Abstract
Tumour necrosis factor (TNF)-receptor-associated periodic syndrome (TRAPS) is a hereditary autoinflammatory disorder involving autosomal-dominant missense mutations in TNF receptor superfamily 1A (TNFRSF1A) ectodomains. To elucidate the molecular effects of TRAPS-related mutations, we transfected HEK-293 cells to produce lines stably expressing high levels of either wild-type (WT) or single mutant recombinant forms of TNFRSF1A. Mutants with single amino acid substitutions in the first cysteine-rich domain (CRD1) were produced both as full-length receptor proteins and as truncated forms lacking the cytoplasmic signalling domain (Δsig). High-level expression of either WT or mutant full-length TNFRSF1A spontaneously induced apoptosis and interleukin-8 production, indicating that the mutations in CRD1 did not abrogate signalling. Consistent with this, WT and mutant full-length TNFRSF1A formed cytoplasmic aggregates that co-localized with ubiquitin and chaperones, and with the signal transducer TRADD, but not with the inhibitor, silencer of death domain (SODD). Furthermore, as expected, WT and mutant Δsig forms of TNFRSF1A did not induce apoptosis or interleukin-8 production. However, whereas the WT full-length TNFRSF1A was expressed both in the cytoplasm and on the cell surface, the mutant receptors showed strong cytoplasmic expression but reduced cell-surface expression. The WT and mutant Δsig forms of TNFRSF1A were all expressed at the cell surface, but a proportion of the mutant receptors were also retained in the cytoplasm and co-localized with BiP. Furthermore, the mutant forms of surface-expressed Δsig TNFRSF1A were defective in binding TNF-α. We conclude that TRAPS-related CRD1 mutants of TNFRSF1A possess signalling properties associated with the cytoplasmic death domain, but other behavioural features of the mutant receptors are abnormal, including intracellular trafficking and TNF binding.
Keywords: autoinflammatory syndrome, mutation, periodic fever syndrome, tumour necrosis factor: tumour necrosis factor receptor, tumour necrosis factor receptor-associated periodic syndrome
Introduction
Tumour necrosis factors (TNF-α and TNF-β) are important inflammatory cytokines that exert a wide range of effects on tissues throughout the body by interacting with two cell-surface receptors: TNF receptor I (TNFRSF1A, TNFR1, p55/p60-TNFR, CD120a) and TNF receptor II (TNFRSF1B, TNFR2, p75/p80-TNFR, CD120b).1 TNF-receptor-associated periodic syndrome (TRAPS; MIM no. 142680), originally termed familial hibernian fever in the prototype family,2,3 is a hereditary autoinflammatory disorder recently shown to involve autosomal-dominant missense mutations in the TNFRSF1A gene4 (reviewed in ref. 5). Both the clinical and the genetic features of TRAPS are distinct from those of familial mediterranean fever (FMF), hyperimmunoglobulinaemia D syndrome (HIDS) and other periodic fever syndromes.5 Diagnostic indicators of TRAPS are recurrent episodes of inflammatory symptoms, often lasting longer than 5 days, which include fever, abdominal pain, myalgia with migratory erythematous macular rashes, conjunctivitis and periorbital oedema, chest pain, arthralgia or monoarticular synovitis; ≈ 15% of patients develop amyloidosis.5
The cell-surface TNFRSF1A protein is a single polypeptide consisting of four cysteine-rich ectodomains (CRD1-4), each of which contains three cysteine–cysteine disulphide bonds, a transmembrane region and a large intracellular region that interacts with signalling molecules (reviewed in ref. 5). The intracellular region includes a death domain (DD) that can initiate signalling cascades for both apoptosis (via caspase activation), and cytokine production and other inflammatory effects (via nuclear factor-κB activation).6 The membrane distal CRD1, also known as the preligand assembly domain (PLAD), is thought to undergo homologous interactions to form TNFRSF1A homotrimers;7 CRD2 and CRD3 interact with homotrimers of TNF-α or TNF-β.8 Over 30 different single nucleotide mutations of the TNFRSF1A gene have been identified in patients with TRAPS, which cause single amino acid substitutions mainly in CRD1, CRD2 or CRD3, about half of these mutations affect the highly conserved cysteine residues that are involved in disulphide bond formation, and one splice mutation has been identified that results in the insertion of four amino acids in CRD2.4,5,9–22 A TRAPS-associated missense mutation has also recently been described that involves an amino acid mutation at the base of the extracellular region of TNFRSF1A (I199N), proximal to the transmembrane region and close to the cleavage site for induced receptor shedding.23
Patients with TRAPS have low blood levels of soluble TNFRSF1A (sTNFRSF1A) that do not increase above the normal range during inflammatory attacks (contrasting with rheumatoid arthritis, where sTNFRSF1A levels start in the normal range and can increase twentyfold).4 Also, the leucocytes of some patients show reduced shedding of TNFRSF1A upon stimulation.4,9 These findings have led to the hypothesis that TNF is not adequately neutralized by the low levels of sTNFRSF1A in TRAPS, resulting in exaggerated inflammatory effects. However, not all the TRAPS-related TNFRSF1A mutations result in defective receptor shedding by leucocytes, indicating that other pathophysiological mechanisms may also be involved.9 The importance of understanding these mechanisms extends beyond TRAPS, as there is evidence that certain of the TNFRSF1A mutations (e.g. R92Q) are also associated with much more common inflammatory diseases, such as rheumatoid arthritis.9
It is possible that some of the TRAPS-related mutations have significant structural effects on TNFRSF1A conformation that may affect the subcellular distribution and/or functional properties of the receptor. Indeed, a growing number of diseases are now understood to result from the pathological effects of misfolded proteins. These ‘protein conformational disorders’ include Alzheimer's disease, Huntington's disease and Parkinson's disease (that affect the central nervous system), and α1-antitrypsin deficiency (affecting the liver).24,25
In order to elucidate the molecular effects of TRAPS-related mutations in the TNFRSF1A gene, we produced cell lines stably expressing high levels of either wild-type (WT) or single-mutant recombinant forms of TNFRSF1A. Mutants with the following single amino acid substitutions in CRD1 were produced both as full-length receptor proteins and as truncated forms representing the extracellular and transmembrane regions, but lacking the cytoplasmic signalling domain (Δsig): C33Y, C52F and T50M. These constructs enabled us to investigate the effects of the mutations on receptor function, distribution and TNF binding. The results indicate that, while the mutant receptors retain signalling properties associated with the cytoplasmic death domain, there are other profound effects of the mutations on the properties of the TNFRSF1A protein.
Materials and methods
Production of recombinant WT and mutant TNFRSF1A DNA clones
The full-length TNFRSF1A coding sequence was polymerase chain reaction (PCR) amplified from cDNA using flanking primers designed from previously published sequences (GenBank accession number: NM_001065). DNA was amplified using Elongase (Invitrogen Life Technologies, Paisley, UK), cloned into vector pcDNA4TO (Invitrogen) and sequenced using Big Dye 2 terminators on an ABI Prism 310 (both Applied Biosystems, Warrington, UK). To remove the cytoplasmic signalling domain, a mutant (Δsig) was constructed using site-directed mutagenesis (Qwikchange Kit; Stratagene, Amsterdam, the Netherlands), with a stop codon introduced at residue 215 (numbered from the start of the mature TNFRSF1A sequence) leaving a 10-residue cytoplasmic tail. Mutant receptors (C33Y, C52F, T50M) were produced from both full-length and Δsig variants, using site-directed mutagenesis. All products were sequenced along the full length of the TNFRSF1A coding region to ensure that only the desired mutation(s) had been introduced. Plasmid isolation from bulk cultures for transfection into eukaryotic cells was carried out using an Endofree Plasmid maxiprep kit (Qiagen, Crawley, UK).
Transfection and cloning of cell lines expressing recombinant TNFRSF1A
The tetracycline-regulated expression (T-Rex™) human embryonic kidney (HEK)-293 cell line was employed for transfections. The HEK-293 cell line expresses low levels of TNF receptors, predominantly TNFRSF1A:26 in our hands, the expression of TNFRSF1A and TNFRSF1B by HEK-293 cells was essentially undetectable by flow cytometry. The HEK-293 cells were stably transfected with the WT and single-mutation constructs using FuGENE-6 (Roche Diagnostics, Lewes, UK) according to the manufacturer's protocols. A FuGENE 6 Reagent/DNA ratio of 3 : 1 was used. Ninety-six hours post-transfection, the T-Rex™ HEK-293 cells were switched to selective medium [Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 10% fetal calf serum (FCS), 100 U/ml penicillin, 10 µg/ml streptomycin, 5 µg/ml Blasticidin S HCl (Invitrogen Life Technologies), 400 µg/ml Zeocin (Invitrogen Life Technologies), 2 mml-glutamine and 10 mm HEPES buffer]. Initial transfection success was assessed using pcDNA4/TO LacZ control plasmid expression and detected using a β-galactosidase staining kit (Invitrogen Life Technologies). Primary selection of transfected cells was for 21 days, after which the cells were induced to express receptor. Cultured cells were split 1 : 2 and 1 µg/ml doxycycline (a tetracycline derivative) (Sigma, Poole, UK) was added. Twenty hours postinduction, the cells were assayed for surface-expressed TNFRSF1A by flow cytometry using mouse anti-human TNFRSF1A-conjugated phycoerythrin (PE) (R & D Systems, Abingdon, UK), or mouse IgG1-PE negative control (Dako, Ely, UK), and assayed on an EPICS-XL flow cytometer (Beckman Coulter, High Wycombe, UK). Clones of the WT and mutant full-length and Δsig transfectants were produced by limiting dilution and subsequent selection of individual colonies.
