Abstract
We previously reported that mouse NK1.1 Ag+ T (NKT) cells activated by interleukin-12 (IL-12) act as anti-tumour/anti-metastatic effectors. However, IL-12 reportedly induces a rapid disappearance of liver NKT cells by activation-induced apoptosis. In the present study, however, we show that injection of IL-12 into mice merely down-regulates the NK1.1 expression of liver NKT cells and Vβ8+ intermediate T-cell receptor cells and CD1d/α-galactosylceramide (α-GalCer)-tetramer reactive cells in the liver remained and did not decrease. Furthermore, when IL-12-pretreated (24 hr before) mice were injected with α-GalCer, not only serum interferon-γ but also serum IL-4 concentrations increased several-fold in comparison to the control α-GalCer-injected mice. However, IL-12 pretreatment markedly up-regulated serum ALT levels and Fas-ligand expression on NKT cells after α-GalCer injection in middle-aged mice only. Consistently, the liver mononuclear cells (MNC) from IL-12-pretreated mice stimulated with α-GalCer in vitro produced much greater amounts of interferon-γ and IL-4, and also showed a more potent cytotoxicity against tumour targets than those from mice pretreated with phosphate-buffered saline. Liver MNC from middle-aged mice, but not from young mice pretreated with IL-12, also showed increased cytotoxicity following in vitroα-GalCer stimulation against cultured hepatocytes. Furthermore, IL-12 treatment of middle-aged mice enhanced tumour necrosis factor receptor 1 mRNA expression in liver Vβ8+ T cells, and in vitro experiments also revealed that IL-12 pretreatment of liver MNC from middle-aged mice enhanced their tumour necrosis factor-α production after α-GalCer stimulation. Synthetic ligand-mediated functions of NKT cells, including IL-4 production, are thus enhanced by IL-12 pretreatment.
Keywords: α-galactosylceramide, hepatotoxicity, interferon-γ, interleukin-12, murine models, NKT cells, NK1.1
Introduction
Natural killer T (NKT) cells are dependent on the major histocompatibility complex (MHC) class Ib molecule, Cd1d, for their development. They are distinct lineage cells from conventional T cells, B cells and NK cells and mainly use Vα14 Jα281/Vβ8.2 gene products for their T-cell receptors.1–3 We previously reported that interleukin-12 (IL-12) induces liver NKT cells to produce interferon-γ (IFN-γ) and acquire a potent anti-tumour cytotoxicity, and that IL-12-activated NKT cells inhibited liver, lung and kidney metastases of tumours.4–8 These results were confirmed in NKT-cell-deficient mice9,10 in which IL-12 injection could not effectively inhibit liver and lung metastases of tumours. However, IL-12 was reported to induce rapid apoptosis of liver NKT cells and thereby such cells disappeared from the liver.11,12 Therefore, the behaviour of NKT cells after IL-12 injection still remains controversial.
On the other hand, a synthetic ligand of NKT cells, α-galactosylceramide (α-GalCer), activates liver NKT cells to produce large amounts of IFN-γ and IL-4, and IFN-γ then activates NK cells to acquire a potent anti-tumour cytotoxicity.13 For effective IFN-γ production by α-GalCer-activated NKT cells, production of IL-12 by macrophages/dendritic cells is required.14,15 However, α-GalCer-activated NKT cells themselves induce hepatocyte injury through the Fas/Fas-ligand (FasL) pathway13 especially in older mice16 whereas NK cells are not significantly involved in hepatocyte injury.13 On the other hand, α-GalCer reportedly promotes the activation-induced apoptosis of liver NKT cells.17,18 In addition, NKT cells may also undergo apoptosis after activation by stimulation through the CD3/T-cell receptor (TCR) complex.12 These findings led researchers to think that activation-induced apoptosis was an essential characteristic of NKT cells. However, if NKT cells truly undergo rapid apoptosis after IL-12 injection, then our previous findings, which demonstrated that IL-12 injection 1 day before tumour administration activated NKT cells and inhibited metastases of NK-resistant tumours in the liver and lung, are thus highly unlikely.4–6
However, it has very recently been reported that spleen and liver NKT cells did not undergo apoptosis after α-GalCer stimulation, but instead became transiently undetectable by the down-regulation of NK1.1 and the internalization of TCR.19,20 We also herein demonstrate that IL-12 does not induce apoptosis of liver NKT cells but only down-regulates their NK1.1 antigen expression while also greatly enhancing the subsequent ligand-mediated activation of NKT cells. Nevertheless, at the same time, we also herein have to state that our previous observation that IL-12 injection increased the NK1.1 antigen expression levels of liver NKT cells4–6 might not be always reproducible.
Materials and methods
Mice
C57BL/6 (B6) mice at 6 weeks and 25 weeks of age were obtained from Japan SLC Inc. (Hamamatsu, Japan) as young and middle-aged mice, respectively.
Reagents
α-GalCer [(2S,3S,4R)-1-O-(α-d-galactopyranosyl)-2-(N-hexacosanoyl-amino)-1,2,4-octadecanetriol (KRN7000)], was provided by the Pharmaceutical Research Laboratory of Kirin Brewery Company.21,22 The original solution of α-GalCer (220 μg/ml) was prepared with 0·5% polysorbate 20 (Nikko Chemical, Tokyo, Japan) in saline and was subsequently diluted either with this solution (vehicle) or with saline. The mice were injected intravenously with 100 μg/kg of body mass of α-GalCer. Mouse recombinant IL-12 was purchased from R & D (Minneapolis, MN). Twenty-four hours before α-GalCer injection, 25 μg/kg of IL-12 was injected intraperitoneally. Cy-Chrome-conjugated human CD1d/α-GalCer tetramer,23 which equivalently binds mouse Vβ8 NKT cells and some mouse Vα14Vβ8 NKT cell hybridomas as compared to mouse CD1d/α-GalCer tetramer, was also provided by the Pharmaceutical Research Laboratory of the Kirin Brewery Company.
