Abstract
Orthotopic liver transplants (OLT) performed in certain combinations of donor and recipient rat strains, such as DA (RT1a) to PVG (RT1c), without immunosuppressive drugs could completely overcome major histocompatibility complex barriers. Although other organs transplanted in a similar fashion within the same combination have been promptly rejected, 60 day post-OLT serum (POD 60) has been proven competent in rapidly reversing the established rejection in animal models. In order to understand the functional role of tolerogenic serum proteins and their involvement with immune response regulation, a comprehensive analysis surveying global changes in complex OLT systems by proteomic techniques was applied. The results display the varying protein expressions in sera extracted from naïve and transplanted animals on POD 60 with regard to immunosuppression. Among these proteins, haptoglobin (Hp) which is related to inhibition of T-cell proliferation was found to be up-regulated following OLT. In addition, the transcriptional expression level and intracellular localization of Hp correlated with the immune events. Hp also exhibited a strong in vitro immunosuppressive effect on the mixed lymphocyte reaction. In conclusion, the presence of Hp may play an important role in modulating the spontaneous tolerance of liver transplantation. Furthermore, the serum proteome map could provide guidance with respect to discovering potential protein targets in OLT tolerance and eventually prolong hepatic allograft survival in the future.
Keywords: proteomics, orthotopic liver transplantation, allograft tolerance, haptoglobin, mixed lymphocyte reaction
Introduction
It is a well-established maxim of organ transplantation that rejection of the allograft will occur unless donors' and recipients' major histocompatibility complex (MHC) are matched in human liver transplantation.1 In contrast, it has been shown that rat models of tolerance could be induced spontaneously in orthotopic liver transplantation (OLT) between different strains.2,3 In addition, OLT is accompanied promptly by a loss of the ability of the recipient to reject other allografts from the liver donor origin. Although there have been several possible mechanisms reported, the properties responsible for the tolerogenic phenomenon is not fully understood. According to previous clinical studies, some several factors from OLT animals mandate life-long administration of immunosuppression that is more potent than that achieved with immunosuppressive drugs.4–7
Understanding the basis of allograft tolerance in OLT is of great clinical relevance since the prevalence of immunosuppressive treatment-related morbidity and mortality may be reduced.8,9 However, the multifactor phenomena concerned with tolerogenic liver transplantation makes it complicated to elucidate the clear physio-mechanisms of the rat OLT model. Hence, adequate methods designed to deal with this complexity are performed for well-characterized molecular cascades involved in the OLT tolerance. In the present work, agents aimed at targeting serum proteins with powerful technologies are capable of monitoring simultaneous changes that occur in a cell at the DNA, RNA and protein levels.10 Functional proteomics provides a superior opportunity over other techniques to identify and analyse modified proteins that are involved in the multiple networks of the living cell and/or body fluid, as well as making an unbiased approach at uncovering new factors that are essential for regulation of cell function, especially in disease and for therapy.11
Functional proteomics is a technology that integrated two-dimensional polyacrylamide gel electrophoresis (2-DE), mass spectrometry and bioinformatics.12,13 The areas of interest extend to establishing a proteomic platform wherein the mysteries of spontaneous tolerance can be revealed and provide information about potential mechanisms of tolerance. In this study, serum proteins from the OLT rat models were dissected with proteomics; 26 protein spots were identified as changing in volume by using N-terminal sequencing and mass spectrometry and we were finally able to align the protein sequences with the database. To further confirm the advanced biological role of varied proteins in OLT tolerance, experiments were evaluated with reverse transcription–plymerase chain reaction (RT–PCR), immunohistochemistry and mixed lymphocyte reaction. The primary goal of this study was to describe the protein profile of rat serum before and after OLT regimens in the hope that it would allow for effective, drug-free acceptance of allografts.
The results indicate that haptoglobin (Hp) may be an important essence referent to the immunologically privilege in OLT model. This novel function of Hp not only facilitates solving the immunological puzzle but is also helpful in enhancing the survival rate after transplantation.
Materials and methods
Animals
Inbred strains of male rats, DA (RT1a) and PVG (RT1c) weighing 200–250 g, were housed at the SPF animal facility in the Chang Gung Memorial Hospital Kaohsiung, and allowed free access to water and standard rat chow.
Orthotopic liver transplantation (OLT)
OLT was performed under ether anaesthesia using Kamada's method, with a few modifications.14 A combination of DA to PVG rats were used as a rejection tolerant model without any immunosuppressive treatment.
Sample preparations
Blood samples (1 ml) were taken from each of the transplanted animals at various days after OLT treatment for a total of 60 days. Samples were also taken from PVG and OLT rats transplanted with syngenic combinations as controls. Serum was separated from the blood by centrifugation at 3000 g for 10 min and stored at −20° until use.
