Abstract
Injection of antigen into the ocular anterior chamber (AC) of a mouse eye (an immunologically privileged site) induces the activation of immunoregulatory NK1.1+, CD4– CD8–, T-cell receptor (TCR) αβ+ thymocytes. These thymocytes transfer the suppression of delayed-type hypersensitivity (DTH) when injected into mice sensitized to the same antigen but do not effect the suppression of DTH. On the other hand, the immunized recipients of these transferred thymocytes produce splenic CD8+ T cells that effect the suppression of DTH. However, it is unclear whether the thymocytes transferred from the AC-injected donor differentiate into and/or activate CD8+ T-splenic suppressor cells. We therefore sought to determine the origin of splenic suppressor cells produced in the recipients of immunoregulatory thymocytes transferred from donors that receive an injection of antigen into the AC. CD45.1+ thymocytes from mice that received an AC injection of 2,4,6-trinitrobenzene sulphonic acid (TNP)-bovine serum albumin (BSA) were transferred to congenic CD45.2+ TNP-BSA-immune recipients. Spleen cells from the recipients were then sorted based on anti-CD45.1 or -CD45.2 antibody binding and assayed for suppressor cells. This was done by the injection of separated spleen cells into the footpad of TNP-BSA-immunized mice, concurrent with the induction of footpad swelling (contact sensitivity) of the footpad elicited by an epicutaneous application of picryl chloride. The systemic distribution of antigen after the injection of antigen into the AC was demonstrated by the injection of fluorescein or 125I-labelled TNP-BSA into the AC. The results demonstrate that (i) splenic CD8+ T-suppressor cells produced in the immunized recipients of immunoregulatory thymocytes are derived from the CD45.2 recipient of the CD45.1+ thymocytes; (ii) the induction of recipient splenic suppressor T cells by the transferred immunoregulatory thymocytes requires that the recipient be immunized to the same antigen as that used to induce immunoregulatory thymocytes; (iii) antigen is introduced to the thymus after an injection of antigen into the AC; (iv) although the transfer of the suppression of DTH by regulatory thymocytes was not dependent on interleukin-4 (IL-4), CD4+ NK1.1– regulatory thymocytes from AC-injected donors enhanced the production of immunoglobulin G1 antibodies to TNP-BSA by an IL-4-dependent mechanism. These observations suggest that the adult thymus plays an active role in the induction and maintenance of anterior chamber-associated immune deviation as manifested by the generation of the suppression of cell-mediated immunity to exogenous antigen and the antigen-induced production of IgG1 antibodies.
Keywords: anterior chamber, immune deviation, regulatory T cells, thymocytes
Introduction
Innate and adaptive immune systems protect the eye from pathogenic invaders and/or tumours. ‘Collateral damage’ or autoimmunity associated with these protective mechanisms could potentiate impaired vision. Fortunately, the eye is protected from such pathologic effects by an ocular environment that is not supportive of immune-mediated reactions. In addition to an immunosuppressive environment, a key feature of this protection is the systemic down-regulation of cell-mediated immunity that occurs when antigen is introduced into the eye's anterior chamber (AC). This alteration of a systemic immune response, known as anterior chamber-associated immune deviation (ACAID) suppresses the induction of cell-mediated immunity and the production of immunoglobulin G2 (IgG2) antibodies specific to the antigen injected into the AC but does not suppress the production of IgG1 antibodies to the antigen.1–3 ACAID is imposed on the immune system by cellular interactions in which ocular-derived antigen presenting cells emigrate to the spleen and interact with CD4+ natural killer T (NKT) cells and CD8+ T lymphocytes. The latter become (suppressor) T lymphocytes that effect the suppression of the production of interferon-γin vitro and delayed-type hypersensitivity (DTH) in vivo.4–15
The activation of suppressor T cells in the spleen after the injection of antigen into the AC requires the participation of CD4+ T cells5,10,14–18 that bear the NK1.·1 marker17 B cells13 and γ/δ T cells.19,20 An intact thymus is essential for the induction of ACAID in adult mice by the injection of antigen into the AC or the transfer of F4/80+ peripheral blood cells from AC-injected mice to recipient mice.6,9 The thymocytes from AC-injected donors that transfer the suppression of delayed-type hypersensitivity to other mice are NK1.1+ CD4− CD8− (DN) but do not effect directly the suppression of DTH.6,9 However, because splenic suppressor T cells are detected in the recipients of thymocytes from AC-injected donors at least one week after the injection of the thymocytes it is uncertain whether thymocytes from AC-injected donors participate in the activation of CD8+ suppressor T cells or become these cells.
We have used the adoptive transfer of immunoregulatory CD45.1 thymocytes from AC-injected donors to immunized CD45.2 recipients to determine directly whether the immunoregulatory thymocytes become splenic suppressor T cells. Moreover, because the immunoregulatory thymocytes transfer the suppression of DTH induced by the injection of antigen into the AC we also investigated the role of the thymocytes in the humoral aspects of ACAID. Our results demonstrate directly that immunoregulatory thymocytes from AC-injected donors activate splenic CD8+ suppressor T cells produced by immunization with the same antigen as that used to produce immunoregulatory thymocytes. In addition, the immunoregulatory thymocyte population contains a CD4+ NK1.1– population that promotes the production of IgG1 antibodies specific to the AC-injected antigen by an IL-4-dependent mechanism. Therefore, the immunoregulatory thymocytes participate in both the cell-mediated and humoral aspects of ACAID.
Materials and methods
Mice
Female BALB/c, BALB/c IL-4tm2Nnt (IL-4−/−), C57BL/6j (CD45.2) B6Ly5.2/Cr (CD45.1) congenic to C57BL/6 mice, 6–8 weeks old were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were maintained in the Center for Laboratory Animal Care of the University of Connecticut Health Center. All animals were treated according to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Antigens
2,4,6-Trinitrobenzene sulphonic acid (TNP), bovine serum albumin (BSA), and ovalbumin (OVA) were purchased from Sigma Chemical Co. (St. Louis, MO). TNP-BSA was prepared as described.6 Picryl chloride (PCl), 2-chloro-1,3,5-trinitrobenzene (the antigenic equivalent of TNP used to elicit contact sensitivity) was purchased as 2-chloro-5-triphtane from Chemical Alta Ltd (Edmonton, Alberta, Canada).
