Abstract
Plasmid DNA continues to attract interest as a potential vaccine-delivery vehicle. However, the mechanisms whereby immune responses are elicited by plasmids are not fully understood. Although there have been suggestions regarding the importance of CpG motifs in plasmid immunogenicity, the molecular mechanisms by which CpG motifs enhance immune responses to DNA vaccines are not well understood. As Toll-like receptor 9-deficient (TLR9−/−) mice fail to respond to the adjuvant effects of CpG oligonucleotides, we used these mice to determine the effect of CpG motifs in plasmids used for DNA immunization. In the study described below, we report that DNA immunization was as effective in eliciting antigen-specific antibody and at stimulating antigen-specific interferon-γ (IFN-γ)-secreting cells in TLR9−/− mice as in TLR9+/+ mice. This study illustrates that DNA vaccines elicit immune responses by multiple mechanisms and demonstrates that TLR9 is not essential for the induction of immune responses following DNA immunization.
Keywords: CpG, DNA vaccine, gene expression, TLR9
Introduction
DNA vaccines continue to attract a great deal of interest in both academia and industry. However, although DNA vaccines have been shown to be effective in numerous mouse models,1,2 the results in humans3,4 and large animals5 have not been as encouraging. The need to improve immune responses to DNA vaccines has caused researchers to investigate protocols to increase the potency of DNA vaccines. There are several ways to increase the immune responses elicited by DNA immunization, such as using delivery methods for increasing gene expression,6,7 adding costimulatory molecules;8 and modifying plasmids, including altering promoters, altering codon biases9 and adding additional CpG motifs to the plasmid.10
It has been hypothesized, by several research groups, that CpG motifs found in plasmids, in particular motifs located in the β-lactamase gene, might be responsible for the T helper 1 (Th1) type responses induced by DNA vaccines. Indeed, it has been suggested that without CpG motifs, DNA vaccines would not work.11,12
Previously, the only way to characterize the effects of CpG motifs on DNA vaccination was to add or remove CpG motifs from the plasmid. However, the results of these studies could not be clearly interpreted as it is impossible to create a plasmid without any immunostimulatory CpG motifs or immunoinhibitory motifs.13 Several studies, where additional CpG motifs were inserted into plasmids, have been conducted in independent laboratories. Some experiments showed that the addition of a CpG motif(s) enhanced the immune responses to some DNA vaccines,14,15 but other investigators reported no significant difference in immune responses elicited by the addition of CpG motifs.16,17 The mixed results of these studies raise questions about the importance of CpG motifs in plasmid vectors. Understanding the importance of CpG motifs in DNA vaccines may be critical to advance the use of DNA vaccines in the field.
Toll-like receptor 9 (TLR9) is considered to be the CpG receptor, as only cells that express TLR9 are activated directly by CpG oligonucleotide (ODNs), and transfection of the TLR9 gene allows cells, previously unresponsive to CpG ODNs, to respond to CpG.18 In addition, TLR9–/– mice do not respond to the adjuvant effects of CpG ODNs.19 These mice offer a unique system for investigating the effect of CpG motifs during DNA immunization. The purpose of this study was to determine whether immune responses to a DNA vaccine would be quantitatively or qualitatively altered in TLR9–/– mice. In the present study, we demonstrate that TLR9–/– mice elicit both antibody and cellular immune responses to DNA vaccines that are similar in magnitude and type to those induced by wild-type mice.
Materials and methods
Mice
TLR9–/– mice were generated by Hemmi et al.,19 by disrupting the TLR9 gene with homologous recombination, and bred on a C57Bl/6 genetic background. Breeding pairs of TLR9–/– mice, developed by Hemmi et al., were obtained as a gift from Dr Heather Davis (Coley Pharmaceutical Group, Ottawa, ON, Canada). TLR9–/– mice were bred at the Vaccine and Infectious Disease Organization (Saskatoon, SK, Canada). C57Bl/6 mice were purchased from Charles River (Montreal, QC, Canada). Animals were treated in compliance with the regulations of the Canadian Council for Animal Care.
