Abstract
Histamine is a major inflammatory molecule released from the mast cell, and is known to activate endothelial cells. However, its ability to modulate endothelial responses to bacterial products has not been evaluated. In this study we determined the ability of histamine to modulate inflammatory responses of endothelial cells to Gram-negative and Gram-positive bacterial cell wall components and assessed the role of Toll-like receptors (TLR) 2 and 4 in the co-operation between histamine and bacterial pathogens. Human umbilical vein endothelial cells (HUVEC) were incubated with lipopolysaccharide (LPS), lipoteichoic acid (LTA), or peptidoglycan (PGN) in the presence or absence of histamine, and the expression and release of interleukin-6 (IL-6), and NF-κB translocation were determined. The effect of histamine on the expression of mRNA and proteins for TLR2 and TLR4 was also evaluated. Incubation of HUVEC with LPS, LTA and PGN resulted in marked enhancement of IL-6 mRNA expression and IL-6 secretion. Histamine alone markedly enhanced IL-6 mRNA expression in HUVEC, but it did not stimulate proportional IL-6 release. When HUVEC were incubated with LPS, LTA, or PGN in the presence of histamine marked amplification of both IL-6 production and mRNA expression was noted. HUVEC constitutively expressed TLR2 and TLR4 mRNA and proteins, and these were further enhanced by histamine. The expression of mRNAs encoding MD-2 and MyD88, the accessory molecules associated with TLR signalling, were unchanged by histamine treatment. These results demonstrate that histamine up-regulates the expression of TLR2 and TLR4 and amplifies endothelial cell inflammatory responses to Gram-negative and Gram-positive bacterial components.
Keywords: endothelial cells, histamine, interleukin-6, Toll-like receptor 2, Toll-like receptor 4
Introduction
Histamine is a major secretory product of mast cells, and is generally implicated in allergic and hypersensitivity reactions. This amine and other mast cell products are known to regulate vasodilatation and bronchoconstriction1,2 and to modulate the functions of a variety of cell types, including monocytes/macrophages,3,4 eosinophils,5,6 T cells,7 neutrophils8 and endothelial cells.9 A direct relationship between histamine and vascular inflammation is evident from the observation that the coronary arteries of patients with ischaemic heart disease contain more mast cells and histamine than normal vessels.10 The possible role of histamine in vascular disease is further supported by the presence of elevated levels of histamine in the coronary circulation of patients with variant angina.11 The ability of histamine to induce the production of cytokines such as interleukin-6 (IL-6) and IL-8 by endothelial cells9,12,13 suggests that this mast cell mediator can act as an important inflammatory signal in addition to its well-recognized function as a vasoactive substance. The physiological processes exerted by histamine are mediated through a family of G-protein-coupled receptors, H1, H2, H3 and H4.14 H1 receptors are highly expressed in smooth muscle cells and endothelial cells, and by signalling through these receptors histamine modulates inflammatory and hypersensitivity responses.9,15 H2 receptors participate in the stimulation of gastric acid secretion in the gut and in the regulation of cytokine production by many cell types in cardiac and smooth muscle tissues, and the immune system.3,16–18 H3 receptors are predominantly found in the brain, where they function as presynaptic autoreceptors on histamine-containing neurons.19,20 H4 receptors, which share approximately 40% homology with H3 receptors, are highly expressed in the bone marrow and in leucocytes, and are moderately expressed in spleen, thymus, lung, small intestine, colon and heart.21–24 Reports from our laboratory have demonstrated that histamine-induced IL-6 and IL-8 production by human vascular endothelial cells are mediated through H1 receptors,9,25 which suggested a major role for this receptor subtype in endothelial cell functions.
The role of infection in atherosclerosis has been proposed and bacterial pathogens are widely recognized as inflammatory stimulants.26 Lipopolysaccharide (LPS), a major cell wall component of Gram-negative bacteria, induces the production of pro-inflammatory cytokines and the expression of adhesion molecules on endothelial cells.27 The Gram-positive cell wall components, peptidoglycan (PGN) and lipoteichoic acid (LTA) also induce endothelial activation.28,29 The response to bacterial pathogens via pattern recognition receptors on endothelial cells is important for innate immune defence but their amplified activation may lead to persistent vascular inflammation. The innate immune system recognizes bacterial pathogens through a family of receptors called Toll-like receptors (TLRs). Mammalian cells express at least 10 TLRs, and among them, TLR4 is the major LPS receptor30–32 and TLR2 recognizes both PGN and LTA.33–35 Further evidence for the possible role of TLRs in coronary disease is the substantial increase in TLR2 and TLR4 message in the endothelium of human atherosclerotic lesions.36–38
A previous report from our laboratory has shown that LPS-induced production of IL-6 and IL-8 by human endothelial cells is greatly enhanced by the presence of histamine.9 These results suggest that the co-operative action between histamine and Gram-negative bacterial components lead to amplified inflammatory responses in vascular endothelium. The mechanism by which histamine enhances sensitivity to the bacterial component is unknown. The objective of this study was to first test whether a similar co-operation also exists between Gram-positive bacterial cell wall components and histamine, and then to define the possible mechanisms of synergy between histamine and the bacterial pathogens in endothelial cell activation. The results presented in this report demonstrate that histamine amplifies endothelial cell responsiveness to both Gram-negative and Gram-positive cell wall components and the synergy between histamine and bacterial pathogens is associated with enhanced expression of TLR2 and TLR4.
Materials and methods
Materials
Human umbilical endothelial cells (HUVEC), endothelial cell growth medium (EGM-2 MV), trypsin–ethylenediaminetetraacetic acid (EDTA) and trypsin neutralizing solution were purchased from Cambrex (San Diego, CA). Escherichia coli (0111:B4) LPS, LTA, PGN, polymyxin B and protease inhibitors were supplied by Sigma Chemical Co. (St Louis, MO). Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 were purchased from R & D Systems (Minneapolis, MN). TLR2 (N-17, H-175) and TLR4 (H-80) antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA), and Cy3 goat anti-rabbit and normal goat serum were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). TRIzol Reagent, RNAse-free DNAse1, Superscript™ II RNase H– Reverse Transcriptase System, and Taq polymerase were obtained from Life Technologies (Rockville, MD). The gene-specific primers used in reverse transcription–polymerase chain reaction (RT-PCR) were purchased from Invitrogen Life Technologies (Carlsbad, CA). Nuclear and cytoplasmic extraction reagents (NE-PER™) were product of Pierce-Endogen (Rockford, IL) and poly(dI-dC) was obtained from Pharmacia (Piscataway, NJ).