ACAID
All mice tested for ACAID were immunized 7 days before the injection of antigen into the anterior chamber. Mice were sensitized to TNP-BSA, or ovalbumin (OVA) by the subcutaneous (s.c.) injection of 200–400 µg antigen in complete Freund's adjuvant (CFA, Sigma). In some experiments mice were sensitized with 400 µg TNP-BSA s.c. alone or 400 µg TNP-BSA + 300 µg of the adjuvant polyadenylic:polyuridylic acid complexes (poly AU).21 Poly AU was prepared by the mixture of equal volumes of 0·5 ml 1·5 mg polyadenylic acid (Sigma) with 1·5 mg of polyuridylic acid (Sigma) in PBS. TNP-BSA was then added to the poly AU such that 100 µl contained 300 µg poly AU and 400 µg TNP-BSA. Seven days after immunization mice were anaesthetized by intraperitoneal (i.p.) injection of ketamine (75 mg/kg)/xylazine (15 mg/kg). Under a dissecting microscope a transcorneal paracentesis in one eye was made and 3 µl (4 µg) of antigen was injected into the AC with a 30-g needle and a manually controlled Hamilton syringe.
Preparation of thymocytes from AC-injected donors
Thymocytes from mice that received an injection of antigen into the AC were prepared as described.6,9 Briefly, naïve mice received an injection of 4 µg TNP-BSA into the AC. One−2 days after AC injection the mice were killed by cervical dislocation and thymi removed. Thymi were dissected and expressed through a 70-µm nylon mesh into phosphate-buffered saline (PBS pH 7·2), washed twice and suspended at 1 × 107 cells/ml in PBS.
Subsets of thymocytes from AC-injected donors
Thymocytes from AC-injected donors were separated based on the expression of CD4 using immunomagnetic beads as described.6 NK1.1+ and NK1.1− thymocyte populations were incubated with biotinylated anti-mouse NK1.1 monoclonal antibody9 at 10 µl/107 cells (Pharmingen, San Diego, CA) for 10–20 min at 4°. The cells were washed twice with labelling buffer (PBS+2 mm ethylenediaminetetraacetic acid (EDTA)) and then incubated with supra-paramagnetic streptavidin microbeads (10 µl/107 cells), washed twice with separation buffer. The cells–bead mixture was then separated into positive and negative populations as described above.
Splenic suppressor T cells
Mice used to produce splenic suppressor T cells were immunized 7 days before receiving an injection of thymocytes from AC-injected donors. The mice were sensitized to TNP-BSA, or ovalbumin (OVA) by the s.c. injection of 200–400 µg antigen in CFA. One week after AC injection or receipt of thymocytes from AC-injected donors, spleens were removed from the mice, diced and expressed through a 40-µm nylon mesh into PBS. The cells were washed twice and suspended in PBS at 1 × 108 cells/ml. Spleen cells from C57BL6j (CD54.2) mice receiving CD45.1 thymocytes from AC-injected donors were separated based on the expression of CD45.2 by incubation of the spleen cells with biotinylated anti-CD45.1 or anti-CD45.2 monoclonal antibodies (Pharmingen) at their optimum titre based on the manufacturer's instructions, 10 µl/107 cells at 4° for 10–20 min The cells were washed twice with 20× volume labelling buffer, resuspended in 90 µl labelling buffer/107 cells. Ten cµl/107 cells magnetic-activated cell sorting (MACS) streptavidin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were added and the mixture incubated for 15 min at 6–12°. The cells were washed with separation buffer (PBS + 0·5% BSA, 2 mm EDTA) and suspended in 500 µl separation buffer/1 × 108 cells. The cells were then loaded on a positive selection column (LS+ Miltenyi Biotec) or a depletion column (BS, Vario, MACS, Miltenyi Biotec) to separate the cells. The cells were washed twice in PBS and resuspended in PBS for injection.
CD45.2+ CD8+ spleen cells were purified by incubation of the C57BL/6j cells with fluoroscein isothiocyanate (FITC) Isomer-1 conjugated-anti-CD45.2 antibody (Pharmingen) at 4° for 10–20 min. The cells were washed with 20× volume labelling buffer and resuspended with labelling buffer (90 µl/107 cells), mixed with MultiSort anti-FITC microbeads (Miltenyi Biotec, 10 µl/107 cells) and incubated 15 min at 6–12°. CD45.2+ cells were collected by loading the cells on a positive selection column (LS+ Miltenyi Biotec). Twenty µl MACS Multisort Release Reagent (Miltenyi Biotec)/ml cell suspension was added and the mixture incubated 10 min at 6–12°. The cells were washed twice and the cell pellet suspended in PBS in a final volume of 50 µl/107 cells. Thirty µl of MACS multisort stop reagent/50 µl cell suspension was added. The microbeads were removed by centrifugation, the cells washed twice and resuspended in 90 µl separation buffer/107 cells. Ten µl/107 cells MACS CD8 microbeads were added and the mixture incubated 15 min at 6–12°. The cells were washed twice with separation buffer and resuspended in 500 µl separation buffer/108 cells. The cell–bead mixture was loaded on an MS+ positive selection column to separate CD8+ CD45.2+ spleen cells.
Adoptive transfer assays (Fig. 1)
Figure 1.
Adoptive transfers and assays (a) Activation of splenic suppressor cells by regulatory thymocytes from AC-injected donors. To prepare regulatory thymocytes (AC-Thy) (I) naïve mice receive an injection of TNP-BSA into an AC. (II) Two days later thymocyte suspensions are prepared and thymocytes injected i.v. into CD45.2+, TNP-BSA-immune receipients. One week after the mice received AC-Thy (III) spleen cells (SPL) are prepared, separated on the basis of the CD45 allele and/or CD8 are injected into the footpads of TNP-BSA immunized mice immediately after epicutaneous challenge with PC1. Footpad swelling is measured 24 hr later and serum collected at the time of challenge, 7, 14, 21 days later to assay for IgG1 antibodies to TNP-BSA. (b) Assay of immunoregulatory thymocytes. One-2 days after thymocyte donors received an injection of TNP-BSA into the AC thymocytes (unseparated) NK1.1+, CD4+ were injected i.v. into TNP-BSA-immunized mice. One week later, the mice received epicutaneous PC1 to a footpad. Swelling of the footpad was measured 24 hr later and the mice were bled 1 or 2 weeks after challenge.
Thymocytes from AC-injected donors (AC-Thy) Thymocyte transfer to suppressor cell donors: TNP-BSA immunized mice that were the recipients of thymocytes were challenged with epicutaneous PCl. Footpad swelling was measured 24 hr later. In some experiments the mice were bled by cardiac puncture at the time of PCl challenge, as well as 7, 14 or 21 days after challenge to prepare serum for enzyme-linked immunosorbent assay (ELISA) for IgG1 anti TNP-BSA antibodies. Assay of the induction of splenic suppressor cells by regulatory thymocytes: Twenty-four−48 hr following AC-injection of TNP-BSA, thymocytes were isolated and 1 × 107 thymocytes in 0·2–0·3 ml PBS were injected into the tail vein of immunized mice. One week following this transfer, spleens were removed and spleen cell suspensions were prepared for adoptive transfer to recipient mice, naïve mice, or immunized mice.