Genotyping of TLR9-deficient mice
To confirm that the TLR9–/– mice were truly deficient, total cellular DNA was extracted from the tail tissues of mice using a Dneasy™ Tissue kit, according to the manufacturer's instructions (Qiagen Inc., Mississauga, ON, Canada). Cellular DNA extracted from 1·2 cm (tip section) of rodent tail was stored in 200 µl of the supplied storage buffer at −20° until further use.
The TLR9 mutated allele was polymerase chain reaction (PCR) amplified using a primer specific for the TLR9 gene downstream of the targeting construct (5′-GCAATGGAAAGGACTGTCCACTTTGTG-3′) and a primer specific for the neomycin-resistant gene used for the targeting construct (5′-ATCGCCTTCTATCGCCTTCTTGACGAG-3′). The resultant 1·2-kbp fragment was PCR amplified using Advantage® 2 DNA polymerase mix (BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer's instructions. The PCR reaction volumes of 25 µl contained 25 pmoles each primer, 0·2 mm dNTP mix and 1 µl of the DNA template. PCR amplification was conducted by an initial denaturation at 94° for 1 min followed by 35 cycles of denaturation at 94° for 30 seconds, annealing at 67° for 45 seconds, and extension for 1 min at 72°, with a final 10-min extension at 72°. TLR9 gene-specific primers were used as controls to PCR amplify the wild-type TLR9 allele using the above-mentioned protocol. The TLR9 gene-specific primers used were a primer specific for the TLR9 gene downstream of the targeting construct (5′-GCAATGGAAAGGACTGTCCACTTTGTG-3′) and a TLR9-specific ‘wild-type’ primer (5′-GAAGGTTCTGGGCTCAATGGTCATGTG-3′).
Plasmids
The plasmid vector, pCAN1, expressing the full-length glycoprotein D (gD) protein from bovine herpesvirus type 1 (BHV-1) under the cytomegalovirus (CMV) promoter pCAN-gD (pgD), was used in the immunization experiments.20 This plasmid contains CpG motifs in the ampicillin resistance gene of the vector. The firefly luciferase gene was amplified by PCR using the following primers (5′-AAT-GGAAGACGCCAAAAC-3′) and (5′-AT-TTTACAATTTGGACTTTCCG-3′) from pMAS-luc.13 The vectors pMASIA, pBISIA24, pBISIA40 and pBISIA88,21 containing 24, 40 and 88 additional tandem CpG motifs, respectively, were cut with SmaI and the luciferase gene was blunt-end ligated into the vectors to create pMASIA-luc, pBISIA24-luc, pBISIA40-luc and pBISIA88-luc. Plasmids were purified using endotoxin-free plasmid kits from Qiagen.
Immunization
Male TLR9–/– and TLR9+/+ C57B mice were immunized by intradermal injection in the back with 0·1, 1 or 10 µg of pCANgD in 10 µl of phosphate-buffered saline (PBS) or with 1 µg of gD protein and 10 µg of CpG ODN (1826). Mice were immunized on day 1 and received a second immunization 4 weeks following the primary immunization. Naïve, unvaccinated mice were used as negative controls.
Characterization of immune responses
BHV-1 gD-specific antibody responses were determined using an enzyme-linked immunosorbent assay (ELISA), as previously described, on sera 2 weeks after the final immunization.22 Briefly, Immunlon 2 (Dynex, Chantilly, VA, Canada) ELISA plates were coated with a truncated version of gD (tgD)22 (1 µg/ml) overnight at 4°. Glycoprotein D-specific immunoglobulin G (IgG)1 and IgG2a levels in serum were determined by using biotinylated goat anti-mouse immunoglobulin specific to IgG1 and IgG2a subclasses (Caltag, Toronto, ON, Canada) followed by staining with streptavidin-conjugated alkaline phosphatase (Jackson Immuno-research Laboratories, West Grove, PA). The alkaline phosphatase activity was determined by p-nitrophenol phosphate (PNPP) (Sigma, St Louis, MO). The absorbance was read after incubation for 15–20 min at 405 nm (Bio-Rad, Mississauga, ON, Canada). Glycoprotein D-specific titres were calculated as the inverse of the serum dilution that gave an absorbance (A) value two standard deviations higher than the values for serum from naive mice.