2-DE
Approximately 10 µl of plasma samples were solubilized in the rehydration buffer containing 8 m urea, 2% 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulyonate (CHAPS), 0·002% bromophenol blue, 2% IPG buffer pH 3–10 linear and 65 mm dithioerythreitol (DTE). The samples were then separated by 13 cm Immobiline DryStrip 3–10 linear on the IPGphor Isoelectric Focusing System (Amersham Bioscience, Uppsala, Sweden) in the first dimension. The running condition of the IEF was as follows: 30 V, 12 hr; 100 V, 1 hr; 250 V, 1 hr; 500 V, 0·5 hr; 1000 V, 0·5 hr; 4000 V, 0·5 hr; 6000 V, up to 18 kVh. Before 2-D sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), the IPG strips were then equilibrated for 15 min in a solution containing 50 mm Tris-HCl pH 8·8, 6 m urea, 2% SDS, 30% glycerol, 2% DTE and a trace of bromophenol blue, followed by 15 min further in the same solution that replaced DTE with 2·5% iodoacetamide. The IPG gel strips were embedded on top of the gels containing 0·5% agarose. 2-D SDS–PAGE was carried out on 10–20% acrylamide gradient gels (Hoefer SE600; Amersham Bioscience) at 24 mA/gel until the bromophenol blue dye front reached the bottom of the gel. After approximately 5 hr, all of the gels were visualized by a silver staining method (Amersham Bioscience) or left overnight with Coomassie Brilliant Blue (CBB) and then scanned using the Hewlett Packard Scan Jet 4100 C. Protein isoelectric point (pI) and molecular weight was assigned by pI calibration markers and molecular weight markers (Bio-Rad), respectively. Protein spots were quantified using the ImageMaster 2D Elite software (Amersham Bioscience). All experiments were repeated more than twice.
N-terminal amino acid sequencing and protein identity
The serum proteins (100 µl) separated on the SDS–PAGE gel was electroblotted onto a polyvinylidene difluoride (PVDF) membrane in a blotting buffer containing 10 mm CHAPS and 10% methanol for 3 hr at 1 mA/cm2 in the semidry electroblotter. The proteins on the PVDF membrane were stained with a 0·1% CBB solution in 50% aqueous methanol for 10 min, and distained with 40% methanol and 10% acetic acid. Selected protein spots were excised from PVDF membrane and submitted to the cartridge of a ABI Model 477A pulsed-liquid protein sequencer (Applied Biosystem, Foster City, CA) and the sequence of 6–12 amino acid residues from the N-terminus was carried out according to the sequencing manual. The amino acid sequence obtained was matched to a similar sequence on the conventional FASTA program from SWISS-PROT.15
Tryptic in-gel digestion of 2-D PAGE-resolved proteins
Protein bands or spots were excised from the polyacrylamide gel, washed twice with 100 µl of 50% acetonitrile/25 mm ammonium bicarbonate buffer pH 8·0 for 15 min, washed once with 100 µl of 100% acetonitrile and dried using a SpeedVac concentrator. The dried gel pieces were swollen in 10 µl of 25 mm ammonium bicarbonate containing 0·1 µg trypsin (Sigma, St Louis, MO). The gel pieces were then crashed with siliconized blue stick and incubated at 37° for at least 16 hr. Peptides were subsequently extracted with 50 µl of 50% acetonitrile/5% trifluoroacetic acid, and dried once again with the SpeedVac concentrator. The peptides or pellets were then resuspended in 20 µl of 0·1% trifluoroacetic acid and the suspended solutions were purified using Zip-Tips (Millipore, Bedford, MA) according to the manufacturer's instructions. The obtained peptides were stored at −20° until analysis.
MALDI-TOF mass spectrometry
A matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometer equipped with a nitrogen laser and operated in a reflectron model was used to identify proteins. These proteins (typically 1/10th) from non-separated tryptic digests were cocrystallized in a matrix of α-cyano-4-hydroxycinnamic acid and analysed using a TofSpec SE (Micromass, Manchester, UK) and Reflex/Biflex™ (Bruker Daltonics, Bremen, Germany). The measured monoisotopic masses of peptide were analysed using World Wide Web search programs such as Mascot, provided by European Molecular Biology Laboratory (EMBL) (http://www.matrixscience.com), with the National Center for Biotechnology Information database. Precursor ion gating was employed to selectively transmit an individual peptide and its metastable fragment ions to the reflectron for post-source delay (PSD) sequencing.16 The search was performed using a mass uncertainty of ± 1 MW and a molecular weight range of ±10% from the MW determined from 2-DE. The output consisted of a list of proteins ranked by statistical score.
