Abstract
The potential involvement of apoptosis in the pathogenesis of visceral leishmaniasis (VL) was examined by studying spontaneous and Leishmania antigen (LAg)-induced apoptosis using cryopreserved peripheral blood mononuclear cells (PBMC) of Sicilian patients with VL. Results indicate that monocytes and T lymphocytes from acute VL patients show a significantly higher level of apoptosis compared with that observed in healed subjects. The percentage of apoptotic cells was higher in monocytes than in T lymphocytes. T cells involved in programmed cell death (PCD) were mainly of the CD4+ phenotype. In particular, the T helper 1-type (Th1) subset, as evaluated by chemokine receptor-5 (CCR5) expression, is involved in this process. Cell death in Th1-type uses a CD95-mediated mechanism. Furthermore, Th1-type CCR5+ cells are prone to cell suicide in an autocrine or paracrine way, as attested by enhanced expression of CD95L in acute VL patients. The reduction in Th1-type cells by apoptosis was confirmed by the decrease in interferon-γ secretion. In conclusion, apoptosis of monocytes, CD4+ and CD4+ CCR5+ T cells could be involved in the failure of cell mediated immunity that is responsible for severe immune-depression in VL.
Keywords: CD4+ CCR5+, CD4+ CCR3+, monocytes, apoptosis, leishmaniasis
Introduction
Visceral leishmaniasis (VL) is a disease caused by the protozoan parasite Leishmania donovani (L. donovani). In particular, in the Mediterranean basin the species of Leishmania infantum (L. infantum) has long been recognized as the causative agent of VL.1Leishmania parasites replicate in macrophages and challenge most of the immune cells in a complex manner.2,3 Chronicity and high mortality is generally associated with circulating lymphopenia and the impairment of T-cell mediated immunity, evaluated as alteration of delayed-type hypersensitivity, of lymphocyte proliferation and of the cytokine network.2,3 The outcome of the infection depends on differential CD4+ T-cell responses, in particular, on the polarization of T helper (Th) cells into Th1 or Th2 on the basis of the cytokines they produce: interferon-(IFN)-γ for Th1 and interleukin (IL)-4, IL-5 and IL-13 for Th2.3,4 At the effector level the balance of Th1/Th2 subsets and cytokines determines the induction of resistance to Leishmania in macrophages from mice and human.2,3
Recently, Th1 and Th2 lymphocytes have been identified on the basis of the expression of chemokine receptors (CCRs) on their surface. Thus, differentiation of T cells into Th1 is associated with chemokine receptors CCR5 and CXCR3, and differentiation in Th2 with CCR3, CCR4 and CCR8.5,6 T-cell maturation from naive to memory cells is characterized by a reduction of CXCR4 and an increase in CCR5.7 The dichotomy of Th1 and Th2 responses induced by Leishmania are clearly showed in the murine model of cutaneous leishmaniasis by L. major, where IFN-γ and IL-12 are critical in Th1 cell development and in the control of infection, whereas IL-4 in Th2 cell development and exacerbation of disease.2,3,8 In experimental visceral leishmaniasis by L. donovani the disease progression is caused by the failure of an appropriate Th1 response, rather than the Th2 cell proliferation.9 On the other hand, Th1 protective responses are dependent on IL-12 production.10 In humans infected with L. donovani, the polarization of T-cell responses into Th1- and Th2-type is less clear, but could also be relevant. In fact, the control of infection or complete recovery is associated with an increased production of IL-2 and IFN-γ.11–14 Furthermore, IL-10 production correlates with the progression of VL15 and neutralization of IL-10 with a specific monoclonal antibody (mAb) restored T-cell proliferation and IFN-γ production in PBMC from acute VL patients.16In vitro studies have also demonstrated that IL-12 shifts the responses toward a Th1-type and enhances IFN-γ production.17 In humans, the balance of cytokines at the site of primary activation of the Leishmania-specific cells appears to be of major importance for the development of Th1 and Th2 responses3,4 even though other unknown factors might influence the cellular immune response.
Although data indicate that L. donovani parasites cause alterations of immune system with immune-depression, the exact mechanism by which the parasites induce immune-depression is not clear. Previously, it has been demonstrated that apoptosis plays a fundamental role in many normal biological processes as well as several disease states.18,19 It has been shown both in mice and humans that the induction of T-cell apoptosis could be involved in the defective host-cellular responses to challenge with pathogenic infectious agents.20–24 In experimental visceral leishmaniasis it has been demonstrated that the infection of a susceptible host results in CD4+ T cell apoptosis and a decrease in Th1 cytokine production.25,26 Mononuclear phagocytes also undergo apoptosis, although the regulation of this pathway is less well established than in lymphocytes. However, recent reports suggest that programmed death cell may play a crucial role in the regulation of monocyte differentiation.27,28 It has been reported in experimental models that some intracellular pathogens, which multiply within macrophages, induce apoptosis.29,30 In particular, it has been demonstrated that resistance to L. major depends on apoptotic mechanisms, mainly operating through the Fas (APO-1/CD95) pathway, and singeneic gld and lpr mice lacking a functional Fas system, fail to heal their lesions.30 Furthermore in mice infected with L. donovani parasites an increased incidence of T-cell apoptosis in liver and spleen was also observed.31
In this study, using cryopreserved peripheral blood mononuclear cells (PBMC), we have investigated the involvement of leucocyte apoptosis and cytokine modifications in patients with VL in acute and healed phases of disease.
Materials and methods
Subjects
Nine Sicilian adult patients with characteristic clinical signs and symptoms of active VL were studied. All patients had irregular fever, hepatosplenomegaly, anaemia, leucopenia, thrombocytopenia and hypergammaglobulinaemia. The diagnosis was confirmed by the presence of amastigotes in the spleen or bone marrow aspirates, by a positive antileishmania titre evaluated by indirect immune fluorescence (>1/100) and by counterimmunoelectrophoresis.32 Length of illness before diagnosis was less than 6 weeks. PBMC were collected at different stages of the disease: at the moment of diagnosis without specific treatment and after clinical recovery. The control group consisted of nine blood donors from the same endemic area. The treatment of disease was carried out with conventional therapy using N-methyl-glucamine antimonate; the drug dosage was based on the body surface area (bsa) according to the formula: 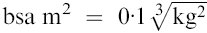 . The drug was given four times a day intramuscularly reaching the daily dose chosen.