BHV-1-specific neutralizing antibody titres in serum were determined as previously described.23 The titres were expressed as the reciprocal of the highest dilution of serum that caused a 50% reduction in viral plaques relative to the virus control.
Enzyme-linked immunosorbent spot-forming cell (ELISPOT) assays for interferon-γ (IFN-γ) and interleukin (IL)-4 were performed, as previously described, using cells isolated from individual mouse spleens 2 weeks after the final immunization.24 Briefly, 1 × 106 cells/well were placed in 96-well culture plates, with or without tgD (1 µg/ml), in AIM-V media (Gibco, Life Technologies, Burlington, ON, Canada) and incubated at 37°, in an atmosphere of 5% CO2, for 24 hr. Cells were resuspended in fresh media and seeded onto nitrocellulose plates (Millipore, Nepean, ON, Canada) coated with either murine IFN-γ- or IL-4-specific capture antibody (2 µg/ml) (PharMingen, San Diego, CA). Biotinylated anti-mouse IFN-γ- or IL-4-specific antibodies (PharMingen) were added at 2 µg/ml to each well followed by streptavidin-alkaline phosphatase (Jackson Immuno-research Laboratories). The plates were developed using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) substrate tablets (Sigma). Spots representing gD-specific cytokine-secreting cells were counted and expressed as the number of cytokine-secreting cells per 1 × 106 cells. Concanavalin A (Con A)-stimulated cells were used as a positive control.
Luciferase assay
Mice were injected intradermally with 10 µg of the luciferase-encoding plasmids containing different numbers of CpG motifs (pMASIA-luc, pBISIA24-luc, pBISIA40-luc, and pBISIA88-luc). Forty-eight hours later, the skin injection sites (≈ 1 cm2 area) were excised and homogenized in 500 µl of lysis buffer (Promega, Madison, WI), using a Polytron homogenizer (Brinkmann Instruments, Rexdale, ON), to produce protein extracts. Luciferase activity in the protein extracts was determined using Promega's luciferase assay system. Samples were counted for 30 seconds on a Packard Picolite Luminometer (Packard Instruments Canada Ltd, Mississauga, ON, Canada). Tissues from untreated animals were used to determine the background level of light units (LUs).
Statistics
Differences in immune responses among vaccine groups were analysed by Prism Graphpad statistical software (GraphPad Software, Inc., San Diego, CA), using a one way analysis of variance (anova) followed by Tukey's multiple comparison test and t-test for antibody responses, and the Kruskal–Wallis one-way anova followed by Dunn's multiple comparison test for cellular responses.
Results
Genotyping of TLR9-deficient mice
Prior to conducting immunization studies, it was important to confirm that TLR9–/– mice did not express TLR9 and that no accidental introduction of the TLR9 gene had occurred between the generation of the mice and their subsequent use. The expected 1·2-kbp fragment was PCR amplified (using the neomycin-specific primer and the primer specific for the TLR9 gene downstream of the targeting construct) from the two TLR9–/– breeding pairs, as well as from all offspring, thus showing the presence of the mutated allele. In addition, no fragment was amplified from any of these mice using the TLR9 gene-specific primers, confirming the absence of the wild-type allele. All TLR9–/– mice were confirmed, using PCR, to be TLR9 knockout. Indeed, the entire colony was tested and all mice were TLR9–/–. In contrast, all wild-type mice carried the TLR9 gene.
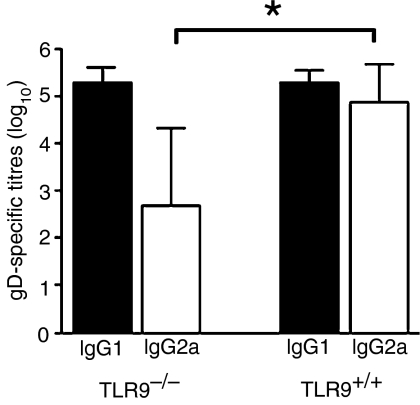
Furthermore, TLR9 has been shown to be important in shifting the immune response to a Th1-like response, with an increased prevalence of IgG2a.19Figure 1 clearly demonstrates that following immunization with gD protein and CpG, both TLR9+/+ and TLR9–/– mice generate similar gD-specific IgG1 titres. However, TLR9+/+ mice generated significantly higher gD-specific IgG2a responses compared with TLR9–/– mice. These results confirm the absence of functional TLR9 expression in TLR9–/– mice.