Semiquantitative RT–PCR
Samples of livers were frozen quickly in liquid nitrogen and stored at −80°. Total RNA was subjected to reverse transcription polymerase reaction for interleukin-2 (IL-2) with the forward and reverse cytokine primers employed as previously described.17 In addition, a partial Hp cDNA was also prepared by RT–PCR.18 The following Hp-specific primers19 were used for PCR: 5′-GTGGAATTGGGCAATGATGCCACA-3′ (sense) and 5′-GTCACTGATCACTGTGGCC CCAGT-3′ (antisense). The condition consisted of denaturing at 94° for 1 min, annealing at 55–58° (55° for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) for 1 min, and extending at 72° for 1 min. PCR was performed at 30 cycles for cytokines and Hp, and 25 cycles for GAPDH. PCR products were separated by 2% agarose gel electrophoresis and stained with ethidium bromide. The target bands were analysed densitometrically by using a GS-700 Imaging Densitometer (Bio-Rad, Hercules, CA). All experiments were repeated more than twice.
Immunohistochemistry
The paraffin-embedded tissue blocks were sectioned in 2-µm slices and placed on slides coated with poly l-lysine. Following overnight incubation in a 60° oven, the slides were dewaxed, rinsed in phosphate-buffered saline (PBS) and then blocked for 5 min with 3% hydrogen peroxide to deprive the endogenous peroxidase activity. After antigen retrieval, Hp (1 : 100 dilution in PBS) was applied to the specimens and incubated for 30 min at room temperature. The sections were then washed with PBS and incubated with HRP/Fab Polymer Conjugate (PicTure™ Bulk Kit; Zymed, San Francisco, CA) for 30 min at room temperature in a humid chamber. After extensive washing, peroxidase substrate diaminobenzidine (Sigma) was added to the specimens and incubated for 5 min. Thereafter, the sections were counter-stained with Mayer's haematoxylin for 2 min, washed in running water for 5 min, dehydrated with serial ethyl alcohol and cleared with xylene. Finally, the slides were mounted with mounting medium and evaluated under the microscope.
Mixed lymphocyte reaction (MLR)
MLR assays were performed three times as previously described with minor modifications.20,21 Splenic lymphocytes from normal PVG rats and X-ray irradiated at 2000 rad. DA cells were used as responder and stimulators, respectively. Equal numbers (5 × 105 cells each) were calculated in a total volume of 200 µl per well in 96 wells for 3 days. Subsequently, 10 µl of Alarmar blue was added to each well and panel, and then incubated for an additional 3 hr. Panels were then read spectrophotometrically with absorbance at 570 nm and 600 nm. Recombinant protein concentrations of 0·5, 5, 50 µg/ml were added to the MLR on the first day of cultivation.
The inhibitory effect of Hp on splenic cells was calculated by the following formula: Cell proliferation index = [(OD570 − OD600) of test agent dilution]/[(OD570– OD600) of control].
Results
Displayed protein spots from sera samples of pre- and post-OLT operation
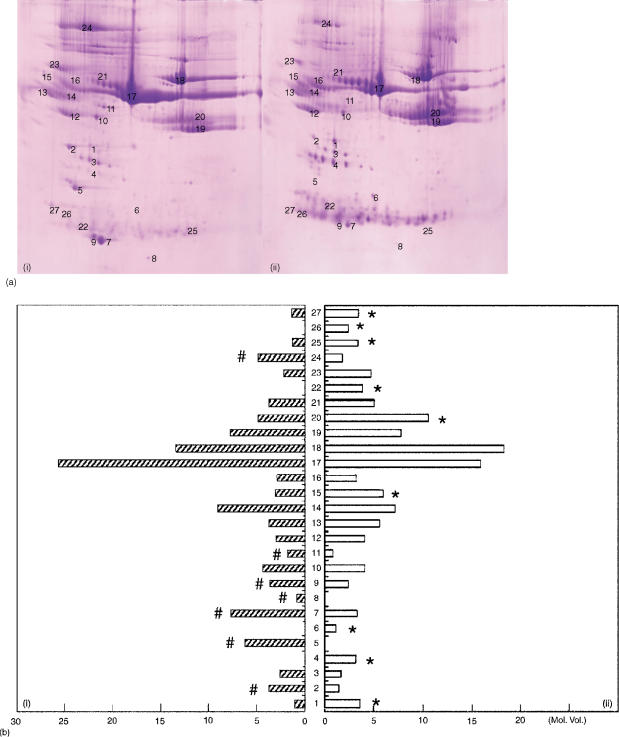
The mechanisms involved with the spontaneous tolerance of the rat model remain largely unclear. However, based on the fact that serum from the OLT drug-free tolerant rat could avoid the immune attack, we hypothesize that some seral protein may be exerted in the cascades of inducing tolerance. In order to evaluate this hypothesis, displayed protein spots were analysed from serum. 2-DE with immobilized pH gradients was applied to separate proteins solubilized in naïve and OLT sera at different periods after OLT. Because the strongest spontaneous tolerant response occurred on 60 day post-OLT serum (POD 60) (Fig. 1a), 2-DE maps were delineated from naïve (a) and POD60 (b) samples, respectively. By using the ImageMaster 2D Elite computerized program, a total of 114 common protein spots were counted for both naïve and POD 60 samples. Of note, 10 spots which appeared in naive samples were markedly reduced or absent in the POD 60 samples. Conversely, the other 16 spots were found in more abundant quantity for POD 60 but markedly decreased or absent in naïve serum samples. These 26 protein spots were quantified and outlined as a bar graph (Fig. 1b). Among these 26 spots, there were 15 spots which displayed significantly different intensities following Coomassie blue staining labelled with an asterisk in Fig. 1(b).