. The drug was given four times a day intramuscularly reaching the daily dose chosen.
Reagents
Tissue culture medium consisted of RPMI-1640 (Hyclone Laboratories, Logan, UT) supplemented with glutamine (2 mm), antibiotics (50 µg/ml penicillin; 50 U/ml streptomycin) plus 10% fetal calf serum (FCS; Hyclone). Endotoxin levels in the FCS and RPMI were less than 0·03 EU (<10 ng/ml) as assessed by the limulus amoebocyte lysate assay. Soluble leishmanial antigen (LAg) was prepared by 10 cycles of freezing and thawing of a suspension of 2 × 108 parasites/ml in phopshate-buffered saline (PBS). Schneider's Drosophila medium was obtained from Gibco (Paisley, UK) and tissue culture plasticware was provided by NUNC (Roskilde, Denmark).
Cells
PBMC were separated from heparinized blood over Ficoll-Hypaque (Nycomed Pharma AS, Oslo, Norway) and centrifuged at 400 g for 30 min at room temperature. Cells recovered were washed twice in RPMI-1640 containing 10% FCS and cryopreserved in liquid nitrogen according to the following criteria.11 PBMC (107 cells) were resuspended in 0·9 ml 100% FCS in a 2-ml screw cap plastic ampoule (NUNC). 0·1 ml of dimetylsulphoxide (Sigma Chemical Co., St. Louis, MO) were added immediately before the ampoule was stored at −70° overnight and then transferred to liquid nitrogen. On each occasion, one or more PBMC preparations from healthy controls were also cryopreserved in parallel experiments to serve as controls. The cells were thawed rapidly in a 37° water bath and washed in RPMI 25% FCS. The content was centrifuged at 600 g for 5 min and washed twice. Viable cells (almost 80%) from each frozen batch were carefully counted by trypan blue exclusion.
Production and evaluation of cytokines
Cryopreserved PBMC were adjusted to 106/ml in medium plus 5% FCS and incubated for 24 hr with or without LAg (2 × 107 equivalent promastigotes). In some experiments PBMC were incubated also with 50 µg/ml of diphtheria toxin (DT; Sigma). Culture supernatants were collected, filtered through 0·22 µm Millex filters (Millipore S.A., Molshiem, France) and tested for IL-4, IFN-γ and IL-10 synthesis. Cytokine production was determined by enzyme-linked immunosorbent assay (ELISA) commercial kits (R & D Systems Inc., Minneapolis, MN) which employ the multiple antibody sandwich principle.
Detection of apoptosis
Several techniques were used to detect apoptosis and at least two different methods were used for studying each experimental sample.
Cell viability
The percentage of viable monocytes and PBMC was determined by trypan blue exclusion.
Acridine orange/ethidium bromide
The percentage of apoptotic cells was measured under the fluorescence microscope by staining the cells with acridine orange and ethidium bromide. 100 µg/ml acridine orange was mixed with 100 µg/ml ethidium bromide (Molecular Probes, Eugene, OR) in PBS. Dye (1 µl) was mixed with 25 µl of cell suspension (5 × 105). The cells counted as apoptotic included cells with characteristic nuclear chromatin condensation and fragmentation.
TdT-mediated biotin–dUTP nick-end labelling (TUNEL) assay
For labelling the DNA breaks in the apoptotic nuclei, the TUNEL technique (TdT-FraEL DNA Fragmentation detection kit, Calbiochem, Cambridge, MA) was applied. Briefly, the cells were fixed on chamber slides in 4% paraformaldehyde, permeabilised in 0·1% Triton-X-100, 0·1% sodium citrate. After washing in PBS, free DNA-3′-OH termini were labelled with terminal nucleotidyl transferase (TdT) for 1 hr at 37°. TdT catalyses the addition of biotin-labelled and unlabelled deoxynucleotides. Biotinylated nucleotides are detected using a streptavidin–horseradish peroxidase conjugate. Diaminobenzidine (DAB) reacts with labelled sample to generate an insoluble substrate at the site of DNA fragmentation. Cells were counterstained with methyl green. A dark brown DAB signal indicates positive staining while shades of blue-green to greenish tan signifies a nonreactive cell.
Cytofluorimetric evaluation of apoptosis
In this assay apoptosis was evaluated on the basis of progressive lose of its semipermeability using propidium iodide (PI) as vital dye. The cells were first incubated with the appropriate amount of monoclonal antibody to identify the cell of our interest (CD4+, CD8+, CD14+, CD4+ CCR3 CCR5+) as described above. Then the cells were incubated with PI (5 µg/ml) for 10 min at room temperature. At least 15 000 events were acquired for each sample. In the analysis the percentage of apoptotic cells were calculated on CD4+ CCR3 or CD4+ CCR5+ cells, considered as 100%. Apoptotic cells were PI positive but with a medium uptake of PI according to Ferlini.33 The cells with high uptake of PI may be necrotic or in the late phase of apoptosis. Apoptosis was also evaluated by the cytometric analysis on CD4+ or CD8+ lymphocytes as described34 and was performed using a commercial kit APO-DirectTM (PharMingen, San Diego, CA) according to the manufacturer's instructions. Briefly, 5 × 105 cells were labelled with the appropriate antibody (anti-CD4 or -CD8), thereafter washed and fixed with 1% (w/v) paraformaldehyde in PBS for 30 min at room temperature, washed and re-suspended in the solution containing TdT and dUTP (fluoroscein isothiocyanate (FITC) labelled), centrifuged and treated for 30 min at room temperature with Rnase (25 µl). At the end of the treatment at least 5000 cells were acquired and detected by dual colour analysis using FACScan. All measurements were made with the same instrument setting and at least 104 cells were analysed using Lysis II software (Becton Dickinson, San Jose, CA).