Figure 1.
Glycoprotein D (gD)-specific antibody isotypes in mice immunized with gD. Toll-like receptor 9 (TLR9)–/– and TLR+/+ mice were immunized with gD protein (1 µg) plus CpG 1826 (10 µg). Immunizations were performed on day 1 and 4 weeks later. Two weeks after the secondary immunization, mouse sera were assessed for gD-specific immunoglobulin G (IgG)1 and IgG2a by enzyme-linked immunosorbent assay (ELISA). n=5. *P < 0·05, Student's t-test.
TLR9−/− mice produce antibody responses following DNA immunization
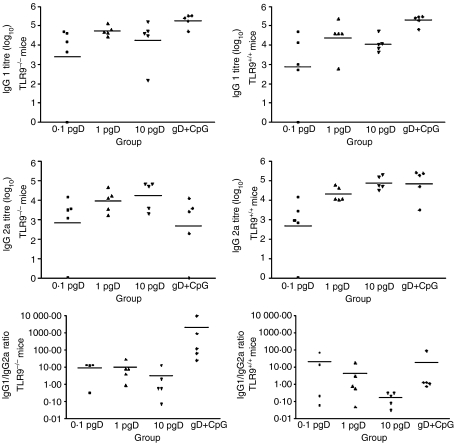
As CpG may be detected at suboptimal levels of plasmid administration, a dose–response experiment was performed using 0·1, 1 and 10 µg of pgD. No significant differences were observed in gD-specific antibody responses between TLR9–/– mice and TLR9+/+ mice at any of the plasmid doses used (Fig. 2). There appeared to be a similar dose-dependent response to pgD in both TLR9–/– and TLR9+/+ mice, but there were no significant differences in titres among the doses. Furthermore, the class of gD-specific antibody (IgG1 and IgG2a) was similar in both TLR9–/– and TLR+/+ mice at all vaccine doses. Unvaccinated control mice were negative for gD-specific antibody (titre < 1), demonstrating that the responses were antigen specific (data not shown).
Figure 2.
Glycoprotein D (gD)-specific antibody isotypes in mice immunized with pgD (plasmid vector pCAN1 expressing the full-length glycoprotein D protein from bovine herpesvirus type 1 under the cytomegalovirus promoter). Toll-like receptor 9 (TLR9)–/– and TLR+/+ mice were immunized with pgD (at doses of 0·1 µg, 1 µg or 10 µg) or gD protein (1 µg) plus CpG 1826 (10 µg). Primary immunization was on day 1 and the secondary immunization was 4 weeks later. Two weeks after the secondary immunization, mouse sera were assessed for gD-specific immunoglobulin G (IgG)1 and IgG2a titres by enzyme-linked immunosorbent assay (ELISA). Data presented are values for individual mice and the bar represents the group mean (n = 5). P < 0·001: TLR9–/– and TLR+/+ mice versus control for both IgG1 and IgG2a by one-way analysis of variance (anova) followed by Tukey's multiple comparison test. P < 0·05: IgG2a titre. P < 0·01: IgG1/IgG2a ratio by t-test comparing gD protein with CpG oligonucleotide (ODN)-immunized TLR9–/– mice versus gD protein with CpG ODN-immunized TLR9+/+ mice. Unvaccinated control mice remained negative (titre < 1). There were no significant differences in antibody titres between TLR9–/– and TLR+/+ mice.
Studies using TLR9–/– and TLR9+/+ mice immunized with gD protein and CpG as a positive control confirmed that TLR9–/– mice did not respond to CpG ODNs (Fig. 2). TLR9–/– mice immunized with gD protein and CpG ODN had significantly lower gD-specific IgG2a titres, and thus a significantly higher IgG1/IgG2a ratio, than TLR9+/+ mice. These results confirm the absence of functional TLR9 expression in TLR9–/– mice (see also Fig. 1).
