Abstract
There are reports of immaturity of the neonatal immune system, which may explain the low incidence of graft-versus-host-disease (GVHD) after cord blood transplantation. The CD40 ligand (CD40L)–CD40 interaction is important in regulating the cellular immune response. We hypothesized that the neonatal immune system may show immaturity in this interaction. We studied the function of the CD40L–CD40 interaction in the T-cell interaction with B cells and monocytes in cord blood compared with adult blood in vitro. Consistent with previous reports, CD4+ T cells do not express CD40L after T-cell activation. In whole blood, adult monocytes, but not neonatal monocytes, were activated following T-cell activation. However, the activation of adult monocytes was not dependent on the CD40L–CD40 interaction. Using the CD40L trimer (Lt), we showed that cord B cells have comparable responses to CD40 ligation to those of the adult B cells. Both cord and adult monocytes do not respond as well as B cells and this is probably related to low density of expression of CD40. However, interferon-γ up-regulated CD40 on adult monocytes but not on cord monocytes. This potentiated the adult monocyte response to CD40 ligation by CD40Lt. Our findings suggest that the neonatal CD40L–CD40 pair is immature in the cellular immune response involving monocytes and that interferon-γ fails to activate neonatal monocytes for a response to CD40L. These findings suggest that in the inflammatory microenvironment of cord blood transplantation neonatal monocytes may play a minor role in the effector arm of the immune response. This finding may be one of several mechanisms for the low incidence of GVHD that is observed following cord blood transplantation. Also the ligand-receptor immaturity may contribute to the increased susceptibility of newborns to certain infections.
Keywords: B cells, macrophages/monocytes, neonatal, signalling/signal transduction, transplantation
Introduction
A successful activation of resting T cells to effector T cells in an immune response requires co-ordinated interactions of several ligand–receptor pairs between T cells and antigen-presenting cells (APCs). The important APCs are B cells, monocytes, or dendritic cells. An important costimulatory pair is the B7–CD28 pair that is crucial in stimulating proliferation, cytokine secretion and prevention of T-cell anergy.1 Other APC T-cell signalling molecular pairs are the adhesion molecules CD58 (LFA-3)–CD2 and CD54 (ICAM-1)–CD11a. Although these can provide the second signal for proliferation they do not prevent the induction of peripheral anergy2 but are more important in lowering the threshold for T-cell activation.3 These molecules mediate the physical interaction between the T-cell and the APC, leading to the formation of the immunological synapse. Within this synapse interaction of the T-cell receptor with the agonist major histocompatibility complex–peptide occurs, leading to the formation of a central cluster of these complexes.4
A third costimulatory pathway involves the CD40 ligand (CD40L)–CD40 pair, members of the tumour necrosis factor(TNF)/TNF receptor (TNFR) superfamilies of molecules, respectively.5,6 This ligand–receptor pair regulates multiple phases of the immune response6 and is important in triggering resting B cells to proliferate, to switch immunoglobulin class, and to up-regulate costimulatory molecules CD80/CD86.5,6 Furthermore, this pair is critical in the development of a competent cellular effector immune response through activation of dendritic cells and monocytes/macrophages.7,8 T cells, in turn, through their interaction with monocytes/macrophages via this pathway, are activated to a type 1 response.7 In addition, several studies have confirmed the importance of the CD40L–CD40 pathway in the in vivo immune response and its importance in the control of viral infections and intracellular killing of pathogens, graft-versus-host reaction and the generation of anti-leukaemia effects.7–11 Blockage of the interaction of this ligand–receptor pair has resulted in a decrease in both the incidence and the severity of graft-versus-host-disease (GVHD) and inflammatory diseases but stimulation of their interaction has enhanced anti-leukaemia or anti-tumour effects.12–14
Following cord blood transplantation the incidence and severity of GVHD are less. We hypothesize that a possible mechanism is an immature neonatal CD40L–CD40 interaction compared with the adult interaction. The immaturity may be related to a reduced up-regulation of CD40L on neonatal T cells, which has been described.15,16 Recent reports, however, showed that CD40L expression on neonatal T cells depends on the strength of the T-cell stimulus. A sustained CD40L expression can follow a T-cell stimulus supplemented by interleukin-2 (IL-2) and/or IL-4 or an allogeneic stimulation by dendritic cells.17,18 In this situation, it is possible that any immaturity of the pathway may be the result of a poor response to CD40 ligation on APCs.
We tested this hypothesis and this paper reports on and compares the CD40L–CD40 interaction of T–B cells and T monocytes from adult and cord sources. It was found that monocytes of both adult and cord blood showed a reduced response to CD40L stimulation compared to B cells and that neonatal monocytes were less responsive than adult monocytes to CD40L stimulation. Interferon-γ (IFN-γ) enhanced the response of adult monocytes, but not of neonatal monocytes, to CD40L ligation. These data indicate that the functional immaturity of the CD40L–CD40 interaction of neonatal monocytes would be more apparent in the context of an inflammatory response.