Figure 1.
(a) 2-DE of serum from female PVG rats (i, control; ii, 60 day after OLP treatment). Serum (8 µl) were focused on a linear 3–10 IPG, and then migrated at a right angle in SDS–PAGE on a 10–20% polyacrylamide gradient. The protein patterns were stained with CBB. (b) Changes in the levels of protein expression during different time course of liver transplantation. Each spot volume was determined and quantified from the intensity of spots derived from the sliver-stained 2-DE (ImageMaster 2D Elite software). #, high expression of protein in naive serum; *, high expression of protein in POD 60 serum.
Identification of sera proteins by sequence homology
In order to explore the protein factor of OLT tolerance, the 15 target protein spots were selected and identified with a combination of peptide mass fingerprinting (PMF) and N-terminal sequencing (Fig. 2a). These proteins were further characterized by performing a postsource decay analysis of the mass peptide fragments (Fig. 2b). The 15 protein spots were subsequently identified by this method and grouped into two categories according to volume expression before and after OLT. Group A, which contained ApoAIV (2) ApoE (5) ApoAI (7,9) VTDB(GC-globulin) (11), and albumin17 was down-regulated after OLT. On the other hand, group B, which contained MAP1 (1), haptoglobin (4) complement C (6), α2-HS-glycoprotein1 (5) immunoglobulin heavy chain (20) immunoglobulin light chain (22,25) and C-reactive protein (26,27) was up-regulated at POD60 following OLT (Table 1).
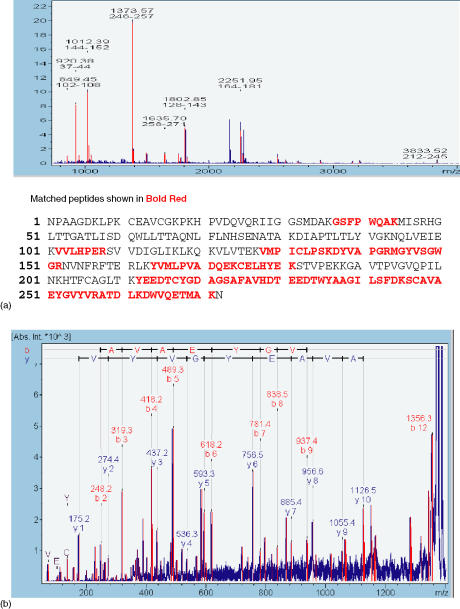
Figure 2.
Identification of haptoglobin. A MALDI-TOF spectrum (a) obtained by in-gel trypsin digestion of spot marked 5 in Table 1. By means of Mascort 8 peptides with m/z-values could be matched to accession No. AAA41349 rat hepatoglobin from the SWISS-PROT database. (b). PSD spectrum. A sequence was confirmed from the labelled b- and y-ions in the spectrum.
Table 1.
List of identified protein spots
| Spot no. | Protein name | Swiss-Prot accession number | Identified by |
|---|---|---|---|
| Proteins with decreasing spot intensity after liver transplantation (group A) | |||
| 2 | ApoA4 | P02651 | N |
| 5 | ApoE | P02650 | N/M |
| 7 | ApoA1 | P04639 | N |
| 9 | ApoA1 | P04639 | N |
| 11 | Gc-globulin (VTDB) | P04276 | N |
| 17 | Albumin | P02770 | N |
| Proteins with increasing intensity after liver transplantation (group B) | |||
| 1 | MAP 1 | AAA41570 | M |
| 4 | Haptoglobin | AAA41349 | M/N |
| 6 | Complement C3 | AAA40837 | M |
| 15 | α2-HS-glycoprotein | P24090 | N |
| 20 | Heavy chain | N | |
| 22 | Ig light chain | N | |
| 25 | Light chain | N | |
| 26 | C-reactive protein | AAA42579 | M |
| 27 | C-reactive protein | ||
M, mass spectrometry analysis of tryptic peptides; N, N-terminal sequencing.