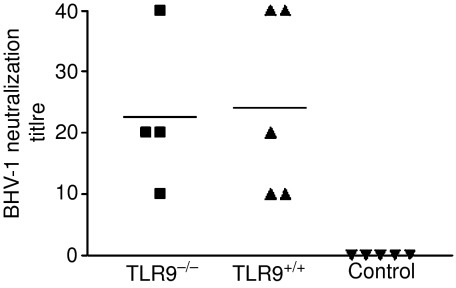
To further test whether the antibody responses were biologically identical, antibodies were assessed for their ability to neutralize BHV-1 (Fig. 3). As expected, no BHV-1 neutralization activity was detected in the sera of naive mice. In contrast, sera from both TLR9–/– mice and TLR9+/+ mice had BHV-1 neutralization titres of similar magnitude. There was no statistical difference between antibody responses induced in TLR9–/– mice and TLR9+/+ mice.
Figure 3.
Bovine herpesvirus type 1 (BHV-1) neutralization titre of sera from Toll-like receptor 9 (TLR9)–/– and TLR9+/+ mice immunized with 10 µg of pgD (plasmid vector pCAN1 expressing the full-length glycoprotein D protein from BHV-1 under the cytomegalovirus promoter) on day 1 and 4 weeks later. Two weeks after the secondary immunization, mouse sera were assayed for BHV-1 neutralization. Data presented are values for individual mice and the bar represents the group mean. n = 4 for TLR9–/– mice and n = 5 for TLR9+/+ mice. TLR9–/– and TLR+/+ versus control, P < 0·05, by one-way analysis of variance (anova) followed by Tukey's multiple comparison test.
TLR9−/− mice produce cellular responses following DNA immunization
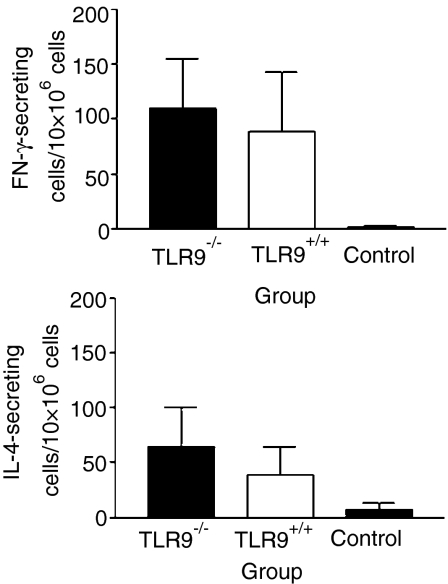
To determine the type of immune responses induced by DNA immunization of TLR9–/– mice, gD-specific lymphocytes were analysed for their ability to secrete IFN-γ and IL-4. Splenocytes from both TLR9–/– and TLR9+/+ mice, immunized with 10 µg of pgD, produced significantly higher numbers of IFN-γ- and IL-4-secreting cells when compared with naive mice, following in vitro stimulation with tgD (Fig. 4). Both TLR9–/– and TLR9+/+ mice produced approximately twice as many gD-specific IFN-γ-secreting cells than gD-specific IL-4-secreting cells, suggesting a bias towards a Th1-type response. There were no significant differences between TLR9–/– and TLR9+/+ mice in either the magnitude or type of cellular immune response elicited at each of the different doses of plasmid used for immunization (data not shown).
Figure 4.
Glycoprotein D-specific cytokine-secreting cells. Toll-like receptor 9 (TLR9)–/– and TLR+/+ mice were immunized with 10 µg of pgD (plasmid vector pCAN1 expressing the full-length glycoprotein D protein from bovine herpesvirus type 1 under the cytomegalovirus promoter) on day 1 and 4 weeks later. Two weeks after the secondary immunization, mouse splenocytes were assessed for the frequency of interferon-γ (IFN-γ)- and interleukin-4 (IL-4)-secreting cells. Error bars represent the standard deviation (SD). n = 4 for TLR9–/– mice and n = 5 for TLR+/+ mice. IFN-γ-secreting cells of TLR9–/– and TLR+/+ mice versus control, P < 0·05. IL-4-secreting cells, TLR9–/– versus control, P < 0·01, using Kruskal–Wallis one-way analysis of variance (anova) and Dunn's multiple comparison test.