Cytofluorimetric evaluation of CCR3 and CCR5 expression; CD95 and CD95L expression on CCR3 and CCR5+ cells
100 µl of freshly collected peripheral blood or 3 × 105Leishmania antigen-activated cells were treated with the appropriate amount of anti-CD4 mAb IS3-conjugated (Immunosource) anti-CD95 PE-conjugated (Caltag Laboratories, Burlingame CA) or biotin–streptavidin PE-conjugated anti-human CD95L mAb (PharMingen) and with anti-CCR3 FITC-conjugated antibody (R & D Systems) or anti-CCR5 FITC-conjugated antibody (PharMingen). After 20 min of incubation in ice, only the blood was treated with lysant (Ortholyse; Ortho Diagnostic Systems, Raritan, NS) of red cells to reduce their interference in the acquisition whereas cultured cells were rinsed in PBS twice. Then, at least 15 000 events were acquired by a FACScan cytometer. The percentage of CCR5 and CCR3 positive cells was calculated on CD4 positive cells of a scatter versus side scatter gated lymphocyte population. The analysis of percentage of CD95 or CD95L positive cells were calculated on CD4+ CCR3+ cells and on CD4+ CCR5+ cells.
Statistical analysis
Standard deviation (SD) and standard error (SE) were calculated and statistical significance analysed by Student's t-test by variance analysis (Student–Newmann–Keuls test). Variance analysis was determined by analysis of variance (anova). Differences between groups were calculated by the Mann–Whitney U-test; P < 0·05 was considered statistically significant.
Results
Basal and LAg-induced apoptosis in monocyte and T lymphocyte from VL acute patients
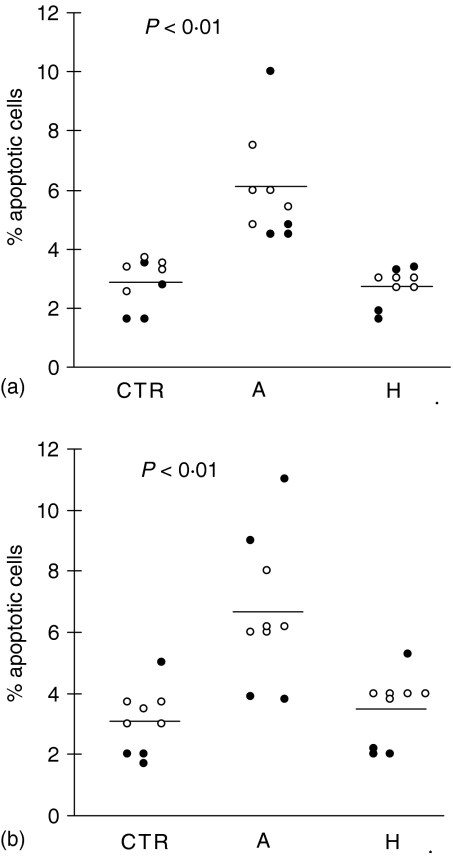
To examine apoptosis involvement in L. donovani infection, we evaluated cell death in acute and healed patients immediately after blood withdrawal. Apoptosis of PBMC was detected using both acridine orange/ethidium bromide technique and anti-CD14 or anti-CD3 mAbs and propidium iodide. In the latter the percentage of apoptotic cells was evaluated by double positive staining. Data presented in Fig. 1(a) clearly indicate that monocytes from acute VL patients show a significantly higher level of apoptosis compared with that observed in healed subjects and in healthy controls. Also T lymphocytes from acute VL patients showed an increased percentage of apoptosis with a profile similar to that detected in monocytes (Fig. 1b). Although we observed in acute VL patients a relative lymphocytosis associated with pancytopenia (anaemia, leucopenia), the total absolute number of lymphocytes was reduced compared with the value detected in the same patients after healing (1710 ± 350 versus 2150 ± 195) and in healthy controls (1710 ± 350 versus 2950 ± 450; P < 0·05).
Figure 1.
Basal values of monocyte and T lymphocyte apoptosis in VL patients. Blood samples were collected from healthy controls (CTR) and 9 VL patients at the time of acute disease (A) and after healing (H) (paired samples) and, hence, evaluated for spontaneous basal apoptosis by FACScan cytometer analysis using propidium iodide (PI) and anti-CD14 mAb to evaluate monocytes (a), and anti-CD3 to estimate lymphocytes (b). Each circle represents a subject. Open circles indicate those patients that were followed through in later studies and showed in other figures and tables. Bars represent the mean value in each group. P < 0·01: significant differences from the mean value of the same patients studied in healed phase of disease and from healthy controls. Basal percentage of monocytes: (a) 16 ± 5; (H) 15 ± 2; (CTR) 14 ± 4. Basal percentage of lymphocytes: (a) 78 ± 10; (H) 70 ± 5; (CTR) 77 ± 9.
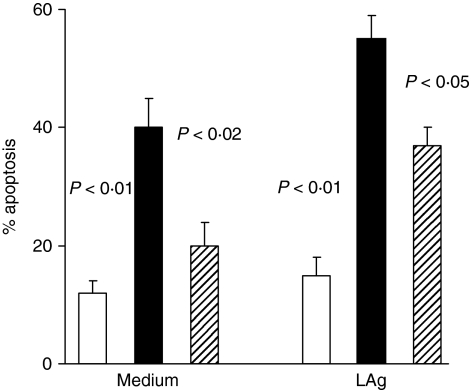
Afterwards, we studied with the same methods monocyte apoptosis, in a short-time culture (24 hr) of PBMC in the presence of LAg, to simulate the conditions observed in vivo where there is a persistent activation by parasite antigens. As shown in Fig. 2, monocytes from acute VL patients underwent, in un-stimulated cultures (medium alone), significant apoptosis compared with healed ones (P < 0·02) and healthy controls (P < 0·01). However, increased apoptosis was also detected in healed subject monocytes compared with healthy control cultures even though in not significant way. The LAg activation was able to determine a significant increase in monocyte programmed cell death (PCD) in acute VL patients and healed subjects (Fig. 2) compared with unstimulated cultures (P < 0·05 for both groups). Furthermore, the value in acute VL was significantly enhanced (P < 0·05) compared with healed ones. However, the activation with LAg was capable to further increase PCD in acute patients (P < 0·01) and healed subjects (P < 0·05) compared with healthy controls.