Culture of HUVEC
HUVEC were grown in EGM-2 MV containing 1 μg/ml hydrocortisone acetate, 50 ng/ml gentamycin, 50 μg/ml amphotericin B, and the recommended concentrations of human epidermal growth factor, vascular endothelial growth factor, human fibroblast growth factor-B, recombinant insulin-like growth factor-1 (R3I) growth factor, ascorbic acid and 5% fetal bovine serum. At confluence, the cells were detached from the culture flasks using trypsin–EDTA, washed twice, and resuspended in EGM-2 MV. The cells used in all experiments were between three and five passages.
Assay of IL-6 production
HUVEC (2 × 104) were plated on to each of the wells of a 96-well microtitre plate and allowed to adhere for 24 hr. Following adherence, selected concentrations of the activating stimuli or medium were added to the monolayers and incubated at 37° in 5% humidified CO2 for 24 hr. After the incubation, culture supernatants were harvested and assayed for IL-6 levels by ELISA.
RT-PCR
Total RNA was isolated from HUVEC treated with medium or appropriate agonists, using TRIzol reagent and treated with RNAse-free DNAse I. For a reverse transcription reaction, Superscript™II RNase H– Reverse Transcriptase system was employed. PCR amplification was performed with Taq polymerase for 32 cycles at 95° for 45 seconds, 54° for 45 seconds, and 72° for 1 min (for TLR2, TLR4 and GAPDH), 95° for 45 seconds, 60° for 45 seconds, and 72° for 1 min (for IL-6), 95° for 30 seconds, 52° for 45 seconds, and 72° for 45 seconds (for MD-2), 94° for 30 seconds, 60° for 40 seconds, and 70° for 2 min (for MyD88). PCR products were electrophorosed on 2% agarose gel. The oligonucleotide primers used for RT-PCR are given in Table 1.
Table 1.
Gene-specific primers used in RT-PCR
| Gene | Primer sequence (5′→3′) | PCR product (size, bp) |
|---|---|---|
| IL-6 | ATGAACTCCTTCTCCACAAGCGC | |
| GAAGAGCCCTCAGGCTGGACTG | 620 | |
| TLR2 | GCCAAAGTCTTGATTGATTGG | 347 |
| TTGAAGTTCTCCAGCTCCTG | ||
| TLR4 | TGGATACGTTTCCTTATAAG | |
| GAAATGGAGGCACCCCTTC | 548 | |
| MD-2 | GAAGCTCAGAAGCAGTATTGGGTC | |
| GGTTGGTGTAGGATGACAAACTCC | 422 | |
| MyD88 | TAAGAAGGACCAGCAGAGCC | |
| CATGTAGTCCAGCAACAGCC | 200 | |
| GAPDH | TGATGACATCAAGAAGGTGGTGAAG | |
| TCCTTGGAGGCCATGTGGGCCAT | 240 |
Immunofluorescent staining
HUVEC (20 000/well) grown on chamber slides were incubated either with medium or histamine (10 μm) for 4, 8, 16 and 24 hr. The cells were then fixed with 4% paraformaldehyde, washed and treated with blocking solution (1% bovine serum albumin, 5% normal goat serum and 0·3% Triton X-100 in phosphate-buffered saline) and stained with rabbit anti-human TLR2 (H-175) or TLR4 (H-80). After overnight staining, the cells were washed, and incubated for 1 hr with the secondary antibody (Cy3 goat anti-rabbit). The slides were then viewed under a fluorescence microscope and imaged.
Western blot
Confluent HUVEC monolayers were incubated with histamine (10 μm) for 16 hr at 37°. The cells were then lysed in buffer containing 20 mm Tris–HCl (pH 7·4), 137 mm NaCl, 10% glycerol, 1% Triton X-100, 2 mm EDTA, 25 mmβ-glycerolphosphate, 2 mm sodium pyrophosphate, 1% protease inhibitor cocktail and 0·5 mm dithiothreitol at 4° for 30 min. Cell debris was removed by centrifugation of the lysate at 13 000 g for 10 min. Aliquots of supernatants normalized for protein concentrations were mixed with equal volumes of 2 × sodium dodecyl sulphate sample buffer and heated to 100° for 5 min. Samples were resolved on 10% sodium dodecyl sulphate–polyacrylamide gel elcetrophoresis and transferred onto a nitrocellulose membrane. After blocking for 2 hr in TBST (20 mm Tris–HCl, 150 mm NaCl, 0·1% Tween-20) containing 5% non-fat milk, membranes were washed thrice in TBST and probed for 1 hr at 4° with anti-TLR4 (H-80) and for 18 hr at 4° for TLR2 using anti-TLR2 antibody (N-17). After washing thrice, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and washed five times and bands were detected using enhanced chemiluminescence reagents (Bio-Rad, Hercules, CA).