Materials and methods
Blood samples
Cord blood was collected from healthy term babies into 20 U/ml of preservative-free heparin. Adult blood for comparative study was similarly collected from healthy individuals into heparin following institutional ethics approval. Functional studies were performed with diluted whole blood or with mononuclear cell preparation after density separation on Lymphoprep™ (Nygaard, Denmark). Isolated cells were resuspended in RPMI-1640 supplemented with insulin/transferrin/selenium (Gibco BRL, Grand Island, NY), 2 × 10−5 m 2-mercaptoethanol and 30% of either adult AB blood serum or cord serum depending on cell source. Serum was used at a concentration of 30% as this was found to minimize apoptosis of monocytes and B cells during culture.19
Flow cytometry analysis
The expression of costimulatory and adhesion molecules on resting and stimulated cells was determined by flow cytometry using a fluorescence-activated cell sorter scan (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, CA). Fluorescein isothiocyanate (FITC) or phycoerythrin (PE) or PECy5 (PC5) conjugated monoclonal antibodies (mAb) against CD4, CD19, CD40, CD40L, CD54, CD58, CD86 (Coulter-Immunotech, Brea, CA), CD8, CD11a, CD25, HLA-ABC (Dakopatt A/S, Copenhagen, Denmark), CD69 and CD154 (BD Biosciences, San Jose, CA, USA) were used. Negative controls were stained with the relevant conjugated γ1/γ2a mAbs from BD Biosciences. Phytohaemagglutinin (PHA) was obtained from Sigma Chemicals (St Louis, MO). Unlabelled αCD28 was obtained from Coulter-Immunotech. Unlabelled αCD3 (Orthoclone OKT*3) was obtained from Janssen-Cilag (North Ryde, NSW, Australia).
T- and B-cell and monocyte interaction in whole blood
Whole blood was used for study where possible as this in vitro model may mimic the in vivo situation best.19 Heparinized whole blood was diluted 1 : 1 with RPMI containing 10% AB serum for adult blood or 10% cord serum for cord blood. PHA (10 μg/ml) and αCD28 (10 μg/ml) were added to stimulate T cells and up-regulate CD40L both on the surface of T cells and internally, costimulatory molecules CD80/CD86 and adhesion molecules CD11a, CD58 on B cells and monocytes. These were examined using immunofluorescence flow cytometry. To determine whether the expression of these molecules on B cells and monocytes was up-regulated through CD40L–CD40 interaction, 10 μg/ml of anti-CD40L mAb was added to the blood prior to the addition of the stimuli. The changes in the expression of these molecules were monitored using flow cytometry at 24, 48 and 72 hr of incubation at 37° in an atmosphere of air containing 5% CO2. The changes in the surface expression of the molecules studied on the various cell subsets were identified by FACS ‘gating’ using αCD3-PC5, αCD19-PC5 and αCD14-PC5. Cord blood samples contain significant numbers of nucleated red cells. Gating of a minimum of 10 000 events based on PC5 versus side-scatter gating allowed exclusion of all nucleated red cells.20 The up-regulation or down-regulation of the studied molecules was assessed by calculating the difference of percentage (%) expression between stimulated and unstimulated (control) samples: % increase or decrease=% expression on stimulated − % expression on control cells at the stated time points.
Up-regulation of costimulatory molecules using isolated MNCs
The use of whole blood has certain drawbacks, a requirement to use higher doses of immune stimuli and the difficulty of lysing red cells with prolonged incubation at 37°. Experiments were also performed using a 106/ml MNC preparation stimulated with αCD3/αCD28 at 200 ng/ml and 200 ng/ml, respectively. Conditions of incubation were as for whole blood studies. MNCs were suspended in RPMI containing 30% AB serum or cord serum depending on the MNC source. Cells were harvested at 24 hr, 48 hr and, in some studies, at 72 hr for phenotypic study using flow cytometry. The changes in the surface expression of the molecules studied on the various cell subsets were identified by FACS ‘gating’ using αCD3-PC5, αCD19-PC5 and αCD14-PC5. The percentage difference was calculated as indicated above.
CD40L trimer (CD40Lt) stimulation of B cells and monocytes
The direct effect of CD40Lt (Immunex, Seattle, WA) stimulation of B cells and monocytes was examined using MNCs isolated by density gradient centrifugation. MNCs were resuspended at 2 × 106 cells/ml and stimulated with CD40Lt at 1·0 μg/ml. Cells were harvested at 24 hr, 48 hr and, in some studies, at 72 hr for phenotypic study using flow cytometry.
Intracellular staining for cytokine and other antigens
When whole blood was studied, cells were fixed and rendered permeable using the following reagents in sequence: 2 ml FACS lysing solution for 10 min followed by centrifugation and then the addition of 500 μl FACSPerm to the cell pellet. Five microlitres of Intragam™ (60 g/l IgG, CSL, Melbourne, Australia) was added to the cell suspension to block non-specific binding. After 10 min of blocking, the appropriate conjugated antibody was added and incubated for 30 min. The cells were then washed twice with 0·5% bovine serum albumin in Isoton II (Coulter/Immunotech). Cells were resuspended in the same buffer and analysed by flow cytometry.