Induction and detection of surface and intracellular TNFRSF1A expression
WT and mutant cell lines were induced to express TNFRSF1A as described above. All the following procedures were performed on ice where possible. Cells were harvested and washed in DMEM containing 1% FCS, then in phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA), and resuspended in PBS containing 0·5% BSA. Cells were mixed with mouse anti-human TNFRSF1A-PE or mouse IgG1-PE negative control and incubated on ice for 30 min in the dark. Cells were subsequently washed twice with PBS containing 0·5% BSA, resuspended in 0·5% formaldehyde fixative and analysed by flow cytometry.
In order to determine the cytoplasmic expression of WT and mutant TNFRSF1A, cell lines were induced with doxycycline as described above. Cells were then harvested and washed in PBA (PBS containing 0·5% BSA and 0·1% sodium azide) and fixed in 2% formaldehyde fixative for 5 min at room temperature. Cells were then washed in saponin buffer [PBA containing 0·1% saponin (Sigma) and 10 mm glucose], centrifuged at 300 g for 5 min, then washed in saponin buffer (10% FCS), centrifuged and resuspended. Cells were mixed with mouse anti-human TNFRSF1A-PE or mouse IgG1-PE negative control and incubated on ice for 2 hr in the dark, with periodic mixing. Cells were subsequently washed three times with saponin buffer, resuspended in 0·5% formaldehyde fixative and analysed by flow cytometry. Duplicate cells were stained for the surface expression of TNFRSF1A for comparison with intracellular levels.
Examination of cell death
HEK-293 cell lines transfected with WT or mutant full-length or Δsig TNFRSF1A were split 1 : 2 and either left untreated or 1 μg/ml doxycycline added. Duplicate samples were also incubated with 10 mm Z-Val-Ala-Asp-CH2F (Z-VAD.fmk), a caspase inhibitor (CN Biologicals, Nottingham, UK) or 5 mm dimethyl sulphoxide (DMSO), the solvent for Z-VAD.fmk. Twenty hours postinduction, the cells were harvested, washed with PBA and fixed with ice-cold 70% ethanol and stored at −20° until use. The cells were then washed twice with PBA, resuspended in PBA containing 100 mg/ml RNAse A (Sigma) and 50 mg/ml propidium iodide solution (Sigma), and incubated at 37° for 20 min. Cell samples were then assayed on an EPICS-XL flow cytometer. Propidium iodide fluorescence is emitted at 600–700 nm and was detectable in the FL3 detector of the EPICS-XL. The FL3 peak and integrated signals using linear amplification were collected. Plotting FL3 area versus FL3 peak was then used to gate single cells to produce an FL3 histogram showing cell cycle peaks and subdiploid DNA-containing cells.
Investigating cytokine production
HEK-293 cell lines transfected with WT or mutant full-length or Δsig TNFRSF1A were split 1 : 2 and either left untreated or 1 μg/ml doxycycline added, and then incubated with 10 mm Z-VAD.fmk or 5 mm DMSO. Seventy-two hours postinduction, the culture supernatants were collected for analysis. Samples were assayed using a human inflammation cytometric bead array (CBA) (BD Biosciences, Oxford, UK) according to the manufacturer's protocol. The beads were analysed on an EPICS-Altra flow cytometer/sorter (Beckman Coulter, High Wycombe, UK).
Confocal microscopy
Dual indirect staining was performed using primary antibodies of different isotypes and the appropriate Alexa Fluor-conjugated anti-isotype secondary antibodies. HEK-293 cell lines transfected with WT or mutant full-length or Δsig TNFRSF1A were induced to express TNFRSF1A, as described above. Cells were washed in PBA and fixed in 4% formaldehyde fixative for 5 min at room temperature. Cells were then washed in PBA, followed by washing in saponin buffer [PBA containing 0·1% saponin (Sigma) and 10 mm glucose] and then in saponin buffer containing 10% FCS. The first primary antibody was added to the cells and incubated for 30 min at room temperature in the dark. Cells were then washed twice in saponin buffer. The corresponding Alexa Fluor-conjugated anti-isotype (Molecular Probes Europe, Leiden, the Netherlands) was added to the cells and incubated for 30 min at room temperature in the dark. Cells were then washed twice in saponin buffer. Cells were incubated with 10% mouse serum (Sigma) in PBA for 10 min at room temperature in the dark. They were then washed twice in saponin buffer. The second primary antibody was added to the cells and incubated for 30 min at room temperature in the dark. Cells were washed twice in saponin buffer. The corresponding Alexa Fluor conjugated anti-isotype was added to the cells and incubated for 30 min in the dark. Cells were then washed twice in saponin buffer. If nuclear staining was required, cells were incubated with Hoechst 33258 (Sigma) for 10 min at room temperature in the dark. Cells were then washed twice in saponin buffer and stored in PBS before being viewed on a Leica SP2 confocal laser scanning microscope using a ×63 objective. The primary monoclonal antibodies (mAbs) and the Alexa Fluor anti-isotype conjugates employed are listed in Table 1. For staining with monodansylcadaverine (MDC) (Sigma), following indirect staining with antibody and washing, cells were incubated with 0·05 mm MDC in PBS at 37° for 10 min and then washed four times with PBS.
Table 1.
Antibodies employed for confocal microscopy
| Primary antibody | Species and isotype/preparation | Source | Secondary conjugate* |
|---|---|---|---|
| Anti-TNFRSF1A | Mouse mAb† IgG1 | R & D Systems Europe Ltd, Abingdon, Oxon, UK | Alexa Fluor 555 goat anti-mouse IgG1 |
| Anti-HLA-ABC | Mouse mAb IgG2a | Serotec, Oxford, UK | Alexa Fluor 488 goat anti-mouse IgG2a |
| Anti-Hsc70 | Mouse mAb IgM | Affinity Bioreagents (through Cambridge Biosciences, Cambridge, UK) | Alexa Fluor 647 goat anti-mouse IgM |
| Anti-BiP/GRP78 | Mouse mAb IgG2a | BD Biosciences, Cowley, Oxford, UK | Alexa Fluor 488 goat anti-mouse IgG2a |
| Anti-HDJ2 | Mouse mAb IgG1 | Abcam, Cambridge, UK | Alexa Fluor 488 goat anti-mouse IgG (H + L) |
| Anti-TCP1 | Rat mAb IgG2a | Abcam, Cambridge, UK | Alexa Fluor 647 goat anti-rat IgG (H + L) |
| Anti-Ubiquitin | Rabbit Ig fraction | Dakocytomation Ltd, Ely, Cambs, UK | Alexa Fluor 488 goat anti-rabbit IgG (H + L) |
| Anti-SODD | Rabbit polyclonal IgG | Upstate Biotechnologies UK, Milton Keynes, UK | Alexa Fluor 647 goat anti-rabbit IgG (H + L) |
| Anti-TRADD | Mouse mAb IgG2a | Upstate Biotechnologies UK, Milton Keynes, UK | Alexa Fluor 488 goat anti-mouse IgG2a |
All Alexa Fluor conjugates were purchased from Molecular Probes Europe, the Netherlands.
mAb, monoclonal antibody.
SODD, silencer of death domain; TNFRSF1A, tumour necrosis factor receptor superfamily 1A; TRADD, TNF receptor-associated death domain.
Analysis of TNF-α binding
WT and mutant Δsig-TNFRSF1A cell lines were induced with doxycyclin to express TNFRSF1A, as described above. Recombinant human TNF-α (R & D Systems, Abingdon, UK) was added at a final concentration of 50 ng/ml and the cells incubated at 37° for 2 hr. Cells were harvested and washed in PBS containing 0·5% BSA and stained with primary antibodies [mouse anti-human TNF-α (Biosource International, Nivelles, Belgium), mouse IgG2a negative control (Dako)] for 30 min on ice. Cells were washed twice with PBS containing 0·5% BSA and mixed with goat anti-mouse immunoglobulin F(ab′)2–fluorescein isothiocyanate (FITC) (Dako) for 30 min on ice. Cells were washed twice, resuspended in 0·5% formaldehyde fixative and analysed by flow cytometry. Duplicate cells were stained for the surface expression of TNFRSF1A, as described above, to confirm doxycycline induction. Controls showed very little non-specific staining of the cells by anti-human TNF-α if TNF-α was not added, or if TNF-α was added but TNFRSF1A expression was not induced with doxycyclin.