Figure 2.
Monocyte apoptosis in VL patients cultured in vitro with or without specific antigen. Frozen PBMC from five VL patients, studied in acute and healed phases of disease, and from five healthy controls were cultured for 24 hr in medium alone and in the presence of LAg. Cells were stained with anti-CD14 mAb and PI and percentage of apoptotic cells among CD4+ evaluated by double staining. Histograms represent median values of the percentage of apoptotic monocytes among CD14+ cells. Healthy controls (white columns); acute VL patients (black columns); healed subjects (shaded columns). Significant differences between acute VL patients and other two groups are shown.
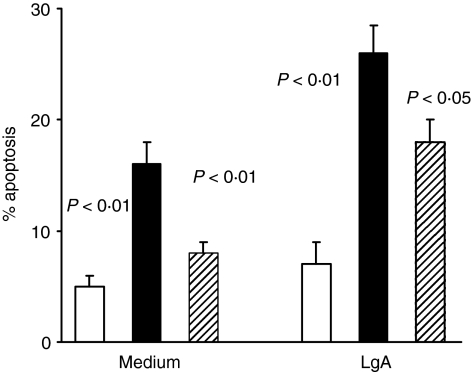
When the same analysis was carried out with CD3+ lymphocytes similar behaviour was observed, even though the percentage values of PCD were below the average detected in monocytic populations (Fig. 3). Data indicate that exogenous LAg exerted a further activation of apoptotic pathway, even though the cells were already strongly stimulated. The enhancement was significant (P < 0·01) compared with those observed in unstimulated cultures.
Figure 3.
CD3+ T-cell apoptosis in VL patients cultured in vitro with or without specific antigen. Frozen PBMC from five VL patients, studied in acute and healed phases of disease, and from five healthy controls were cultured for 24 hr in medium alone and in the presence of LAg. Cells were stained with anti-CD3+ mAb and PI and percentage of apoptotic cells evaluated by double staining. Histograms represent median values of the percentage of apoptotic monocytes among CD3+ cells. Healthy controls (white columns); acute VL patients (black columns); healed subjects (shaded columns). Significant differences between acute VL patients and other two groups are shown. The activation with LAg increased significantly (P < 0·01) PCD in acute and healed patients compared with that detected in unstimulated cultures.
Evaluation of apoptosis in CD4+ and CD8+ T-cell populations in basal conditions and after LAg activation
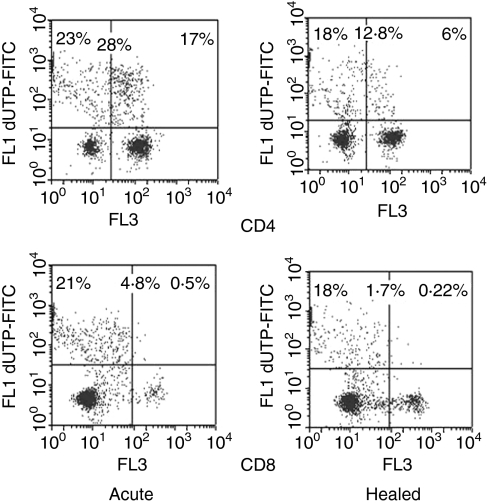
To identify apoptosis of T-cell subpopulations we used, together with acridine orange/ethidium bromide and PI techniques, also the Tdt method associated with anti-CD4 and anti-CD8 mAbs. Table 1 indicates that, immediately after blood withdrawal, a significant (P < 0·05) increased spontaneous apoptosis was detected in CD4+ lymphocytes from acute VL acute patients in comparison with healed ones and controls. The LAg activation of PBMC cultures promoted a significant enhancement in PCD in the CD4+ T-cell subpopulation. On the contrary, CD8+ lymphocytes were not modified neither in basal conditions nor after LAg stimulation (Table 1). This behaviour is clearly reported in Fig. 4, where a representative dot plot from a patient in acute phase of infection and after healing is shown.
Table 1.
Evaluation of apoptosis in CD4+ and CD8+ T-cell populations using Tdt method
Frozen PBMC from five VL patients, studied in acute and healed phases of disease, were cultured for 24 hr in medium alone (–) and in the presence of LAg (+) (2 × 107 equivalent promastigotes). Afterwards, cells were treated with appropriate IS3-conjugated antibody (anti-CD4 or anti-CD8) and treated with Tdt-FITC staining. Data are expressed as mean percentage ± SE.
P < 0·05: significantly different from healed subjects. Percentage of PCD in five healthy un-stimulated control (CTR) PBMCs: CD4+ (4 ± 2); CD8+ (2 ± 1). Percentage of CD4+ T cells: (A) 35 ± 8; (H) 42 ± 5; (CTR) 44 ± 8. Percentage of CD8+ T cells: (A) 30 ± 7; (H) 33 ± 5; (CTR) 34 ± 6.
Figure 4.
CD4+ and CD8+ apoptosis in a VL patient. FACScan dot plots from a representative patient analysed in acute phase of disease and after healing. Frozen PBMC were cultured for 24 hr in the presence of LAg, stained with anti-CD4 or anti-CD8 mAbs IS3-conjugated and than treated with TdtFITC staining. Dot plots show the percentage of CD4+ or CD8+ apoptotic cells double positive after in vitro LAg activation. The values in bold are the percentage of Tdt positive cells among CD4+ and CD8+ lymphocytes considering them as 100%.
