Abstract
The cysteinyl leukotrienes (cysLTs) are potent lipid mediators in allergic disease, acting through a receptor (cysLT1-R) which can be targeted in rhinitis and asthma. We investigated the effects of cysLT1-R antagonism in experimental allergic rhinitis, focusing on bone marrow eosinophil progenitor responses. BALB/c mice were sensitized, then given daily intranasal ovalbumin for 2 weeks, with montelukast sodium (5 mg/kg or 2·5 mg/kg) or placebo by gavage. Bone marrow eosinophil/basophil colonies were enumerated, and colony cells were morphologically assessed as indices of eosinophil differentiation and maturation. Montelukast treatment resulted in a significant decrease of eosinophils in the nasal mucosa, and in either bone marrow interleukin (IL)-5-, but not IL-3-, or granulocyte-macrophage colony-stimulating factor-responsive eosinophil/basophil colony-forming units, and IL-5-stimulated eosinophil maturation. These results indicate that cysLT1-R antagonism in vivo limits both IL-5-responsive eosinophilopoiesis, acting at several stages of eosinophil differentiation and maturation. The anti-allergic effects of cysLT1-R antagonists are consistent with the concept that cysLTs and IL-5 act together in the recruitment of eosinophils and eosinophil progenitors from the marrow during upper airway allergic inflammation.
Keywords: allergic rhinitis, cysteinyl leukotriene receptor antagonist, eosinophils, haemopoiesis
Introduction
It is evident that eosinophils play a prominent role in the development of allergic inflammation.1,2 Eosinophils are derived from eosinophil/basophil (Eo/Baso) progenitor cells in the bone marrow and, when activated, they represent a cellular source of cysteinyl leukotriene (cysLT) production.3,4 It is also known that both mature eosinophils and immature eosinophil progenitors express the cysLT1 receptor (cysLT1-R).5 The cysLTs are pro-inflammatory mediators which include LTC4, LTD4 and LTE4, with LTD4 being the most potent of the group. CysLT1-R activation by LTD4 contributes to bronchial smooth muscle contraction and proliferation, mucus secretion, tissue oedema and eosinophil recruitment, all of which are observed in airway allergic diseases.6–9 One way to block the action of leukotrienes, in terms of clinical therapy, is to inhibit the cysLT1-R by using one of the selective antagonists of the LTD4 receptor.10 Recent studies in murine models, including those which we have developed, indicate that the extent of the tissue eosinophilic response is related, at least in part, to eosinophil differentiation in the bone marrow.11,12 Given this, and the evidence of expression of cysLT1-R by CD34+ progenitors,5 targeting the haemopoietic response may be an important mechanism underlying the anti-inflammatory effects of leukotriene antagonists.
Materials and methods
Animals and ovalbumin sensitization
Under pathogen-free conditions, mice were sensitized to induce pure upper airway allergic inflammation using ovalbumin (OVA) antigen, as previously described.12 Briefly, OVA sensitization was achieved by administering 40 µg/kg OVA (Sigma, St Louis, MO), diluted in sterile normal saline with aluminum hydroxide gel (alum adjuvant, 40 mg/kg), to animals, four times by intraperitoneal (i.p.) injection on days 1, 5, 14 and 21. This was followed by daily intranasal challenge to conscious animals (an anaesthetic drug was not used in order to ensure antigen delivery only to the nasal cavity) with OVA, diluted in sterile normal saline, (20 µl of 25 mg/ml OVA per mouse) from day 22 to day 35 (Fig. 1). Age-matched (eight- to 10-week old) BALB/c mice (female, Charles River, St Constant, QC, Canada) were placed into one of three treatment groups, as follows:
the montelukast (ML) 5 mg/kg group, which was OVA-sensitized then challenged daily, for the next 14 days, intranasally with OVA; montelukast sodium (5·0 mg/kg diluted in 150 µl of sterile water) was administered by gavage, using an oral administration tube (Animal feeding tube, Popper®; Fisher Scientific, Nepean, ON, Canada) 1 hr before each challenge;
the ML 2·5 mg/kg group, which was OVA-sensitized similarly to the ML 5 mg/kg group, but given 2·5 mg/kg of montelukast sodium instead of 5 mg/kg; and
the placebo group, which was OVA-sensitized, then challenged daily, for the next 14 days, with intranasal OVA in the same schedule as the ML groups with administration of sterile water (instead of montelukast sodium) by gavage.