Evaluation of haptoglobin and IL-2 mRNA levels of rat liver after OLT
Hp (protein spot number 4) has been reported to provide an inhibitory effect on T-cell proliferation following OLT. In order to prove simultaneous Hp production during the immunoregulatory reaction, mRNA levels were investigated with RT–PCR. At the same time, we analysed IL-2, which is up-stream of gene expression and regulates the activation of Hp. The results of RT–PCR for IL-2 cytokine and Hp in the liver of allogeneic (DA into PVG) OLT combinations on days 2, 7, 14, 21, 40, and 60 are shown in Fig. 3. From days 2–7 following OLT, the expression of both IL-2 and Hp is modest. Soon after day 14 after OLT, the strongly increasing intensity of IL-2 indicated activation and expression of Hp. This regulatory cycle recruited another high-level of IL-2 on POD 40 resulting in the up-regulation of Hp on POD 60. In the OLT (DA to PVG) model, Hp in DA livers peaked both on days 14 and 60 after liver transplantation, facilitating spontaneous tolerance during these two particular dates. However, rejection occurred on POD 14 which could be a result of the abundant Hp transcript had not been converted to protein. Additional quantification of Hp protein level in OLT serum was performed. The Hp protein stayed at low levels during days 2–21, and suddenly increased on days 40 and 60 (data not shown). According to the Hp curve after OLT, a correlation regarding tolerance or rejection with Hp confirmed the previous hypothesis about RT–PCR results on days 14 and 60.
Figure 3.
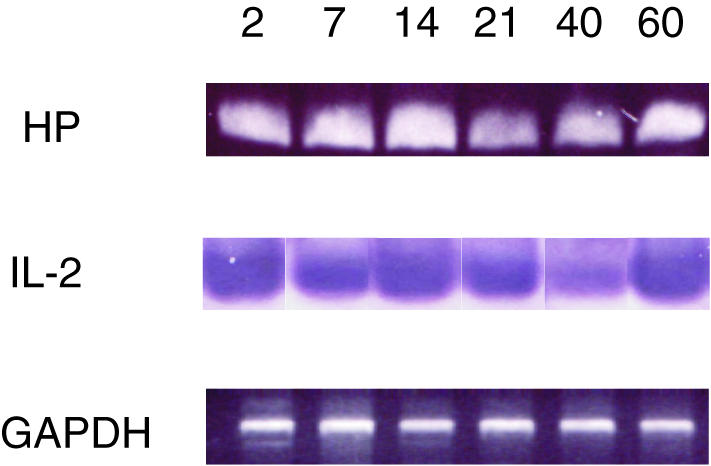
RT–PCR of IL-2 and haptoglobin mRNA expression in the allogeneic (DA-PVG) model. Glyceraldehyde-3-phosphate dehydrogenase mRNA expression (GAPDH) was used to control for equal gel loading.
Immunohistochemistry of haptoglobin expression in OLT liver
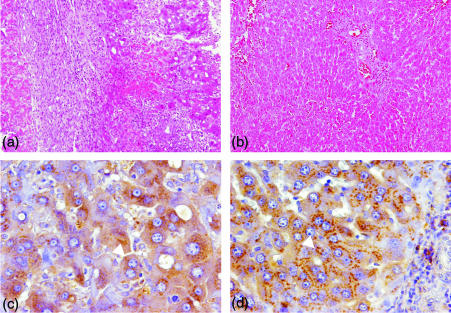
Histological examination revealed in situ immunological conditions following OLT (DA-PVG). As shown in Fig. 4(a), serious rejection was confirmed by dense cellular infiltration on day 14 and focal necrosis of hepatocytes scattered throughout the sinusoids. Alternatively, rejection was overcome and tolerance was induced at POD 60 of OLT (Fig. 4b). This was identified by regression to normal histological liver architecture without signs of inflammation.18 The study demonstrated a significant difference in Hp localization between the rejection and tolerant periods. Hp was scattered throughout the inside of the hepatocyte during rejection on day 14 (Fig. 4c). In the tolerogenic liver, Hp appeared to be highly concentrated spots and gather in the hepatocyte Golgi organelle on POD 60 (Fig. 4d).
Figure 4.