NF-κB activation
Confluent HUVEC monolayers were treated with LPS (100 ng/ml), PGN (100 μg/ml), or LTA (10 μg/ml) in the absence or presence of histamine (10 μm) for 2 hr at 37°. After the incubation, nuclear proteins were extracted using nuclear and cytoplasmic extraction reagents (NE-PER™) according to the manufacturer's protocol. An electrophoretic mobility shift assay (EMSA) of nuclear proteins was carried out utilizing the oligonucleotide probe, 5′-AGTTGAGGGGACTTTCCCAGGC-3′. The specific oligonucleotide and its complimentary strand were annealed and then end-labelled using γ-[32P]ATP (6000 Ci/mmol; New England Nuclear, Boston, MA) and T4-polynucleotide kinase (Amersham, Piscataway, JS), as described.39,40 Nuclear proteins (8 μg) were mixed with 3 μg poly(dI-dC) and ∼2 ng (100 000–400 000 counts per minute) of end-labelled DNA in 30 μl of 50 mm Tris–HCl buffer (pH 7·5) containing 250 mm NaCl, 5 mm EDTA, 5 mm dithiothreitol and 25% glycerol, and incubated for 30 min at 25°. Following the initial binding reaction, 20 μl of the mixture were electrophoresed on a 6% native polyacrylamide gel, and then processed for autoradiography.
Statistical analysis
The data were analysed by one-way analysis of variance with subsequent Newman–Kuel test. All results were expressed as mean ± SD and P < 0·05 was considered significant.
Results
Histamine amplifies LPS, LTA and PGN-induced endothelial cell production of IL-6
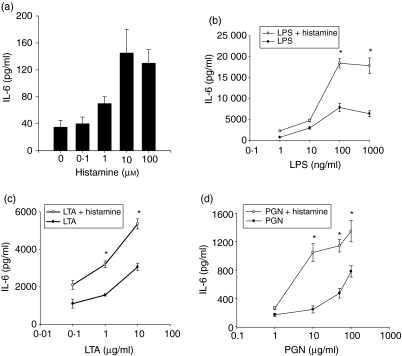
To examine the direct effect of histamine on endothelial cell production of IL-6, HUVEC monolayers were incubated with different concentrations of histamine (0·1–100 μm) for 24 hr, and the levels of secreted IL-6 in the culture media were quantified. The results presented in Fig. 1(a) reveal that histamine is capable of inducing IL-6 production in a dose-dependent manner. The effect of histamine begins at a concentration of 1 μm and achieved a plateau at a concentration of 10 μm.
Figure 1.
Amplification of LPS, LTA and PGN-induced IL-6 production by histamine. HUVEC (1 × 104 cells) were plated on to each of the wells of a 96-well culture plate and allowed to adhere for 18–20 hr. The monolayers were then incubated with the different doses of histamine (0·1–100 μm)(a), and histamine (10 μm) in the presence of LPS (1–1000 ng/ml) (b), LTA (0·1–10 μg/ml) (c), or PGN (1–100 μg/ml)(d). After a 24-hr incubation, IL-6 levels in the culture media were assayed by ELISA. Each value presented is the mean ± SD of quadruplicate determinations. The results presented are representatives of four independent experiments. An asterisk indicates P < 0·01 when compared to the value for the TLR agonist alone.
To evaluate the modulatory effect of histamine on endothelial cell responses to Gram-negative and Gram-positive cell wall components, HUVEC monolayers were incubated with different doses of LPS, LTA, or PGN in the presence or absence of histamine (10 μm) for 24 hr and the secreted IL-6 levels were quantified. Figure 1(b) demonstrates that LPS induced significant stimulation of IL-6 production in a dose-dependent fashion, which achieved a plateau at a dose of 100 ng/ml. Simultaneous presence of histamine markedly enhanced LPS-induced IL-6 production at all concentrations of LPS-tested.
Gram-positive bacterial cell wall components, LTA and PGN, also stimulated endothelial cells to generate IL-6 in a dose-dependent manner. When compared to the effect of LPS, the responses by LTA and PGN were of a lesser magnitude whether compared on a weight or molar basis. As in the case of LPS, LTA- and PGN-induced IL-6 production was also amplified by histamine (Fig. 1c,d). The effects of LTA and PGN were resistant to polymyxin B treatment, demonstrating that the effects were not the result of LPS contamination in the LTA and PGN preparations (data not shown).
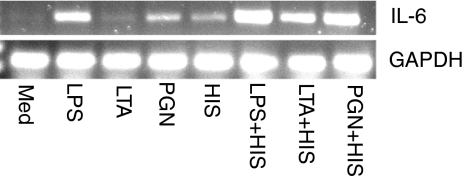
Increased IL-6 mRNA expression after stimulation with LPS, LTA, PGN and histamine
To examine whether the amplified release of IL-6 by combined effects of histamine and bacterial cell wall components reflects in enhanced gene expression, IL-6 mRNA expression was examined (Fig. 2). Unactivated HUVEC expressed relatively low levels of IL-6 mRNA. IL-6 mRNA expression was significantly increased when HUVEC were stimulated with LPS, LTA, PGN and histamine. LPS was found to be the most potent inducer of IL-6 mRNA expression. A further enhancement of LPS, LTA and PGN-induced IL-6 mRNA expression was noted in the presence of histamine.
Figure 2.
Enhancement of IL-6 mRNA expression by histamine and bacterial cell wall components. HUVEC (1 × 106 cells) were allowed to adhere in each of the wells of six-well culture plates for 18–20 hr. The monolayers were then incubated with LPS (100 ng/ml), LTA (10 μg/ml), PGN (100 μg/ml), or histamine (HIS, 10 μm), and in combinations of histamine + LPS, histamine + LTA, or histamine + PGN. After a 2-hr incubation, total RNA was isolated and subjected to RT-PCR using IL-6 primers (22 cycles) and the amplified products were electrophoresed on a 2% agarose gel. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression was used for normalization. The results presented are representatives of four independent experiments.
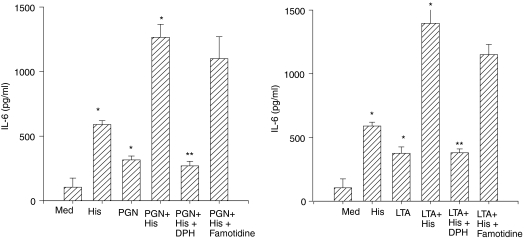
H1 receptor antagonist inhibits histamine-mediated enhancement of IL-6 production
Diphenhydramine (10 μm), an H1 receptor antagonist, completely abrogated histamine-induced potentiation of IL-6 production by LTA- and PGN-stimulated HUVEC (Fig. 3). Famotidine, an H2 receptor antagonist, had no effect on histamine-induced potentiation of IL-6 production. A concentration of 10 μm diphenhydramine is sufficient to block the effect of histamine in endothelial cells whereas famotidine, even at 100 μm, did not have any effect.9
Figure 3.