There were 10 mice in each ML group and 14 mice in the placebo group.
Figure 1.
Sensitization and challenge protocol. Schematic representation of the murine experimental allergic rhinitis model. Mice were killed on day 36 and replicate bone marrow and nasal tissue samples were taken for analyses. OVA, ovalbumin.
Tissue samples
The mice in each group were killed by deep anaesthesia using a solution containing Ketamine Hydrochloride (Ketalean®; Bimeda-MTC, Animal Health Inc., Cambridge, ON, Canada) and Xylazine (Rompun®; Bayer Inc. Agriculture Division, Animal Health, Toronto, ON, Canada) diluted in normal saline, at 24-hr postintranasal provocation on day 35. Nasal mucosa and bone marrow tissues were taken and processed immediately, as described below.
All procedures were performed in accordance with the ethical guidelines of the Canadian Council on Animal Care's Guide to the Care and Use of Experimental Animals and approved by the Animal Ethics Committee of McMaster University.
Tissue preparation
Nasal tissues were fixed in 10% formalin followed by decalcification, embedded in paraffin and then cut into 4-µm thin slices for histological analysis. Bone marrow cells were obtained from femoral bone marrow, which was suspended in McCoy's 3+ culture medium [modified McCoy's 5A medium and 15% fetal calf serum (FCS) (Gibco BRL, Grand Island, NY) containing 1% penicillin/streptomycin and 0·35% 2-mercaptoethanol (2-ME)], as previously described.12 Both total bone marrow cells and mononuclear cells, which were separated by density-gradient centrifugation over LymphoPrep (NYCOMED Pharma, Oslo, Norway) for 25 min at 960 g at room temperature, were incubated in plastic flasks for 2 hr at 37° in 5% CO2 to remove adherent cells, and recruited for methylcellulose culture assays. Duplicate or triplicate samples were prepared and counted for each animal and time-point.
Bone marrow methylcellulose cultures and cell differentiation analysis
To investigate the effects of in vivo administration of the cysLT1-R antagonist on eosinophil progenitor proliferation, differentiation and maturation, ex vivo analysis of bone marrow colony-forming assays in methylcellulose, an established functional assay of progenitor numbers and responsiveness to specific differentiation-inducing cytokines in humans and several animal models,12–16 was performed. Non-adherent mononuclear cells (NAMNC) were cultured in 35 × 10-mm tissue culture dishes (Falcon Plastics, London, ON, Canada) in culture medium which comprised 0·9% methylcellulose (The Dow Chemical Company, Midland, MI), 20% FCS and Iscove's Dulbecco's medium (containing 1% penicillin/streptomycin, 0·35% 2-ME and 0·1% bovine serum albumin) and the following recombinant mouse (rm) cytokines (R & D Systems Inc., Minneapolis, MN): rm interleukin (IL)-5 (5 ng/ml) with 1 × 105 NAMNC, rmIL-3 (1 ng/ml) with 5 × 104 NAMNC, or rm granulocyte–macrophage colony-stimulating factor (GM-CSF) (1 ng/ml) with 2·5 × 104 NAMNC. After 6 days, colonies of greater than 40 cells were counted using inverse microscopy, and Eo/Baso-colony-forming units (CFU) were classified using morphological and histological criteria (tight, compact, round refractile cell aggregates). To identify the differentiated cells from colonies as Eo/Baso-CFU, sample cells in each 10-day culture were evaluated; 3 ml of phosphate-buffered saline (PBS) was added to the sample in each culture dish, then the sample was centrifuged at 345 g for 10 min at 4°. After the sample was resuspended in 3 ml of PBS, cytocentrifuge slides were created on APTEX-coated glass slides, and DiffQuick staining was performed (DiffQuick®; Baxter, McGaw Park, IL) for morphological analysis of maturation by differential counting. Immature eosinophilic cells, mature eosinophils and other types of cells on each slide were enumerated by light microscopy: 100 cells were counted, and the result was expressed as a percentage of total cells. The classification of eosinophil maturation was performed by following previously published morphological criteria.17
In vitro experiments were additionally performed to confirm whether cysLT1-R antagonism affects the proliferation of IL-5-driven bone marrow cells. Bone marrow-derived NAMNCs from OVA-sensitized mice were grown in the presence of an optimal concentration of IL-5 (5 ng/ml), with or without LTD4 (0·1 and 1 μm) and with or without montelukast (1, 10, and 100 μm). Bone marrow cultures for Eo/Baso CFU were performed, as described above. LTD4 was purchased from Caymen Chemical (Ann Arbor, MI) in powder form, diluted in ethanol and stored at −80°.
Analysis of nasal mucosal inflammation
In the lamina propria of the nasal mucosa, total numbers of eosinophils were enumerated after DiffQuick staining (DiffQuick®, Baxter). The area of the nasal tissue was measured, excluding glands, using an eyepiece with a grid, and the cell count results were expressed as the number of cells/0·01 mm2 of lamina propria. In addition, mucosal thickness was measured as a hallmark of nasal mucosal swelling; nasal septum mucosa was taken from three locations (upper, middle and lower areas from the bottom of the nostrils) and the thickness was measured using a microscopic scale with an eyepiece.
Nasal histamine responsiveness
Nasal histamine responsiveness (NHR) was measured by determining the concentration of histamine that caused symptoms (sneezing and itching) as measured on day 35. NHR was expressed as the limiting concentration of histamine (log10 pg/ml), as previously described.12
Statistics
For all cell counts of stained slides, the slides were read randomly and in a blinded manner. Eo/Baso CFU counts were also obtained by two independent observers who were blinded to plate conditions. Analysis of variance (anova), followed by the Student's Newman-Keuls test, were employed for comparison of data among groups.
Results
Eo/Baso-CFU formation
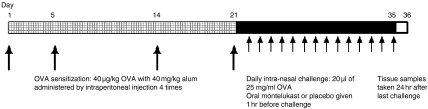
Figure 2 depicts Eo/Baso-CFU data obtained from methylcellulose cultures after 2 weeks of in vivo treatment with montelukast sodium. Eo/Baso-CFU numbers were decreased significantly compared to the placebo group in the presence of IL-5 (Fig. 2a), but not in the presence of IL-3 or GM-CSF (Fig. 2b).
Figure 2.
Eosinophil/basophil colony-forming unit (Eo/Baso-CFU) formation in methylcellulose culture assays in the presence of (a) recombinant murine interleukin-5 (rmIL-5) (5 ng/ml), and (b) interleukin-3 (IL-3) (1 ng/ml)+granulocyte–macrophage colony-stimulating factor (GM-CSF) (1 ng/ml). The numbers of Eo/Baso-CFU per 1 × 105 non-adherent mononuclear cells (NAMNC) plated are shown for each group for each cytokine. Error bars represent the standard error of the mean (SEM). ML, montelukast.