Statistics
A two-way analysis of variance (anova) was used to analyse and compare the adult and cord data. The computer program used was obtained from graphpad Software, San Diego, CA.
Results
Expression and up-regulation of CD40L molecule on T cells
Resting CD4+ and CD8+ T cells from both adult and cord blood did not express CD40L. On stimulation with PHA and αCD28, expression of CD40L was up-regulated on adult CD4+ T cells but not CD8+ in a time-dependent manner, with a peak at 24 hr. The percentage of CD4+ T cells expressing surface CD40L was 36·61 ± 3·12% at 24 hr (n = 3) and 27·38 ± 3·45% at 48 hr (n = 3). The percentage of CD4+ T cells expressing intracellular CD40L was 10·47 ± 1·05% at 24 hr (n = 3) and 17·72 ± 4·05% at 48 hr (n = 3). In contrast, up-regulation of CD40L was very minimal on cord CD4+ cells. Similar findings were observed with either adult MNCs or cord MNCs stimulated with αCD3/αCD28 (data not shown). As IL-2 is poorly produced by cord T cells compared to adult, exogenous IL-2 was added to cord MNCs to determine if it would enhance CD40L expression following αCD3/αCD28 stimulation. Addition of IL-2 at 100 U/ml enhanced the expression of CD40L on cord CD4 T cells but not to the incidence of positivity of adult CD4 T cells stimulated with αCD3/αCD28 without the addition of IL-2: CD40L (cord with IL-2) 11·96 ± 9·32% at 24 hr (n = 3); CD40L (cord with IL-2) 9·77 ± 8·76% at 48 hr; compared to adult CD40L (without IL-2) 35·0 ± 1·14% at 24 hr, (n = 3); CD40L (without IL-2) 21·60 ± 7·7% at 48 hr, (n = 3), respectively.
CD40L is an important regulator of B cells rather than monocytes following T-cell stimulation in whole blood
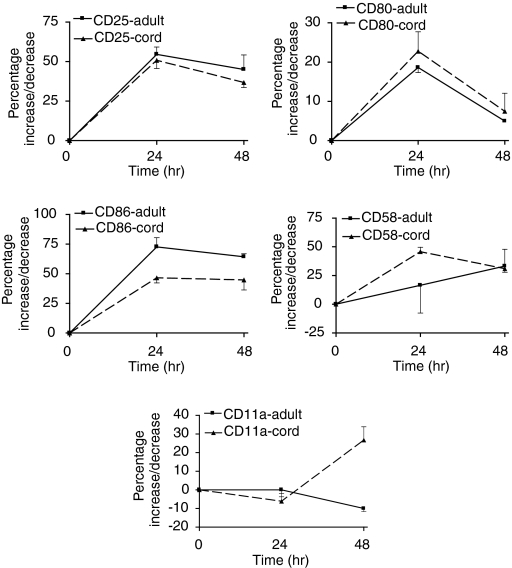
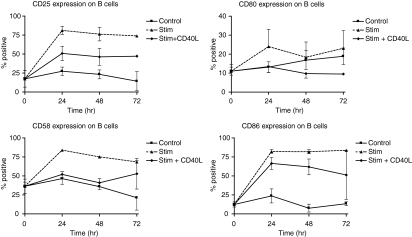
To study further the role of the CD40L–CD40 receptor ligand pair in the multi-step interaction of T cells with APCs, blood from either source was stimulated with PHA and αCD28. Following T-cell activation the up-regulation of costimulatory CD80/CD86 (B7 molecules) and adhesion molecules CD11a, CD58 on B cells and monocytes was determined. The expression of these molecules is given in Table 1. Up-regulation of CD25 was used as an indication of cell activation. Baseline expression of CD25 on adult B cells was 24·64 ± 6·74%, (n = 5) and that of cord B cells was 6·76 ± 1·96%, (n = 5). Baseline expression of CD25 on both cord and adult monocytes was less than 2%. In the presence of αCD40L up-regulation of CD25 was inhibited on B cells of adult blood indicating an inhibition of B-cell activation. There was an initial (24 hr) increase in the expression of the CD80/CD86, CD58 on B cells and a subsequent decrease in their expression. CD11a which was highly expressed on resting B cells, decreased following T-cell stimulation in adult blood (Fig. 1). In the presence of αCD40L, the changes in their expression were inhibited (Fig. 2).
Table 1.