H1 receptor antagonist, but not H2 receptor antagonist, abrogates histamine-induced enhancement of PGN and LTA on IL-6 production. HUVEC (1 × 104 cells) were plated on to each of the wells of a 96-well culture plate and allowed to adhere for 18–20 hr. HUVEC monolayers were subsequently incubated with 100 μg/ml PGN (left panel) or 10 μg/ml LTA (right panel) in the presence and absence of histamine (10 μm). Wherever applicable, 10 μm diphenhydramine (DPH, H1R antagonist), or 10 μm famotidine (H2R antagonist) was added simultaneously with the agonists. After a 24-hr incubation, the culture media were assayed for IL-6 levels by ELISA. Each value presented is the mean ± SD of the quadruplicate determinations. The results presented are representative of four independent experiments. Asterisk indicates a statistically significant difference at P < 0·05 when compared to HUVEC incubated with medium alone. Double asterisk indicates a statistically significant effect of DPH at P < 0·05 when compared to the effect of histamine plus LTA or histamine + PGN.
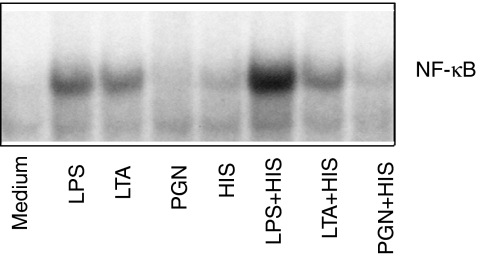
Increased NF-κB activation by histamine and bacterial cell wall components
The exposure of endothelial cells to LPS or LTA for 2 hr substantially increased translocation of NF-κB proteins to the nuclei (Fig. 4). Incubation of HUVEC with LPS in the presence of histamine further enhanced nuclear translocation of NF-κB, although histamine alone was a weak stimulant of NF-κB translocation. In contrast to the combined effect of histamine and LPS, histamine did not amplify NF-κB activation in LTA or PGN-treated HUVEC.
Figure 4.
Increased nuclear NF-κB proteins in HUVEC treated with histamine and bacterial cell wall components. HUVEC (2·5 × 106 cells) were plated in 60-mm culture dishes and allowed to adhere for 18–20 hr. The cell monolayers were then incubated with LPS (100 ng/ml), LTA (10 μg/ml), PGN (100 μg/ml) or histamine (HIS, 10 μm), and in combinations of histamine+LPS, histamine+ LTA, or histamine+PGN. After a 2-hr incubation, the nuclear proteins extracted from the cells were incubated with 32P-labelled oligonucleotide containing NF-κB binding sites. The samples were then subjected to electrophoresis on 6% native polyacrylamide gels and processed for autoradiography. The autoradiograph shown is the representative of three separate experiments.
Histamine induces TLR2 and TLR4 mRNA expression
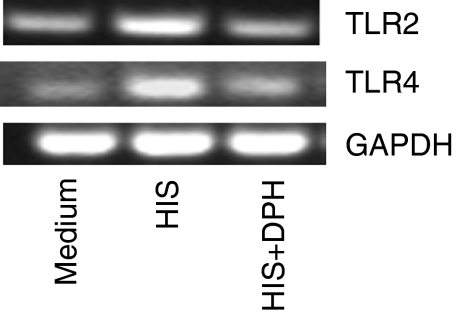
Gram-positive and Gram-negative bacterial cell wall components are recognized by TLR2 and TLR4, respectively. To assess the role of these receptors in histamine-mediated amplification of the effects of LPS, LTA and PGN, the expression of TLR2 and TLR4 mRNA was assessed. The results demonstrate that histamine (10 μm) induced both TLR2 and TLR4 mRNA expression in HUVEC after 2 hr of stimulation (Fig. 5). The histamine-induced TLR2 and TLR4 mRNA expression was also blocked by the H1 receptor antagonist, diphenhydramine (Fig. 5).
Figure 5.
Histamine induces TLR2 and TLR4 mRNA expression. HUVEC (1 × 106 cells) were allowed to adhere in each of the wells of a six-well culture plates for 18–20 hr. Cell monolayers were then incubated with histamine (10 μm) alone or histamine+diphenhydramine (DPH, 10 μm) for 2 hr. After the incubation, total RNA was isolated and subjected to RT-PCR using TLR2 and TLR4 primers. The amplified products were electrophoresed on a 2% agarose gel. GAPDH mRNA expression was used for normalization. The results presented are representatives of four independent experiments.