Eosinophil maturation
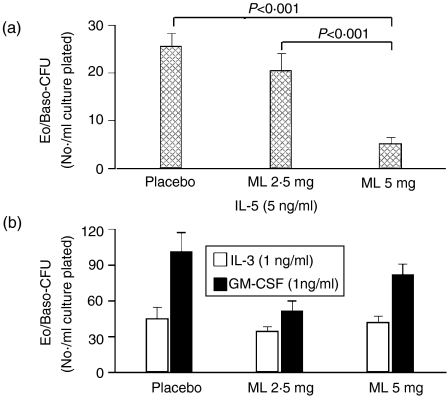
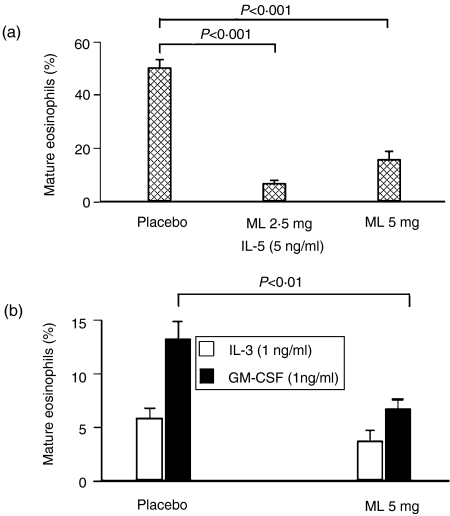
In the presence of IL-5, the percentage of mature eosinophils in colonies at 10 days of methylcellulose culture was significantly decreased in both the ML 2·5 mg/kg and ML 5 mg/kg groups after 2 weeks of treatment (day 36) (Fig. 3a), while other cell types were increased significantly (Fig. 4a) (mean ± SEM: 31·5 ± 3·4 and 65·1 ± 5·1 for the placebo and ML 5 mg/kg groups, respectively, P < 0·001). On the other hand, when marrow progenitors from ML-treated animals were cultured ex vivo in the presence of IL-3, there was no significant decrease in the percentage of mature eosinophils in colonies compared with placebo (5·8 ± 1·0 and 3·7 ± 1·0 for the placebo and ML 5 mg/kg groups, respectively) (Fig. 3b). In the presence of GM-CSF ex vivo, significant differences again were observed (13·3 ± 1·6 and 6·8 ± 0·8 for the placebo and ML 5 mg/kg groups, respectively, P < 0·01) (Fig. 3b). There were no differences in the numbers of other cell types for IL-3- or GM-CSF-stimulated cultures (Fig. 4b). Cultures were not performed on the ML 2·5 group for IL-3 or GM-CSF.
Figure 3.
Ex vivo cell maturation: Eosinophils. The percentage of mature eosinophils in 10-day methylcellulose culture assays are shown for (a) interleukin-5 (IL-5), and (b) interleukin-3 (IL-3)+granulocyte–macrophage colony-stimulating factor (GM-CSF). Error bars represent the standard error of the mean (SEM). ML, montelukast.
Figure 4.
Ex vivo cell maturation: Other cells. The percentage of non-eosinophilic cells (i.e. all cells apart from mature and immature eosinophils) in 10-day methylcellulose culture assays are shown for (a) interleukin-5 (IL-5), and (b) interleukin-3 (IL-3) + granulocyte–macrophage colony-stimulating factor (GM-CSF). Error bars represent the standard error of the mean (SEM). ML, montelukast.
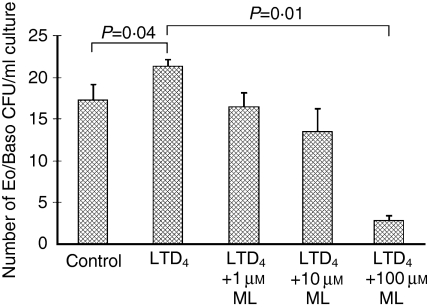
The addition of LTD4 in vitro at 1 µm significantly increased the number of bone marrow Eo/Baso CFUs in the presence of IL-5, from 17·3 ± 1·8 to 21·3 ± 0·8/1 × 105 NAMNC (P = 0·04) (Fig. 5). The number of bone marrow Eo/Baso CFUs grown in the presence of IL-5 and 1 µm LTD4 was significantly suppressed by ML at 100 µm, from 21·3 ± 0·8 to 2·8 ± 0·5/1 × 105 NAMNC (P = 0·01). A similar trend was observed for ML at 1 and 10 µm (16·5 ± 1·5 and 13·5 ± 2·7/1 × 105 NAMNC, respectively) (Fig. 5). The addition of ML at 1, 10 and 100 µm induced 24·8, 35·7, and 86·7% suppression, respectively.