Suppressor-cell assay
Suppressor cell donor mice were killed by cervical dislocation, spleens removed, diced and expressed through a 70-µm nylon mesh into PBS. The cells were washed twice with PBS and resuspended in PBS as described.9 Suppressor cells were assayed by a modification9 of the Local Adoptive Transfer14 (LAT) assay. Spleen cells (2·5 × 104 cells at 5 × 106/ml) from naïve mice or the donors of suppressor T cells were injected s.c. into the footpad of immunized mice immediately following epicutaneous challenge with PCl. Footpad swelling was measured 24 hr later.
DTH contact sensitivity (CS) assay for TNP-BSA-sensitized mice Fifteen µl of 1% PCl (in acetone: olive oil, 4 : 1) was applied to one footpad. The CS response was graded by measuring footpad thickness with an engineer's digital micrometer (Mitatoyo, Tokyo, Japan) 24 and 48 hr after challenge with PCl. Micrometres of swelling were determined by computing the difference in thickness between the challenged footpad and the unchallenged footpad before and after challenge. Each measurement was then corrected by subtracting the 24-hr difference in the footpads of challenged and unchallenged footpads of naïve mice.
Measurement of serum antibodies
IgM and IgG1 antibodies were measured by ELISA. Twenty-four well microtitre plates (Nunc-Fisher Scientific, Los Angeles, CA) were coated overnight at room temperature with 1 µg/well TNP-BSA or BSA as described.22 One hundred µl dilutions of immune or naïve mouse serum obtained by cardiac puncture was added to the plates and after washing, a 1 : 2000 dilution of monoclonal biotinylated anti-mouse IgG1 or rabbit anti-mouse IgM was added and the plates incubated for 1 hr at 37°. After washing the plate 5× alkaline phosphatase-conjugated avidin or sheep anti-rabbit IgG (Sigma) was added and the plate incubated 1 hr at 37°. The plate was then washed five times and p-nitropheynyl phosphate substrate then added. Optical density was determined (usually after 10–20 min) by spectrophotometric absorption with a Thermolabsystems Multiskan™ spectrometer. The titre of the serum is taken as the highest dilution of the serum to give an OD twice that of wells that received no murine serum.
Statistics
Statistical significance was calculated by one-way anova (Primer). P-values <0·05 were considered significant.
Results
Thymocytes transferred from AC-injected donors activate recipient splenic suppressor cells
Although AC-Thy do not effect the suppression of DTH9 we reasoned that as recent thymic emigrants to the spleen they may differentiate into and/or activate CD8+ splenic suppressor T cells. To determine the origin of the recipient splenic suppressor cells, naïve CD45.1+ CD45.2– donor mice received an AC injection of TNP-BSA. Thymocytes were prepared two days after the injection of TNP-BSA into the AC and then injected into congenic TNP-BSA-immunized, CD45.1− CD 45.2+ recipients. In four separate experiments flow cytometry revealed that approximately 0·4–1·4% of the spleen cells recovered from the recipients of CD45.1+ AC-Thy were CD45.1+(Fig. 2a). Anti-CD45.1 stained approximately 0·05–0·12% of the C57Bl/6j spleen cells in mice that did not receive the thymocytes (data not shown).To detect the splenic suppressor cells in the recipients of CD45.1 AC-Thy, the spleen cells of the CD45.2 recipients were incubated with biotinylated anti-CD45.1 or CD45.2 antibodies and streptavidin-paramagnetic beads. The CD45.1– population of the spleen cells of recipients of CD45.1 AC-Thy contained >90% CD45.2+ cells and 0·12% CD45.1+ cells (Fig. 2b). Some of the CD45.1– cells were separated from the streptavidin-microbeads and then incubated with anti-CD8 paramagnetic beads and the CD8+ cells recovered. These separated cells were injected into the footpads of TNP-BSA-sensitized mice concurrent with an epicutaneous application of PCl. Footpad swelling was measured 24 hr later. The results of one experiment are shown in Fig. 3(a). Because these experiments were performed four times, the pooled data in Fig. 3(b) of four experiments is expressed as the percent suppression of swelling (DTH). In these experiments the swelling ranged from 600 to 1100 µm in separate experiments. The footpad swelling of naïve (control) mice challenged with PCl was 50–250 µm. The footpad swelling of the recipients of CD45.2+ spleen cells and CD45.1– CD8+ populations from the recipients of CD45.1+ thymocytes from AC-injected donors was reduced significantly (70–90%) below controls compared with mice that were injected with no spleen cells, naïve spleen cells or CD8– spleen cells. In one experiment splenic suppressor cells were detected in the CD45.1+ spleen cells enriched to 90%. However, repeated attempts to purify and assay the CD45.1+ population recovered from the CD45.2 recipients of CD45.1+ thymocytes was frustrated by difficulties in removing all of the CD45.2+ (recipient) cells.
Figure 2.
Spleen cells of recipients of CD45.1+ thymocytes contain CD45.1+ cells. Naïve CD45.1+ thymocyte donor mice received an injection of TNP-BSA into the AC. Twenty-four h later thymocytes were prepared and injected i.v. into CD45.2+ recipient mice. (a) One week later, spleen cells from the recipient C57Bl/6 mice (CD45.2+) were stained with PE-anti-CD45.2 and FITC anti-CD45.1 antibodies. (b) Spleen cells from C57Bl/6 mice that did not receive CD45.1+ thymocytes were stained with anti-CD45.2 and CD45.1 antibodies.
Figure 3.
Thymocytes from AC-injected donors activate splenic suppressor cells.Three CD45.1+ thymocyte donors received an injection of TNP-BSA into an AC. Twenty-four hr later thymocyte suspensions were prepared and injected i.v. into CD45.2+, TNP-BSA-immunized recipients. After 1 week, recipient spleen cells (SPL) were incubated with biotinylated anti-CD45.1 or anti-CD45.2 and separated with biotin-paramagnetic beads. CD45.2+ cells were released enzymatically from the anti-CD45.2 beads and incubated with anti-CD8 paramagnetic beads. 2·5 × 104 positively or negatively selected-recipient cells or naïve C57 BL/6j (CD45.2+) spleen cells were injected into the footpad of TNP-BSA-immunized mice immediately after epicutaneous PCl challenge. Footpad swelling was measured 24 hr later. (a) One representative experiment (of four). Data shows the mean ± SEM swelling of four mice/group. *P < 0·02. (b) The data represents the mean percentage suppression ± SEM of 12 mice/group in four separate experiments. *P = <0·0.01. % suppression =1 − (µm injected with SPL − µm naïve control) × (100 µm no SPL − µm naïve control). Footpad swelling of immunized mice that were challenged but were injected with naïve spleen cells or no spleen cells ranged from 600 to 1100 µm. Swelling of naïve mice ranged from 50 to 250 µm.