Results
High expression of both WT and mutant recombinant forms of full-length TNFRSF1A induce apoptosis and cytokine production
HEK-293 cells with T-Rextm™ were transfected with WT and mutant forms of the full-length TNFRSF1A gene (i.e. including the cytoplasmic signalling region) in the plasmid vector pcDNA4TO (see the Materials and methods for details). HEK-293 cells naturally express a low level of TNF receptors, predominantly TNFRSF1A:26 in our hands, TNFRSF1A and TNFRSF1B expression by HEK-293 cells was essentially undetectable by flow cytometry. Thus, the vast majority of TNFRSF1A expressed in the transfected cell lines was derived from the recombinant WT or mutant constructs upon induction with doxycyclin (see below for detection of receptor expression).
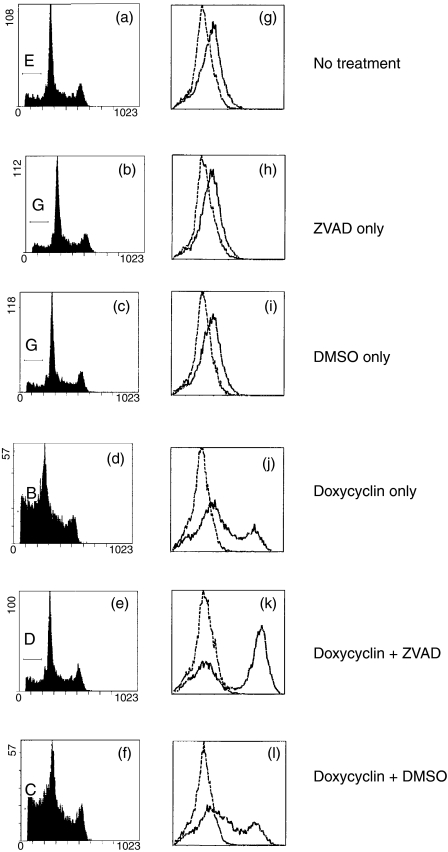
The transfected HEK-293 cell lines were induced to express recombinant full-length TNFRSF1A by treatment with doxycyclin for 16–18 hr, and then stained with propidium iodide and analysed by flow cytometry to detect the cellular DNA content. Dying and dead cells (early and late apoptotic cells) were defined as those with a subdiploid fractional DNA content, i.e. with fluorescence intensity less than the dominant, sharp G1 peak (Fig. 1a–1f). For nondoxycyclin-induced transfected cells (i.e. not expressing the TNFRSF1A transgene), typically 9–15% of cells were in the subdiploid fraction (Fig. 1a and Table 2). However, following treatment with doxycyclin, the proportion of cells in the subdiploid fraction increased substantially in the cell lines expressing either the WT form or any of the mutant forms (C33Y, C52F, T50M) of TNFRSF1A (Fig. 1d and Table 2). This induction of cell death by high-level expression of WT or mutant recombinant TNFRSF1A was inhibited by the addition of Z-VAD.fmk to the cultures at the same time as doxycyclin (Fig. 1e and Table 2). Cell death was not inhibited by the solvent for Z-VAD.fmk, DMSO (Fig. 1f and Table 2); conversely, death was not induced by Z-VAD.fmk or DMSO alone in the absence of doxycyclin (Fig. 1b,1c and Table 2). Doxycyclin did not induce the death of non-transfected HEK-293 cells (data not shown), indicating that death was a consequence of TNFRSF1A expression.
Figure 1.
Detection of apoptosis and tumour necrosis factor receptor superfamily 1A (TNFRSF1A) expression by HEK-293 cells transfected with wild-type (WT), full-length TNFRSF1A. Figure 1(a)–1(f) shows the flow cytometry profiles of cells stained with propidium iodide to indicate DNA content: cells with a subdiploid DNA content (to the left of the sharp G1 peak) were defined as apoptotic. Figure 1(g)–1(l) shows flow cytometry profiles of permeabilized cells stained for the expression of TNFRSF1A (solid line) compared with the isotype-control staining (dashed line). DMSO, dimethyl sulphoxide; ZVAD, Z-Val-Ala-Asp-CH2F.
Table 2.
Percentage apoptotic transfected HEK-293 cells not induced, or induced with doxycyclin to express wild-type (WT) or mutant tumour necrosis factor receptor superfamily 1A (TNFRSF1A) as either the full-length (F-L) or Δsig constructs: inhibition of apoptosis is shown with Z-Val-Ala-Asp-CH2F (Z-VAD.fmk) [dimethylsulphoxide (DMSO) was used as the solvent control]
| Cell line | No treatment | ZVAD only | DMSO only | Doxycyclin only | Doxycyclin + ZVAD | Doxycyclin + DMSO |
|---|---|---|---|---|---|---|
| WT (F-L) | 17·1 | 15·2 | 16·9 | 39·9 | 16·0 | 38·4 |
| C33Y (F-L) | 10·9 | 8·0 | 16·2 | 19·7 | 11·5 | 18·9 |
| C52F (F-L) | 11·4 | 10·0 | 13·5 | 41·2 | 10·4 | 39·1 |
| T50M (F-L) | 16·0 | 16·2 | 15·1 | 23·6 | 14·2 | 27·4 |
| WT (Δsig) | 20·9 | 19·8 | 21·7 | 26·4 | 25·2 | 22·5 |
| C33Y (Δsig) | 8·3 | 5·3 | 5·8 | 8·3 | 5·9 | 6·1 |
| C52F (Δsig) | 24·5 | 9·9 | 21·7 | 16·9 | 12·2 | 18·3 |
| T50M (Δsig) | 24·6 | 15·9 | 24·8 | 18·9 | 13·6 | 16·2 |
Z-VAD is a highly specific, cell-permeable, irreversible inhibitor of caspases-1, -3, -6 and -7.27 Therefore, both the WT and mutant recombinant forms of TNFRSF1A spontaneously induce apoptosis when expressed at high levels in the transfected HEK-293 cells. Z-VAD.fmk does not inhibit the TNFRSF1A expression induced by doxycyclin (Fig. 1k), which might otherwise be an explanation for the reduction of cell death by Z-VAD.fmk; indeed, expression of either WT or mutant forms of TNFRSF1A was enhanced in the presence of Z-VAD.fmk, presumably because the reduction of TNFRSF1A expression that would accompany apoptosis did not occur (Fig. 1k). Another caspase inhibitor, Boc-Asp(OMe)-CH2F, had a very similar inhibitory effect on cell death induced by WT or mutant TNFRSF1A expession to that of Z-VAD.fmk described above (data not shown): this further supports the conclusion that high-level expression of either WT or mutant forms of TNFRSF1A induces apoptosis.
As TNFRSF1A can signal for cell activation and cytokine production as well as apoptosis,6 we tested culture supernatants from the TNFRSF1A-transfected HEK-293 cell lines for secreted cytokines by flow cytometry using the Becton Dickinson inflammatory CBA system (see the Materials and methods for details). Induction of a high-level expression of either WT, or any of the mutant, forms of recombinant TNFRSF1A resulted in greatly augmented production of the chemokine interleukin-8 (IL-8) (Table 3).
Table 3.
Interleukin-8 production (pg/ml) by transfected HEK-293 cells not induced, or induced with doxycyclin to express wild-type (WT) or mutant tumour necrosis factor receptor superfamily 1A (TNFRSF1A) as either the full-length (F-L) or Δsig constructs
| Cell line | No doxycyclin | With doxycyclin |
|---|---|---|
| WT (F-L) | 104·8 | 3914·1 |
| C33Y (F-L) | 86·9 | 1435·7 |
| C52F (F-L) | 112·4 | 5000·0 |
| T50M (F-L) | 117·7 | 4920·2 |
| WT (Δsig) | 150·9 | 184·1 |
| C33Y (Δsig) | 122·5 | 126·0 |
| C52F (Δsig) | 33·8 | 33·1 |
| T50M (Δsig) | 93·4 | 81·3 |
IL-8 levels were measured by flow cytometry using the Cytokine Bead Array in culture supernatants collected 3 days after the addition of doxycyclin.