Figure 5.
In vitro effects of montelukast (ML) on the number of LTD4- and interleukin-5 (IL-5)-mediated eosinophil/basophil colony-forming units (Eo/Baso-CFU). The number of Eo/Baso-CFU are shown in the presence of 1, 10 and 100 μm ML added to 1 μm LTD4 and 5 ng/ml IL-5. Error bars represent the standard error of the mean (SEM).
Allergic inflammation in the nasal mucosa and mucosal thickness
In both the ML 2·5 mg/kg and ML 5 mg/kg groups, the numbers of eosinophils in the nasal mucosa were significantly decreased by treatment compared to the placebo group; however, there was no significant difference in nasal mucosal thickness among the three groups (Table 1).
Table 1.
Eosinophilia in the nasal mucosa
| Eosinophils* | Mucosal thickness† | |
|---|---|---|
| Placebo | 142·8 ± 7·9 | 0·88 ± 0·05 |
| ML 2·5 mg/kg | 107·8 ± 5·9‡ | 0·87 ± 0·04 |
| ML 5 mg/kg | 98·2 ± 8·6‡ | 0·97 ± 0·07 |
Cell number/0·01 mm2.
mm2.
P < 0·01 compared with the placebo group.
ML, montelukast.
Nasal histamine responsiveness
When the limiting concentrations of histamine (log10 pg/ml) for the placebo and ML 5 mg/kg groups were compared, significantly less NHR was observed in the treatment group (−9·2±0·3, −7·3±0·6; P<0·05, for placebo and treatment groups, respectively).
Discussion
Recent studies have demonstrated a close relationship among the secretion of pro-inflammatory lipid mediators, the cysLTs (LTC4, LTD4 and LTE4) and eosinophils. Eosinophils produce cysLTs, as do other allergic inflammatory cells,18 and these have been reported to cause airway reactions such as bronchoconstriction and secretions.6–9 The gene responsible for LTC4 synthesis has been found to be encoded on chromosome 5q13, the same locus of other genes important in allergic inflammation, i.e. IL-4, IL-5 and IL-13.19 It could be speculated that pathophysiological events linking specific T helper 2 (Th2) cytokines, eosinophils and cysLTs lead to characteristic allergic inflammatory responses in tissues.
CysLTs work through binding to cysLT1-R and cysLT2-R, which are 38% homologous; recent studies suggest that cysLT1-R is expressed on haemopoietic progenitor cells and that it plays a major role in acute inflammation, including IgE-mediated mast cell-dependent local anaphylaxis.5,20 Thus, cysLT1-R antagonism could be predicted to have effects on eosinophil differentiation, maturation and trafficking, as well as playing a role in allergic inflammation by acting upon other cell populations.3,5,17,21,22 Indeed, our current study, in an experimental murine allergic rhinitis model, demonstrates that in vivo administration of a cysLT1-R antagonist suppresses eosinophilopoiesis ex vivo in the presence of either IL-5 or GM-CSF, but not IL-3, with effects on indices of both differentiation (numbers of IL-5-responsive Eo/Baso-CFU) and maturation (numbers of mature eosinophils in developing, methylcellulose colonies). Moreover, an in vitro dose-dependent suppression of LTD4 and IL-5-mediated Eo/Baso CFU formation by ML was observed, consistent with the direct or indirect involvement of cysLT1-R in the down-regulation or suppression of IL-5R ligation on eosinophilopoiesis.
