed] [Google Scholar]
- 27.Schneider K, Puehler F, Baeuerle D, Elvers S, Staeheli P, Kaspers B, Weining KC. cDNA cloning of biologically active chicken interleukin-18. J Interferon Cytokine Res. 2000;20:879–83. doi: 10.1089/10799900050163244. [DOI] [PubMed] [Google Scholar]
- 28.Weining KC, Sick C, Kaspers B, Staeheli P. A chicken homolog of mammalian interleukin-1: cDNA cloning and purification of active recombinant protein. Eur J Biochem. 1998;258:994–1000. doi: 10.1046/j.1432-1327.1998.2580994.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneider K, Klaas R, Kaspers B, Staeheli P. Chicken interleukin-6. cDNA structure and biological properties. Eur J Biochem. 2001;268:4200–6. doi: 10.1046/j.1432-1327.2001.02334.x. [DOI] [PubMed] [Google Scholar]
- 30.Bedard P-A, Alcorta D, Simmons DL, Luk K-C, Erikson RL. Constitutive expression of a gene encoding a polypeptide homologous to biologically active human platelet protein in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1987;84:6715–9. doi: 10.1073/pnas.84.19.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugano S, Stoeckle MY, Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987;49:321–8. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 32.Jakowlew SB, Dillard PJ, Sporn MB, Roberts AB. Complementary deoxyribonucleic acid cloning of a messenger ribonucleic acid encoding transforming growth factor-β4 from chicken embryo chondrocytes. Mol Endocrinol. 1988;2:1186–95. doi: 10.1210/mend-2-12-1186. [DOI] [PubMed] [Google Scholar]
- 33.Swaggerty CL, Pevzner IY, Ferro PJ, Crippen TL, Kogut MH. Association between in vitro heterophil function and the feathering gene in commercial broiler chickens. Avian Pathol. 2003;32:483–8. doi: 10.1080/0307945031000154071. [DOI] [PubMed] [Google Scholar]
- 34.National Research Council. Nutrient Requirements for Poultry. 9. Washington DC: National Academy Press; 1994. p. 19. [Google Scholar]
- 35.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiology. 2000;146:3217–26. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 36.Moody A, Sellers S, Bumstead N. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT–PCR. J Virol Methods. 2000;85:55–64. doi: 10.1016/s0166-0934(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser P, Underwood G, Davison F. Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease. J Virol. 2003;77:762–8. doi: 10.1128/JVI.77.1.762-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser P, Rothwell L, Vasicek D, Hala K. A Role for IL-15 in driving the onset of spontaneous autoimmune thyroiditis? J Immunol. 2002;168:4216–20. doi: 10.4049/jimmunol.168.8.4216. [DOI] [PubMed] [Google Scholar]
- 39.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4:s233–42. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151–67. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 41.Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989;121:280–9. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 42.Okamura H, Tsutsui H, Kashiwamura S-I, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 43.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med. 2002;80:147–62. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 44.Dinarello CA, Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187:s370–84. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 45.Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–21. [PubMed] [Google Scholar]
- 46.Barreiros AP, Schirmacher P, Laufenberg-Feldmann R, Meyer Zum Buschenfelde K-H, Schlaak JF. The early immune response in the liver of BALB/c mice infected with S. typhimurium. Scand J Immunol. 2000;51:472–8. doi: 10.1046/j.1365-3083.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 47.Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879–86. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 48.Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukocyte Biol. 2002;72:401–9. [PubMed] [Google Scholar]
- 49.Kogut MH, Lowry VK, Farnell MB. Selective pharmacological inhibitors reveal the role of Syk tyrosine kinase, phospholipase C, phosphatidylinositol-3′-kinase, and p38 mitogen-activated protein kinase in Fc receptor-mediated signaling of chicken heterophil degranulation. Int Immunopharmacol. 2003;2:963–73. doi: 10.1016/s1567-5769(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 50.He H, Farnell MB, Kogut MH. Inflammatory agonist stimulation and signal pathway of oxidative burst in neonatal chicken heterophils. Comp Biochem Physiol. 2003;135:177–84. doi: 10.1016/s1095-6433(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 51.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991;3:21–7. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 53.Kogut MH. Dynamics of a protective avian inflammatory response. The role of an IL-8-like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp Immunol, Microbiol, Infect Dis. 2002;25:159–72. doi: 10.1016/s0147-9571(01)00035-2. [DOI] [PubMed] [Google Scholar]
- 54.Omer FM, de Souza JB, Riley EM. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol. 2003;171:5430–6. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- 55.Omer FM, Kurtzhals JAL, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]