Medium
For the liver mononuclear cell (MNC) culture, RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; 10% RPMI) was used. For the primary hepatocyte culture, we used a hepatocyte growth medium containing 10% FBS (10% HCGM)24 with slight modifications: Dulbecco's modified Eagle's medium (DMEM; Sigma, St Louis, MO), containing 20 mm HEPES, 30 μg/ml l-proline, 10 mm nicotinamide, 0·2 mm Asc-2P (Sigma), 0·5 μg/ml insulin (Sigma), 10−7 m dexamethasone (Sigma), 400 nm glucagon (Sigma), 40 mm NaHCO3 and 100 IU/ml penicillin G and 100 μg/ml streptomycin as antibiotics.
Isolation and culture of MNC
Hepatic MNC were prepared essentially as described previously.4,5 In brief, the livers were minced with scissors and then suspended in 0·05% collagenase-Hanks' balanced salt solution and incubated at 37° for 20 min while shaking. Thereafter, the specimens were washed twice and passed through a 200-gauge stainless steel mesh; thus liver MNC with Kupffer cells were obtained by osmolarity using pH-adjusted 33% Percoll solution containing 100 U/ml heparin and centrifugation at 500 g for 20 min at room temperature. The resulting pellet was resuspended in red blood cell lysis solution and then was washed twice in medium. After counting the number of cells in the pellet, 5 × 105 cells in 200 μl medium per well were cultured with α-GalCer in 96-well flat-bottom plates in 5% CO2 at 37°. Next, these cells were used for further experiments. The culture supernatants were stored for 24 hr after seeding.
Isolation and culture of hepatocytes
Hepatocytes were obtained from 4-week-old mice essentially as described previously.24 Briefly, the portal vein was perfused with 0·05% collagenase (Type I; Sigma), and dispersed cells were filtered through a 40-μm nylon-mesh (Cell Strainer; Falcon 2340; BD Biosciences, San Jose, CA). Based on morphological examinations, approximately 98% of the filtered cells were identified as hepatocytes. The viability of the hepatocytes estimated by the trypan blue dye exclusion test was approximately 95%. In culture, 1 × 105 cells suspended in the HCGM were plated on a 24-well type I collagen-coated plastic dish (Iwaki, Funabashi, Japan). After the hepatocytes adhered to the dish, these cells were used for further experiments.
Flow cytometric analysis
The surface phenotypes of liver MNC were characterized by a two- or three-colour flow cytometric analysis. A fluorescein isothiocyanate (FITC) -conjugated monoclonal antibodies (mAbs) to mouse TCRαβ (H57-597) and Vβ8 (F23.1) and a phycoerythrin (PE) -conjugated mAb to NK1.1 (PK136) were obtained from BD PharMingen (San Diego, CA). Cy-Chrome-conjugated human CD1/α-GalCer tetramer23 was also used. Before staining with antibodies, the MNC were incubated for 10 min at 4° with Fc-blocker (2.4G2; BD PharMingen) to prevent any non-specific binding. For an analysis of the FasL expression, liver MNC were isolated 1 hr after the injection of either α-GalCer or vehicle into B6 mice. Next, the cells were stained with the FITC-conjugated mAb to TCRαβ, the PE-conjugated mAb to NK1.1, and a biotin-conjugated mAb to FasL [Kay-10, immunoglobulin G2b (IgG2b); BD PharMingen]; immune complexes formed by the latter antibody were detected with Cy5-streptavidin. Flow cytometry was performed using the EPICS XL (Coulter, Miami, FL).
Intracellular IFN-γ staining
One hour after α-GalCer injection into mice pretreated with IL-12 or phosphate-buffered saline (PBS; 24 hr before), livers were obtained and 1 × 106 liver MNC were incubated for 1 hr with Golgistop (PharMingen) at 37°. Thereafter the Fc-blocker 2.4G2 was added and the mixture was incubated at 4° for 10 min. PE-anti-αβTCR antibody was added to the liver MNC, the mixture was further incubated at 4° for 20 min. The Cytofix/Cytoperm solution (PharMingen) was added to the liver MNC at 4° for 20 min. Next, the liver MNC were washed twice by a Perm/Wash solution (PharMingen) and incubated with FITC-conjugated anti-IFN-γ antibody at 4° for 30 min. Finally, the liver MNC were washed twice and analysed by the EPICS XL (Coulter).
Cytotoxicity assay against primary-cultured hepatocytes
Primary-cultured hepatocytes were used as target cells.24 Isolated hepatocytes were plated on a 24-well type I collagen-coated microplate containing 10% HCGM for 1 hr. Next, the supernatants, including any dead cells from each well, were eliminated and the adherent hepatocytes were labelled with 10 μCi 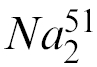 CrO4 in 1 ml 10% HCGM for 8 hr at 37°. After the 51Cr-labelling incubation, the hepatocytes were washed three times with medium alone, and the cells in 500 μl of HCGM were mixed with 50 times as many of fresh liver MNC which had been obtained from each age of mice and were suspended with α-GalCer in 500 μl 10% RPMI in each well. The plate was then centrifuged, and the resulting supernatants were harvested and their radioactivity was determined using a gamma counter. Cytotoxicity was calculated as the percentage of released radioactivity after correcting for the spontaneous release, which was <15% of the maximal release.