The production of splenic suppressor cells by thymocytes transferred from AC-injected donors requires immunization of the donor of the spleen cells
We next sought to determine antigenic requirements for the activation of splenic suppressor cells by thymocytes from AC-injected donors. Naïve spleen cell donor mice or spleen cell donor mice immunized one week previously with TNP-BSA, or OVA were injected with thymocytes obtained from naïve donors that received an injection of TNP-BSA into the AC. One week later, spleen cells obtained from these donors were injected into the footpads of TNP-BSA-immunized mice concurrent with an epicutaneous footpad challenge with PCl. Figure 4 shows that the injection of spleen cells from TNP-BSA-immunized mice that had received thymocytes from mice receiving an injection of TNP-BSA into the AC suppressed footpad swelling by approximately 80%. Spleen cells from OVA-immunized or naïve mice that received thymocytes from donors receiving an injection of TNP-BSA failed to suppress the swelling of footpads elicited by PCl. These results suggested that the production of splenic suppressor T cells activated by thymocytes from AC-injected mice may be amplified by immunization of the donor of the spleen cells. To explore this possibility further we investigated the effects of immunization of the donors of splenic suppressor cells on the generation of splenic suppressor cells induced by AC-injection. First we compared DTH and IgG1 antibody production to TNP promoted by immunization with TNP-BSA ± the adjuvants CFA or poly AU. The footpad swelling of mice immunized with TNP-BSA + CFA was twofold that of mice immunized with TNP-BSA s.c. only or TNP-BSA + poly AU s.c. (Fig. 5a). However, mice receiving TNP-BSA + CFA or poly AU had four- to fivefold greater IgG1 antibody titres to TNP-BSA than mice receiving TNP-BSA only (Fig. 5b). Because the induction of DTH and IgG1 antibody production was differentially influenced by the immunization with CFA or poly AU, we next determined the effects of these immunizations on the generation of splenic suppressor cells after the injection of the immunized mice with TNP-BSA into the AC. Spleen cells recovered 1 week after immunized spleen cell donors received an injection of TNP-BSA into the AC were injected into the footpads of mice immunized with TNP-BSA + CFA immediately after an epicutaneous challenge with PCl. Footpad swelling was measured 24 hr later. Spleen cells recovered from donors immunized with TNP-BSA + CFA that received an AC-injection of TNP-BSA suppressed the swelling of the footpads of the challenged recipients. However, spleen cells from AC-injected mice immunized with TNP-BSA + poly AU failed to suppress the swelling of footpads of the recipients (Fig. 5c).
Figure 4.
Thymocytes from AC-injected donors activate splenic suppressor cells in mice immunized to the same antigen that activated the thymocytes. Thymocyte (THY) donor BALB/c mice (three to four/group) received an injection of TNP-BSA or BSA into an AC. Two days later thymocytes were prepared and injected i.v. into naïve spleen cell donors or donors immunized 1 week previously with 400 µg TNP-BSA/FCA or OVA/FCA. One week after the injection of the thymocytes spleen cell suspensions were prepared and 2·5 × 104 spleen cells (SPL) were injected into a footpad concurrently with an epicutaneous challenge of PCl. Swelling was measured 24 hr later. The reduction of swelling (in µm) is shown as a percentage of the swelling obtained in footpads that did not receive spleen cells (Fig. 3 for calculation) and represents the mean ± SE of 8–12 mice in three separate experiments. *P < 0·001 compared to spleen cells from TNP-BSA-immunized mice that received thymocytes from mice injected with TNP-BSA into the AC.
Figure 5.
The generation of splenic suppressor cells by AC injection depends on immunization of the donor of the spleen cells. BALB/c mice (four/group/experiment) were immunized s.c. with 400 µg TNP-BSA ± CFA or 300 µg poly AU. (a) One week after immunizing a footpad was challenged with epicutaneous PCl and swelling measured 24 hr later. The data represents the mean swelling ± SEM of 12 mice/group mice, three separate experiments. *P < 0·001. The swelling of naïve mice (30 µm) has been subtracted from the swelling of immunized mice.(b) The mice were bled seven days after challenge and the serum of each mouse assayed for IgG1 anti-TNP-BSA antibodies. Data represents the geometric mean titre (1/dilution) of four sera/group in three separate experiments. *P < 0·01. (c) Spleen cell donors from AC-injected mice were immunized with TNP-BSA as shown. One week after immunizing the donors received TNP-BSA in an injection into the AC. One week after the AC injection, spleen cell suspensions from each of four of the donors were pooled and 2·5 × 104 spleen cells were injected into the footpad of a TNP-BSA-immunized mouse concurrent with an epicutaneous challenge with PCl. Swelling was measured 24 hr later. Swelling of naïve mice challenged with PCl was 65 ± 15 µm. The data represents the mean swelling ± SEM of four mice/group. *P < 0·01.
Antigen injected into the anterior chamber appears in the thymus and spleen within 6 hr after injection
We determined the systemic distribution of antigen after injection of antigen into the anterior chamber using FITC-TNP-BSA or 125I-TNP-BSA. After the injection of antigen into the anterior chamber, organs and peripheral blood were assayed for labelled antigen or cell-associated, labelled antigen 1–24 hr after injection. Cells bearing FITC-labelled TNP-BSA were detected in the thymus, spleen, lymph nodes and blood 1–6 hr after injection. The number of FITC-labelled cells peaked in the thymus 6 hr after injection (Fig. 6a). Six hr after the intravenous injection of FITC-TNP-BSA a large number of FITC-bearing cells appeared in the spleen but no FITC-bearing cells appeared in the thymus (Fig. 6b). The systemic distribution of AC-injected antigen was quantified by detecting the distribution of 125I-TNP-BSA after injection into the AC. Six hr after the injection of antigen approximately 0·3% of the AC-injected 125I-TNP-BSA was detected in the thymus and the liver (Figs 7a, b). Radiolabelled antigen decreased in the eye within 6 hr after injection of 125I-TNP-BSA into the anterior chamber (Fig. 7c).
Figure 6.