Baseline expression of surface molecules on cord and adult B cells and on cord and adult monocytes
| CD80 | CD86 | CD11a | CD58 | CD40 | |
|---|---|---|---|---|---|
| B cells* | |||||
| Adult | |||||
| % | 6·9 ± 4·9 | 31·6 ± 7·9 | 93·7 ± 1·6 | 35·0 ± 10·3 | 97 ± 4·5 |
| MFI | 42·3 ± 21·1 | 4·1 ± 0·2 | 32·6 ± 6·2 | 6·9 ± 1·0 | 1300 ± 490 |
| Cord | |||||
| % | 0 | 23·9 ± 3·1 | 97·0 ± 0·9 | 38·8 ± 5·9 | 99 ± 1 · 2 |
| MFI | 0 | 2·3 ± 0·5 | 39·8 ± 7·6 | 7·1 ± 2·8 | 1200 ± 190 |
| Monocytes* | |||||
| Adult | |||||
| % | 0 | 98·4 ± 0·7 | 98·0 ± 1·8 | 99·1 ± 0·3 | 84 ± 10 |
| MFI | 0 | 104·6 ± 29·6 | 456·5 ± 96·6 | 147·0 ± 20·4 | 110 ± 69 |
| Cord | |||||
| % | 0 | 93·8 ± 1·8 | 99·0 ± 0·1 | 99·1 ± 0·1 | 64 ± 11 |
| MFI | 0 | 48·7 ± 7·4 | 175·5 ± 42·4 | 137·2 ± 2·7 | 86 ± 48 |
% indicates the percentage of cells expressing the surface molecules; MFI, mean fluorescence intensity.
Results of n = 3 experiments±1SD.
Figure 1.
Comparison of the up-regulation of B7 costimulatory molecules and adhesion molecules CD11a and CD58 on adult and neonatal B cells following stimulation of T cells by PHA (10 μg/ml) and αCD28 (10 μg/ml) in whole blood. Both adult and cord B cells responded similarly. CD25 was used as a marker of B-cell activation. Adult B cells compared to neonatal B cells responded differently with respect to CD86 and CD11a; P = 0·0031 and P = 0·002, respectively.
Figure 2.
The incidence of expression of B7 costimulatory molecules and adhesion molecules CD11a and CD58 on adult B cells following T-cell activation in whole blood. Addition of αCD40L inhibited the up-regulation of these molecules on B cells.
Expression of CD80/CD86 on adult monocytes in whole blood was up-regulated initially (24 hr) and subsequently decreased. The expression of CD11a and CD58 remained unchanged at 24 hr and then decreased subsequently (Fig. 3). However, the addition of αCD40L did not inhibit the changes in expression of the studied molecules on monocytes (data not shown) suggesting that factors other than CD40L, possibly cytokines, are more important in the activation of monocytes. Generally the changes in expression of these molecules were greater on B cells than monocytes.
Figure 3.
Comparison of the up-regulation of B7 costimulatory molecules and adhesion molecules CD11a and CD58 on adult and neonatal monocytes following T-cell activation in whole blood. Cord monocytes were not activated in whole blood following T-cell activation whereas adult monocytes were (CD25 up-regulation, P = 0·0001). Adult monocytes up-regulated CD80 significantly compared with cord monocytes, P = 0·0001.
The up-regulation of the B7 and the adhesion molecules, CD11a and CD58, on cord blood B cells was similar to adult B cells following T-cell stimulation except for CD86 and CD11a. The increase in expression of CD86 on cord B cells was less than that of adult B cells. A late increase in expression of CD11a on cord blood B cells was observed (Fig. 1). However, the maximal increase in density (MFI) of CD80 and CD86 was higher on adult B cells being 16- and 54-fold compared to two- and 40-fold on cord B cells, respectively. The changes in density of CD58 and CD11a were comparable for adult and cord B cells, being five-fold for CD58 but unchanged for CD11a. There was no activation of cord monocytes and CD80 was up-regulated minimally (Fig. 3). The changes in expression of molecules CD86, CD58 and CD11a on cord blood monocytes generally paralleled the changes observed in adult blood but with cord blood showing a greater decrease in expression except for CD58 (Fig. 3). In summary, the degree of changes observed was similar in adult and neonatal B cells and monocytes. The exceptions were CD86, CD11a on B cells and CD80 on monocytes (Figs 1–3). The inhibitory effect of αCD40L was not examined as CD40L was not up-regulated on neonatal T cells stimulated by PHA and αCD28.
Direct effects of CD40 ligation by CD40L on B cells and monocytes
The lower degree of up-regulation of costimulatory and adhesion molecules on cord B cells and monocytes following T-cell stimulation is not surprising as CD40L is not up-regulated well on T cells in both our in vitro systems using the current stimuli. Cord T cells can up-regulate CD40L with additional stimuli such as IL-2 or staphylococcal endotoxin A (SEA) (data not shown) or in an allogeneic induced response.16 We hypothesized that apart from minimal up-regulation of CD40L there could be a poor response to CD40 ligation, and therefore, the response of B cells and monocytes to direct CD40 Lt stimulation was examined.