CrO4 in 1 ml 10% HCGM for 8 hr at 37°. After the 51Cr-labelling incubation, the hepatocytes were washed three times with medium alone, and the cells in 500 μl of HCGM were mixed with 50 times as many of fresh liver MNC which had been obtained from each age of mice and were suspended with α-GalCer in 500 μl 10% RPMI in each well. The plate was then centrifuged, and the resulting supernatants were harvested and their radioactivity was determined using a gamma counter. Cytotoxicity was calculated as the percentage of released radioactivity after correcting for the spontaneous release, which was <15% of the maximal release.
Measurement of IFN-γ, IL-4, tumour necrosis factor-α (TNF-α) and serum alanine aminotransferase (ALT)
The mice pretreated with IL-12 or PBS were given α-GalCer intravenously. The peripheral blood of individual mice was collected at the indicated time points from the retro-orbital sinus. The IFN-γ, IL-4 and TNF-α levels of the sera or culture supernatants were measured using cytokine-specific enzyme-linked immunosorbent assay (ELISA) kits (PharMingen). The activity of ALT in the serum was determined with a DRICHEM 3000 V device (Fuji Medical Systems, Tokyo, Japan).
RT-PCR analysis
The sequences of oligonucleotide primers for reverse transcription–polymerase chain reaction (RT-PCR) analysis were as follows: For mouse TNF receptor 1 (TNFR1)25 antisense (CAAGATAACCAGGGGCAACAG) and sense (CCAACGCTACCTGAGTGAGA) primers were used. For mouse IL-12 receptor β1 (IL-12Rβ1)10 antisense (AACCTTGTACATCACTCGGCTG) and sense (GAACCACACACACTGTACCCTG) primers were used. For mouse GAPDH, antisense (GTCCAGGGTTTCTTACTCCT) and sense (ATGACCACAGTCCATGCCAT) primers were used. For quantification, the band intensity was analysed using the National Institutes of Health (Bethesda, MD) image software package (version 1·60). PCR bands of IL-12Rβ1 and TNFR1 were quantified based on the ratio of the signal intensity of the PCR products to those of the internal controls (GAPDH). The linearity of an RT-PCR analysis was confirmed in advance, and an estimation of each band was performed within a linear range.
Statistical analysis
In each experiment, the results were expressed as the mean ± SD. Where appropriate, Student's t-test was employed to compare the data of two different groups. P-values of less than 0·05 were considered to be significant.
Decrease of NK1.1 antigen expression of NKT cells after IL-12 administration
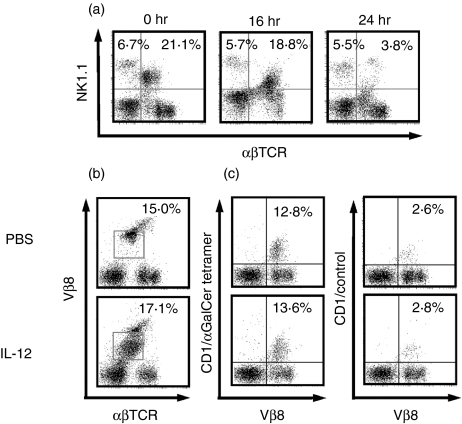
After IL-12 administration, the NK1.1 antigen expression levels of NKT cells were substantially decreased 16 hr after IL-12 administration while NK1.1 antigen had almost disappeared at 24 hr after IL-12 administration (Fig. 1a). However, Vβ8+T cells with intermediate TCR, which comprise 60–70% of liver NKT cells, did not decrease (Fig. 1b), although the Vβ8 intensity became lower and broad.6 Furthermore, CD1d/α-GalCer tetramer binding Vβ8+T cells were also present (Fig. 1c).
Figure 1.
NK1.1+T cells in the liver became undetectable after IL-12 injection into mice, whereas Vβ8+T cells with lower TCR and CD1d/α-GalCer tetramer binding Vβ8+T cells remain detectable. (a) NK1.1 expression of liver αβT cells. The middle-aged (25-week-old) mice were injected intraperitoneally with IL-12 and livers were obtained at the indicated times after IL-12 injections. Liver MNC were stained and analysed. Numbers of upper quadrants represent percentage of NK cells and NK1.1+T cells in the liver MNC. (b) Vβ8 expression of liver αβT cells. Numbers within left panels represent percentage ofVβ8T cells with intermediate TCR in whole liver MNC (indicated by squares). (c) CD1/α-GalCer tetramer binding Vβ8+T cells. Livers were obtained from middle-aged mice 24 hr after IL-12 injection and then the liver MNC were stained and analysed.