Effect of TLR9 on gene expression
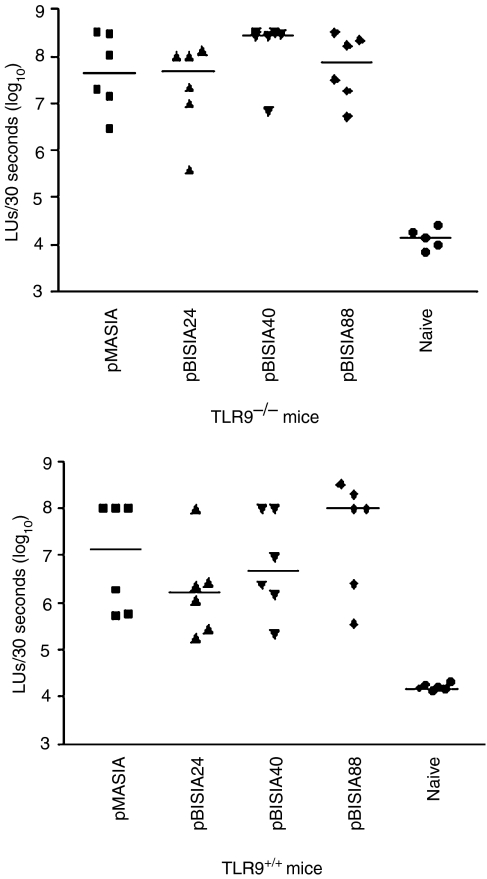
As there were no detectable differences in immune responses between TLR9–/– and TLR9+/+ mice following immunization with a gD-expressing plasmid, we further investigated whether the absence of TLR9 had an impact on the level of plasmid gene expression in vivo. Plasmids containing different numbers of CpG motifs were constructed and the luciferase gene was cloned in these plasmids. These plasmids were tested for expression in vitro by transfection of COS7 cells. All plasmids, regardless of the number of CpG motifs, expressed similar levels of luciferase following transfection in vitro (data not shown). It is possible that CpG motifs in plasmids are able to influence gene expression in vivo by stimulating TLR9, leading to changes in gene expression, and that this is influenced by the number of CpG motifs present in the plasmid. To test this, plasmids were injected intradermally into mice to determine whether CpG motifs influenced the level of gene expression in vivo. Figure 5 demonstrates that the level of luciferase expression in TLR9–/– and TLR+/+ mice was similar and that there was no significant difference in expression between plasmids with different numbers of CpG motifs. Thus, our results indicate that TLR9 is not critical for either efficiency of transfection or for the induction of immunity by DNA vaccines.
Figure 5.
Luciferase gene expression in skin following the administration of plasmids encoding luciferase in Toll-like receptor 9 (TLR9)–/– and TLR+/+ mice. Plasmids encoding luciferase and containing different numbers of CpG motifs were administered intradermally and, 2 days later, skin samples were assessed for expression of the luciferase gene. Data represent luciferase expression for injection sites of individual mice and the bar represents the median. All plasmids induced significant gene expression versus naive tissue (P < 0·05), as determined using one-way analysis of variance (anova) followed by Tukey's multiple comparison test. There were no significant differences among the different plasmids or between TLR9–/– and TLR+/+ mice. LUs, light units.