Distribution of FITC-antigen after injection of TNP-BSA into the anterior chamber. Three BALB/c naïve mice received an injection of four µg FITC-TNP-BSA into an anterior chamber. (a) 1–24 hr after injection, blood and organs were removed. Cells suspensions were prepared and analysed by flow cytometry. Data represents the mean percent of fluorescent cells minus the mean percentage of fluorescent cells from non-injected mice ± SEM of six to eight separate experiments. (b) Cell suspensions from organs obtained 6 hr after the injection of antigen i.v. or into the AC were compared. Data represents the mean ± SEM percent of fluorescent cells of six separate experiments. *P < 0·05.
Figure 7.
Distribution of 125I-labelled TNP-BSA after injection into the anterior chamber. Four µg 125I-labelled TNP-BSA was injected into the anterior chamber of 3 naïve BALB/c mice and 1–168 hr after injection organs or blood were removed. (a,b) Whole individual organs were counted for 125I. Peripheral blood cells were prepared from whole blood by centrifugation of 1 ml of blood/mouse. The cell pellet was washed three times and counted for 125I. The data represents the mean counts/10 min of 15 samples in five separate experiments (three samples/experiment). (c) 125I-TNP-BSA in the eye of each of four mice.
Thymocytes transferred from AC-injected donors promote the antigen-induced production of IgG1 antibodies
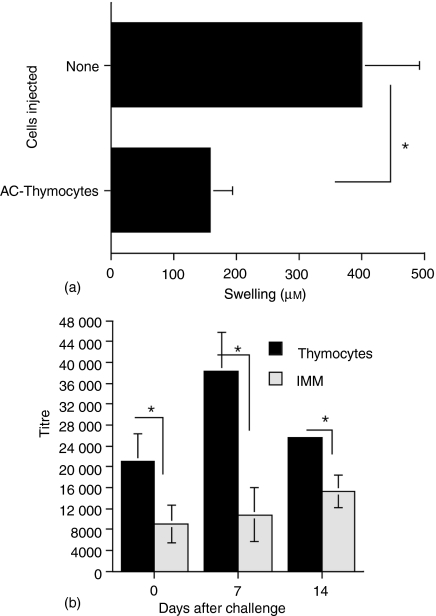
Because ACAID is characterized by the maintenance of IgG1 antibodies specific to the antigen injected into the anterior chamber while the production of IgG2 antibodies and DTH to the AC-injected antigen is suppressed we determined whether thymocytes from donors that received an injection of TNP-BSA into the AC would also influence the recipients' humoral antibody response. C57BL/6 mice immunized one week previously with TNP-BSA received an i.v. injection of thymocytes from naïve mice that had received an AC injection of TNP-BSA. One week later a footpad of the thymocyte recipients was challenged with epicutaneous PCl. Swelling was measured 24 hr after the challenge. To obtain sera, the mice were bled 24 hr, 7 and 14 days after the challenge with PCl. Twenty-four hr following the challenge, the footpad swelling of the recipients of the thymocytes transferred from AC-injected mice was significantly lower than that of immunized mice that did not receive thymocytes (Fig. 8a). Also, the challenged footpad swelling of mice that received thymocytes from non-AC-injected (naïve) mice was the same as that of immunized mice that were not injected with thymocytes (data not shown). On the day of footpad challenge (2 weeks after sensitization with TNP-BSA) the IgG1 antibody titre to TNP-BSA was approximately threefold greater in mice receiving thymocytes from AC-injected donors (Fig. 8b). Seven days after the challenge the titre of serum IgG1 anti-TNP antibodies was more than fourfold higher in mice that received the thymocytes from AC-injected donors. However, 14 days after challenge the titre of IgG1 anti-TNP antibodies had decreased (from that of 7 days after the challenge) in mice receiving thymocytes but still significantly higher than that of mice that did not receive thymocytes. Assays of the pooled sera of mice used in studies published previously6 showed a similar pattern. Seven days after the epicutaneous challenge with PCl, Balb/c mice that received thymocytes from AC-injected donors had four- to fivefold higher titres of IgG1 anti-TNP-BSA antibodies than mice that received no thymocytes, naïve thymocytes or thymocytes from immunized donors. Moreover, the recipients of an injection of TNP-BSA into the AC or thymocytes from AC-injected donors did not produce IgG2 anti-TNP antibodies (data not shown). In addition, none of the mice produced antibodies to BSA.
Figure 8.
Thymocytes from AC-injected mice promote the antigen-induced production of IgG1 antibodies. C57BL/6j mice immunized 1 week previously with TNP-BSA received i.v. thymocytes prepared 24 hr after the naïve donor mice received an injection of TNP-BSA into the AC (AC-thymocytes). One week after the injection of the thymocytes, the recipient mice were bled to collect sera and challenged with epicutaneous PCl to a footpad. (a) Footpad swelling was measured 24 hr later. Data represents the mean ± SEM of swelling of eight TNP-BSA-immunized mice, four mice/group, two experiments. Footpad swelling of naïve mice was 110 ± 17 µm. *P < 0·01 (b). The mice were bled at the time of challenge and 7 and 14 days after challenge. IgG1 antibodies to TNP-BSA were assayed by ELISA using doubling dilutions of antiserum and biotinylated anti-IgG1 and alkaline phosphatase-conjugated streptavidin added to microtitre trays coated with 1 µg/well of antigen. The data represents the geometric mean titre ± SEM of the pooled sera in duplicate assays of three mice/group in two separate experiments. The titre of naïve mice was 40 ± 20. *P < 0·01 IMM-immunized mice that did not receive thymocytes.
NK1.1– CD4+ thymocytes from AC-injected donors promote antigen-induced IgG1 production
Because NK1.1+ thymocytes from AC-injected donors transfer the suppression of DTH9 we therefore sought to determine the NK1.1 phenotype of thymocytes from AC-injected mice that promote the production of IgG1 antibodies. Thymocytes recovered from C57BL/6 mice 2 days after AC injection were separated by immunomagnetic beads based on NK1.1 expression. The positively selected cells were approximately 88% NK1.1+ (FACS), the NK1.1– fraction was approximately 3·4% NK1.1+. The separated cells were injected i.v. into TNP-BSA-immunized mice. One week later the mice that received the thymocytes were challenged with epicutaneous PCl and footpad swelling was measured 24 hr later. In addition, the mice were bled 7 days after the challenge to collect sera to be assayed for IgG1 anti-TNP-BSA antibodies. Footpad swelling in the recipients of only 1 × 105 NK1.1+ thymocytes from AC-injected mice was 75% less than that of mice receiving no thymocytes or 5 × 106 NK1.1– thymocytes from AC-injected mice (Fig. 9a). Seven days after the challenge, mice receiving 5 × 106 NK1.1– or unseparated thymocytes recovered from AC-injected donors had three- to fourfold higher titres of IgG1 anti-TNP-BSA antibodies than immunized, challenged mice that received NK1.1+ thymocytes from AC-injected donors or mice that did not receive thymocytes (Fig. 9b).