Number of CD4+ T cells expressing chemokine receptors and their apoptosis in VL patients
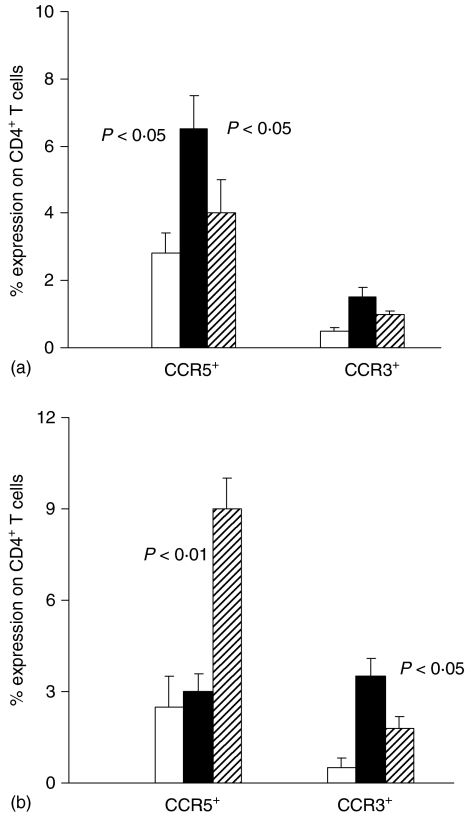
By fluorescence-activated cell sorting (FACS)-analysis we studied the expression of CCR5 and CCR3 on CD4+ T lymphocytes cultured in medium alone (Fig. 5a) or in the presence of LAg (Fig. 5b) to evaluate Th1- and Th2-type subsets, respectively. CCR5+ CD4+ T cells were increased in unstimulated CD4+ lymphocytes from acute VL patients compared with healed subjects and healthy controls (Fig. 5a). In vitro T-cell receptor (TCR) activation by LAg (Fig. 5b) caused a reduction in the percentage of CD4+ T cells expressing CCR5 in acute VL patients (from 6 ± 1 to 3 ± 0·5) and an increase of them in healed ones (from 4 ± 0·5 to 9 ± 1) compared with the data observed in unstimulated cultures. On the contrary, 24-hr cultures in medium alone did not determine any significant modification of CCR3+ CD4+ T cells (Fig. 5a). Antigen stimulation caused an increase in CCR3+ CD4+ T lymphocytes in acute VL patients compared with the unstimulated ones. Whereas in healed subjects TCR activation by LAg caused a slight not significant increase in CCR3+ CD4+ T cells compared with the unstimulated cultures (Fig. 5b).
Figure 5.
Surface expression of chemokine receptors (CCR5 and CCR3) on CD4+ lymphocyte subsets in the peripheral blood of VL patients cultured in vitro with or without specific antigen. Frozen PBMC from five VL patients, studied in acute and healed phases of disease, and from five healthy controls were cultured for 24 hr in medium alone (a) and in the presence of LAg (b). Cells were stained with anti-CD4+, anti-CCR5 and anti-CCR3 mAbs and PI. Histograms represent the mean percentage of positive CD4+ cells expressing CCR5 or CCR3. Healthy controls (white columns); acute VL patients (black columns); healed subjects (shaded columns). In (a) significant differences between acute VL patients and other two groups are shown. In (b) significant differences between acute VL patients and the value detected in the same patients studied in healed phase of disease are shown.
Next, we analysed PCD in these two CD4+ T-cell subpopulations using triple staining. As shown in Table 2, we detected a significant increase in apoptosis only in CD4+ CCR5+ T lymphocytes of acute VL patients stimulated with LAg, whereas no variations were observed in healed subjects. Data in medium alone are not shown because apoptosis was not detectable.
Table 2.
Evaluation of apoptosis percentage in CD4+ /CCR5+ and CD4+ CCR3+ T lymphocytes inside the total CD4+ CCR5+ and CD4+ CCR3+ T populations using PI method after TCR activation by Lag
| % apoptosis | ||||
|---|---|---|---|---|
| CD4+ /CCR5+ | CD4+ /CCR3 | |||
| − | + | − | + | |
| Acute (A) | 10·0 ± 2 | 35·0 ± 8* | 2·5 ± 0·8 | 1·5 ± 0·5 |
| Healed (B) | 7·5 ± 1 | 5·0 ± 1 | 2·0 ± 1·0 | 2·5 ± 1·0 |
Frozen PBMC from five VL patients, studied in acute and healed phases of disease, were cultured for 24 hr in medium alone (–) or in the presence of LAg (+) (2 × 10 7 equivalent promastigotes). Cells were treated with IS3-conjugated antibody anti-CD4, with anti-CCR5 or anti-CCR3 FICT-conjugated antibodies and incubated with PI. Data are expressed as mean percentage ±SE.
P < 0·05: significant different from healed subjects.
To support in somehow the phenotype Th1- and Th2-type undergoing apoptosis we studied the cytokine contents in the supernatants of same experiments shown in Fig. 5 and in Table 2.
The results in Table 3 demonstrated that PBMC from acute VL patients produced higher amounts of IFN-γ in unstimulated cultures than those LAg-treated in agreement with the higher CCR5 expression (see Fig. 5). The activation with LAg determined a reduction of IFN-γ synthesis. These data are in line with the observed increase in the apoptosis of CD4+ CCR5+ T cells (Table 2) and the reduction in T cells expressing CD4+ CCR5+ (Fig. 5). Opposite profile was observed for IL-4 and IL-10 cytokines. In healed subjects the LAg stimulation was responsible of an increase in the synthesis all three cytokines object of our study, compared with the untreated cultures, in agreement with the increase of both CD4+ CCR5+ and CD4+CCR3+ T lymphocytes (Fig. 5) and the absence of detectable apoptosis (Table 2).
Table 3.
Mean values of cytokines in the culture supernatants from acute (A) and healed (B) VL patients
| Cytokines −(pg/ml) | A | B | ||
|---|---|---|---|---|
| + | − | + | − | |
| IFN-γ | 20 ± 3 | 12 ± 2* | 20 ± 4 | 60 ± 6† |
| IL-10 | 25 ± 4 | 40 ± 9** | 23 ± 5 | 40 ± 3** |
| IL-4 | 5 ± 2 | 13 ± 3** | 15 ± 2 | 20 ± 3 |
Frozen PBMC from five VL patients, studied in acute and healed phases of disease, were cultured for 24 hr in medium alone (–) or in the presence of LAg (+) (2 × 107 equivalent promastigotes). Supernatants were filtered (25 µm) and tested for cytokine contents. Cytokine production by frozen PBMC from five healthy controls: IFN-γ: 6 ± 2 pg/ml; IL-10: 4 ± 1 pg/ml; IL-4: 0·8 ± 0·2 pg/ml. Data are expressed as mean ± SE.