Localization of haptoglobin in the tolerogeneic rat OLT model. Hp was not detected at POD 2 (a) but appeared at POD 14 (b), POD 40 (c) and POD 60 (d) 120×). Higher magnification of hepatocytes showed different expression patterns during rejection and tolerance (240×).
Haptoglobin inhibits proliferation of lymphocyte in MLR
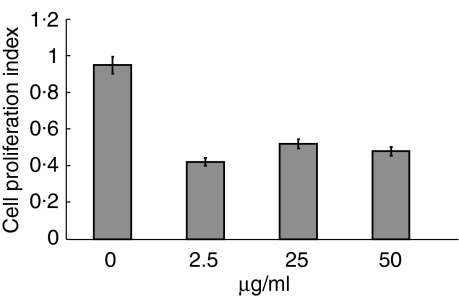
The immunologically suppressive property of haptoglobin on cell proliferation was assayed with MLR. Commercialized Hp at a dose of 2·5 µg/ml was found to significantly inhibit lymphocytes proliferation and showed non-specific suppression by 60% without dose dependence (Fig. 5), while irradiated lymphocytes from donors (DA) caused anergy in immunity, and lymphocytes from recipients (PVG) acted as responders. Based on these findings, Hp could be considered a potential inhibitor of immunological reaction. In addition, the toxicity of Hp was examined by trypan blue exclusion against cultured cells (data not shown).
Figure 5.
Effect of haptoglobin on cell proliferation in alloantigen-stimulated DA spleenocyte. The results demonstrated the ratio of absorption to each stimulated control without haptoglobin. The mean ± SD of three different experiments are shown.
Discussion
2-DE, mass spectrometry, immunohistochemistry and MLR were used to investigate the potential protein factors related to spontaneous tolerance in rat liver transplantation. By means of 2-DE analysis of OLT animal models, the status of protein expression was evaluated, revealing 15 proteins with significant changes after OLT. It has felt that those proteins might be associated with tolerance or rejection. According to their expressive time-course during OLT, the 15 proteins were classified into two groups (Table 1). Lord et al. had demonstrated that expression of novel immunosuppressive protein- LSF-1, a 40 000 MW protein, was highly expressed in postoperative serum after 60 days.21 Fortunately, 2-DE and mass spectrometry analysis showed Hp with similar molecular weight increased significantly on POD 60, indicating a potential candidate for modulation of spontaneous tolerance, although Hp is considered an acute-phase reactant with several biological functions.22
Because of the relative inefficiency and lack of specificity of acute-phase reactants as immunoregulatory substances, the physiological importance of these reactants in vivo has been largely neglected throughout the past decade. Apffel and Peters23 have proposed that carbohydrate-rich plasma proteins produced by the liver, named ‘symbodies’, located on the surface of cancer cells, allow them to escape the immunological attack of the host. These carbohydrate-rich plasma proteins are now identified as acute-phase reactants. Interestingly, although Hp's major function is to bind to haemoglobin, it has been found to be potentially suppressive on many cell-mediated immune responses in cancer patients.24 Arredouani et al. have shown that Hp specifically interacts with both resting and activated CD4+ and CD8+ T cells. The regulation of Hp has also been reported to be essential in the testis sertoli cell for maintaining immune privilege.25 Our previous results also demonstrated that T-cell apoptosis may contribute to the control of the immune response in the drug-free tolerant OLT model.26 This evidence directed attention to Hp as an immunological suppressor in OLT rather than the other 14 proteins that also changed in quantity during orthotopic liver transplantation. Through a series of experiments, we could conclude that Hp might be responsible for spontaneous tolerance for OLT.
The results demonstrate that Hp was highly expressed during post-transplantation day 14 and day 60. However, the induced consequences are contrasted. On POD 14 after OLT, although the mRNA level of Hp increased and concentrated within granulocytes and monocytes in considerable quantities, there was less Hp protein detected in serum and therefore could not reverse the rejection derived from recipients' immune system. The reason for this may be that the Hp appearing during the acute phase of transplantation was neutralized by haemoglobin. At the same time, IL-2 was abundantly produced and released at day 14 leading to remarkable shifts of immune response towards a dominant T helper 1 cellular response also enhancing the rejection response. Hp expression was significantly detectable at POD 60, too. Not only was the high concentration noted in mRNA, but a large quantity of Hp protein product was also found in the serum of the rat with OLT. This Hp expression induces a suppressive signal from the graft to the lymphocyte through CD11b, resulting in spontaneous allograft tolerance. Similar responses were seen in patients with cancer, high Hp serum concentrations were found to inhibit phytohaemagglutinin (PHA)-induced blastogenesis of lymphocytes, suggesting that the inhibition caused the protection of tumours against immune attacks.21,27 Finally, two different transplanted liver Hp localizations were confirmed with our immunohistochemistry results. This evidence explains Hp's dual role in the tolerogenic model, one as an acute-phase reagent and the other as an escape from immune surveillance, allowing for liver tolerance. We could therefore conclude that Hp inhibits cell proliferation in allogenic transplantation and has immunoregulatory activity.