We observed a dose-dependent effect of ML in vitro on Eo/Baso CFU differentiation (Fig. 2a), and equipotent suppression of eosinophil maturation at both doses (Fig. 3a). One possible explanation for the degree of suppression of CFU and mature eosinophils by ML is that there might be several (at least two) stages of eosinophilopoiesis responsive to the LT-IL-5 pathway. Differential expression of cytokine receptors, or varying functional responses of cysLT1-R at these different stages of eosinophilopoiesis, may thus underlie this observation.
Previous reports, in which in vitro suppression by a cysLT1-R antagonist of eosinophil differentiation and/or maturation has been shown, include a study of eosinophil lineage commitment of an HL-60 cell line22 and a recent report from our group on the role of cysLT1-R antagonism in abrogation of LTD4- and GM-CSF-induced Eo-Baso-CFU formation by marrow or blood progenitors taken from human asthmatic subjects.23 Our results, from this murine study, provide the first evidence that administration of cysLT1-R antagonist can cause changes in eosinophil accumulation in vivo, as well as ex vivo effects on eosinophilopoiesis. A similar ex vivo effect on nascent eosinophil cytokine (GM-CSF and IL-5) generation was seen in a study we performed in human atopic asthmatic subjects treated in vivo with inhaled corticosteroids.24 The increases in other (non-eosinophilic) cell types in ex vivo cultures, in the context of suppression of a maximal eosinophil response in vivo and ex vivo following ML administration, might mean either that there is direct suppression of pluripotent haemopoietic stem cell commitment to the eosinophil lineage, or that cysLT1-R antagonism favours lineage skewing to progenitors for other cells, such as mast cells. To clarify this point further, studies examining murine mast cell differentiation need to be carried out in this model.
While the exact mechanisms underlying the ex vivo and in vitro effects on eosinophilopoiesis remain to be clarified, they could include down-regulation of relevant cytokine receptors (such as IL-5R), linked to effects of ML on cysLT1-R, or alterations in cytokine generation and (autocrine) responsiveness within maturing (colony-derived) eosinophils, also through effects of cysLT1 blockade. Regarding potential mechanisms of suppression of eosinophilopoiesis via cysLT1-R antagonism, our previous report supports a direct connection between cysLT actions and cytokine-driven haemopoiesis.23 Also, a recent study demonstrated that a cysLT1-R antagonist, given in vivo during antigen sensitization, blocks eosinophil recruitment to airways via blockade of several Th2 cytokines in the lung, including IL-5;25 this could be a mechanism underlying the down-regulation of eosinophilopoiesis that we observed in the current study.
However, these findings do not address the nature of the effects of cysLTs on signalling pathways mediated by ligation of the cysLT1-R in eosinophil progenitors. In this regard, the effect of IL-5 on the production, by progenitors, of LTs through binding of the IL-5 receptor needs to be explored,22,26 along with studies of cysLT1-R antagonists on this mechanism. Our recent finding of increased IL-5Rα expression on murine progenitor cells in murine allergic rhinitis, and its down-regulation in IL-5-deficient animals,13 suggests that the effects on eosinophilopoiesis of cysLT1-R antagonism seen in the current study may be mediated by ‘cross-talk’ between IL-5R and cysLT1-R-related signalling events.13 Studies exploring these possibilities are underway.