P < 0·01: significantly different from healed subjects.
P < 0·05: significantly different from unstimulated cultures.
P < 0·01: significantly different from unstimulated cultures.
CD4+ and CD8+ T-cell apoptosis after TCR activation with LAg and DT
To evaluate if apoptosis was specific to LAg stimulation or it was a result of a normal consequence of an immune response, we carried out experiments by culturing PBMC from VL patients either in the presence of specific LAg or in the presence of an unrelated antigen (DT). Data reported in Table 4 indicate that the exposure at both antigens is able to induce apoptosis in acute VL patients, whereas in healthy controls only DT is capable to determine PCD in CD4+ and CD8+ T cells. These data indicate that apoptosis appear to be a normal response following TCR activation by antigens.
Table 4.
Evaluation of apoptosis in CD4+ cells after LAg and DT exposure
Frozen PBMC from three VL patients, studied in acute and healed phases of disease (paired samples), were cultured for 24 h in medium alone (–) and in the presence of LAg (+) (2 × 107 equivalent promastigotes) or DT (50 µg/ml). Afterwards, cells were treated with appropriate IS3-conjugated antibody (anti-CD4) and with Tdt-FITC staining. Data are expressed as mean percentage of increase in apoptosis of CD4+ in three patients. The variation of mean percentage of apoptosis in the two patients was less than 10%.
P < 0·05: significantly different from unstimulated or stimulated cultures with LAg.
P < 0·05: significantly different from unstimulated cultures.
CD95 and CD95L expression on CD4+ CCR3+ and CD4+ CCR5+ lymphocytes
Since TCR activation induces CD95 cell surface receptor35,36 we investigated the cell membrane expression of CD95 on CD4+ CCR5+ and CD4+ CCR3+ T-cell subsets. Analysis using flow cytofluorometry indicated that the percentage of Th1-and Th2-type cells expressing cell surface CD95, in unstimulated cultures, was not significantly modified both in acute and healed VL patients compared with healthy controls. After LAg activation a very significant increase (P < 0·01) in CD95 cell surface expression was detected particularly in CD4+ CCR5+ population in acute VL patients, but it was also detected in the CD4+ CCR3+ subset (P < 0·05) (Table 5).
Table 5.
CD95 and CD95L expression on CD4+ CCR5+ and CD4+ CCR3+ T lymphocytes
| CD4+ CCR5+ | CD4+ CCR3+ | |||
|---|---|---|---|---|
| − | + | − | + | |
| CD95 expression | ||||
| Acute (A) | 1·3 ± 0·3 | 16·7 ± 1·5* | 1·5 ± 0·4 | 3·2 ± 0·5* |
| Healed (H) | 1·8 ± 0·5 | 4·1 ± 1·9 | 1·2 ± 0·2 | 2·0 ± 0·3 |
| CD95L expression | ||||
| Acute (A) | 3·6 ± 0·5* | 5·2 ± 0·8* | 1·0 ± 0·4 | 11·2 ± 1·1* |
| Healed (H) | 1·9 ± 0·3 | 0·8 ± 0·2 | 1·1 ± 0·8 | 2·5 ± 1·1 |
Frozen PBMC from five VL patients, studied in acute and healed phases of disease, were cultured for 24 hr in medium alone (–) and in the presence of LAg (+) (2 × 107 equivalent promastigotes). Cells were treated with anti-CD4+ monoclonal antibody IS3-conjugated, anti-CD95 PE-conjugated or biotin–streptavidin PE-conjugated anti-human CD95L, and anti-CCR3 or anti-CCR5 FITC-conjugated antibodies. Data are expressed as mean percentage ± SE.
P < 0·05: significantly different from healed subjects. Percentage of CD95 expression in five healthy controls (CTR): CD4+ CCR5+ (1·2 ± 0·3); CD4+ CCR3+ (0·2 ± 0·01). Percentage of CD95L expression in CTR: CD4+ CCR5+ (0·3 ± 0·03); CD4+ CCR3+ (0·5 ± 0·01).
The next step was to evaluate CD95L expression on CD4+ CCR5+ and on CD4+ CCR3+ cells. An enhancement of CD95L CD4+ CCR5+ cells from unstimulated cultures of acute VL patients, compared with healed subjects and healthy controls, was found. CD95L increase was also observed in Th1 CCR5+ cells from healed subjects respect to healthy controls (1·9 ± 0·3 versus 0·3 ± 0·03; P < 0·05), even though it was less significant that observed in acute VL (Table 5, 0·01). In CD4+ CCR3+ cells from acute VL patients, incubated in medium alone, CD95L expression was not significant increased compared with that detected in healed subjects and healthy controls (Table 5). After LAg TCR activation a significant increase in CD95L expression (P < 0·01) both in CD4+ CCR5+ and CD4+ CCR3+ T lymphocytes in acute VL patients was observed (Table 5).