Recombinant full-length TNFRSF1A has the appearance of inclusion bodies, or aggregates, in transfected cell lines
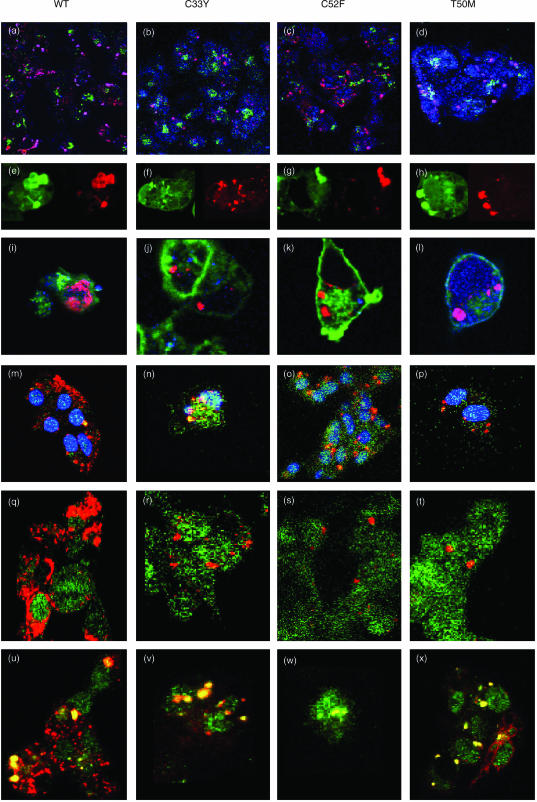
The finding that high-level expression of recombinant TNFRSF1A in transfected cell lines spontaneously induced both apoptosis and IL-8 production is consistent with previous reports.28,29 Our findings, that these effects were induced by expression of the C33Y, C52F and T50M mutants, as well as the WT receptor, indicate that these mutations in CRD1 do not abrogate the signalling potential of the DD. Indeed, Boldin et al. observed that overexpression of just the DD per se induced apoptosis and IL-8 production, and this ligand-independent signalling was proposed to result from spontaneous self-association of DDs when expressed at high levels.28 In order to visualize whether similar self-association of WT or mutant forms of TNFRSF1A might explain the induction of apoptosis and IL-8 production in our experiments, the HEK-293 cell lines transfected with full-length WT or mutant TNFRSF1A were induced with doxycyclin to give high-level expression of recombinant TNFRSF1A and were fluorescently stained, following permeabilization, for detection of constituents by confocal microscopy. mAbs were used to detect TNFRSF1A, golgin as a marker of the Golgi apparatus, HLA class I as a cell-surface protein, and several markers of protein processing (see the Materials and methods for details). In most cases, TNFRSF1A induction was performed in the presence of Z-VAD.fmk to inhibit apoptosis so that a greater number of cells remained available for staining and visualization: the treatment with Z-VAD.fmk was shown not to alter the pattern of staining with anti-TNFRSF1A. The results shown in Fig. 2 indicate that the recombinant WT and mutant forms of TNFRSF1A were detected as discrete cytoplasmic particles that were distinct from the Golgi apparatus (Fig. 2a–2d). These cytoplasmic particles had the appearance of inclusion bodies or aggregates that are formed by misfolded proteins.24,25 Such aggregates have been extensively studied in other transfection systems. For example, some misfolded mutant proteins that are associated with protein conformational disorders dislocate from the endoplasmic reticulum (ER) and form cytoplasmic aggregates that coalesce into ‘aggresomes’.30,31 These misfolded proteins become labelled with ubiquitin, as found with mutant cystic fibrosis transmembrane conductance regulator,30 and/or associate with chaperone proteins of the heat shock protein (HSP)-40 and HSP-70 series, as observed with mutant p115 membrane transport factor.31 These modifications mark the mutant proteins for degradation by 26S proteasomes (although the aggregated proteins are resistant to degradation). Staining of the HEK-293 transfectants, expressing the mutant forms of TNFRSF1A, with anti-ubiquitin showed some co-localization of TNFRSF1A and ubiquitin, although not all of the TNFRSF1A aggregates appeared to be ubiquitinated (Fig. 2m–2p). However, it has been observed previously that some misfolded protein aggregates show little, or no, association with ubiquitin, but are strongly associated with HSP chaperones.31 Consistent with this, we observed that the aggregates of WT or mutant TNFRSF1A were strongly stained by antibodies to Hsc-70 (a member of the HSP-70 family) (Fig. 2a–2d) and HDJ-2 (a member of the HSP-40 family) (Fig. 2e–2h). On the other hand, the TNFRSF1A aggregates did not consistently co-stain with the chaperonin TCP-1 (Fig. 2i–2l). Hence, the aggregated TNFRSF1A presumably has exposed hydrophobic regions, resulting in interactions with chaperones, but is not always amenable to refolding in the cage-like structures formed by chaperonins.
Figure 2.
Detection of immunofluorescent staining by confocal microscopy in permeabilized HEK-293 cells transfected with full-length constructs of wild-type (WT) or mutant tumour necrosis factor receptor superfamily 1A (TNFRSF1A): WT TNFRSF1A, panels (a), (e), (i), (m), (q), (u); C33Y TNFRSF1A, panels (b), (f), (j), (n), (r), (v); C52F TNFRSF1A, panels (c), (g), (k), (o), (s), (w); T50M TNFRSF1A, panels (d), (h), (l), (p), (t), (x). Figure 2(a)–2(d) shows cells stained with anti-Golgin (green), anti-TNFRSF1A (red) and anti-Hsc70 (blue); in these overlay images the purple/magenta staining of aggregates indicates co-staining for TNFRSF1A and Hsc-70. Figure 2(e)–2(h) shows cells stained with anti-HDJ-2 (green, left panels), and the same cells stained with anti-TNFRSF1A (red, right panels), indicating that aggregates co-stain for both markers. Figure 2(i)–2(l) shows cells stained with anti-HLA class I (green), anti-TNFRSF1A (red) and anti-TCP-1 (blue); in these overlay images, co-staining of aggregates for TNFRSF1A and TCP-1 is purple/magenta. Figure 2(m)–2(p) shows cells stained with anti-TNFRSF1A (red), anti-ubiquitin (green) and nucleus (blue); in these overlay images, aggregates co-stained for TNFRSF1A and ubiquitin are yellow/orange. Figure 2(q)–2(t) shows cells stained with anti-TNFRSF1A (red) and anti-silencer of death domain (anti-SODD) (green). Figure 2(u)–2(x) shows cells stained with anti-TNFRSF1A (red) anti-TNF receptor-associated death domain (anti-TRADD)(green); in these overlay images, aggregates co-stained for TNFRSF1A and TRADD are yellow.
Addition of the proteasome inhibitor, acetyl-leucyl-leucyl-norleucinal (ALLN), to the transfected cell lines during the induction of TNFRSF1A expression was found to increase the size of the TNFRSF1A aggregates formed, suggesting that a proportion of the misfolded receptor may undergo proteasomal degradation (Fig. 3).
Figure 3.
Detection of immunofluorescent staining by confocal microscopy in doxycyclin-treated HEK-293 cells transfected with full-length constructs of wild-type (WT) or C33Y mutant tumour necrosis factor receptor superfamily 1A (TNFRSF1A). Cells stained with anti-human leucocyte antigen (HLA) class I (green) and with anti-TNFRSF1A (red). Panels (a) and (b) are WT transfectants treated without (a), or with (b), acetyl-leucyl-leucyl-norleucinal (ALLN). Panels (c) and (d) are C33Y transfectants treated without (c), or with (d), ALLN.
Some misfolded proteins (e.g. mutants of α1-antitrypsin and huntingtin) have been found to be targeted for disposal by autophagy (i.e. the intracytoplasmic formation of phagosomes) rather than by proteasomal degradation.32,33 Autophagosomes are stained by MDC.32 However, there was no clear staining by MDC in the transfectants expressing the full-length mutant forms of TNFRSF1A (data not shown).
Although we detected a small proportion of WT TNFRSF1A on the cell surface by flow cytometry (see below), this was not readily seen by confocal microscopy, presumably because of the lower sensitivity of this latter technique.
Thus, high-level production of full-length TNFRSF1A by the transfected HEK-293 cells led to the formation of cytoplasmic receptor aggregates that appeared to spontaneously trigger the signalling pathways for apoptosis and cytokine production. Both WT and TRAPS-related mutant forms of TNFRSF1A have these signalling properties. This signalling is presumably initiated by DD interactions in the receptor aggregates.28 Under normal circumstances, the DDs of TNFRSF1A are associated with silencer of death domain (SODD) that prevents spontaneous aggregation and binding of the signal transducer TNF receptor-associated death domain (TRADD): the interaction of the TNF ligand with the receptor trimer induces the release of SODD, allowing binding of TRADD.34 We therefore investigated, by using confocal microscopy, whether there was co-localization of SODD and/or TRADD with the receptor aggregates in the full-length TNFRSF1A transfectants. The results shown in Fig. 2(q)–2(t) indicate that there was no co-localization of staining for TNFRSF1A and SODD in the transfectants. By contrast, Fig. 2(u)–2(x) show strong co-localization of TNFRSF1A and TRADD in both the WT and mutant transfectants. This apparent interaction of TNFRSF1A and TRADD is consistent with the spontaneous signalling for apoptosis and cytokine production described above. Interestingly, TRADD co-localized mainly with the larger aggregates of TNFRSF1A: this is particularly apparent in Fig. 2(u).
WT and mutant forms of Δsig-TNFRSF1A do not induce apoptosis or cytokine production
As the cytoplasmic region of TNFRSF1A influences both its subcellular localization29,35–37 and its signalling properties,28,29 we examined the properties of WT and mutant (C33Y, C52F, T50M) receptors lacking the cytoplasmic signalling region (here referred to as Δsig-TNFRSF1A). Recombinant WT and mutant TNFRSF1A clones were prepared in pcDNA4TO with a stop codon introduced at residue 215 (numbered from the start of the mature TNFRSF1A sequence), leaving a 10-residue cytoplasmic tail. These were transfected into the T-Rex™ HEK-293 cells. Clones of these cell lines were selected on the basis of showing surface expression of the Δsig-TNFRSF1A upon induction with doxycyclin (see below).