Figure 9.
Phenotype of IgG1-enhancing thymocytes from AC-injected mice. Thymocytes from C57BL/6 mice were prepared by AC injection of TNP-BSA and were separated by immunomagnetic beads into NK1.1+ and NK1.1– cells. 1 × 105 NK1.1+or 5 × 106 NK1.1– thymocytes were injected i.v. into TNP-BSA-immunized mice. One week after the injection of the thymocytes, the mice were challenged by epicutaneous PCl to a footpad. (a) Swelling of the footpad was measured 24 hr later. Footpad swelling of naïve mice challenged with PCl was 35 ± 5 µm. The data represents the mean swelling ± SEM of four mice/group. *P < 0·01. (b) 7 days after the challenge the mice were bled and the sera assayed individually for IgG1 antibodies to TNP-BSA. The data represents the geometric mean titre ± SEM of three to four mice/group in two separate experiments. Unseparated, unseparated thymocytes. (c) CD4+ thymocytes promote antigen-induced IgG1 antibody production. Thymocytes prepared 2 days after AC injection of TNP-BSA were separated by immunomagnetic beads into CD4+ and CD4– cell populations. 5 × 105 or 1 × 107 thymocytes were injected i.v. into TNP-BSA-immunized recipients. The mice were challenged 7 days after the injection of AC-Thy and bled 1 week after the challenge. Data represents the geometric mean titre ± SEM of four individual sera/group *P < 0·01.
In similar experiments we determined the effect of transferred CD4+ and CD4– thymocytes from AC-injected Balb/c donors on the production of IgG1 anti-TNP-BSA antibodies in immunized thymocyte recipients. Two days following the injection of TNP-BSA into the AC of the thymocyte donors, thymocytes and thymocyte suspensions from these donors were recovered and the cells were incubated with anti-CD4 antibodies. The thymocytes were then separated by immunomagnetic beads into CD4+ and CD4– cells. The separated thymocytes were injected i.v. into BALB/c TNP-BSA-immunized mice. Seven days later the mice were bled to obtain sera. The IgG1 anti-TNP-BSA titres of immunized mice receiving 5 × 105 CD4+ thymocytes from AC-injected donors were more than fourfold that of mice receiving no thymocytes or 5 × 106 CD4– thymocytes from AC-injected donors (Fig. 9c).
The production of IgG1 antibodies promoted by thymocytes from AC-injected donors is dependent on IL-4
The role of IL-4 in the enhancement of IgG1 antibody production by thymocytes was investigated by preparing thymocytes from BALB/c IL-4+/+ and IL-4–/– mice 2 days after these naïve donor mice received an injection of TNP-BSA into the AC. The thymocytes were injected i.v. into Balb/c TNP-BSA-immunized mice. Seven days after the injection of the thymocytes, a footpad was challenged epicutaneously with PCl. The mice were bled 1 week after the challenge. The titre of IgG1 anti-TNP-BSA antibodies was approximately fourfold higher in the recipients of thymocytes from AC-injected IL-4+/+ donors than the titer in recipients of thymocytes from IL-4–/– donors or mice that received thymocytes from naïve mice that received no AC injection (Fig. 10). However, footpad swelling was reduced significantly to the same extent in the recipients of thymocytes from IL-4+/+ or IL-4–/– donors of the thymocytes (data not shown).
Figure 10.
Thymocytes from AC-injected, IL-4–/– mice do not enhance the production of IgG1 antibodies. BALB/c IL-4–/– and +/+ mice received an injection of TNP-BSA into the AC. Two days later, thymocytes from these donors were injected into TNP-BSA-immunized mice. One week after the injection of the thymocytes, the recipients were challenged on one footpad with epicutaneous PCl. Swelling of the footpad was measured 24 hr later and 7 days after the challenge the mice were bled intracardially. Sera were assayed by ELISA individually for IgG1 anti-TNP-BSA antibodies. IL-4+/+: sera from recipients of IL-4+/+ thymocytes, IL-4–/–: sera from recipients of IL-4–/– thymocytes, Naïve: sera from mice that received naïve thymocytes from donors that did not receive an AC injection of TNP-BSA. The data are expressed as the geometric mean titre (one/dilution) of eight sera, four mice/group in two experiments. *P < 0·05.
Discussion
Protection from ‘collateral damage’ by an immune response may be based on physical barriers; an environment not conducive to the activation or the effector function of immunocompetent cells; or a relationship to the immune system that induced immunoregulatory T lymphocytes.1,2 In effect, the ACAID phenomenon expresses the immune privilege of the eye. It employs both the intraocular environment and its relationship with the central and peripheral immune system. This relationship may result in the down-regulation of an immune response that could otherwise damage the eye if an agent that incites an immune response is present in the eye. Transforming growth factor-β within aqueous humor in the anterior chamber is required for the antigen-induced activation of F4/80+ cells in the iris/ciliary body. These cells are able to migrate to the systemic immune system and participate in the recruitment and activation of regulatory T cells.7,8,26
Preliminary evidence from our laboratory further indicates that after the injection of antigen into the AC (i) F4/80+ iris/ciliary body cells induce immunoregulatory thymocytes and (ii) ACAID and (iii) home to both the thymus and spleen.27 In this way, the ocular environment and the iris/ciliary body F4/80+ cells are a link to both the central and peripheral immune system.
The injection of antigen into the anterior chamber of naïve mice induces F4/80+ cells from the iris/ciliary body that transfer the suppression of DTH to other mice regardless of whether the recipient mice are immunized prior to the injection of antigen into the AC.7–9 Circulating F4/80+ cells from AC-injected mice migrate to the spleen.7 Six hr after the injection of fluoresceinated or radioiodinated antigen into the AC of naïve mice, we detected cell-associated, labelled antigen in the thymus and spleen. Preliminary evidence suggests that the FITC-labelled TNP-BSA is associated with relatively large cells. However, we did not determine if the FITC is still protein-associated. Similarly, we do not know if the 125I is still protein associated. However, it is unlikely that 125I-tyrosine was released extrathymically (e.g. in the blood) because intravenously injected antigen did not reach the thymus (Fig. 6b), although antigen is detected in the spleens of mice receiving intravenous antigen. These data suggest that approximately 0·005% of AC-injected antigen is delivered to the thymus (in the present study approximately 200 pg) as cell-associated antigen. Preliminary evidence suggests that F4/80+ iris/ciliary body cells induced by the injection of antigen into the AC migrate to the thymus and spleen, and induce immunoregulatory thymocytes. That such cells internalize antigen has been demonstrated.23,24 The intrathymic presentation of antigen induces immunoregulatory T cells and a tolerant state.28 Therefore, we have suggested that AC-injected antigen is delivered to the thymus by F4/80+ cells and is likely presented to CD1d-dependent T cells27 to induce immunoregulatory thymocytes that, in turn, migrate to the spleen and participate in the induction of tolerance to the intrathymic antigen.