Up-regulation of TLR2 and TLR4 proteins by histamine
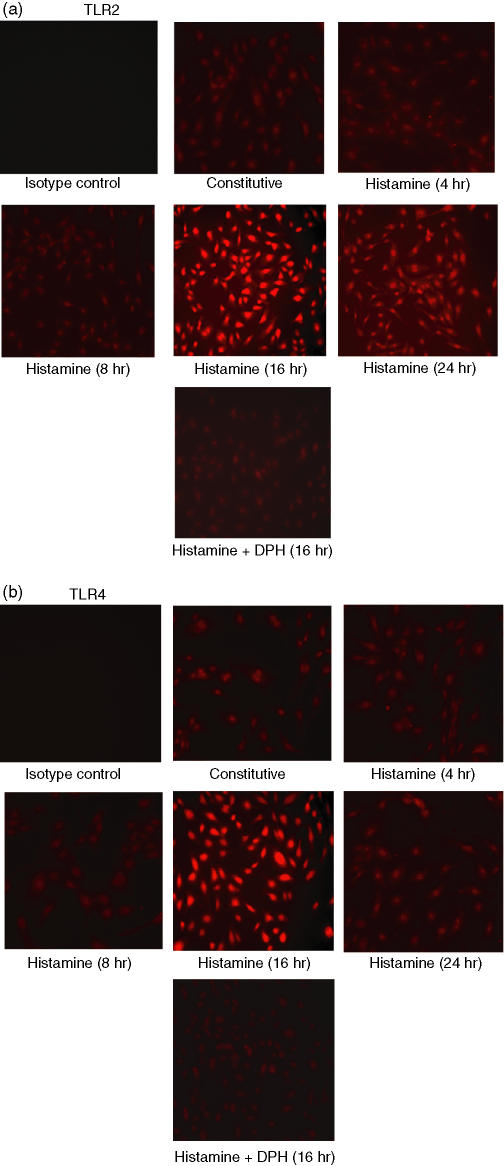
To determine whether histamine-induced TLR2 and TLR4 mRNA expression is associated with increased expression of proteins, immunofluorescence and Western blot analyses were carried out. Immunofluorescence analyses revealed detectable levels of expression of TLR2 (Fig. 6a) and TLR4 (Fig. 6b) proteins which were markedly enhanced when HUVEC were incubated with histamine for 16 hr and was completely inhibited by the H1 receptor antagonist, diphenhydramine. Histamine-induced TLR2 and TLR4 expression was transient as it returned to constitutive levels by 24 hr even in the continued presence of histamine.
Figure 6.
Histamine induces expression of TLR2 (a) and TLR4 (b) proteins as determined by immunofluorescence. HUVEC (2 × 104 cells) were plated on each of the wells of a chamber slide and allowed to adhere for 18–20 hr. The cells were incubated with medium (constitutive expression), histamine (10 μm) for 4, 8, 16 and 24 hr, and with histamine plus diphenhydramine (DPH, 10 μm) for 16 hr. After stimulation, the cells were fixed with paraformaldehyde and stained with the respective antibodies, or isotype immunoglobulin G (control). After extensive washing, the cells were incubated with rhodamine-conjugated secondary antibodies and viewed under a fluorescence microscope for imaging.
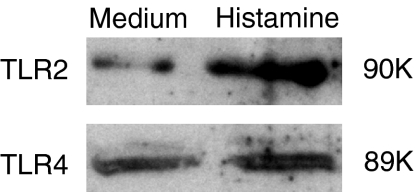
The constitutive expression of TLR2 and TLR4 proteins and their up-regulation by histamine were further verified by Western blot analysis. As evident from Fig. 7, treatment of HUVEC with histamine for 16 hr resulted in enhanced expression of TLR2 and TLR4 proteins.
Figure 7.
Histamine up-regulates the expression of TLR2 and TLR4 as determined by Western blot. HUVEC (2 × 106 cells) were plated in 60-mm culture dishes and allowed to adhere for 18–20 hr. Cell monolayers were then incubated with medium or histamine (10 μm) for 16 hr. After washing, total cell lysates were prepared as described in the Materials and methods section. Protein samples were separated by sodium dodecyl sulphate–polyscrylamide gel electrophoresis (10%), transferred to nitrocellulose membranes, and probed with anti-human TLR2 and TLR4 antibodies. The signals were detected by standard enhanced chemiluminescence technique after labelling with horseradish peroxidase-conjugated secondary antibodies.
Priming with histamine enhances the responsiveness of HUVEC to TLR2 and TLR4 ligands
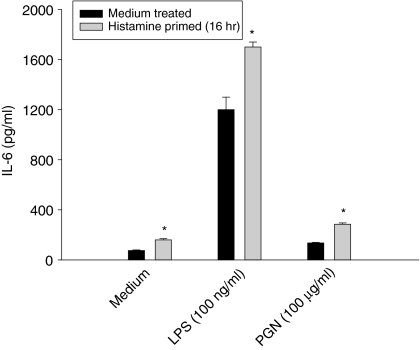
The results presented in Figs 6 and 7 demonstrated that exposure of HUVEC for 16 hr results in a maximum level of expression of TLR2 and TLR4. To relate the histamine-induced expression of TLRs with enhanced sensitivity to the bacterial cell wall components, HUVEC were primed with histamine for 16 hr and subsequently challenged with LPS and PGN, and IL-6 production was measured. As shown in Fig. 8, histamine priming significantly enhanced the responsiveness of cells to both LPS and PGN. These results further suggest that the newly expressed receptors are functionally active and contribute to the amplified inflammatory responses.
Figure 8.
Histamine priming enhances the responsiveness of endothelial cells to TLR2 and 4 ligands. HUVEC (1 × 104 cells) were plated on to each of the wells of a 96-well culture plate and allowed to adhere for 18–20 hr. HUVEC monolayers were incubated with medium or histamine (10 μm) for 16 hr, and then activated with LPS (100 ng/ml), or PGN (100 μg/ml) for an additional 24 hr. After the incubation, IL-6 levels in the culture media were assayed. Each value presented is the mean ± SD of quadruplicate determinations. The results presented are representatives of three independent experiments. An asterisk indicates P < 0·01 when compared to value for medium-treated cells.
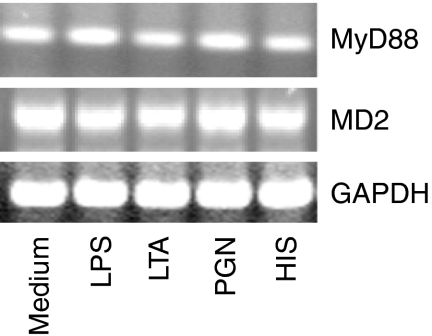
MD-2 and MyD88 mRNA expression is not altered by histamine or by the bacterial cell wall components
MD-2 is an accessory protein, which is required by TLR4 to respond to LPS.31,41,42 Similarly, MyD88 is an adapter molecule that is required for TLR-mediated signal transduction and NF-κB activation.43,44 To evaluate the role of MD-2 and MyD88 in histamine-mediated amplification, their mRNA expressions were determined by RT-PCR. The results demonstrate that treatment of HUVEC with LPS, LTA, PGN, or histamine for 2 hr did not affect MD-2 and MyD88 mRNA expression (Fig. 9).