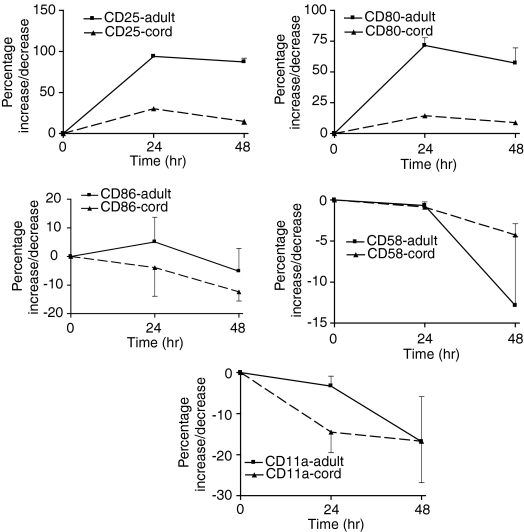
Cord B cells responded as well as adult B cells to direct CD40Lt stimulation (Fig. 4). Table 1 shows the baseline expression of the surface molecules on B cells and monocytes. CD40L ligation of B cells increased the expression of CD80, CD86, and CD58. These molecules were expressed in a small percentage of resting B cells (Table 1). However, CD11a, which is expressed on nearly all B cells, was down-regulated (Fig. 4). The expression of these molecules on cord B cells was similarly increased or decreased by CD40Lt stimulation except for a smaller increase of CD86 and CD58 (Fig. 4). The MFI of CD80, CD86 and CD58 on adult B cells was increased 3-, 30- and 10-fold, respectively. MFI of CD11a remained unchanged. In contrast, the MFI of these molecules was hardly increased on cord B cells, being <2-fold.
Figure 4.
Comparison of the up-regulation of B7 costimulatory molecules and adhesion molecules on adult and cord B cells following stimulation by CD40Lt (1·0 µg/ml). Both adult and cord B cells responded similarly. CD25 used as a marker of B cell activation. The response of adult and neonatal B cells was not significantly different.
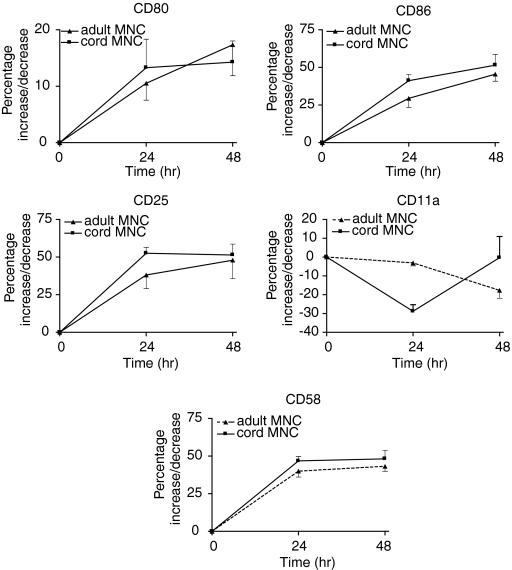
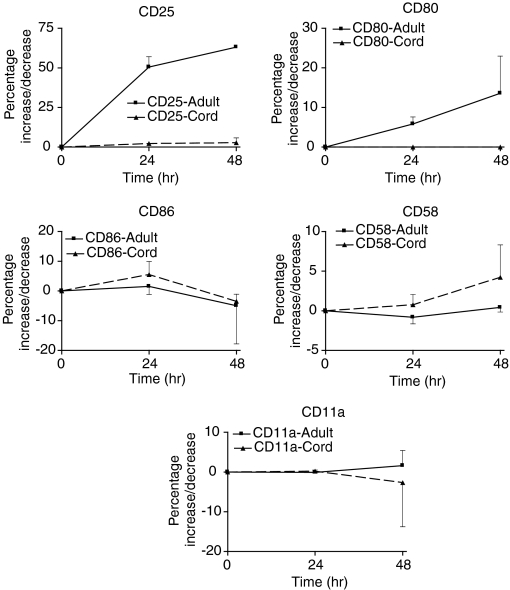
In contrast, adult and cord monocytes responded differently to direct CD40Lt stimulation from B cells. CD86, CD58 and CD11a were expressed on nearly all adult and cord monocytes (Table 1). Following cell culture (24 hr) expression of CD86 was down-regulated in the absence of CD40Lt stimulation of monocytes. However, the expression of CD86 on monocytes was increased slightly by the presence of CD40Lt (Fig. 5) and then decreased. CD80, which was not expressed on monocytes, was minimally up-regulated on adult and cord monocytes. Adult monocytes increased its expression slightly better than cord monocytes (Fig. 5). There was little change in CD11a expression but CD58 was up-regulated in both adult and cord monocytes. Both adult and cord monocytes expressed low levels of CD40 compared with B cells which could explain their poorer response (Table 1). We examined the effect of priming monocytes first with IFN-γ to increase CD40 expression. The addition of IFN-γ increased minimally the expression of CD40 on cord monocytes: change in percentage expression of 22·3 ± 18·4% (n = 3); MFI of 227 ± 190 after 24 hr incubation. In contrast, although adult monocytes increased the percentage of expression by 17·8 ± 1·2%, (n = 3); MFI increased by 4569 ± 1195. In the presence of soluble CD40Lt the up-regulation of costimulatory molecules CD80 and CD86 on the cell surface of cord monocytes was reduced compared with adult monocytes despite priming by IFN-γ (Table 2).
Figure 5.
Comparison of the up-regulation of B7 costimulatory molecules and adhesion molecules on adult and cord monocytes following stimulation by CD40Lt (1·0 μg/ml). Both adult and cord monocytes responded poorly. CD25, used as a marker of monocyte activation, showed significant activation of adult monocytes, P = 0·0001. CD80 was up regulated on adult monocytes (P = 0·05) but CD86 was up-regulated on neonatal monocytes, P = 0·0072.