Finally, our study also showed that tissue (mucosal) eosinophilia and nasal histamine hyper-responsiveness were both suppressed by administration of the cysLT1-R antagonist. The mechanism underlying this suppression could be the direct attenuation of eosinophil proliferation in the bone marrow, leading to decreased eosinophil accumulation in the nasal mucosa and/or blockade of eosinophil progenitor or mature eosinophil trafficking by the cysLT1-R antagonist. As we found time-dependent differences in effects on eosinophilopoiesis in our preliminary studies (data not shown), it is possible that there is a critical time for exposure which is necessary to suppress both eosinophil proliferation and maturation by blockade of the cysLT1-R. This might involve a cascade of effects on several cellular populations in the bone marrow. Indeed, the induced translocation of 5-lipooxygenase to the nucleus by IL-5, and the expression of LTC4 synthase, are potentially critical events to examine in understanding the effects of cysLT1-R antagonism in our model.27
The discrepancy between the degree of suppression of eosinophil counts in the nasal mucosa and bone marrow Eo/Baso CFU results primarily from time differences in the experimental protocol: bone marrow tissue and nasal mucosa from an individual mouse were taken at the same time, at the end-point of sensitization. It would take some time (several days) for cells to differentiate from CFUs to mature eosinophils; thus, the nasal eosinophil count at that point is thought to be the result of suppression of bone marrow eosinopoiesis only between days 28 and 35. Supporting this notion, the suppression of nasal eosinophils was not significant after 1 week of treatment (results not shown). In ex vivo eosinophil-maturation studies, suppression of eosinophilopoiesis after 1 week of treatment was ≈ 200% (results not shown), which would be compatible with the degree of suppression seen in nasal mucosal eosinophil counts. A second explanation is that mature and immature eosinophil populations are differentially affected by cysLT1 antagonists.
In conclusion, this study illustrates a new mechanism and treatment for the pathogenesis of airway allergic disorders through the haemopoietic actions of cysLT1-R antagonism.
Acknowledgments
Lynne Larocque's expert help with the manuscript is gratefully acknowledged. This study was supported by the Merck Medical School Grants programme. Dr Saito was the recipient of a joint Research Fellowship of the Canadian Society of Allergy and Clinical Immunology and Merck Frosst Canada.
Abbreviations
- CFU
colony-forming units
- cysLT
cysteinyl leukotriene
- cysLT1-R
cysLT1 receptor
- Eo/Baso
eosinophil/basophil
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL
interleukin
- ML
montelukast
- NAMNC
non-adherent mononuclear cells
- NHR
nasal histamine responsiveness
- OVA
ovalbumin
- rm
recombinant mouse
- Th2
T helper 2
References
- 1.Denburg JA. Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammation. Immunol Today. 1999;20:111–3. doi: 10.1016/s0167-5699(98)01423-6. [DOI] [PubMed] [Google Scholar]
- 2.Denburg JA, Sehmi R, Saito H, Jeong P-S, Inman MD, O'Byrne PM. Systemic aspects of allergic inflammatory disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–S246. doi: 10.1067/mai.2000.110156. [DOI] [PubMed] [Google Scholar]
- 3.Baatjes AJ, Sehmi R, Saito H, Cyr MM, Dorman SC, Inman MD, O'Byrne PM, Denburg JA. Anti-allergic therapies: effects on eosinophil progenitors. Pharmacol Ther. 2002;95:63–72. doi: 10.1016/s0163-7258(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 4.Cyr MM, Denburg JA. Systemic aspects of allergic disease: the role of the bone marrow. Curr Opin Immunol. 2001;13:727–32. doi: 10.1016/s0952-7915(01)00286-2. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa DJ, Breyer RM, Defoe SK, et al. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Respir Crit Care Med. 2001;163:226–33. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- 6.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–6. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–55. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 8.Piper PJ. Formation and actions of leukotrienes. Physiol Rev. 1984;64:744–61. doi: 10.1152/physrev.1984.64.2.744. [DOI] [PubMed] [Google Scholar]