The effect of IL-12 pretreatment on the elevation of serum cytokines and ALT in the mice injected with α-GalCer
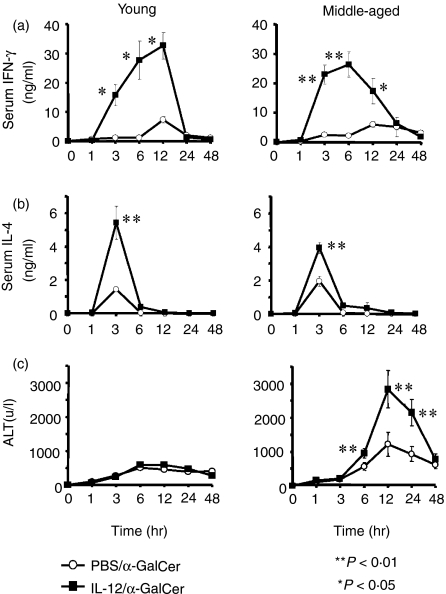
IL-12 pretreatment induced several fold greater levels of serum IFN-γ and IL-4 after α-GalCer injection in young and middle-aged mice (Fig. 2a,b). However, although IL-12 pretreatment did not enhance the elevation of serum ALT levels after α-GalCer injection in young mice, it greatly up-regulated the ALT levels in middle-aged mice (Fig. 2c).
Figure 2.
The serum IFN-γ, IL-4 and ALT levels after α-GalCer administration in young (6 weeks) and middle-aged (25 weeks) mice pretreated with IL-12 or PBS. Young and middle-aged mice pretreated with IL-12 or PBS 24 hr before were administered with α-GalCer intravenously and peripheral blood samples from five individual mice were collected at the indicated time points from the retro-orbital plexus. The data are expressed as the mean ± SE from five mice of each group.
In vitro evidence of the augmentation of the ligand-mediated NKT cell function by IL-12
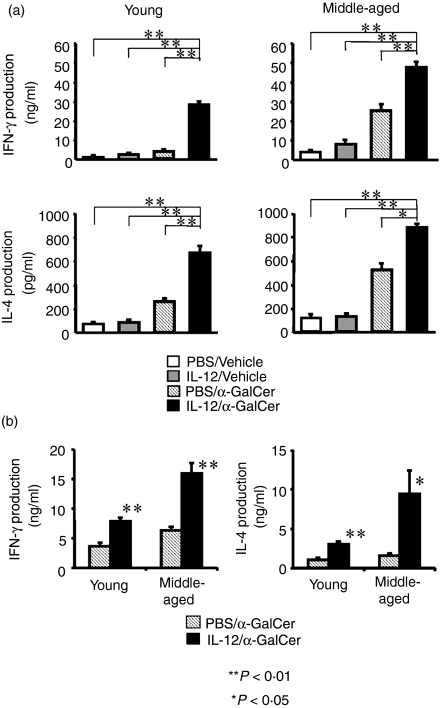
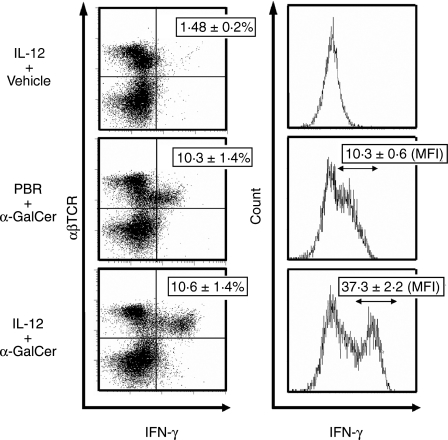
When liver MNC isolated from mice injected with IL-12 or PBS (control) 24 hr before were stimulated with α-GalCer in vitro, liver MNC from IL-12-treated young and middle-aged mice produced significantly greater amounts of both IFN-γ and IL-4 (Fig. 3a). When isolated liver MNC were stimulated with IL-12 in vitro for 24 hr followed by additional in vitro culture with α-GalCer for 24 hr, liver MNC also produced larger amounts of IFN-γ and IL-4 than liver MNC without IL-12 stimulation. In addition, the results of intracellular IFN-γ staining clearly demonstrated that although IL-12 pretreatment did not increase the proportion of IFN-γ-producing T cells with intermediate TCR (NKT cells), it did augment IFN-γ production from intermediate TCR cells stimulated with α-GalCer, as evidenced by the increase of mean fluorescence intensity of intracellular IFN-γ (10·3 versus 37·3) (Fig. 4). In contrast to IL-12 injection into mice, in vitro culture of liver MNC with IL-12 for 24 hr did not decrease either the expression of NK1.1 of NKT cells or the proportion of NKT cells (not shown). IL-4 pretreatment had no effect on either serum IFN-γ or serum IL-4 levels after α-GalCer administration (data not shown). IL-12 pretreatment also augmented the cytotoxicity of α-GalCer-activated liver MNC against Yac-1 and EL-4 tumour cells (data not shown).
Figure 3.
(a) In vitroα-GalCer-stimulated IFN-γ and IL-4 production from liver MNC of mice pretreated with IL-12 or PBS. Liver MNC were obtained from young or middle-aged mice pretreated 24 hr before with IL-12 or PBS were stimulated with α-GalCer in vitro for 24 hr and culture supernatants were subjected to ELISA. (b) In vitroα-GalCer-stimulated IFN-γ and IL-4 production from liver MNC pretreated with IL-12 (25 ng/ml). Liver MNC were obtained from young or middle-aged mice and cultured with medium supplemented with IL-12 (in 5 μl PBS, final concentration; 25 ng/ml)or 5 μl PBS for 24 hr and thereafter were stimulated with α-GalCer in vitro for 24 hr and culture supernatants were subjected to ELISA. The data are expressed as the mean ± SE from four mice of each group.
Figure 4.