Unlike the HEK-293 cells transfected with full-length TNFRSF1A, induction of Δsig-TNFRSF1A expression (either WT or mutant) by treatment with doxycyclin for 16–18 hr did not stimulate apoptosis, as judged by propidium iodide staining (Table 2), or IL-8 production (Table 3). Thus, as expected, expression of the cytoplasmic region is necessary for functional activity of both the WT and mutant receptors. These findings also exclude the possibility that the induction of either apoptosis or cytokine production is a consequence of doxycyclin treatment per se, or the induction of high-level transgene expression, irrespective of the nature of the recombinant protein.
Recombinant WT and mutant forms of full-length TNFRSF1A show differences in surface and intracellular expression in transfected cell lines
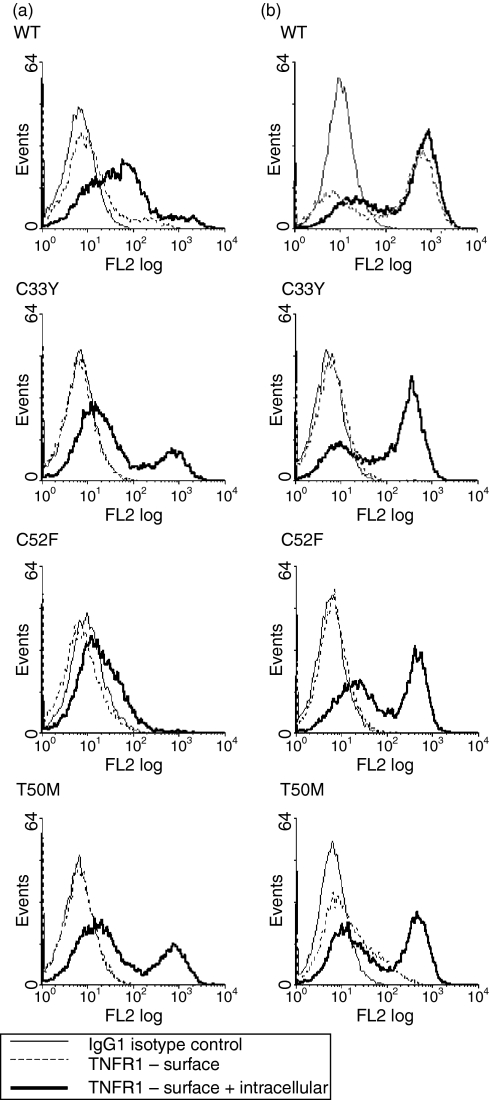
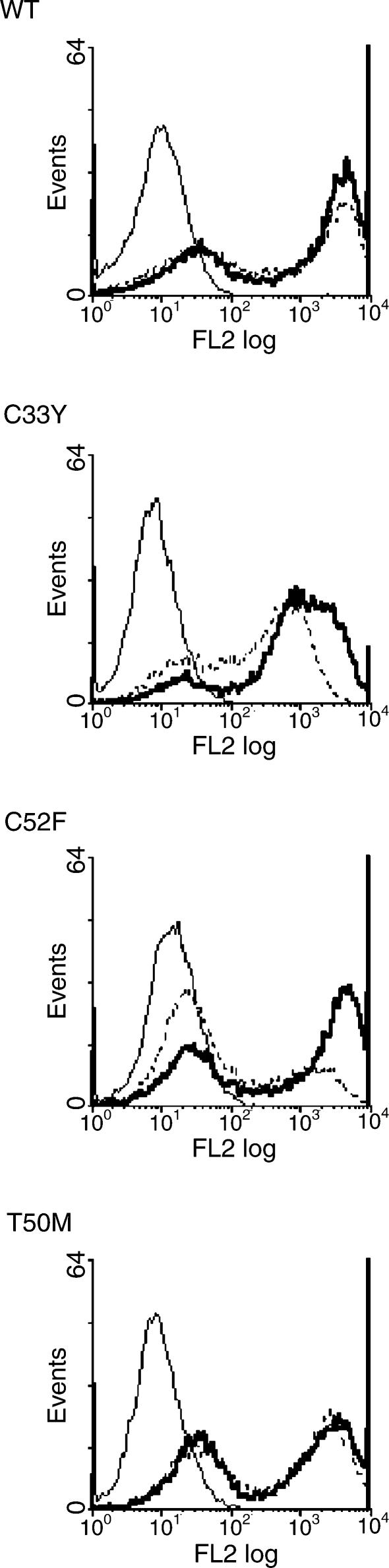
Following induction of recombinant full-length TNFRSF1A expression by treatment of the transfected cell lines with doxycyclin for 16–18 hr, staining with fluorochrome-labelled anti-TNFRSF1A mAb and flow cytometric analysis revealed the surface expression of only the WT receptor by a minority of the cells; no surface expression of TNFRSF1A with the T50M, C33Y or C52F mutations was observed (Fig. 4a). We therefore stained transfected cells with anti-TNFRSF1A mAb following permeabilization to detect intracellular TNFRSF1A. As expected, the WT line showed enhanced staining following permeabilization, indicating detection of both surface and cytoplasmic TNFRSF1A (Fig. 4a). However, even stronger staining for intracellular TNFRSF1A was detected in the C33Y and T50M cell lines, and to a lesser extent in the C52F line, despite their complete lack of surface TNFRSF1A expression (Fig. 4a). [This detection of intracellular WT and mutant TNFRSF1A by flow cytometry is consistent with the confocal microscopy results described above (Fig. 2).]
Figure 4.
Surface and intracellular expression of recombinant full-length tumour necrosis factor receptor superfamily 1A (TNFRSF1A). Transfected HEK-293 cell lines (a), or clones (b), induced with doxycyclin to express the wild-type (WT) or different mutant full-length TNFRSF1A were stained with phycoerythrin-labelled anti-TNFRSF1A, without permeabilization, for surface staining, or with permeabilization for surface plus cytoplasmic staining, followed by flow cytometric analysis. Thin solid line, IgG1 isotype control; dashed line, surface staining with anti-TNFRSF1A; bold solid line, staining of permeabilized cells with anti-TNFRSF1A.
In order to obtain a more detailed picture of the surface and cytoplasmic expression of full-length TNFRSF1A, clones of the cell lines that gave the results shown in Fig. 4(a) were produced and analysed in the same way. The results obtained with representative clones are shown in Fig. 4(b). In the clone expressing full-length WT TNFRSF1A, surface expression of the receptor was observed by the majority of cells, with only a slight increase in fluorescence when the intracellular receptor was also stained. As in the cell lines, the clones expressing the C33Y and C52F mutant receptors showed intracellular expression, but essentially no surface expression. Interestingly, the clone expressing T50M mutant TNFRSF1A showed strong intracellular expression and also a low level of surface expression, although substantially less than WT (Fig. 4b). Thus, full-length WT TNFRSF1A was definitely expressed on the cell surface, whereas T50M showed much less surface expression and the cysteine mutants were only detected in the cytoplasm.
Mutant, as well as WT, forms of Δsig-TNFRSF1A are expressed on the cell surface
If the abnormalities indicated above were caused by the mutations in CRD1 of the mutant forms of TNFRSF1A, then these would be expected to affect the Δsig forms of the receptor, as well as full-length TNFRSF1A. Thus, the cellular distribution of WT and mutant forms of Δsig-TNFRSF1A was examined. Following induction of recombinant Δsig-TNFRSF1A expression by treatment of the transfected cell clones with doxycyclin for 16–18 hr, staining with fluorochrome-labelled anti-TNFRSF1A mAb and flow cytometric analysis revealed surface expression of not only WT, but also the mutant receptors – i.e. C33Y, C52F and T50M – by a proportion of the cells (Fig. 5). Staining of permeabilized cells to detect both cytoplasmic and surface TNFRSF1A expression, revealed a level of staining only slightly greater than that seen for just surface expression with WT and T50M, but generally higher expression than for surface alone with the C33Y and C52F mutants (Fig. 5). Thus, in contrast to the full-length receptor, the absence of the cytoplasmic signalling region facilitates the surface expression of the TNFRSF1A mutants as well as the WT receptor. This suggests that the mutations in CRD1 inhibit surface expression only of receptors with intact cytoplasmic regions in the transfected HEK-293 cells. However, the cysteine mutant receptors lacking the cytoplasmic region still showed a higher proportion of intracellular expression than the WT receptors.
Figure 5.
Surface and intracellular expression of recombinant Δsig-tumour necrosis factor receptor superfamily 1A (TNFRSF1A). Transfected HEK-293 cell lines induced with doxycyclin to express the wild-type (WT) or different mutant Δsig-TNFRSF1A were stained with phycoerythrin-labelled anti-TNFRSF1A without permeabilization for surface staining, or with permeabilization for surface plus cytoplasmic staining, followed by flow cytometric analysis. Thin unbroken line, IgG1 isotype control; dashed line, surface staining with anti-TNFRSF1A; bold unbroken line, staining of permeabilized cells with anti-TNFRSF1A.