- 9.Lynch KR, O'Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 10.Busse WW, McGill KA, Horwitz RJ. Leukotriene pathway inhibitors in asthma and chronic obstructive pulmonary disease. Clin Exp Allergy. 1999;29(Suppl. 2):110–5. doi: 10.1046/j.1365-2222.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Marcos L, Schuster A. New perspectives for asthma treatment: anti-leukotriene drugs. Pediatr Allergy Immunol. 1999;10:77–88. doi: 10.1034/j.1399-3038.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Howie K, Wattie J, Denburg A, Ellis R, Inman MD, Denburg JA. Allergen-induced murine upper airway inflammation: local and systemic changes in murine experimental allergic rhinitis. Immunology. 2001;104:226–34. doi: 10.1046/j.0019-2805.2001.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Matsumoto K, Denburg AE, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002;168:3017–23. doi: 10.4049/jimmunol.168.6.3017. [DOI] [PubMed] [Google Scholar]
- 14.Inman MD, Denburg JA, Ellis R, Dahlback M, O'Byrne PM. Allergen-induced increase in bone marrow progenitors in airway hyperresponsive dogs: regulation by a serum hemopoietic factor. Am J Respir Cell Mol Biol. 1996;15:305–11. doi: 10.1165/ajrcmb.15.3.8924277. [DOI] [PubMed] [Google Scholar]
- 15.Inman MD, Ellis R, Wattie J, Denburg JA, O'Byrne PM. Allergen-induced increase in airway responsiveness, airway eosinophilia and bone-marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol. 1999;21:473–9. doi: 10.1165/ajrcmb.21.4.3622. [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer R, Denburg JA. Immunolocalization of CD34 in nasal polyposis. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–97. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Robertson T, Smith J, Kilfeather S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am J Respir Crit Care Med. 2000;161:1881–6. doi: 10.1164/ajrccm.161.6.9907054. [DOI] [PubMed] [Google Scholar]
- 18.Sampson AP. The role of eosinophils and neutrophils in inflammation. Clin Exp Allergy. 2000;30(Suppl. 1):22–7. doi: 10.1046/j.1365-2222.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 19.Penrose JF, Baldasaro MH, Webster M, Xu K, Austen KF, Lam BK. Molecular cloning of the gene for mouse leukotriene-C4 synthase. Eur J Biochem. 1997;248:807–13. doi: 10.1111/j.1432-1033.1997.00807.x. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–4. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 21.Bautz F, Denzlinger C, Kanz L, Mohle R. Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood. 2001;97:3433–40. doi: 10.1182/blood.v97.11.3433. [DOI] [PubMed] [Google Scholar]
- 22.Thivierge M, Doty M, Johnson J, Stankova J, Rola-Pleszczynski M. IL-5 up-regulates cysteinyl leukotriene 1 receptor expression in HL-60 cells differentiated into eosinophils. J Immunol. 2000;165:5221–6. doi: 10.4049/jimmunol.165.9.5221. [DOI] [PubMed] [Google Scholar]
- 23.Braccioni F, Dorman SC, O'Byrne PM, et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- 24.Gauvreau GM, O'Byrne PM, Moqbel R, Velazquez J, Watson RM, Howie KJ, Denburg JA. Enhanced expression of GM-CSF in differentiating eosinophils of atopic and atopic asthmatic subjects. Am J Respir Cell Mol Biol. 1998;19:55–62. doi: 10.1165/ajrcmb.19.1.2871. [DOI] [PubMed] [Google Scholar]
- 25.Wu AY, Chik SC, Chan AW, Li Z, Tsang KW, Li W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin Exp Allergy. 2003;33:359–66. doi: 10.1046/j.1365-2222.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 26.Adachi T, Alam R. The mechanism of IL-5 signal transduction. Am J Physiol. 1998;275:C623–C633. doi: 10.1152/ajpcell.1998.275.3.C623. [DOI] [PubMed] [Google Scholar]
- 27.Boyce JA, Lam BK, Penrose JF, Friend DS, Parsons S, Owen WF, Austen KF. Expression of LTC4 synthase during the development of eosinophils in vitro from cord blood progenitors. Blood. 1996;88:4338–47. [PubMed] [Google Scholar]