Intracellular IFN-γ expression of liver MNC after α-GalCer administration. One hour after α-GalCer injection into middle-aged mice pretreated with IL-12 or PBS, liver MNC were isolated and stained with PE-antiαβ TCR antibody or FITC-anti-IFN-γ antibody as described in the Materials and Methods. Proportions (%) of IFN-γ-expressing cells in whole liver MNC are shown in left panels, while the mean fluorescence intensities (MFI) are shown in right panels (mean ± SE of three mice in each group). Similar results were obtained in two additional experiments.
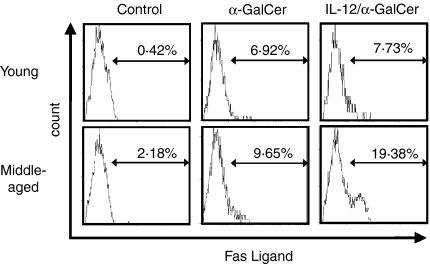
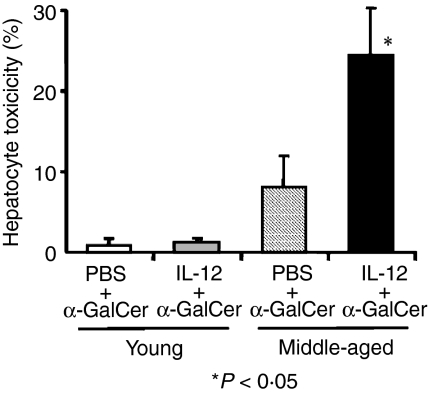
IL-12 pretreatment increases FasL expression of liver NKT cells after α-GalCer injection and increases cytotoxicity of liver MNC against cultured hepatocytes
Young and middle-aged mice pretreated with IL-12 or PBS (24 hr before) were injected with α-GalCer and the expression of FasL was examined 1 hr after α-GalCer injection. The results showed that while the pretreatment of IL-12 did not significantly affect the FasL expression of liver NKT cells in young mice, it did increase the proportion of FasL-expressing NKT cells in middle-aged mice (Fig. 5). IL-12 pretreatment also increased the cytotoxicity of the liver MNC from middle-aged mice stimulated with α-GalCer in vitro against cultured hepatocytes (Fig. 6). α-GalCer-stimulated liver MNC from young mice with or without IL-12 pretreatment did not induce any significant cytotoxicity against cultured hepatocytes (Fig. 6).
Figure 5.
The effect of IL-12 pretreatment in mice on the FasL expression on liver NKT cells after α-GalCer administration. One hour after α-GalCer administration, liver MNC were isolated and stained as described in the and analysed. Similar results were obtained in two additional experiments.
Figure 6.
The effect of IL-12 pretreatment in middle-aged and young mice on the cytotoxicity of liver MNC against cultured hepatocytes stimulated with α-GalCer in vitro. The data are expressed as the mean ± SE from three independent experiments.
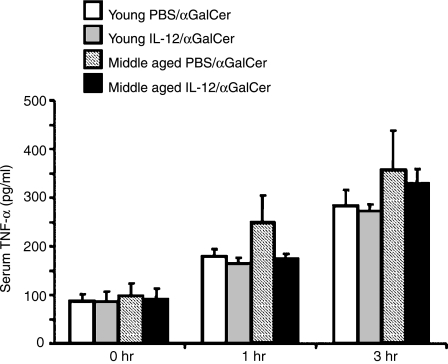
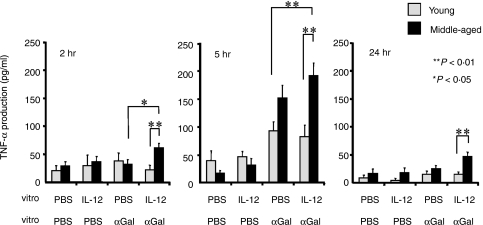
Serum TNF-α levels in mice with/without IL-12 pretreatment after α-GalCer-injection and the effect of in vitro IL-12 pretreatment on the TNF-α production from the liver MNC in vitro
We recently found that TNF-α is required for hepatotoxicity of NKT cells in aged mice after α-GalCer injection because neutralization of TNF-α induced a complete inhibition of hepatic injury accompanied by partial blocking of the FasL expression of NKT cells (T. Inui et al. submitted for publication). Therefore, the serum TNF-α levels of young and middle-aged mice pretreated with IL-12 or PBS (24 hr before) were examined 1 and 3 hr after α-GalCer-injection. The results showed that serum TNF-α levels did not significantly differ among the mouse groups (Fig. 7). However, when isolated liver MNC from middle-aged mice were pretreated with/without IL-12 in vitro for 24 hr and further stimulated with α-GalCer in vitro, IL-12 pretreatment significantly enhanced TNF-α production at 2 hr after α-GalCer stimulation as compared to either that produced by IL-12-pretreated young liver MNC or that produced by PBS-treated middle-aged liver MNC (Fig. 8). In contrast, IL-12 pretreatment did not affect the TNF-α production from liver MNC of young mice (Fig. 8). In addition, amounts of TNF-α produced by IL-12-pretreated liver MNC from middle-aged mice 5 hr after α-GalCer-stimulation were significantly larger than those from young liver MNC with or without IL-12 pretreatment (Fig. 8). IL-12 pretreatment tended to increase the TNF-α production from liver MNC of middle-aged mice as compared to that from those without IL-12 pretreatment but the difference was not statistically significant (Fig. 8).