In this study, we also showed the obviously immunosuppressive effects of Hp on the allogenic DA spenlocyte in vitro. The inhibition was affected by 60%, and rat haptoglobin revealed 68% homology to human haptoglobin. Thus, we propose that transplanted tolerance in the liver must be achieved at threshold and it affected several factors in addition to Hp. Kamrin has used these acute-phase proteins which modulate immune functions to prolong the survival of random bred rats after skin grafts.19 Of these proteins, particularly tumour-derived haptoglobin, may account for some of the depressed cell-mediated immunities seen in cancer patients.28 A report from Zuo et al. has indicated that antithrombin III significantly inhibits allograft rejection in a highly histoincompatiable model of rat lung transplantation and concanavalin A-stimulated rat spleen cell proliferation in vitro.29 Pratt et al. demonstrated that local tissue production of C3 is important in renal graft survival.30 According to our findings, complement C3 and antithrombin III were both expressed in increased amounts on POD 60. Two of the up-regulated candidate proteins – immunoglobulin light and heavy chains – have shown their immunosuppressive effect by using pooled serum 2–4 months after OLT in MLR; this suggested that inhibition was caused mainly by immunoglobulin, particularly class II antibodies reactive with DA stimulator.31 Based on the above results, we could propose that the tolerance of OLT after 60 days should be multifactorial.
In conclusion, proteomics permitted a logical analysis and direct detection of differentially expressive protein patterns associated with OLT tolerance, including Hp, IgG, complement, and glycoprotein. By means of molecular, cellular and histologic techniques, we certified that Hp is the important molecule in regulating the immune response and tolerogenic liver transplantation without the need for immunosuppressive therapy. Our results offer the opportunity of making Hp a clinical biomarker for transplantation. The link between Hp, the tolerogenic model, and the pharmacological effect of Hp in vivo still require further experimental exploration.
Acknowledgments
This work was supported by a grant from the National Science Council and Chang Gung Memorial Hospital, Taiwan. We would like to thank the colleagues in Academia Sinica and Chang Gung University for their excellent skills in carrying out the MALDI-TOF.
References
- 1.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue in transplant immunity. Proc R Soc. 1954;143:58–80. doi: 10.1098/rspb.1954.0054. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann FA, Davies HS, Knoll PP, Gokel JM, Schmidt T. Orthotopic liver allografts in the rat. The influence of strain combination on the fate of the graft. Transplantation. 1984;37:406–10. doi: 10.1097/00007890-198404000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Kamada N, Davies HS, Brons G. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–2. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 4.Kamada N, Sumimoto R, Baguerizo A, Yoshimatsu A, Teramoto K, Yamaguchi A. Mechanisms of transplantation tolerance by liver grafting in rats: involvment of serum factors in clonal deletion. Immunology. 1988;64:315–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Goto S, Lord R, Kobayashi E, Vari F, Edwards-Smith C, Kammada N. Novel immunosuppressive proteins purified from the serum of liver retransplantation rats. Transplantation. 1996;61:1147–51. doi: 10.1097/00007890-199604270-00004. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. The lost chord: microchimerism and allograft survival. Immunol Today. 1996;17:577. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop GA, McCaughan GW, Sun J, Aheil AG. Microchimerism and transplant tolerance. Immunol Today. 1997;18:455–6. doi: 10.1016/s0167-5699(97)82722-3. [DOI] [PubMed] [Google Scholar]
- 8.Riordan SM, Williams R. Tolerance after liver transplantation: does it exist and can immunosuppression be withdrawn? J Hepatol. 1999;31:1106–19. doi: 10.1016/s0168-8278(99)80326-2. [DOI] [PubMed] [Google Scholar]
- 9.Goddard S, Adams DH. New approaches to immunosuppression in liver transplantation. J Gastrol Hepatol. 2002;17:116–26. doi: 10.1046/j.1440-1746.2002.02633.x. [DOI] [PubMed] [Google Scholar]