Table 2.
Up-regulation of CD80 and CD86 following priming with interferon-γ followed by CD40Lt
| CD80* | CD86* | |||
|---|---|---|---|---|
| CD80* |
CD86* |
|||
| IFN-γ† | IFN-γ+CD40Lt‡ | IFN-γ† | IFN-γ + CD40Lt‡ | |
| Adult | 59·6 ± 8·0 | 22·9 ± 12·1 | 32·6 ± 3·8 | 11·6 ± 10·4 |
| Cord | 0 | 0 | 6·7 ± 0·7 | 0 |
Results of n = 3 experiments±1SD.
Increase from control level.
Increase from IFN-γ stimulated level.
Discussion
The B7–CD28 interaction is closely linked to CD40L–CD40 interaction. These two ligand-receptor pairs are part of a sequential multisteps T-cell activation involving APCs like B cells and monocytes.21 CD40L–CD40 plays an important role in regulating APC function.5 We examined in parallel the role of CD40L–CD40 interaction in maturing B cells or monocytes to competent APCs in adult and cord whole blood and in the MNC fraction. In whole blood the stimulation of adult T cells with PHA and αCD28 was associated with the up-regulation of B7 molecules and CD58 on adult B cells. CD11a initially remained stable and subsequently decreased. The changes in the expression of these molecules on B cells were dependent on CD40L as αCD40L inhibited their up- or down-regulation. The inhibition, however, was not complete, suggesting other factors are involved in regulating or modifying their expression. MNCs were also used to examine more directly the role of CD40L–CD40 interaction in adult B cells. The response by adult B cells to direct CD40L ligation in MNCs paralleled that seen in whole blood (Figs 1 and 2). However, we found that neonatal B cells in whole blood showed parallel changes to that of adult blood for B7 molecules, CD58 and CD11a following T-cell stimulation. This was unexpected as CD40L was poorly expressed on cord blood T cells following T-cell stimulation in agreement with other reports.15,16 These findings suggest that CD40L independent factors could replace CD40L in neonatal B-cell activation. The molecular mechanisms may involve cytokines or other ligand–receptor pair interactions possibly other members of the TNF–TNFR superfamilies. Although neonatal T cells produce cytokines poorly22 neutrophils and platelets, which do express adhesion molecules and produce cytokines, are present in whole blood23 and these may potentially modify B-cell and monocyte responses. Also a recent report using CD40L-deficient T-cell clones showed that a T cell can activate B cells via CD40L-independent mechanisms.24 The relevance of the CD40L-independent activation of B cells for the in vivo situation needs further exploration.
Neonatal T cells are not intrinsically deficient but they do differ qualitatively from adult T cells when stimulated under standard conditions.25 Under certain conditions CD40L were up-regulated on neonatal T cells. Although stimulation by αCD3 and costimulation with αCD28 up-regulated only marginally the expression of CD40L on cord T cells, the addition of IL-2 did enhance the expression of CD40L, the expression was still less than that of the adult CD4+ T cells in the absence of added IL-2. Also certain other stimuli like Staphylococcal Endotoxin A (data not shown), agents that trigger protein kinase A26 or allogeneic stimulation18 can produce sustained CD40L up-regulation. Furthermore, priming of neonatal T cells enhanced CD40L expression on neonatal T cells with subsequent T-cell stimulation.16, 17 The expressed CD40L on activated neonatal T cells can regulate neonatal B cells.17 In agreement with these reports, we showed that B cells from cord blood responded to CD40Lt stimulation similarly to B cells from adult cells (Fig. 2). Taken together these findings would confirm that the immaturity of neonatal B-cell functions relates to more uneducated neonatal T cells in contradistinction to adult T cells. Should CD40L be up-regulated, the T-cell regulation of B-cell function via CD40L would be reasonably intact in the neonates. However, there is a slight subtle difference between adult and cord B cells in that the changes with respect to density of B7 molecules, adult B cells showed a higher fold increase in both whole blood and MNC preparations.
Adult monocytes were similarly activated and matured to competent APCs as evidenced by up-regulating CD25 and CD80 following T-cell stimulation in whole blood. Surface molecules such as CD86, CD58 and CD11a, which are highly expressed in the resting state, decreased with time. This may reflect their down-regulation following their interaction with their respective cognate ligand suggesting a molecular activation of these molecules. Surprisingly, αCD40L had no inhibitory effect on the up-regulation of B7 molecules on adult monocytes. This observation suggests that CD40L has little role in activating adult monocytes and that cytokines, for example, IFN-γ or other membrane receptor–ligand interactions, may play a more functional role. Our findings in whole blood studies suggest that the CD40L–CD40 ligand-pair possibly plays a lesser role in regulating monocyte function. This is consistent with our findings that CD40Lt up-regulated B7 molecules poorly on adult monocytes in MNC preparations.