Discussion
The mechanisms whereby DNA vaccines induce immune responses are not completely understood. Previously, it was hypothesized that CpG motifs contribute significantly to the induction of immune responses to DNA vaccines.10,12 This was a reasonable hypothesis because CpG-containing ODNs are potent adjuvants that elicit Th1-type responses to protein antigens in various species.25–27
As DNA vaccines induce immune responses without a conventional adjuvant, it is possible that CpG motifs in plasmids might act as pattern-recognition molecules and thereby function as adjuvants for DNA vaccines. With the discovery that TLR9 is the pattern-recognition receptor for CpG19 and the recent development of TLR9 knockout mice, which fail to respond to CpG (as measured by proliferation of splenocytes, production of pro-inflammatory cytokines, and the ability of CpG-containing ODNs to act as a Th1 adjuvant in vivo),19 it was possible to directly test this theory. The intradermal route was chosen because it elicits Th1 responses to DNA vaccines, allowing the effects of TLR9 to be determined on the induction of immunity. The immune assays in the present investigation support the conclusion that TLR9 does not contribute significantly to the induction of humoral and cellular immune responses to DNA vaccines. As gD-specific T-cell responses were essentially identical between TLR9–/– and TLR9+/+ mice, it is not surprising that gD-specific antibody responses were also essentially identical between TLR9–/– and TLR9+/+ mice. Our data build on the recent observation that DNA immunization elicits equivalent cytotoxic CD8+ responses in TLR9–/– and TLR9+/+ mice.18
Currently, there are 10 TLRs known to recognize various microbial components, leading to the stimulation of innate immunity, with TLR9 being identified as the receptor that can recognize bacterial CpG motifs.19 Other unidentified TLRs that recognize DNA are probably not involved in bypassing the need for TLR9, as it was recently shown that plasmids can activate dendritic cells exclusively via TLR9.28 Alternatively, other non-TLRs, such as scavenger receptors, may also play a role in DNA uptake and recognition, and may influence the immunogenicity of DNA vaccines. This would not be a surprise in view of the redundancy of the immune system, with numerous receptors and cytokines being able to compensate for a loss or reduction in the expression of certain receptors in vivo. Our present results support the conclusion that induction of an immune response by DNA vaccines is not significantly influenced by the recognition of CpG motifs by TLR9, and additional CpG motifs in the plasmid did not alter gene expression following plasmid DNA administration. Therefore, the mechanism by which increasing the number of CpG motifs in plasmids enhanced immune responses elicited by DNA vaccines14,15 must be reconsidered.
It is possible that DNA vaccines might work through mechanisms other than the TLR9 receptor. These mechanisms could involve other danger signals, resulting from cell transfection and altered gene regulation in the cell. This possibility has not been investigated in much detail to date, but may be an important mechanism regarding how DNA vaccines elicit immune responses without a conventional adjuvant. The availability of various gene knockout mice, and a better definition of the plasmid backbone, should provide a better understanding of how DNA vaccines work in vivo.
Acknowledgments
We thank the animal care unit at VIDO for the care and handling of the animals, as well as Valaria Alcon and Elaine Van Moorlehem for technical assistance. Financial support was provided by the Canadian Institute for Health Research.
Abbreviations
- BHV-1
bovine herpesvirus type 1
- CMV
cytomegalovirus
- gD
glycoprotein D
- ODN
oligonucleotide
- PCR
polymerase chain reaction
- Th1
T helper 1
- TLR9
Toll-like receptor 9
References
- 1.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 2.Manickan E, Yu Z, Rouse RJ, Wire WS, Rouse BT. Induction of protective immunity against herpes simplex virus with DNA encoding the immediate early protein, ICP 27. Viral Immunol. 1995;8:53–61. doi: 10.1089/vim.1995.8.53. [DOI] [PubMed] [Google Scholar]
- 3.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 4.Ugen KE, Nyland SB, Boyer JD, et al. DNA vaccination with HIV-1 expressing constructs elicits immune responses in humans. Vaccine. 1998;16:1818–21. doi: 10.1016/s0264-410x(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 5.van Drunen Littel-van den Hurk S, Gerdts V, Loehr BI, Pontarollo R, Rankin R, Uwiera R, Babiuk LA. Recent advances in the use of DNA vaccines for the treatment of diseases of farmed animals. Adv Drug Deliv Rev. 2000;43:13–28. doi: 10.1016/s0169-409x(00)00074-0. [DOI] [PubMed] [Google Scholar]