Discussion
Data reported clearly indicate that in acute VL patients monocytes and CD4+ T cells undergo significant level of apoptosis compared with that observed in healed persons and in healthy controls. Since the progression of infection is related to the impairment of cell mediated immunity (CMI)2,3 the detection of CD4+ T-cell and monocyte apoptosis could contribute to inefficient cell immune responses during L. infantum infection. The observation of a further enhancement of apoptosis in PBMC from acute VL patients stimulated in vitro with LAg, compared with healed subjects, should indicate that the leucopenia could somehow be due to accelerated rates of T-cell and macrophage apoptosis, caused by the continuous Leishmania antigen load and, hence, by the chronic antigen stimulation.29,37
The macrophage PCD may have opposite effects on the outcome of Leishmania infection. In fact, the prompt death of macrophages that are using their destructive potential against such pathogens, could be beneficial for the host, because it deprives the parasite of their natural habitat.38 On the contrary, the pathogens, inducing macrophage apoptosis, can overcome the microbicidal arsenal of the phagocyte.38 On the other hand, Leishmania amastigotes might avoid activating macrophages by mimicing apoptotic cells and, therefore, inhibit macrophage activity by exposing phosphatidylserine.39
The high frequency of apoptotic events observed in CD4+ compared with CD8+ lymphocytes from acute VL patients under in vitro LAg stimulation, in line with a previous report40 may offer an explanation of the immune depression. Even though, results indicate that apoptosis appear to be a physiological response following TCR activation by LAg as well as unrelated antigen (DT; see Table 4), it may be secondary expression of other immune mechanisms, or to reflect persistent immune activation in vivo, thus favouring CD4+ T cell depletion. Furthermore, the lowest level of CD4+ T cell apoptosis in healed subjects compared with acute VL ones supports the hypothesis that the low T-cell response detected in patients with acute severe forms of Leishmania infections2,3,12,13 could be depend on the depletion of Leishmania-reactive CD4+ T cells.41 The highest presence of CCR5+ CD4+ T cells in basal condition (Fig. 5a) and their reduction after TCR activation by LAg (Fig. 5b) in acute VL patients compared with healed ones shows that in disease state Th1-type cells are already activated and the further stimulation induces cell death, the expected fate of the activated cells.18,19,21 The data suggest that these patients had a better response to antigen during acute VL than after healing, that is rather unexpected. In reality, we detected an increase in apoptosis (Tables 1,2 and 5) and not in protective cytokines (Table 3) under specific antigen stimulation in acute VL patients, whereas in healed subjects, activation with production of Th1 cytokines rather than increases in PCD is dominant. This means that, even though apoptosis is a normal fate of antigen-activated T cells, the detected increase in apoptosis of Th1, but not Th2, in acute VL patients is specific of those cells undergo a rapid Fas/FasL-mediated activation induced cell death (AICD) upon reactivation with antigen.42 Therefore, the significant increase in apoptosis of the Th1-like subset (15–20% of CD4+ cells) is in line with the studies carried out in susceptible hosts showing an enhancement of CD4+ T-cell apoptosis, particularly of Th1-type cells, associated with a decrease in Th1 cytokine production.25,26 Because Th1 cytokines seem to be involved in the protection against leishmaniasis, both in experimental models2,3,8,25,26 and in human infections11–16,29 this deletion of CD4+ Th1-type cells could contribute to the depressed CMI in acute VL patients.
Th1- and Th2-type cells from VL subjects undergo apoptosis using CD95-mediated mechanisms41 and the TCR stimulation by LAg further up-regulates CD95 expression.43 The enhanced expression of CD95 in frozen CD4+ CCR5+ cells (Th1-type) in acute VL patients after LAg activation compared with the expression of it in CD4+ CCR3+ cells (Th2-type) (16·7±1·5 vs 3·2±0·5) could indicate that Th1-type cells are more susceptible than Th2-type cells to cell suicide in an autocrine or paracrine way, as observed in other cell models.44,45 However, the enhancement of Fas and FasL in CD4+ CCR5+ T cells may contribute to the highest sensitivity of Leishmania-specific T cells to apoptosis. Our observation of Th1 cells expressing more Fas and less FasL compared with Th2 is in contrast with data of Ramsdell et al.,46 indicating that Th1 express more FasL, whereas Th2 cells show high level of Fas. Ramsdell uses cloned cells; on the contrary we use PBMC collected from VL patients, frozen and activated in vitro for very short time. Therefore, our system is less stable and under the influence of all the variables of an in vivo stimulation. Furthermore, our data are in line with those of Zhang et al.42 obtained using fresh activated mouse cells. In fact, both observe that Th1, but not Th2, effectors undergo a rapid Fas/FasL-mediated AICD upon reactivation with antigen. This difference between Th1 and Th2 effectors may be responsible for the predominance of the Th2 subset in our acute VL patients, that can have a role somehow in the disease progression.
Taken together data indicate that in our Sicilian patients with acute VL the apoptosis involves infected monocytes and uninfected CD4+ T cells, and PCD could have a role in the progressive failure of CMI and in the immune depression, even though it could be a normal consequence of the immune response during infection. Because PCD may be modulated by therapeutic strategies, a better definition of the role of apoptosis in VL will aid the design of novel drugs able to control this infectious disease.
Acknowledgments
We are grateful to Dr Sheila McIntyre for helping in the preparation of the manuscript and to Matthew Sweet for helpful discussion and criticism.
References
- 1.Gradoni L, Gramiccia M. Leishmania infantum tropism: strain genotype or host immune status? Parasitol Today. 1994;7:264–6. doi: 10.1016/0169-4758(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 2.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 3.Kemp M, Theander TG, Kharazmi A. The contrasting roles of CD4+ T cells in intracellular infections in humans: leishmaniasis as an example. Immunol Today. 1996;17:13–6. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 4.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zingoni A, Soto H, Hedrick JA, et al. The chemokine receptor CCR8 is preferentially expressed Th2 but not Th1 cells. J Immunol. 1998;161:547–51. [PubMed] [Google Scholar]
- 7.Bleul C, Wu L, Hoxie J, Springer T, Mackay C. The HIV receptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew FY, Wei XQ, Proudfoot L. Cytokines and nitric oxide as effector molecules against parasitic infections. Phil Trans R Soc Lond B. 1997;352:1311–5. doi: 10.1098/rstb.1997.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaye PM, Curry AJ, Blackwell JM. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine- induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991;146:2763–70. [PubMed] [Google Scholar]