Figure 7.
The serum TNF-α levels in mice after α-GalCer injection. Young and middle-aged mice pretreated with IL-12 or PBS 24 hr before were administered intravenously with α-GalCer and peripheral blood samples from mice were collected at the indicated time points from the retro-orbital plexus. The data are expressed as the mean ± SE from five mice of each group.
Figure 8.
In vitro TNF-α production from liver MNC stimulated with α-GalCer. Liver MNC were isolated from livers of young and middle-aged mice and cultured with IL-12 (PBS as a control) for 24 hr and were further stimulated with α-GalCer and cultured for 24 hr. Culture supernatants at 2, 5 and 24 hr after α-GalCer stimulation were collected and were subjected to ELISA. The data are expressed as the mean ± SE from four mice of each group.
IL-12 receptor and TNF-receptor mRNA expressions of liver MNC
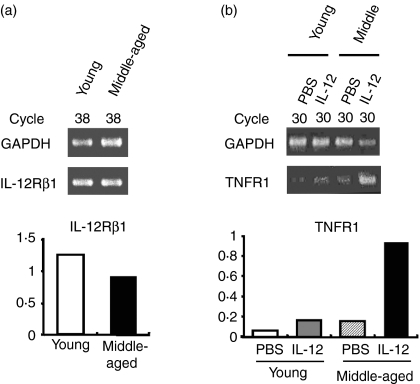
Vβ8+T cells sorted by magnetic beads from liver MNC obtained from young and middle-aged mice did not show a significant difference in mRNA expressions of IL-12Rβ1 in liver MNC (Fig. 9a). Since IL-12Rβ1 is expressed by resting NKT cells but not by conventional T cells,10 IL-12Rβ1-expressing Vβ8+T cells were suggested to be Vβ8+ NKT cells. However, IL-12 treatment of mice enhanced TNFR1 mRNA expression of liver Vβ8+T cells in middle-aged mice but not in young mice (Fig. 9b).
Figure 9.
IL-12 receptor and TNF-α receptor expressions of liver Vβ8+T cells. (a) IL-12Rβ1 mRNA expression in Vβ8+liver MNC from young and middle-aged mice (upper panels) and mRNA expression levels relative to those of GAPDH (lower panels). (b) TNFR1 mRNA expression in Vβ8+ liver MNC from young and middle-aged mice pretreated with IL-12 or PBS (control) (upper panels) and the mRNA expression levels relative to those of GAPDH (lower panels). PCR bands of IL-12Rβ1 and TNFR1 were quantified based on the ratio of the signal intensity of the PCR products to those of the internal controls (GAPDH) as described in the Materials and Methods.
Discussion
In the present study, we show that while IL-12 induces a down-regulation of NK1.1 antigen in liver NKT cells, NKT cells did not undergo apoptosis. IL-12 pretreatment of mice enhances not only IFN-γ production but also IL-4 production from α-GalCer-stimulated NKT cells, both in vivo and in vitro. A several-fold greater increase of the serum IFN-γ and IL-4 levels after α-GalCer injection was observed in IL-12-pretreated mice than in the control α-GalCer-injected mice. In contrast, IL-4 pretreatment did not affect α-GalCer-mediated elevation of either cytokine. However, the increases of serum ALT concentrations, FasL expression on liver NKT cells and cytotoxicity against cultured hepatocytes of liver MNC induced by α-GalCer injection were observed only in IL-12 pretreated middle-aged mice but not in young mice. Although the serum TNF-α levels after α-GalCer injection did not significantly differ among the mouse groups, the in vitro TNF-α production from α-GalCer-stimulated liver MNC of middle-aged mice (but not of young mice) was significantly enhanced by the in vitro IL-12 pretreatment and was larger than that of liver MNC of young mice. In addition, the TNFR1 mRNA expression of Vβ8+T cells was also enhanced by IL-12 treatment only in middle-aged mice.
NKT cells were found to be potent and prompt producers of both IFN-γ and IL-4 by TCR/CD3 cross-linking.26,27 We also reported that NKT cells are potent IFN-γ producers and anti-metastatic effector cells by the stimulation of IL-124,5,28 and suggested that NKT cells are mainly involved in the T helper type 1 (Th1) immune responses. Vβ8+NKT cell-deficient NC/Nga mice demonstrated an impaired IFN-γ production in response to bacterial components and showed high IgE levels. They also developed dermatitis which resembled human atopic dermatitis.29 In addition, NKT cells acquire a potent anti-tumour cytotoxicity via IFN-γ produced by NK cells in mice injected with a Gram-negative bacterial component, lipopolysaccharide.7,30 However, because NKT cells also produce both IFN-γ and IL-4 by the stimulation of their synthetic ligand, α-GalCer31–33 it has been hypothesized that NKT cells are involved in either the Th1 or Th2 immune response.