Figure 9.
MD-2 and MyD88 mRNA expression. HUVEC (1 × 106 cells) were allowed to adhere in each of the wells of a six-well culture plate for 18–20 hr. HUVEC monolayers were then incubated with LPS (100 ng/ml), LTA (10 μg/ml), PGN (100 μg/ml) and histamine (HIS, 10 μm) for 2 hr. After the incubation, total RNA was isolated, and subjected to RT-PCR using MD-2 and MyD88 primers. The amplified products were electrophoresed on a 2% agarose gel. GAPDH mRNA expression was used for normalization. The results presented are representatives of four independent experiments.
Discussion
The results of the present study demonstrate that histamine, a major secretory product of the mast cell, is not only able to stimulate endothelial cells to synthesize and secrete IL-6, but can also amplify the stimulatory effects of Gram-positive and Gram-negative bacterial cell wall components. The ability of histamine to induce both IL-6 mRNA and protein expression suggests that the amplifying effect was not solely the result of histamine-induced secretion of pre-formed cytokine but is the result of enhanced gene expression and protein synthesis. The fact that histamine by itself is a strong stimulant of IL-6 mRNA expression suggests that the primary effect of histamine in the amplification cascade is induction of IL-6 gene expression, whereas the combined action of histamine and bacterial pathogens involves both transcriptional and post-transcriptional regulation.
Toll-like receptors are involved in innate immune recognition and cellular activation in response to microbial antigens.34,35,45–48 The present results show that histamine-induced amplification of the effects of the bacterial components was associated with enhanced expression of TLR2 and TLR4 mRNA and proteins. Although TLR2 and TLR4 mRNA expression is seen in 2 hr, the maximum protein expression was noted at 16 hr after activation with histamine. Histamine-induced TLR2 and TLR4 expression returned to constitutive levels by 24 hr, which suggests that the up-regulation of receptors is transient. To our knowledge, this is the first study, which demonstrates the ability of histamine to up-regulate TLR2 and TLR4 expression and function, and modulate cytokine secretion in endothelial cells.
The modulation of the expression of the TLRs by histamine may provide a critical role for this amine in endothelial cell response to microbial pathogens and activation of inflammatory responses. The histamine-mediated enhancement of IL-6 production, as well as TLR2 and TLR4 expression, was inhibited by the H1 receptor antagonist diphenhydramine and not by famotidine, demonstrating that the effect is mediated via H1 receptors. It appears that the surface expression of TLR2 and TLR4 is relatively low when compared to the level of mRNA expression. The low level surface expression of TLR2 and TLR4 suggests the requirement of only a limited number of functional receptors and the stringent regulation of pathogen-mediated inflammatory responses in the vessel wall. The low level surface expression of TLRs in endothelial cells is consistent with the recent report by Zeuke et al.49 who failed to detect TLR4 surface proteins in human coronary artery endothelial cells despite the presence of substantial amounts of mRNA. It is noteworthy that TLR4 remains functional even at extremely low receptor numbers in several cell types including immature dendritic cells and neutrophils.50
Since histamine induces TLR2 and TLR4 expression, it is reasonable to postulate that the amplified responsiveness of endothelial cells to bacterial products is the result of enhanced or sustained signalling via newly expressed TLR2 and TLR4. This contention is supported by the finding that endothelial cells primed with histamine for 16 hr, which induced maximum levels of TLR2 and TLR4, produced significantly higher amounts of IL-6 when challenged with LPS or PGN. It is noteworthy that although TLR2 and TLR4 are expressed at low levels on human endothelial cells49 a substantial increase of their messages was noted in the endothelium covering human atherosclerotic lesions.37 These findings, together with the presence of an increased number of mast cells in the atherosclerotic lesions,51,52 suggest that the co-operative action of mast cell-derived histamine and bacterial products possibly derived from colonized or circulating pathogens cause persistent vascular inflammation. It is of interest that a TLR4 polymorphism, which diminishes the inflammatory response to Gram-negative pathogens, is associated with decreased risk of atherosclerosis in humans.37
Although TLRs are the primary signalling receptor for the bacterial cell wall components, TLR2 and TLR4 alone are not capable of sensing the presence of LPS. Another accessory molecule MD-2, which is physically associated with TLR4, is required for LPS recognition and signalling.41,53 The present results reveal that histamine did not modulate the expression of MD-2 mRNA in endothelial cells. Based on the constitutive expression of MD-2 in naïve endothelial cells it is presumed that adequate amounts of MD-2 molecules are present for full response to the bacterial pathogens and histamine-mediated amplification does not involve newly expressed MD-2. Similarly, exposure of endothelial cells to histamine did not alter the expression of MyD88, an accessory molecule that is recruited to the intracellular domains of TLR2 and TLR4 after recognition of the pathogens.43,44 It is possible that histamine may enhance the recruitment of both MD-2 and MyD88 from existing pools without enhancing their syntheses. Alternatively, histamine may enhance the release of MD-2 proteins or stabilize the LPS/MD-2 complex, as secreted MD-2 in the absence of LPS is labile.54
Activation of TLR2 and TLR4 involves NF-κB translocation.55,56 Promoters of the gene for IL-6 contain recognition sites for the transcription factor NF-κB57,58 and a number of studies have demonstrated the involvement of NF-κB in the regulation of IL-6 transcription.59,60 In this study, activation of endothelial cells by histamine alone caused only a minimal level of NF-κB translocation although it caused a substantial amount of IL-6 mRNA expression. Interestingly, histamine-induced IL-6 mRNA expression did not reflect proportional levels of IL-6 protein synthesis. The exposure of endothelial cells to LPS for 2 hr substantially increased translocation of NF-κB proteins to the nuclei, which was further amplified by the presence of histamine. Incubation of HUVEC with LTA or PGN also caused nuclear translocation of NF-κB proteins but the effect was of lesser magnitude when compared to that of LPS, and was not enhanced by histamine. Although both LTA and PGN induced signal transduction via TLR2, LTA was more effective in inducing NF-κB activation. It is apparent that the low level of NF-κB translocation in PGN-treated cells is associated with a lesser degree of IL-6 production when compared to LTA.