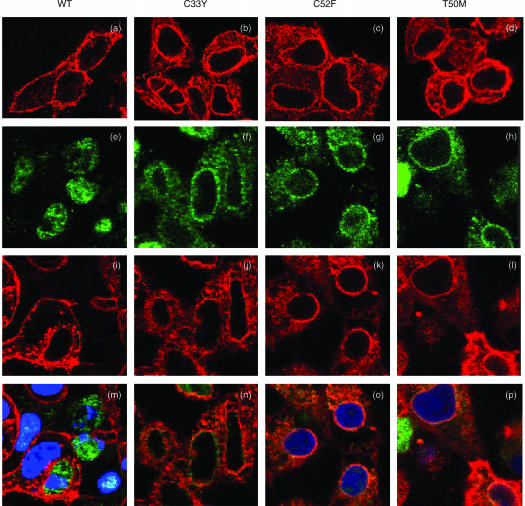
Recombinant mutant, but not WT, forms of Δsig-TNFRSF1A show evidence of retention in the cytoplasm in transfected cell clones
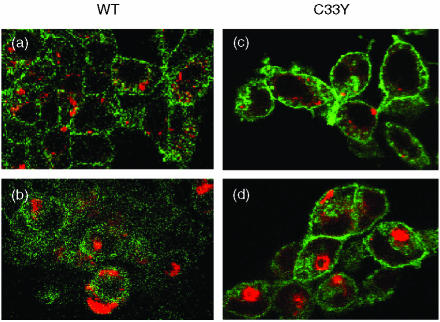
The cellular distribution of WT and mutant forms of Δsig-TNFRSF1A was examined further by confocal microscopy following induction with doxycyclin. Staining with anti-TNFRSF1A indicated that the WT receptors were detected only on the cell surface (Fig. 6a). However, the C33Y, C52F and T50M mutant receptors were stained both at the cell surface and in the cytoplasm, giving both diffuse cytoplasmic and distinctive perinuclear staining (Fig. 6b–6d). The Δsig staining pattern seen by confocal microscopy is clearly different from that described above for the full-length TNFRSF1A, indicating that the presence of the cytoplasmic region influences the subcellular distribution of the receptor. However, the mutant Δsig receptors show a somewhat different distribution from the WT Δsig. The diffuse cytoplasmic and perinuclear staining pattern given by the mutant forms of Δsig-TNFRSF1A is suggestive of localization to the ER. Indeed, mutant α1-antitrypsin has been reported to show prolonged retention in the ER and therefore give co-localization of staining with the ER chaperone BiP/GRP78.38 We similarly observed some co-localization of staining of mutant Δsig-TNFRSF1A and BiP (Fig. 6n–6p), but not WT Δsig-TNFRSF1A and BiP (Fig. 6m).
Figure 6.
Detection of immunofluorescent staining by confocal microscopy in permeabilized HEK-293 cells transfected with Δsig constructs of wild-type (WT) or mutant tumour necrosis factor receptor superfamily 1A (TNFRSF1A): WT TNFRSF1A, panels (a), (e), (i), (m); C33Y TNFRSF1A, panels (b), (f), (j), (n); C52F TNFRSF1A, panels (c), (g), (k), (o); T50M TNFRSF1A, panels (d), (h), (l), (p). Panels (a)–(d) show cells stained with anti-TNFRSF1A (red), panels (e)–(h) show cells stained with anti-BiP (green), and panels (i)–(l) show the same cells stained with anti-TNFRSF1A (red). Panels (m)–(p) show the overlay of the corresponding images, i.e. panels (e)/(i), (f)/(j), (g)/(k), (h)/(l), where orange/yellow indicates co-staining for TNFRSF1A and BiP. (Nuclei are stained blue in panels m, o and p.)
Recombinant mutant forms of Δsig-TNFRSF1A show evidence of defects in binding TNF-α
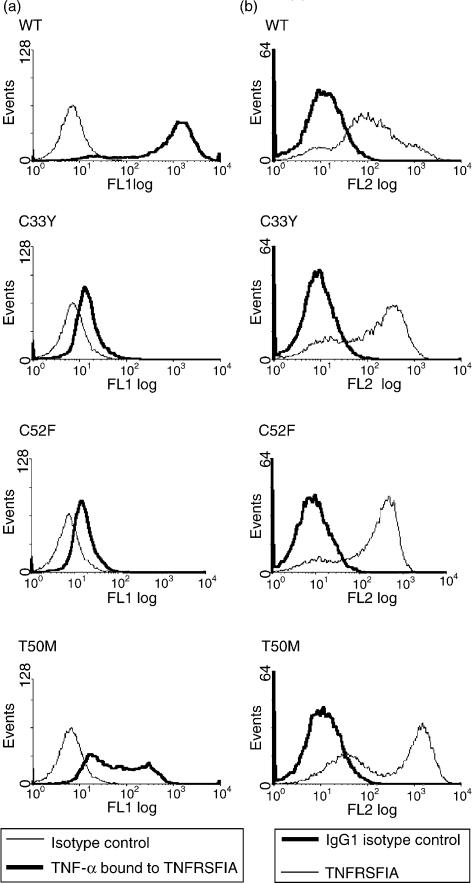
As the WT Δsig-TNFRSF1A, and a proportion of the mutant forms of Δsig-TNFRSF1A, are expressed at the cell surface, we investigated their ability to bind TNF-α. This was assessed by incubating the doxycyclin-induced HEK-293 transfectants with TNF-α, followed by fluorochrome-labelled anti-TNF-α, and flow cytometric analysis. High levels of TNF-α binding were observed with the doxycyclin-induced WT cell clone (Fig. 7a). However, TNF-α binding to the T50M mutant clone was greatly reduced and very little TNF-α binding was detected on the C33Y and C52F mutant clones (Fig. 7a), even though they had high levels of surface TNFRSF1A expression (Fig. 7b). This is consistent with conformational defects in the C33Y, C52F and T50M mutant receptors inhibiting TNF-α binding.
Figure 7.
Tumour necrosis factor-α (TNF-α) binding to recombinant Δsig-tumour necrosis factor receptor superfamily 1A (TNFRSF1A). Transfected HEK-293 cell clones induced with doxycyclin to express wild-type (WT) or different mutant Δsig-TNFRSF1A were either stained with phycoerythrin-labelled anti-TNFRSF1A (b), or were incubated with recombinant TNF-α followed by staining with anti-TNF-α and fluoresceinated anti-immunoglobulin (a). (a) Thin line, isotype control; bold line, surface staining of bound TNF-α. (b) Bold line, isotype control; thin line, surface staining with anti-TNFRSF1A.
Discussion
The findings presented in this study indicate that TRAPS-associated mutations within the CRD1 of TNFRSF1A result in the receptor displaying defective behaviour, but the signalling properties of the cytoplasmic DD of the mutant receptors are not abrogated.
The defects of the CRD1 mutants of TNFRSF1A that we observed in the high-expression HEK-293 transfection system were reduced translocation to the cell surface of the full-length receptor and retention within the cytoplasm (possibly within the ER) of a proportion of the Δsig receptor lacking the cytoplasmic region. In addition, the Δsig mutant receptors that were expressed on the cell surface showed a greatly reduced ability to bind TNF-α. Clearly, the high-level expression of recombinant TNFRSF1A in the HEK-293 transfection system cannot be regarded as physiological and cannot precisely reproduce the subtle, fluctuating cellular disturbances in TRAPS patients. However, it is the subtlety of TRAPS pathophysiology that makes it difficult to investigate in patients, and therefore the model system we have employed is useful to highlight abnormalities in the behaviour of TRAPS-related mutants of TNFRSF1A.
Differences in the behaviour of the full-length and Δsig TNFRSF1A, in terms of their subcellular distribution, were not unexpected because it has been well described that elements of the receptor's cytoplasmic region influence its distribution within the cell and, in particular, the cytoplasmic region is required for accumulation of the receptor in the trans-Golgi apparatus.29,35–37
The cytoplasmic aggregates that were formed by the full-length TNFRSF1A in the transfected cell lines were not associated with the Golgi apparatus, and both WT and mutant receptors formed similar aggregates that were associated with chaperones and whose formation appeared to be enhanced by the inhibition of proteasome function. Thus, the formation of these aggregates, which may be related to the ‘aggresomes’ described for other proteins,30 was not specifically related to the mutations, as the WT receptor behaved similarly. It is probable that the formation of aggregates is a consequence of overloading the transfected cells by the high levels of TNFRSF1A produced upon expression of the transfected gene. Indeed, it is recognized that protein overexpression can itself lead to misfolding.38 However, a key difference in behaviour that we observed between the WT and the mutant full-length receptors was that little or no mutant receptor was expressed at the cell surface. Little is known about how TNFRSF1A trafficks from the cytoplasm to the surface membrane, although there is evidence that the TNFRSF1A stored in the trans-Golgi apparatus serves as a reservoir of receptor for translocation to the cell surface.39 Thus, it is possible that some of the WT, but little of the mutant, TNFRSF1A expressed in the transfectants trafficks to the cell surface via the trans-Golgi apparatus rather than accumulating in the cytoplasmic aggregates.