- 10.Yeatman TJ. The future of cancer management. Translating the genome, transcriptome, and proteome. Ann Surg Oncol. 2003;10:7–14. doi: 10.1245/aso.2003.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy S. Proteomic profiling from human samples: the body fluid alternative. Toxicol Lett. 2001;120:379–84. doi: 10.1016/s0378-4274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 12.Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis. 1998;19:1853–61. doi: 10.1002/elps.1150191103. [DOI] [PubMed] [Google Scholar]
- 13.Patterson SD, Aebersold R. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis. 1995;16:1791–814. doi: 10.1002/elps.11501601299. [DOI] [PubMed] [Google Scholar]
- 14.Kamada N, Kobayashi E, Goto S. Liver transplantation in the rat. In: Green MK, Mandel TE, editors. Experimental Transplantation Models in Small Animals. Chur, Switzerland: Harwood Academic Publishers; 1995. pp. 341–52. [Google Scholar]
- 15.Altshul SF, Madden TL, Schaffer AA, Zang J, Zang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gevaert K, Demol H, Martens L, et al. Protein identification based on matrix assisted laser desorption/ionization-post source decay-mass spectrometry. Electrophoresis. 2001;22:1645–51. doi: 10.1002/1522-2683(200105)22:9<1645::AID-ELPS1645>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Toogood GJ, Rankin AM, Tam PK, Morris PJ, Dallman MJ. The immune response following small bowel transplantation. I. An unusual pattern of cytokine expression. Transplantation. 1996;62:851–5. doi: 10.1097/00007890-199609270-00025. [DOI] [PubMed] [Google Scholar]
- 18.Pan TL, Goto S, Lin YC, et al. The fas and fas ligand pathways in liver allograft tolerance. Clin Exp Immunol. 1999;118:180–7. doi: 10.1046/j.1365-2249.1999.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinkovic S, Baumann H. Structure, hormonal regulation, and identification of the interleukin-6- and dexamethasone-responsive element of the rat haptoglobin gene. Mol Cell Biol. 1990;10:1573–83. doi: 10.1128/mcb.10.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanBuskirk AM, Adams PW, Orosz CG. Nonradioactive alternative to clinical mixed lymphocyte reaction. Hum Immunol. 1995;43:38–44. doi: 10.1016/0198-8859(94)00117-9. [DOI] [PubMed] [Google Scholar]
- 21.Lord R, Kamada N, Kobayashi E, Goto S, Sunagawa M. Isolation of a 40 kDa immunohibitory protein induced by rat liver transplantation. Transplant Immunol. 1995;3:174–9. doi: 10.1016/0966-3274(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 22.Kamrin BB. Role of alpha globulins in immunosuppression: reactive site occlusion hypothesis. Transpl Proc. 1969;1:506–10. [PubMed] [Google Scholar]
- 23.Apffel CA, Peters JH. Tumors and serum glycoproteins. The ‘symbodies.’. Prog Exp Tumor Res. 1969;12:1–54. [PubMed] [Google Scholar]
- 24.Oh SK, Very DL, Walker JE. Interference with immune response at the level of generating effector cells by tumor-associated haptoglobin. J Natl Cancer Inst. 1990;82:934–40. doi: 10.1093/jnci/82.11.934. [DOI] [PubMed] [Google Scholar]
- 25.O'Bryan MK, Grima J, Mruk D, Cheng CY. Haptoglobin is a sertoli cell product in the rat seminiferous epithelium: its purification and regulation. J Androl. 1997;18:637–45. [PubMed] [Google Scholar]
- 26.Arredouani M, Matthijs P, Hoeyveld EV, Kasran A, Baumann H, Ceuppens JL, Stevens E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology. 2003;108:144–51. doi: 10.1046/j.1365-2567.2003.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord R, Goto S, Pan T, Chiang K, Chen C, Sunagawa M. Peak protein expression of IL-2 and IFN-gamma correlate with the peak rejection episode in a spontaneously tolerant model of rat liver transplantation. Cytokine. 2001;13:155–61. doi: 10.1006/cyto.2000.0815. [DOI] [PubMed] [Google Scholar]
- 28.Frings W, Dreier J, Sorg C. Only the soluble form of the scavenger CD163 acts inhibitory on phorbol ester-activated T-lymphocytes, whereas membrane-bound protein has not effect. FEBS. 2002;526:93–6. doi: 10.1016/s0014-5793(02)03142-3. [DOI] [PubMed] [Google Scholar]
- 29.Zuo XJ, Nicolaidou E, Okada Y, Toyoda M, Jordan SC. Antithrombin III inhibits lymphocyte proliferation, immunoglobulin production and mRNA expression of lymphocyte growth factors (IL-2, gamma-IFN and IL-4) in vitro. Transpl Immunol. 2001;9:1–6. doi: 10.1016/s0966-3274(01)00042-9. [DOI] [PubMed] [Google Scholar]
- 30.Sohn JH, Bora PS, Suk HJ, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Mat Med. 2003;9:206–12. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation. 1990;50:678–82. doi: 10.1097/00007890-199010000-00029. [DOI] [PubMed] [Google Scholar]