The results presented here unravel one of the mechanisms by which histamine amplifies endothelial cell sensitivity to TLR2 and TLR4 ligands. Since both bacterial products and histamine were simultaneously present in the culture system, and such an environment could presumably be present in vivo under certain pathological conditions, the modulation of histamine responsiveness by TLR2 and TLR4 ligands is also feasible. Thus, it is conceivable that the amplification of IL-6 production by the combined effects of histamine and bacterial products could also be the result of an enhanced endothelial cell response to histamine secondary to the action of bacterial components. To this effect, we have noted increased H1 receptor mRNA expression in endothelial cells after exposure to LPS, LTA, or PGN (Talreja et al. unpublished data). It remains to be tested whether endothelial cells, which are primed by the bacterial pathogens, express functionally active H1 receptors and whether the enhanced H1 receptor signalling amplifies the inflammatory responses in the endothelium.
In conclusion, the present study demonstrates that incubation of human endothelial cells with LPS, LTA, or PGN results in the stimulation of IL-6 mRNA expression and IL-6 release. The effects of LPS, LTA, or PGN on endothelial activation are further enhanced by the simultaneous presence of histamine. Both the direct and potentiating effects of histamine on endothelial cell activation are completely abolished by an H1 receptor antagonist, and not by an H2 receptor antagonist. The histamine-induced amplification of the effects of the bacterial cell wall components on endothelial cell activation is associated with the up-regulation of TLR2 and TLR4 expression. The results also suggest that histamine enhances cellular responses to LPS via amplified NF-κB translocation. This phenomenon was not observed with LTA and PGN. Studies on the effects of Gram-positive and Gram-negative bacterial pathogens on the modulation of H1 receptor expression and signalling, and their contribution to the synergy are currently underway. Collectively, the results suggest that individuals who have increased levels of circulating histamine may be at a risk for persistent and amplified inflammatory responses to bacterial pathogens.
Acknowledgments
This study was supported by the Joseph and Elizabeth Carey Arthritis Funds, the Hinman Fund, and the Jones Fund from the Kansas University Endowment Association, and by the Lied Endowed Basic Science Research Fund from The University of Kansas Medical Center Research Institute.
References
- 1.Barnes PJ. Histamine receptors in the lung. Agents Action (Suppl.) 1991;33:103–22. doi: 10.1007/978-3-0348-7309-3_9. [DOI] [PubMed] [Google Scholar]
- 2.Hill SJ. Multiple histamine receptors: properties and functional characteristics. Biochem Soc Trans. 1992;20:122–5. doi: 10.1042/bst0200122. [DOI] [PubMed] [Google Scholar]
- 3.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 4.Dileepan KN, Lorsbach RB, Stechschulte DJ. Mast cell granules inhibit macrophage-mediated lysis of mastocytoma cells (P815) and nitric oxide production. J Leukoc Biol. 1993;53:446–53. doi: 10.1002/jlb.53.4.446. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA, Gallin JI, Kaplan AP. The selective chemotactic activity of histamine. J Exp Med. 1975;142:1462–76. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dileepan KN, Simpson KM, Lynch SR, Stechschulte DJ. Dismutation of eosinophil superoxide by mast cell granule superoxide dismutase. Biochem Arch. 1989;5:153–60. [Google Scholar]
- 7.Ogden BE, Hill HR. Histamine regulates lymphocyte mitogenic responses through activation of specific H1 and H2 histamine receptors. Immunology. 1980;41:107–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Beer DJ, Matloff SM, Rocklin RE. The influence of histamine on immune and inflammatory responses. Adv Immunol. 1984;35:209–68. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chi Y, Stechschulte DJ, Dileepan KN. Histamine-induced production of IL-6 and IL-8 by human coronary artery endothelial cells is enhanced by endotoxin and TNF-α. Microvascular Res. 2001;61:253–62. doi: 10.1006/mvre.2001.2304. [DOI] [PubMed] [Google Scholar]
- 10.Kalsner S, Richards R. Coronary arteries of cardiac patients are hyper reactive and contain stores of amines: a mechanism for coronary spasm. Science. 1984;223:1435–7. doi: 10.1126/science.6701530. [DOI] [PubMed] [Google Scholar]
- 11.Sakata YK, Komamura K, Hirayama A, Nato S, Kitakaze M, Hori M, Kodama K. Elevation of the plasma histamine concentration in the coronary circulation in patients with variant angina. Am J Cardiol. 1996;77:1121–6. doi: 10.1016/s0002-9149(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 12.Delneste Y, Lassalle P, Jeannin P. Histamine induces IL-6 production by human endothelial cells. Clin Exp Immunol. 1994;98:344–9. doi: 10.1111/j.1365-2249.1994.tb06148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeannin P, Delneste Y, Gossette P, Molet S, Lassalle P, Hamid Q, Tsicopoulos A, Tonnel AB. Histamine induces IL-8 secretion by endothelial cells. Blood. 1994;84:2229–33. [PubMed] [Google Scholar]
- 14.Hough LB. Genomics meets histamine receptors: new subtypes, new receptors. Mol Pharmacol. 2001;59:415–9. [PubMed] [Google Scholar]
- 15.Walsh GM, Annunziato L, Frossard N, et al. New insights into the second generation antihistamines. Drugs. 2001;61:207–36. doi: 10.2165/00003495-200161020-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kohka H, Nishibori M, Iwagaki H, et al. Histamine is a potent inducer of IL-18 and IFNγ in human peripheral blood mononuclear cells. J Immunol. 2000;164:6640–6. doi: 10.4049/jimmunol.164.12.6640. [DOI] [PubMed] [Google Scholar]
- 17.Caron G, Delneste Y, Roelandts E, Duez C, Bonnefoy JY, Pestel J, Jeannin P. Histamine polarizes human dendritic cells into Th2 cell-promoting effector dendritic cells. J Immunol. 2001;167:3682–6. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- 18.Jutel M, Watanabe T, Klunker S, et al. Histamine regulates T-cell and Ab responses by differential expression of H1 and H2 receptors. Nature (Lond) 2001;413:420–4. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 19.Leurs R, Blandina P, Tedford C, Timmerman H. Therapeutic potential of histamine H3 receptor agonists and antagonists. Trends Pharmacol Sci. 1998;19:177–83. doi: 10.1016/s0165-6147(98)01201-2. [DOI] [PubMed] [Google Scholar]
- 20.Hough LB, Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhlen S, editors. Histamine, in Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven; 1999. pp. 293–313. [Google Scholar]