F4/80+ cells that migrated to the spleen after the AC injection of antigen produce MIP-2 that recruits NKT cells necessary for the induction of ACAID.11 Some of these NKT cells (but likely not all) may be recent thymic emigrants.28 F4/80+ cells also interact with NK1.1+ and NK1.1– T cells in the spleen11 and B cells required to induce CD8+ suppressor cells that suppress DTH.5,8,29,30 Our experiments (Fig. 3) and our observation that CD3– spleen cells from AC-injected donors are not suppressive (unpublished results) provide further documentation for this well-established observation. We found that the activation of splenic CD8+ suppressor T cells by thymocytes from AC-injected donors requires that the donor of the spleen cells be immunized to the same antigen as that used to generate the regulatory thymocytes. The specificity of suppression by the splenic suppressor cells activated by AC-Thy and immunization is similar to that we have observed for the specificity of immunization with TNP-BSA for the TNP epitope.6,15 We have not observed DTH to BSA in mice immunized with TNP-BSA suggesting that trinitrophenylation may destroy T-cell epitopes in BSA (especially if those epitopes contain lysine). Similarly, suppressor T cells generated to BSA do not effect the response to TNP-BSA. Moreover, we observed that the generation of CD8 suppressor T cells that suppress DTH requires immunization that induces a DTH response to the immunizing antigen (Fig. 5). This requirement of homologous antigen for the activation of splenic CD8+ suppressor T cells suggests that ocular-derived F4/80+ cells that present antigen in the spleen may well provide the focus for interactions with B cells as well as NKT cells and CD8+ T cells12,13,29,30 and may thereby participate in the activation of sensitized regulatory CD4+ and CD8+ T cells in ACAID.
Because ACAID is manifested as a down-regulation of DTH and IgG2 antibodies but maintenance of IgG1 antibodies to the AC-injected antigen3 we determined whether thymocytes from AC-injected donors affect the production of IgG1 antibodies as well as the regulation of DTH to the antigen injected into the AC. The enhanced production of IgG1 (and also IgM, data not shown) antibodies induced by thymocytes from AC-injected mice may amplify the role for B cells in ACAID by facilitating the recruitment of NKT cells as shown for the initiation of DTH.31 However, although the enhancement of IgG1 antibodies by thymocytes from AC-injected donors is IL-4-dependent, IL-4 is not required for the regulation of DTH by thymocytes or the induction of ACAID.32 Unlike AC-Thy that activate CD8+ splenic suppressor T cells, the enhancement of IgG1 and IgM antibodies by thymocytes from AC-injected donors is mediated by CD4+ NK1.1– thymocytes. Presumably, the splenic suppressor T cells activated by AC-Thy also suppress IgG2 antibody production. The infusion of IL-4-producing AC-Thy may provide for additional T-cell help for antibody production.
To identify the origin of splenic suppressor T cells in the recipients of regulatory thymocytes from AC-injected mice we used CD45 as a marker for the transferred thymocytes or the spleen cells of the recipient. The specific staining of approximately 0·2–1% of the donor thymocytes in the recipient spleen is consistent with the extent of migration of recent thymic emigrants to the spleen.33 The immunomagnetic beads effectively separated donor and recipient cells so that recipient populations depleted of donor thymocytes could be prepared. We were frustrated in our attempts to repeatedly purify donor populations from the recipient spleen because of >98% recipient cells. Therefore, except for one experiment we are not certain whether a small component of the splenic suppressor cell population is thymus-derived. Despite this, the population of purified thymocyte recipient-derived CD8+ CD45.2+ and CD45.1– spleen cells were effective suppressor cells. The latter population would contain many cell types although only the CD8+ population has been shown to be a suppressor (effector) cell in ACAID.14,18 Moreover, the possible contribution of donor cells (<2%) in 25 000 spleen cells (injected into the footpad) would be approximately <500 cells. We have found that suppression by the injected spleen cells requires a minimum 5000–10 000 cells (X. Li, unpublished observation).
The dependence on immunization for the generation in vivo of detectable splenic suppressor T cells suggests that the number of antigen-specific splenic suppressor cells is increased by immunization. We compared the effects of two adjuvants on this process because we found that although both adjuvants promoted the production of IgG1 antibody to the immunogen, CFA but not poly AU promoted DTH and the generation of splenic suppressor cells induced in ACAID. These observations suggest that the generation of the suppressor cells that regulate DTH may have requirements similar to those for the generation of DTH. The initiation of DTH requires liver-derived NKT cells and B cells.31 The requirement for immunization (in vivo) suggests that during an immune response regulatory cells may be produced that are not activated without certain signals. These signals may be provided by AC-Thy and peripheral NKT cells. In addition, F4/80+ (eye-derived) cells may also ‘convert’ activated CD8+ T cells to a suppressive activity.34
In aggregate, splenic suppressor cells induced by the injection of antigen into the anterior chamber are produced during the course of an immune response but require specific activation signals to function. Recent thymic emigrants to the spleen, activated by the delivery of intraocular antigen to the thymus by ocular-derived cells participate in the transmission of these signals and also influence the production of IgG1 antibodies specific for the antigen introduced into the anterior chamber. Therefore, recent thymic emigrants induced by an injection of antigen into the AC actually transmit ACAID as manifested by the regulation of DTH and a maintenance of IgG1 antibodies specific for the antigen injected into the AC. Therefore, the immune privilege of the eye results in part from a connection between the eye and both the central and peripheral immune systems. In this regard, the thymus plays an active role in the adult in the regulation of an immune response manifested as the generation of immunoregulatory spleen cells humoral antibody response.
Acknowledgments
We thank Jennifer Wegh and Rinette Pelletier for help with preparation of this manuscript. Supported by grant 013243 United States Public Health Service and the Connecticut Lions Eye Research Foundation.