The present results, however, suggest that NKT cells may not fit the Th1/Th2 theory under certain conditions. It is well known that IL-12 drives the Th1 immune response and is a potent IFN-γ inducer from NK cells and NKT cells.28,34,35 NKT cells and their CD1d-dependent IL-4 production have previously been reported to play a crucial role in the IgG production that may suppress protozoal infections, such as Plasmodium and Trypanosoma infections.36 It was also recently reported that NKT cells may be a source of IL-4 production in filarial infection.37 These findings suggest that IL-4 production from NKT cells may thus be a mechanism to induce rapid, MHC-unrestricted antibody responses to pathogens.36 NKT cells stimulated with α-GalCer indeed probably produce both IFN-γ and IL-4 simultaneously.23 Liver NKT cells could be activated by IL-12 produced by Kupffer cells and dendritic cells in bacterial and protozoal infections and may augment their capacity to produce both IFN-γ and IL-4 after recognition of pathogenic antigens. The production of IFN-γ as well as IL-4 by NKT cells may thus play an integral part in the defence against microbes but their IL-4 production does not always play a direct role in the Th2 immune response per se. In other words, they may participate in the development of both cellular immunity and humoral immunity against microbes for the host defence.
We recently showed that ligand-mediated functions of NKT cells including cytokine production, anti-tumour cytotoxicity, FasL expression and hepatocyte toxicity were all enhanced with ageing.16 As shown in the present study, IL-12 pretreatment of young mice enhanced cytokine production and anti-tumour cytotoxicity of ligand-activated NKT cells, whereas it did not induce an increase in the FasL expression on NKT cells and thereby IL-12 pretreatment did not aggravate ligand-mediated hepatic injury in young mice. In contrast, IL-12 pretreatment of middle-aged mice induced an increase in both the FasL expression and hepatocyte-toxicity of NKT cells after α-GalCer stimulation.
We recently found that the neutralization of TNF-α completely inhibited α-GalCer-induced hepatic injury while also partially suppressing the FasL expression of NKT cells in aged mice without attenuating the anti-tumour effect of α-GalCer (T. Inui et al., submitted for publication). Interestingly, the present study showed that amounts of TNF-α produced from the liver MNC (presumably by Kupffer cells) of middle-aged mice after in vitroα-GalCer stimulation increased after in vitro IL-12 pretreatment, whereas TNF-α amounts from liver MNC of young mice did not increase at all with IL-12 pretreatment. Therefore, although serum TNF-α levels after α-GalCer injection did not significantly differ among mouse groups, TNF-α levels in situ of the liver may increase in middle-aged mice, especially in IL-12-pretreated mice. This discrepancy between in vivo and in vitro results may be partly explained by the short serum half-life of TNF-α in mice (10 min).38 In addition, the current study also showed that the TNFR1 expression of liver NKT cells of middle-aged mice was increased by IL-12 pretreatment. Therefore, the increased hepatotoxicity induced by α-GalCer of liver NKT cells in IL-12-pretreated middle-aged mice may be attributable to both the enhanced TNF-α production in situ and the increased responsiveness of NKT cells to TNF-α.
The possibility has been raised that NKT cells in aged mice may strengthen their autoreactive mechanisms including a Fas-FasL/TNF-α system to survey abnormal autologous cells and senescent cells and thus induce their apoptosis to maintain host homeostasis. In fact, a large number of abnormal CD4– CD8– double-negative T cells accumulate in the liver of both FasL-deficient gld/gld mice and Fas-deficient lpr/lpr mice.39–41 It was also reported in normal mice that activated T cells accumulate in the liver and undergo apoptosis42 and NKT cells kill autologous CD4+ CD8+ double-positive thymocytes using the Fas-FasL system.43,44 In addition, agonistic anti-Fas antibody and FasL have been reported to induce fulminant hepatic failure in normal mice.45 However, hepatic failure was not induced in the liver following a partial hepatectomy, in which hepatocytes are newly generated and actively proliferate.46
Some researchers have argued that IL-12 induces a rapid decrease/deletion of liver NKT cells by activation-induced apoptosis.11,12 However, IL-12 did induce IFN-γ production and anti-tumour cytotoxicity of liver MNC in NK cell-depleted mice or NK-deficient beig/beig mice8 but could not induce these effects in either NKT cell-depleted mice6 or genetically NKT cell-deficient mice.10 The present study thus clearly showed that NKT cells in IL-12-injected mice merely down-regulate or modulate NK1.1 antigens from the surface. In line with our findings, recent studies have also shown that liver and spleen NKT cells became transiently undetectable in mice even after α-GalCer injection by the down-regulation of NK1.1 and the internalization of TCR but do not undergo apoptosis.19,20
However, even though the sources of IL-12, mice and antibodies (we previously used biotinylated NK1.1 antibody) differed between us and other groups, we agree that the increase of NK1.1 expression on liver NKT cells after IL-12 injection cannot be reproduced using FITC- or PE-conjugated anti-NK1.1 antibody and instead is down-regulated. While it is known that staining of antigens with biotinylated antibody shows much higher fluorescence intensities than those by conjugated antibody, we cannot precisely explain this discrepancy. However, as we previously pointed out7 using 40% Percoll solution instead of 33% Percoll solution for isolating liver MNC, a significant portion of either activated NK cells or NKT cells, or both, are lost.
Acknowledgments
Collectively, whereas IL-12 treatment down-regulates NK1.1 antigen of liver NKT cells, IL-12 indeed augments ligand-mediated functions of NKT cells.