- 21.Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279:615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- 22.Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–6. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 23.Morse KL, Behan J, Laz TM, et al. Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther. 2001;296:1058–66. [PubMed] [Google Scholar]
- 24.Gantner F, Sakai K, Tusche MW, Cruikshank WW, Center DM, Bacon KB. Histamine (H4) and (H2) receptors control histamine-induced interleukin-16 release from human CD8(+) T cells. J Pharmacol Exp Ther. 2002;303:300–7. doi: 10.1124/jpet.102.036939. [DOI] [PubMed] [Google Scholar]
- 25.Chi L, Bittel LS, Smirnova I, Stechschulte DJ, Dileepan KN. Signal transduction pathways in mast cell granule-mediated endothelial cell activation. Med Inflamm. 2003;12:79–87. doi: 10.1080/0962935031000097682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grayston JT, Kuo CC, Campbell LA, Benditt EP. Chlamydia pneumoniae, strain TWAR and atherosclerosis. Eur Heart J. 1993;14:66–71. [PubMed] [Google Scholar]
- 27.Brown Z, Gerritsen ME, Carley WW, Strieter RM, Kunkel SL, Westwick J. Chemokine gene expression and secretion by cytokine activated human microvascular endothelial cells. Differential regulation of monocyte chemoattractant protein-1 and interleukin-8 in response to interferon-gamma. Am J Pathol. 1994;145:913–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura N, Imanishi N, Koike H, Nakahara H, Phillips L, Morooka S. Lipoteichoic acid-induced neutrophil adhesion via E-selectin to human umbilical vein endothelial cells (HUVECs) Boichem Biophys Res Commun. 1995;217:1208–15. doi: 10.1006/bbrc.1995.2897. [DOI] [PubMed] [Google Scholar]
- 29.Blease K, Chen Y, Hellewell PG, Burke- Gaffney A. Lipoteichoic acid inhibits lipopolysaccharide-induced adhesion molecule expression and IL-8 release in human lung microvascular endothelial cells. J Immunol. 1999;163:6139–47. [PubMed] [Google Scholar]
- 30.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor-4 deficient mice are hyporesponsive to lipopolysaccharide. Evidence for TLR-4 as the lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 31.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR-4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 32.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;166:1938–89. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 33.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–7. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takeda H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 36.Kiechl S, Lorenz E, Reindl M, Weidermann C, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphism and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 37.Edfeldt K, Swedenberg J, Hansson GK, Yan CQ. Expression of Toll-like receptors in human atherosclerotic lesions. A possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- 38.Kleijn DD, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- 39.Ito N, Li Y, Suzuki T, Stechschulte DJ, Dileepan KN. Transient degradation of NF-κB proteins in macrophages after interaction with mast cell granules. Med Inflamm. 1998;7:397–407. doi: 10.1080/09629359890776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muroi M, Muroi Y, Yamamoto K, Suzuki T. Influence of 3′ half-site sequence of NF-κB motifs on the binding of lipopolysaccharide-activable macrophage NF-κB proteins. J Biol Chem. 1993;268:19534–9. [PubMed] [Google Scholar]
- 41.Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol. 2003;3:119–28. doi: 10.1016/s1567-5769(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 42.Talreja J, Dileepan K, Filla M, Dileepan KN, Stechschulte DJ. Lipopolysaccharide unresponsiveness of human conjunctival epithelial cells (HCEC) is associated with the lack of MD2 expression. FASEB J. 2003;17:C57. [Google Scholar]
- 43.Janssens S, Beyaert E. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474–82. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–67. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 45.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 46.Krutzik SR, Sieling PA, Modlin RL. The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol. 2001;13:104–8. doi: 10.1016/s0952-7915(00)00189-8. [DOI] [PubMed] [Google Scholar]
- 47.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 48.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57Bl/10ScCr mice. Mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 49.Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, Heine H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res. 2002;56:126–34. doi: 10.1016/s0008-6363(02)00512-6. [DOI] [PubMed] [Google Scholar]
- 50.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 51.Forman MB, Oates JA, Robertson D, Robertson RM, Roberts LJ, Virmani R. Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. 1985;313:1138–41. doi: 10.1056/NEJM198510313131807. [DOI] [PubMed] [Google Scholar]
- 52.Jeziorska M, McCollum C, Wooley DE. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol. 1997;182:115–22. doi: 10.1002/(SICI)1096-9896(199705)182:1<115::AID-PATH806>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kennedy MN, Mullen GED, Leifer CA, Lee CW, Mazzoni A, Dileepan KN, Segal DM. A complex of soluble MD2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J Biol Chem. 2004;279:34698–704. doi: 10.1074/jbc.M405444200. [DOI] [PubMed] [Google Scholar]
- 55.Faure E, Thomas L, Xu H, Medvedev AE, Equils O, Arditi M. Bacterial lipopolysaccharide and IFNγ induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: role of NF-kB activation. J Immunol. 2001;166:2018–24. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 56.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-κB through Toll-like receptor-4 in cultured human dermal endothelial cells. J Biol Chem. 2000;275:11058–63. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 57.Liberman TA, Baltimore D. Activation of interleukin 6 gene expression through the NF-κB transcription factor. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimazu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K. Involvement of NF-κB in the activation of IL-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990;10:561–8. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Ann Rev Immunol. 1994;12:141–50. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 60.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]