Abbreviations
- AC
anterior chamber
- ACAID
anterior chamber-associated immune deviation
- BSA
bovine serum albumin
- CFA
complete Freund's adjuvant
- CS
contact sensitivity
- DN
double negative
- DTH
delayed-type hypersensitivity
- PBMC
peripheral blood mononuclear cell
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PCl
picryl chloride
References
- 1.Streilein JW. Immunoregulation and the eye. A dangerous compromise. FASEB J. 1987;1:199–208. [PubMed] [Google Scholar]
- 2.Streilein JW, Stein-Streilein J. Does innate immune privilege exist? J Leukoc Biol. 2000;67:479–87. doi: 10.1002/jlb.67.4.479. [DOI] [PubMed] [Google Scholar]
- 3.Wilbanks GA, Streilein JW. Distinctive humoral responses following anterior chamber and intravenous administration of soluble antigen. Evidence for active suppression of IgG2a-secreting B cells. Immunology. 1990;71:566–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–67. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittum JA, Niederkorn JY, McCulley JP, Streilein JW. The role of suppressor T cells in herpes simplex-induced immune deviation. J Virol. 1984;51:556–8. doi: 10.1128/jvi.51.2.556-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Goldschneider I, Foss D, Wu DY, O'Rourke J, Cone RE. Direct thymic involvement in anterior chamber-associated immune deviation. J Immunol. 1997;160:2150–5. [PubMed] [Google Scholar]
- 7.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber-associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4:130–7. [PubMed] [Google Scholar]
- 8.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018–24. [PubMed] [Google Scholar]
- 9.Wang Y, Goldschneider I, O'Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–6. [PubMed] [Google Scholar]
- 10.Sonoda K-H, Exley M, Snapper S, Ball ST, Stein-Streilein J. CD1-reactive natural killer T cells are required for the development of systemic tolerance through an immune privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faunce DE, Sonoda K-H, Stein-Streilein J. MIP-2 mediated recruitment of NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313–21. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 12.Sonoda K-H, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell derived IL-10 is essential for the differentiation of antigen-specific regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 13.D'Orazio TG, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the production of anterior chamber-associated immune deviation. Immunololgy. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederkorn JY, Streilein JW. Characterization of the suppressor cell (s) responsible for anterior chamber-associated immune deviation (ACAID) induced in Balb/c mice by P815 cells. J Immunol. 1985;134:1381–7. [PubMed] [Google Scholar]
- 15.Wang y Ghali W, Pingle P, Traboulsi A, Dalal T, O'Rourke J, Cone RE. Splenic T cells from mice receiving intracameral antigen suppress in-vitro antigen-induced proliferation and interferon-γ production by sensitized lymph node cells. Ocular Immunol Inflamm. 2003;11:39–51. doi: 10.1076/ocii.11.1.39.15578. [DOI] [PubMed] [Google Scholar]
- 16.Skelsey ME, Mayhew E, Niederkorn JY. CD25+, interleukin-10-producing CD4+ T cells are required for the suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology. 2003;110:18–29. doi: 10.1046/j.1365-2567.2003.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Sonoda K-H, Faunce DE, Gumperz J, Yamamura T, Mikake S, Stein-Streilein J. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an immune privileged site. J Immunol. 2003;171:1266–71. doi: 10.4049/jimmunol.171.3.1266. [DOI] [PubMed] [Google Scholar]
- 18.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID). Evidence of two functionally and phenotypically distinct suppressor T cell populations. Immunology. 1990;71:383–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Skelsey ME, Mellon J, Niederkorn JY. γ/δ T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–33. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Kapp JA. γ/δ T cells in in anterior chamber-induced tolerance in CD8+ CTL responses. Invest Ophthamol Vis Sci. 2002;43:3473–9. [PubMed] [Google Scholar]
- 21.Cone RE. The influence of synthetic homoribopolynucleotide complexes on the immune response. Pharmacol Ther. 1980;8:321–37. doi: 10.1016/0163-7258(80)90051-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, O'Rourke J, Cone RE. Serum TABM produced during anterior chamber-associated immune deviation passively transfers suppression of delayed-type hypersensitivity to primed mice. Int Immunol. 1997;9:211–8. doi: 10.1093/intimm/9.2.211. [DOI] [PubMed] [Google Scholar]
- 23.Camelo S, Voon ASP, Blunt S, McMenamin PG. Local retention of soluble antigen by potential antigen resenting cells in the anterior segment of the eye. Invest Ophthamol Vis Sci. 2003;44:5315–20. doi: 10.1167/iovs.03-0181. [DOI] [PubMed] [Google Scholar]
- 24.Becker MD, Planck SR, Crespo S, et al. Immunohistology of antigen-presenting cells in vivo: a novel method for serial observation of fluorescently-labeled cells. Invest Ophthamol Vis Sci. 2003;44:2004–9. doi: 10.1167/iovs.02-0560. [DOI] [PubMed] [Google Scholar]
- 25.Cousins SW, McCabe WM, Danielpour D, Strelein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–11. [PubMed] [Google Scholar]
- 26.Cone RE, Li X, O'Rourke J. Association of Research Vision Ophthamologists Annual Meeting. Fort Lauderdale, FL: 2004. Phenotypic and immunoregulatory characteristics of myeloid cells from the iris ciliary body; p. 2308. Abstract. [Google Scholar]
- 27.Durkin HG, Waksman BH. Thymus and tolerance. Is regulation the major function of the thymus? Immunol Rev. 2001;182:33–57. doi: 10.1034/j.1600-065x.2001.1820103.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldschneider I, Cone RE. a central role for peripheral dendritic cells in the induction of acquired thymic tolerance. Trends Immunol. 2003;24:77–81. doi: 10.1016/s1471-4906(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 29.D'Orazio TJ, Mayhew E, Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen II. Evidence for presentation by Qa-1. J Immunol. 2001;166:26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 30.Skelsey ME, Mayhew E, Niederkkorn J. Splenic B cells act as antigen presenting cells for the induction of anterior chamber-associated immune deviation. Inv Ophthamol Vis Sci. 2003;44:5355–61. doi: 10.1167/iovs.03-0768. [DOI] [PubMed] [Google Scholar]
- 31.Askenase PW. Yes T cells, but three different T cells (alpha beta, gammadelta and NK T cells) and also B-1 cells mediate contact sensitivity. Clin Exp Immunol. 2001;125:345–50. doi: 10.1046/j.1365-2249.2001.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koseiwicz MM, Okamoto S, Miki S, Ksander BR, Shimizu T, Streilein JW. Imposing deviant immunity on the presensitized state. J Immunol. 1994;153:2962–73. [PubMed] [Google Scholar]
- 33.Wu DY, Goldschneider I. Tolerance to cyclosporin-A induced autologous graft-versus-host disease is mediated by a CD4+, CD25+ subset of recent thymic emigrants. Journ Immunol. 2001;166:7158–64. doi: 10.4049/jimmunol.166.12.7158. [DOI] [PubMed] [Google Scholar]
- 34.Kezuka T, Steilein JW. In vitro generation of regulatory CD8+ T cells similar to those in mice with anterior chamber-associated immune deviation. Inv Ophthamol Vis Sci. 2000;41:1803–11. [PubMed] [Google Scholar]