References
- 1.Makino Y, Koseki H, Adachi Y, Akasaka T, Tsuchida K, Taniguchi M. Extrathymic differentiation of a T cell bearing invariant V alpha 14J alpha 281 TCR. Int Rev Immunol. 1994;11:31–46. doi: 10.3109/08830189409061715. [DOI] [PubMed] [Google Scholar]
- 2.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8– T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto W, Takeda K, Anzai R, Ogasawara K, Sakihara H, Sugiura K, Seki S, Kumagai K. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–40. [PubMed] [Google Scholar]
- 5.Takeda K, Seki S, Ogasawara K, et al. Liver NK1.1+ CD4+ alpha beta T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol. 1996;156:3366–73. [PubMed] [Google Scholar]
- 6.Seki S, Hashimoto W, Ogasawara K, Satoh M, Watanabe H, Habu Y, Hiraide H, Takeda K. Antimetastatic effect of NK1+ T cells on experimental haematogenous tumour metastases in the liver and lungs of mice. Immunology. 1997;92:561–6. doi: 10.1046/j.1365-2567.1997.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense. the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 8.Anzai R, Seki S, Ogasawara K, Hashimoto W, Sugiura K, Sato M, Kumagai K, Takeda K. Interleukin-12 induces cytotoxic NK1+ alpha beta T cells in the lungs of euthymic and athymic mice. Immunology. 1996;88:82–9. doi: 10.1046/j.1365-2567.1996.d01-638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, Abo T, Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- 11.Emoto M, Emoto Y, Kaufmann SH. Interleukin-4-producing CD4+ NK1.1+ TCR alpha/beta intermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur J Immunol. 1995;25:3321–5. doi: 10.1002/eji.1830251218. [DOI] [PubMed] [Google Scholar]
- 12.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–53. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa R, Nagafune I, Tazunoki Y, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol. 2001;166:6578–84. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomura M, Yu WG, Ahn HJ, et al. A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 16.Inui T, Nakagawa R, Ohkura S, et al. Age-associated augmentation of the synthetic ligand- mediated function of mouse NK1.1ag(+) T cells: their cytokine production and hepatotoxicity in vivo and in vitro. J Immunol. 2002;169:6127–32. doi: 10.4049/jimmunol.169.11.6127. [DOI] [PubMed] [Google Scholar]
- 17.Leite-De-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol. 2000;165:4367–71. doi: 10.4049/jimmunol.165.8.4367. [DOI] [PubMed] [Google Scholar]
- 18.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–18. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020–7. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 21.Morita M, Motoki K, Akimoto K, et al. Structure–activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi Y, Motoki K, Ueno H, Maeda K, Kobayashi E, Inoue H, Fukushima H, Koezuka Y. Enhancing effects of (2S,3S,4R)-1-O-(alpha-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol (KRN7000) on antigen-presenting function of antigen-presenting cells and antimetastatic activity of KRN7000-pretreated antigen-presenting cells. Oncol Res. 1996;8:399–407. [PubMed] [Google Scholar]
- 23.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–54. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inui T, Shinomiya N, Fukasawa M, Kobayashi M, Kuranaga N, Ohkura S, Seki S. Growth-related signaling regulates activation of telomerase in regenerating hepatocytes. Exp Cell Res. 2002;273:147–56. doi: 10.1006/excr.2001.5446. [DOI] [PubMed] [Google Scholar]
- 25.Walter U, Franzke A, Sarukhan A, Zober C, von Boehmer H, Buer J, Lechner O, Frantzke A. Monitoring gene expression of TNFR family members by beta-cells during development of autoimmune diabetes. Eur J Immunol. 2000;30:1224–32. doi: 10.1002/1521-4141(200004)30:4<1224::AID-IMMU1224>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+ CD4+ CD8– thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–10. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogasawara K, Takeda K, Hashimoto W, et al. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–7. [PubMed] [Google Scholar]
- 29.Habu Y, Seki S, Takayama E, Ohkawa T, Koike Y, Ami K, Majima T, Hiraide H. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J Immunol. 2001;166:5439–47. doi: 10.4049/jimmunol.166.9.5439. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Ogasawara K, Takeda K, et al. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156:2436–42. [PubMed] [Google Scholar]
- 31.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge. activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–7. [PubMed] [Google Scholar]
- 33.Cui J, Watanabe N, Kawano T, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells. J Exp Med. 1999;190:783–92. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12. A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–31. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 36.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–9. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 37.Balmer P, Devaney E. NK T cells are a source of early interleukin-4 following infection with third-stage larvae of the filarial nematode Brugia pahangi. Infect Immun. 2002;70:2215–19. doi: 10.1128/IAI.70.4.2215-2219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flick DA, Gifford GE. Pharmacokinetics of murine tumor necrosis factor. J Immunopharmacol. 1986;8:89–97. doi: 10.3109/08923978609031087. [DOI] [PubMed] [Google Scholar]
- 39.Ohteki T, Seki S, Abo T, Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4–8– double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990;172:7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–17. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 41.Masuda T, Ohteki T, Abo T, Seki S, Nose S, Nagura H, Kumagai K. Expansion of the population of double negative CD4–8– T alpha beta-cells in the liver is a common feature of autoimmune mice. J Immunol. 1991;147:2907–12. [PubMed] [Google Scholar]
- 42.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 43.Arase H, Arase N, Kobayashi Y, Nishimura Y, Yonehara S, Onoe K. Cytotoxicity of fresh NK1.1+ T cell receptor alpha/beta+ thymocytes against a CD4+8+ thymocyte population associated with intact Fas antigen expression on the target. J Exp Med. 1994;180:423–32. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moroda T, Iiai T, Suzuki S, et al. Autologous killing by a population of intermediate T-cell receptor cells and its NK1.1+ and NK1.1– subsets, using Fas ligand/Fas molecules. Immunology. 1997;91:219–26. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 46.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–3. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]