- 6.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 7.Widera G, Austin M, Rabussay D, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–40. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 8.Kim JJ, Nottingham LK, Wilson DM, et al. Engineering DNA vaccines via co-delivery of co-stimulatory molecule genes. Vaccine. 1998;16:1828–35. doi: 10.1016/s0264-410x(98)00177-7. [DOI] [PubMed] [Google Scholar]
- 9.Vinner L, Nielsen HV, Bryder K, Corbet S, Nielsen C, Fomsgaard A. Gene gun DNA vaccination with Rev-independent synthetic HIV-1 gp160 envelope gene using mammalian codons. Vaccine. 1999;17:2166–75. doi: 10.1016/s0264-410x(98)00474-5. [DOI] [PubMed] [Google Scholar]
- 10.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–9. [PubMed] [Google Scholar]
- 11.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–4. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM, Yi AK, Schorr J, Davis HL. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 1998;6:23–7. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM, Wu T, Weeratna R, et al. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci USA. 1998;95:12631–6. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima Y, Xin KQ, Ooki T, et al. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002;20:2857–65. doi: 10.1016/s0264-410x(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Forns X, Gutierrez R, et al. DNA-based vaccination against hepatitis C virus (HCV): effect of expressing different forms of HCV E2 protein and use of CpG-optimized vectors in mice. Vaccine. 2002;20:3263–71. doi: 10.1016/s0264-410x(02)00304-3. [DOI] [PubMed] [Google Scholar]
- 16.Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R, Wolf H, Wagner R. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J Virol. 2001;75:10991–1001. doi: 10.1128/JVI.75.22.10991-11001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Hodge LM, Jones HP, Tabor L, Simecka JW. Co-expression of granulocyte-macrophage colony-stimulating factor with antigen enhances humoral and tumor immunity after DNA vaccination. Vaccine. 2002;20:1466–74. doi: 10.1016/s0264-410x(01)00476-5. [DOI] [PubMed] [Google Scholar]
- 18.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Uwiera RR, Rankin R, Adams GP, Pontarollo R, van Drunen Littel-van den Hurk S, Middleton DM, Babiuk LA, Griebel PJ. Effects of intradermally administered plasmid deoxyribonucleic acid on ovine popliteal lymph node morphology. Anat Rec. 2001;262:186–92. doi: 10.1002/1097-0185(20010201)262:2<186::AID-AR1024>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Pontarollo RA, Babiuk LA, Hecker R, Van Drunen Littel-Van Den Hurk S. Augmentation of cellular immune responses to bovine herpesvirus-1 glycoprotein D by vaccination with CpG-enhanced plasmid vectors. J Gen Virol. 2002;83:2973–81. doi: 10.1099/0022-1317-83-12-2973. [DOI] [PubMed] [Google Scholar]
- 22.Baca-Estrada ME, Snider M, Tikoo SK, Harland R, Babiuk LA, van Drunen Littel-van den Hurk S. Immunogenicity of bovine herpesvirus 1 glycoprotein D in mice: effect of antigen form on the induction of cellular and humoral immune responses. Viral Immunol. 1996;9:11–22. doi: 10.1089/vim.1996.9.11. [DOI] [PubMed] [Google Scholar]
- 23.van Drunen Littel-van den Hurk S, Gifford GA, Babiuk LA. Epitope specificity of the protective immune response induced by individual bovine herpesvirus-1 glycoproteins. Vaccine. 1990;8:358–68. doi: 10.1016/0264-410x(90)90095-4. [DOI] [PubMed] [Google Scholar]
- 24.Baca-Estrada ME, Foldvari M, Snider M, van Drunen Littel-van den Hurk S, Babiuk LA. Effect of IL-4 and IL-12 liposomal formulations on the induction of immune response to bovine herpesvirus type-1 glycoprotein D. Vaccine. 1997;15:1753–60. doi: 10.1016/s0264-410x(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 25.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ioannou XP, Griebel P, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. The immunogenicity and protective efficacy of bovine herpesvirus 1 glycoprotein D plus Emulsigen are increased by formulation with CpG oligodeoxynucleotides. J Virol. 2002;76:9002–10. doi: 10.1128/JVI.76.18.9002-9010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–7. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, Heit A, Wagner H. Vaccination with plasmid DNA activates dendritic cells via toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J Immunol. 2003;171:5908–12. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]