- 10.Murray HW. Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis. 1997;175:1477–9. doi: 10.1086/516482. [DOI] [PubMed] [Google Scholar]
- 11.Cillari E, Liew FY, Lo Campo P, Milano S, Mansueto S, Salerno A. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol. 1988;140:2721–6. [PubMed] [Google Scholar]
- 12.Cillari E, Milano S, Dieli M, et al. Reduction in the number of UCHL-1+ cells and IL-2 production in the peripheral blood of patient with visceral leishmaniasis. J Immunol. 1991;146:1026–30. [PubMed] [Google Scholar]
- 13.Carvalho EM, Badaro R, Reed SG, Jones TC, Johnson WD. Absence of gamma interferon and interleukin-2 production during active visceral leishmaniasis. J Clin Invest. 1985;76:2066–9. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks DL, Lai SL, Shrivastava SN, Blackwell J, Neva FA. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987;138:908–13. [PubMed] [Google Scholar]
- 15.Ghalib HW, Piuvezam MR, Skeiky YAM, et al. Interleukin-10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho EM, Bacelar O, Brownell CE, Regis T, Coffman RL, Reed SG. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 17.Ghalib HW, Whittle JA, Kubin M, et al. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 18.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 19.Vaux DL. Immunopathology of apoptosis – introduction and overview. Springer Semin Immunopathol. 1998;19:271–8. doi: 10.1007/BF00787224. [DOI] [PubMed] [Google Scholar]
- 20.Lopes MF, da Veiga VF, Santos AR, Fonseca MEF, DosReis GA. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas's disease. J Immunol. 1995;154:744–52. [PubMed] [Google Scholar]
- 21.Akbar AN, Borthwick N, Salmon M, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;178:427–38. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez JM, Ameisen JC. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–67. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dockrell DH. Apoptotic cell death in the pathogenesis of infectious diseases. J Infect. 2001;42:227–34. doi: 10.1053/jinf.2001.0836. [DOI] [PubMed] [Google Scholar]
- 24.Sereti I, Herpin B, Metcalf JA, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. AIDS. 2001;15:1765–75. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 25.Das G, Vohra H, Rao K, Saha B, Mishra GC. Leishmania donovani infection of a susceptible host results in CD4+ T-cell apoptosis and decreased Th1 cytokine production. Scand J Immunol. 1999;49:307–10. doi: 10.1046/j.1365-3083.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 26.Das G, Vohra H, Saha B, Agrewala JN, Mishra GC. Leishmania donovani infection of a susceptible host results in apoptosis of Th1-like cells: rescue of anti-leishmanial CMI by providing Th1-specific bystander costimulation. Microbiol Immunol. 1998;42:795–801. doi: 10.1111/j.1348-0421.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–8. [PubMed] [Google Scholar]
- 28.Munn DH, Beall A, Song D, Wrenn RW, Throckmorton DC. Activation-induced-apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony stimulating factor and interferon-γ. J Exp Med. 1995;181:127–36. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas M, Barrera LF, Puzo G, Garcia LF. Differential induction of apoptosis by virulent Mycobacterium tubercolosis in resistant and susceptible murine macrophages. J Immunol. 1997;159:1352–61. [PubMed] [Google Scholar]
- 30.Conceicao-Silva F, Hahne M, Schroter M, Louis J, Tschopp J. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur J Immunol. 1998;28:237–45. doi: 10.1002/(SICI)1521-4141(199801)28:01<237::AID-IMMU237>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Alexander CE, Kaye PM, Engwerda CR. CD95 is required for the early control of parasite burden in the liver of Leishmania donovani-infected mice. Eur J Immunol. 2001;31:1199–210. doi: 10.1002/1521-4141(200104)31:4<1199::aid-immu1199>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Cillari E, Vitale G, Arcoleo F, et al. In vivo and in vitro cytokine profiles and mononuclear cell subsets in Sicilian patients with active visceral leishmaniasis. Cytokine. 1995;7:740–5. doi: 10.1006/cyto.1995.0088. [DOI] [PubMed] [Google Scholar]
- 33.Ferlini C, Di Cesare S, Rainaldi G, Malori W, Samoggia P, Biselli R, Fattorossi A. Flow cytometric analysis of the early phase of apoptosis by cellular and nuclear techniques. Cytometry. 1996;24:106–15. doi: 10.1002/(SICI)1097-0320(19960601)24:2<106::AID-CYTO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas Ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 35.Ju ST, Panka DJ, Cui H, et al. Fas (CD95) /Fas–L interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 36.Toth R, Szegezdi E, Reichert U, et al. Activation-induced apoptosis and cell surface expression of Fas (CD95) ligand are reciprocally regulated by retinoic acid receptor alpha and gamma and involve nur77 in T cells. Eur J Immunol. 2001;31:1382–91. doi: 10.1002/1521-4141(200105)31:5<1382::AID-IMMU1382>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 37.Cenini P, Berhe N, Hailu A, McGinness K, Frommel D. Mononuclear cell subpopulation and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis. 1993;168:986–93. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 38.Marrack P, Kappler J. Subversion of the immune system by pathogens. Cell. 1994;76:323–32. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 39.de Freitas Balanco JM, Moreira ME, Bonomo A, Bozza PT, Amarante-Mendes G, Pirmez C, Barcinski MA. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–3. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 40.Wolday D, Akuffo H, Demissie A, Britton S. Role of Leishmania donovani and its lipophosphoglycan in CD4+ T cell activation-induced human immunodeficiency virus replication. Infect Immun. 1999;67:5258–64. doi: 10.1128/iai.67.10.5258-5264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eidsmo L, Wolday D, Berhe N, et al. Alteration of Fas and Fas ligand expression during human visceral leishmaniasis. Clin Exp Immunol. 2002;130:307–13. doi: 10.1046/j.1365-2249.2002.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Brunner T, Carter L, et al. Unequal death in T helper cell (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837–49. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucek-Szabo C, Andjelic S, Lacy E, Elkon K, Nikolic-Zugic J. T cell Fas receptor/CD95 regulation, in vivo activation, and apoptosis. Activation-induced death can occur without Fas receptor. J Immunol. 1996;156:192–200. [PubMed] [Google Scholar]
- 44.Roskams T, Libbrecht L, Van Damme B, Desmet V. Fas and Fas ligand: strong co-expression in human hepatocytes surrounding hepatocellular carcinoma: can cancer induce suicide in peritumoural cells? J Pathol. 2000;191:150–3. doi: 10.1002/(SICI)1096-9896(200006)191:2<150::AID-PATH612>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 45.Gisslinger H, Hurzrock R, Gisslinger B, et al. Autocrine cell suicide in a Burkitt lymphoma cell line (Daudi) induced by interferon alpha: involvement of tumor necrosis factor as ligand for the CD95 receptor. Blood. 2001;97:2791–7. doi: 10.1182/blood.v97.9.2791. [DOI] [PubMed] [Google Scholar]
- 46.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–53. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]