The spontaneous triggering of apoptosis and IL-8 production upon induction of either WT or mutant full-length TNFRSF1A expression is presumably a result of signalling initiated by DD interactions in the receptor aggregates, as previously observed by others.28 This is strongly supported by our observation that TRADD, but not SODD, co-localized with the large cytoplasmic aggregates of TNFRSF1A. Thus, the reason why the overexpressed receptor aggregates cause spontaneous, ligand-independent, signalling is presumably the result of insufficient interaction with SODD and spontaneous binding of TRADD: it could be the location, conformation, or sheer quantity of TNFRSF1A in the aggregates that results in a lack of inhibition by SODD. Whatever the mechanism, our findings show that, although the mutant forms of TNFRSF1A show certain abnormalities of behaviour (i.e. reduced surface translocation in the transfected cells), they can perform signalling functions for apoptosis and cytokine production by interaction with TRADD.
The experiments with Δsig-TNFRSF1A provide evidence suggestive of conformational abnormalities of the extracellular portion of the mutant receptor as a result of misfolding. Thus, retention in the ER and association with BiP/Grp78, as suggested here for the mutants of Δsig-TNFRSF1A, has been noted in studies of misfolded mutant proteins, including mutants of α1-antitrypsin,40 low-density lipoprotein receptor41 and the receptor tyrosine kinase RET.42 Furthermore, the greatly reduced TNF-α binding observed for surface-expressed mutant Δsig-TNFRSF1A compared with WT is most probably caused by conformational defects that inhibit effective interactions with the ligand. This could be because the mutations in CRD1, which is also the preligand assembly domain, prevent the receptor trimerisation required for TNF binding and/or result in conformational defects in the TNF-binding site in CRD2 and CRD3. This is the subject of further investigation. More generally, misfolding of mutant TNFRSF1A may have functional consequences relevant to the pathophysiology of TRAPS. Misfolding of mutant proteins can result in either gain- or loss-of-function in different protein conformational disorders, including periodic fever syndromes. In the recessive autoinflammatory disorder FMF, there is strong evidence that loss-of-function mutations in pyrin/marenostrin result in loss of its activitiy as a negative regulator of interleukin-1 (IL-1) activation.43 Also, in HIDS, the activity of mutated mevalonate kinase is particularly temperature sensitive, with loss of function associated with increasing temperature and decreased production of anti-inflammatory isoprenoids.44 Thus, in both FMF and HIDS, the loss of function of proteins with anti-inflammatory activities appears to facilitate autoinflammation. A similar scenario in TRAPS seems unlikely given the pro-inflammatory effects of TNFRSF1A and the observations that TNFRSF1A knockout mice have a greatly reduced capacity to generate inflammatory reactions.45,46 We hypothesize a gain-of-function pro-inflammatory consequence of the TNFRSF1A mutations: thus, TNFRSF1A aggregation and ligand-independent signalling may occur under certain circumstances, as is well known to occur for certain growth factor receptors in tumorigenesis.47 The nature of these circumstances remains to be elucidated and is being investigated. In the current study, we have presented evidence that TRAPS-related mutants of TNFRSF1A are conformationally abnormal, but retain signalling functions, although, in the experimental system used here, both mutant and WT TNFRSF1A showed spontaneous signalling, probably as a result of the overexpression of recombinant TNFRSF1A in the transfected cells. Not surprisingly, in view of our current findings, it was previously reported that the peripheral blood mononuclear cells (PBMCs) of patients with TRAPS were not hypersensitive to stimulation by TNF4 and, indeed, we have observed, with both PMBCs and dermal fibroblasts, that the cells of patients with TRAPS tend to actually produce less interleukin-6 (IL-6) than those of normal healthy controls when stimulated with TNF (M. Huggins et al., unpublished observations).
The proposed conformational abnormalities that prevent the interaction of mutant TNFRSF1A with TNF ligand may also prevent interaction with other proteins – for example, the ARTS-1 protein (aminopeptidase regulator of TNFR1 shedding), which is a membrane protein that binds to the extracellular region of TNFRSF1A and promotes shedding.48 Indeed, reduced interaction of mutant TNFRSF1A with ARTS-1 may explain the reduced shedding of TNFRSF1A in TRAPS to produce the TNF-inhibiting soluble receptor.4 On the other hand, our observations, that the mutants of TNFRSF1A show reduced surface expression in the HEK-293 transfectants, could appear to be at odds with the shedding hypothesis. It is possible that the mutant receptors are surface expressed in vivo as distinct from the transfectants. However, others have demonstrated that reduced levels of soluble TNFRSF1A can occur in periodic fever patients who do not have TNFRSF1A mutations,22 suggesting that TNFRSF1A mutations per se are not responsible for reduced shedding of the receptor. Furthermore, the therapeutic benefits in TRAPS of the TNF-binding agent, etanercept,5,49 could be caused by the general anti-inflammatory effects of TNF neutralization (as seen in other inflammatory disease such as rheumatoid arthritis) rather than because it specifically compensates for low levels of soluble TNFRSF1A.
The aim of the current study was to identify specific functional abnormalities of the TNFRSF1A monomer resulting from TRAPS-associated CRD1 mutations, as these have previously not been clearly defined. Thus, the transfections were performed with just the mutant forms of the receptor, and compared with transfectants expressing just the WT receptor, in order to define the functional abnormalities of the mutants, as demonstrated in the experiments described above. (Although the HEK-293 cells spontaneously express very low levels of TNFRSF1A,26 this would be insignificant relative to the amount of transfected receptor expressed.) As the TNFRSF1A mutations in TRAPS are autosomal dominant, patients' cells will express both WT and mutant receptors, presumably at similar levels (although this has not been formally demonstrated). Thus, future studies will involve co-transfections of WT and mutant TNFRSF1A to examine the effects of both being present.
It is of interest to note in the present study that the abnormalities of behaviour were less severe for the T50M mutant TNFRSF1A than the C33Y and C52F mutants. Thus, the T50M mutant receptor showed some surface expression (Figs 4b and 5) and was not totally devoid of TNF-binding capacity (Fig. 7a). This is understandable in structural terms, as the T50M mutation disrupts a non-covalent hydrogen bond, whereas the cysteine mutations remove covalent disulphide bridges. It also appears to relate to clinical severity as most cases of amyloidosis associated with TRAPS are in patients with cysteine, rather than non-cysteine, mutations.5
Acknowledgments
This work was supported by the The Jones 1986 Charitable Trust. We are grateful to Dr Susan Anderson and Mr Ian Ward for assistance with the confocal microscopy.
Abbreviations
- ALLN
acetyl-leucyl-leucyl-norleucinal
- CBA
cytometric bead array
- CRD
cysteine-rich domain
- DD
death domain
- DMSO
dimethyl sulphoxide
- Δsig
lacking the cytoplasmic signalling region
- ER
endoplasmic reticulum
- FMF
familial Mediterranean fever
- HEK
human embryonic kidney
- HIDS
hyperimmunoglobulinaemia D syndrome
- IL
interleukin
- mAb
monoclonal antibody
- MDC
monodansylcadaverine
- PBA
phosphate-buffered saline containing azide
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PLAD
preligand assembly domain
- SODD
silencer of death domain
- TNF
tumour necrosis factor
- TNFRSF
TNF receptor superfamily
- TRADD
TNF-receptor-associated death domain
- TRAPS
TNF receptor-associated periodic syndrome, WT, wild type
- Z-VAD.fmk
Z-Val-Ala-Asp-CH2F
References
- 1.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–25. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 2.Williamson LM, Hull D, Mehta R, Reeves WG, Robinson BH, Toghill PJ. Familial Hibernian Fever. Quart J Med. 1982;51:469–80. [PubMed] [Google Scholar]
- 3.McDermott EM, Smillie DM, Powell RJ. Clinical spectrum of Familial Hibernian Fever: a 14-year follow-up study of the index case and extended family. Mayo Clin Proc. 1997;72:806–17. doi: 10.4065/72.9.806. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFRSF1A, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–44. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 5.Hull KM, Drewe E, Aksentijevich I, Dean IJ, Singh H, Powell RJ, Kastner DL. TNF receptor-associated periodic syndrome: emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81:349–68. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 6.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signalling. 2002;14:477–92. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 7.Chan FKM, Chun HJ, Zheng L, Siegel R, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand independent receptor assembly and signalling. Science. 2000;288:2351–4. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 8.Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55kD TNF receptor-TNFβ complex: implications for TNF receptor activation. Cell. 1993;73:431–55. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 9.Aksentijevich I, Galon J, Soares M, et al. The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype–phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. Am J Hum Genet. 2001;69:301–14. doi: 10.1086/321976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dode C, Papo T, Fieschi C, et al. A novel missense mutation (C30S) in the gene encoding tumor necrosis factor receptor 1 linked to autosomal-dominant recurrent fever with localized myositis in a french family. Arthitis Rheum. 2000;43:1535–42. doi: 10.1002/1529-0131(200007)43:7<1535::AID-ANR18>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Jadoul M, Dode C, Cosyns J-P, Abramowicz D, Georges B, Delpech M, Pirson Y. Autosomal-dominant periodic fever with AA amyloidosis: novel mutation in tumor necrosis factor receptor 1 gene. Kidney Int. 2001;59:1677–82. doi: 10.1046/j.1523-1755.2001.0590051677.x. [DOI] [PubMed] [Google Scholar]