In contrast neonatal monocytes were not activated following T-cell activation in cord whole blood. This could be because of a lack of CD40L stimulation but also indicates that there were no CD40L-independent factors as seen in adult whole blood. Similar to adult monocytes, neonatal monocytes showed little response to CD40Lt stimulation. The poor response to CD40Lt ligation by both adult and neonatal monocytes may be in part explained by the degree of surface expression of CD40 on the monocytes. B cells from both sources had similar expression of CD40. Ninety-nine per cent of B cells in adult and cord blood expressed CD40 with comparable antigen density based on MFI level. A smaller percentage of adult and cord blood monocytes expressed CD40 compared to B cells and at a lower density (Table 1). This may explain the poorer response of adult monocytes to CD40Lt. In the case of neonatal monocytes it may also be the result of naivety or immaturity related to an undeveloped intracellular signal transduction as there was no increase in CD25 up regulation as observed with adult monocytes following CD40Lt ligation.
IFNγ is a potent activator of monocytes. We examined the possibility that priming by IFNγ may improve neonatal monocyte response to CD40Lt. IFNγ up regulated CD40 on adult monocytes but marginally on neonatal monocytes. B7 molecules on adult monocytes were also up regulated and the addition of CD40Lt had an additive effect. As discussed above, neonatal monocytes in whole blood were not activated following T-cell activation, negating a role for other stimuli in activating neonatal monocytes as seen with adult monocytes. This may be because of lower production of cytokines like IFN-γ by neonatal T and NK cells.22 However, IFN-γ also failed to activate or increase expression of CD40 or adhesion molecules on neonatal monocytes. This failure is not the result of a lack of IFN-γ receptors as we have previously shown that the incidence of the IFN-γ receptor was the same on cord and adult monocytes.22 The addition of CD40Lt together with IFN-γ also failed to up-regulate B7 or any other adhesion molecules. This is similar to a report that showed that a functional abnormality of phagocytosis became apparent in cord monocytes following IFN-α stimulation.27 Taken together, these findings suggest a possible functional immaturity of signal transduction in neonatal monocytes and that this becomes more apparent in the presence of an inflammatory stimulus.
In summary, our findings confirm previous observations that neonatal B cells are intrinsically functionally intact.16,17,28 They could be activated by the CD40L–CD40 ligand pair and under certain conditions T-cell help through CD40L is functional in regulating neonatal B cells.16,17 However, the importance of our studies relates to the functional immaturity of neonatal monocytes. Our findings suggest that in the adult, CD40L–CD40L receptor interaction has a lesser role in T-dependent activation of monocytes, however, the pair is active if monocytes are primed by IFN-γ. Similarly, in the neonates, the CD40L–CD40 ligand interaction involving monocytes is deficient but IFN-γ fails to activate neonatal monocytes for response to CD40L. Several studies have shown that CD40L–CD40 interaction is an important regulator of the cellular immune response5,6 and is important in GVHD, graft versus leukeamia (GVL) or graft versus tumour (GVT). Monocytes are considered important in the effector cellular response in GVHD29 and T-cell and monocyte interaction are important in regulating the immune response.30 We have previously shown possible naivety or immaturity of neonatal monocyte interaction with T cells: compared with adult cells, neonatal monocytes produced less inflammatory cytokines like IL-1α/β and TNF-α and neonatal T and NK cells expressed less of the receptors for IL-12 and IL-2.22,31 This report suggests that immaturity of the CD40L–CD40 ligand pair interaction may be an additional mechanism of deficient T-monocyte interaction. Our findings suggest that in an inflammatory microenvironment, e.g. cord blood transplantation neonatal monocytes may play a minor role in the effector arm of immune response. Taken together, our findings suggest that poor T-cell : monocyte interaction may explain the lower incidence of GVHD observed following transplantation of cord blood. It may also predict a poorer GVL or GVT effect following cord blood transplantation. Furthermore, ligand-receptor pair immaturity may contribute to the increase susceptibility of neonates to certain infections.32
References
- 1.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 2.Boussiotis VA, Freeman GJ, Gray G, et al. B7 but not intracellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993;178:1753–63. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann MF, McKall-Faienza K, Schmits R, et al. Distinct roles for LFA-1 and CD58 during activation of naïve T cells: adhesion versus costimulation. Immunity. 1997;7:549–7. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 4.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signalling. Nat Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbaugh D, Ochs HD, Noelle RJ, Ledbetter JA, Aruffo A. The role of CD40 and its ligand in the regulation of the immune response. Immunol Rev. 1994;138:23–37. doi: 10.1111/j.1600-065x.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 6.Grewal IS, Flavell RA. The CD40 ligand: at the centre of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 7.Kiener PA, Moran-Davis P, Rankin BM, et al. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–25. [PubMed] [Google Scholar]
- 8.Stout RD, Suttles J, Xu J, et al. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 9.Blazar BR, Taylor PA, Noelle RJ, Vallera DA. CD4+ T cells tolerized ex vivo to host alloantigen by anti-CD40 ligand (CD40L: CD154) antibody lose their graft-versus-host disease lethality capacity but retain nominal antigen responses. J Clin Invest. 1998;102:473–82. doi: 10.1172/JCI3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durie FH, Aruffo A, Ledbetter J, et al. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chronic forms of graft-versus-host disease. J Clin Invest. 1994;94:1333–8. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Blockade of CD40 ligand–CD40 interaction impairs T cell-mediated alloreactivity by inhibiting mature donor T cell expansion and function after bone marrow transplantation. J Immunol. 1997;158:29–39. [PubMed] [Google Scholar]
- 12.French RR, Chan HTC, Tutt AL, Glennie M. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nature Med. 1999;5:548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 13.Dilloo D, Brown M, Roskrow M, et al. CD40 ligand induces an antileukemia immune response in vivo. Blood. 1997;90:1927–33. [PubMed] [Google Scholar]
- 14.Diehl LTh, Den Boer A, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 15.Durandy A, De Saint Basile G, Lisowska-Grospierre B, et al. Undetectable CD40 ligand expression on T cells and low B cells responses to CD40 binding agonists in human newborns. J Immunol. 1994;154:1560–8. [PubMed] [Google Scholar]
- 16.Nonoyama S, Penix LA, Edwards CP, et al. Diminished expression of CD40 ligand by activated neonatal T cells. J Clin Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Splawski JB, Nishioka J, Nishioka Y, Lipsky PE. CD40 ligand is expressed and functional on activated neonatal T cells. J Immunol. 1996;154:119–27. [PubMed] [Google Scholar]
- 18.Matthews NC, Wadhwa M, Bird C, et al. Sustained expression of CD154 (CD40L) and proinflammatory cytokine production by alloantigen-stimulated umbilical cord blood T cells. J Immunol. 2000;164:6206–12. doi: 10.4049/jimmunol.164.12.6206. [DOI] [PubMed] [Google Scholar]
- 19.Hodge G, Hodge S, Han P. Increased levels of apoptosis of leucocyte subsets in cultured PBMCs compared to whole blood as shown by annexin V binding: relevance to cytokine production. Cytokine. 2000;12:1763–8. doi: 10.1006/cyto.2000.0790. [DOI] [PubMed] [Google Scholar]
- 20.Hodge G, Lloyd JV, Hodge S, et al. Functional lymphocyte immunophenotypes observed in thalassaemia and haemophilia patients receiving current blood product preparations. Br J Haematol. 1999;105:817–25. doi: 10.1046/j.1365-2141.1999.01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 22.Han P, Hodge G. Intracellular cytokine production and cytokine receptor interaction of cord mononuclear cells: relevance to cord blood transplantation. Br J Haematol. 1999;107:450–7. doi: 10.1046/j.1365-2141.1999.01696.x. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Puri RK. Common and uncommon features of cytokines and cytokine receptors: an overview. In: Aggarwal BB, Puri RK, editors. Human Cytokines: Their Role in Disease and Therapy. Melbourne: Blackwell Science; 1995. pp. 3–24. [Google Scholar]
- 24.Lane P, Burdet C, McDonnell F, et al. CD40 ligand-independent B cell activation revealed by CD40 ligand-deficient T cell clones: evidence for distinct activation requirements for antibody formation and B cell proliferation. Eur J Immunol. 1995;25:1788–93. doi: 10.1002/eji.1830250646. [DOI] [PubMed] [Google Scholar]
- 25.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 26.Suarez A, Mozo L, Gayo A, Simo A, Gutierrez C. Induction of functional CD154 (CD40 ligand) in neonatal T cells by cAMP-elevating agents. Immunol. 2000;100:432–40. doi: 10.1046/j.1365-2567.2000.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Mohande AE, Brudno DS, Ahronovich MD. Impaired interferon-alpha enhancement of neonatal monocyte phagocytosis. Biol Neonate. 1990;58:260–3. doi: 10.1159/000243277. [DOI] [PubMed] [Google Scholar]
- 28.Pastorelli G, Rousset F, Pene J, et al. Cord blood B cells are mature in their capacity to switch to IgE-producing cells in response to interleukin-4 in vitro. Clin Exp Immunol. 1990;82:114–19. doi: 10.1111/j.1365-2249.1990.tb05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara JLM, Antin JH. The pathophysiology of graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Haematopoietic Cell Transplantation. Malden: Blackwell Science Inc.; 1999. pp. 305–15. [Google Scholar]
- 30.Doherty TM. T-cell regulation of macrophage function. Curr Opin Immunol. 1995;7:400–4. doi: 10.1016/0952-7915(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 31.Hodge S, Hodge G, Flower R, Han P. Cord blood leucocyte expression of functionally significant molecules involved in the regulation of cellular immunity. Scand J Immunol. 2001;53:72–8. doi: 10.1046/j.1365-3083.2001.00845.x. [DOI] [PubMed] [Google Scholar]
- 32.Lewis DB, Wilson CB. Developmental immunology and role of host defences in neonatal susceptibility to infection. In: Remington JS, Klein WB, editors. Infectious Diseases of the Fetus and Newborn Infant. 5. Philadelphia: W.B. Saunders; 2000. pp. 25–138. [Google Scholar]