- 12.Simon A, Dode C, van der Meer JWM, Drenth JPH. Familial periodic fever and amyloidosis due to a new mutation in the TNFRSF1A gene. Am J Med. 2001;110:313–6. doi: 10.1016/s0002-9343(00)00716-6. [DOI] [PubMed] [Google Scholar]
- 13.Simon A, van Deuren M, Tighe PJ, van der Meer JWM, Drenth JPH. Genetic analysis as a valuable key to diagnosis and treatment of periodic fever. Arch Intern Med. 2001;161:2491–3. doi: 10.1001/archinte.161.20.2491. [DOI] [PubMed] [Google Scholar]
- 14.Rosen Wolff A, Kreth HW, Hofmann S, et al. Periodic fever (TRAPS) caused by mutations in the TNF alpha receptor 1 (TNFRSF1A) gene of three German patients. Eur J Haematol. 2001;67:105–9. [PubMed] [Google Scholar]
- 15.Aganna E, Zeharia A, Hitman GA, et al. An Israeli Arab patient with a de novo TNFRSF1A mutation causing tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2002;46:245–9. doi: 10.1002/1529-0131(200201)46:1<245::AID-ART10038>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Nevala H, Karenko L, Stjernberg S, et al. A novel mutation in the third extracellular domain of the tumor necrosis factor receptor 1 in a Finnish family with autosomal-dominant recurrent fever. Arthritis Rheum. 2002;46:1061–6. doi: 10.1002/art.10224. [DOI] [PubMed] [Google Scholar]
- 17.Dode C, Andre M, Bienvenu T, et al. The enlarging clinical, genetic, and population spectrum of tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2002;46:2181–8. doi: 10.1002/art.10429. [DOI] [PubMed] [Google Scholar]
- 18.Dode C, Hazenberg BP, Pecheux C, et al. Mutational spectrum in the MEFV and TNFRSF1A genes in patients suffering from AA amyloidosis and recurrent inflammatory attacks. Nephrol Dial Transplant. 2002;17:1212–7. doi: 10.1093/ndt/17.7.1212. [DOI] [PubMed] [Google Scholar]
- 19.Stjernberg S, Pettersson T, Karenko L, Pitkanen S, Peterson P, Ranki A. A novel mutation in exon 3 of the TNFRSF1A gene in a Finnish family with TRAPS. Clin Exp Rheumatol. 2002;20(Suppl. 26):S–72. [Google Scholar]
- 20.Frenkel J, Ploos van Amstel JK, Smith B, Lasham C, Gaiser N, Rijkers GT. Non-cysteine TNFRSF1A mutation in Dutch family with periodic fever. Clin Exp Rheumatol. 2002;20(Suppl. 26):S–73. [Google Scholar]
- 21.Obici L, Palladini G, Marciano S, Massa M, Zoppo M, Merlini G. Molecular characterisation of tumor necrosis factor receptor associated periodic syndrome (TRAPS) in Italy: identification of novel and recurrent mutations and evidence for a high frequency of the R92Q allele in the Italian population. Clin Exp Rheumatol. 2002;20(Suppl. 26):S–76. [Google Scholar]
- 22.Aganna E, Hammond L, Hawkins PN, et al. Heterogeneity among patients with tumor necrosis factor receptor-associated periodic syndrome phenotypes. Arthritis Rheum. 2003;48:2632–44. doi: 10.1002/art.11215. [DOI] [PubMed] [Google Scholar]
- 23.Kriegel MA, Huffmeier U, Scherb E, Scheidig C, Geiler T, Kalden JR, Reis A, Lorenz H-M. Tumour necrosis factor receptor-associated periodic syndrome characterised by a mutation affecting the cleavage site of the receptor: implications for pathogenesis. Arthritis Rheum. 2003;48:2386–8. doi: 10.1002/art.11169. [DOI] [PubMed] [Google Scholar]
- 24.Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–9. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 25.Carrell RW. Lomas DA. Alpha1-antitrypsin deficiency – a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 26.McFarlane SM, Pashmi G, Connell MC, Littlejohn AF, Tucker SJ, Vandenabeele P, MacEwan DJ. Differential activation of nuclear factor-kB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 2002;515:119–26. doi: 10.1016/s0014-5793(02)02450-x. [DOI] [PubMed] [Google Scholar]
- 27.Waterhouse NJ, Finucane DM, Green DR, et al. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 1998;5:1051–61. doi: 10.1038/sj.cdd.4400425. [DOI] [PubMed] [Google Scholar]
- 28.Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self association of the ‘Death Domains’ of the p55 tumour necrosis factor (TNF) receptor and Fas/APO1 prompts signalling for TNF and Fas/Apo1 effects. J Biol Chem. 1995;270:387–91. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 29.Gaeta ML, Johnson DR, Kluger MS, Pober JS. The death domain of tumor necrosis factor receptor 1 is necessary but not sufficient for Golgi retention of the receptor and mediates receptor desensitization. Lab Invest. 2000;80:1185–94. doi: 10.1038/labinvest.3780126. [DOI] [PubMed] [Google Scholar]
- 30.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol. 1999;146:1239–54. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teckman JH, Perlmutter DH. Retention of mutant α1-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G961–74. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- 33.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Human Mol Genet. 2002;11:1107–17. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 34.Chan FK-M, Siegel RM, Lenardo MJ. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–22. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 35.Bradley JR, Thiru S, Pober JS. Disparate localization of 55-kd and 75-kd tumor necrosis factor receptors in human endothelial cells. Am J Pathol. 1995;146:27–32. [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SR, Ledgerwood EC, Prins JB, Galbraith J, Johnson DR, Pober JS, Bradley JR. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J Immunol. 1999;162:1042–8. [PubMed] [Google Scholar]
- 37.Storey H, Stewart A, Vandenabeele P, Luzio JP. The p55 tumour necrosis factor receptor TNFR1 contains a trans-Golgi network localization signal in the C-terminal region of its cytoplasmic tail. Biochem J. 2002;366:15–22. doi: 10.1042/BJ20020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders CR, Nagy JK. Misfolding of membrane proteins in health and disease: the lady or the tiger? Curr Opin Struct Biol. 2000;10:438–42. doi: 10.1016/s0959-440x(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Al-Lamki RS, Zhang H, Kirkiles-Smith N, Gaeta ML, Thiru S, Pober JS, Bradley JR. Histamine antagonizes tumor necrosis factor (TNF) signalling by stimulating TNF receptor shedding from the cell surface and Golgi storage pool. J Biol Chem. 2003;278:21751–60. doi: 10.1074/jbc.M212662200. [DOI] [PubMed] [Google Scholar]
- 40.Lin L, Schmidt B, Teckman J, Perlmutter DH. A naturally occurring nonpolymerogenic mutant of α1-antitrypsin characterised by prolonged retention in the endoplasmic reticulum. J Biol Chem. 2001;276:33893–8. doi: 10.1074/jbc.M105226200. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen MM, Jensen ON, Holst HU, Hansen JJ, Corydon TJ, Bross P, Bolund L, Gregersen N. Grp78 is involved in retention of mutant low density lipoprotein receptor protein in the endoplasmic reticulum. J Biol Chem. 2000;275:33861–8. doi: 10.1074/jbc.M004663200. [DOI] [PubMed] [Google Scholar]
- 42.Kjaer S, Ibanez CF. Intrinsic susceptibility to misfolding of a hot-spot for Hirschsprung disease mutations in the ectodomain of RET. Hum Mol Genet. 2003;12:2133–44. doi: 10.1093/hmg/ddg227. [DOI] [PubMed] [Google Scholar]
- 43.Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 44.Houten SM, Frenkel J, Rijkers GT, Wanders RJA, Kuis W, Waterham HR. Temperature dependence of mutant mevalonate kinase activity as a pathogenic factor in hyper-IgD and periodic fever syndrome. Hum Mol Genet. 2002;11:3115–24. doi: 10.1093/hmg/11.25.3115. [DOI] [PubMed] [Google Scholar]
- 45.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55kD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–67. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 46.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–52. [PubMed] [Google Scholar]
- 47.Penuel E, Akita RW, Sliwkowski MX. Identification of a region within the ErbB2/HER2 intracellular domain that is necessary for ligand-independent association. J Biol Chem. 2002;277:28468–73. doi: 10.1074/jbc.M202510200. [DOI] [PubMed] [Google Scholar]
- 48.Cui X, Hawari F, Alsaaty S, et al. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest. 2002;110:515–26. doi: 10.1172/JCI13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drewe E, McDermott EM, Powell PT, Isaacs JD, Powell RJ. Prospective study of anti-tumour necrosis factor receptor superfamily 1B fusion protein, and case study of anti-tumour necrosis factor receptor superfamily 1A fusion protein, in umour necrosis factor receptor associated periodic syndrome (TRAPS): clinical and laboratory findings in a series of seven patients. Rheumatology. 2003;42:235–9. doi: 10.1093/rheumatology/keg070. [DOI] [PubMed] [Google Scholar]