Abstract
Haemophilus influenzae type b (Hib) is one of the leading causes of invasive bacterial infection in young children worldwide. During childhood, acquisition of antibody directed against the polysaccharide capsule of the organism, presumably as a result of asymptomatic carriage, confers protection and disease is much less common after the age of 4 years. Like other polysaccharides, the polyribosyl ribitol phosphate (PRP) of the Hib capsule is a T-independent antigen and not immunogenic when administered as a vaccine in infancy. Because the highest rates of disease occur in the first 2 years of life, efficacious Hib vaccines have been designed by covalently linking the PRP capsule to a carrier protein that recruits T-cell help for the polysaccharide immune response and induces anti-PRP antibody production even in the first 6 months of life. Introduction of Hib protein–polysaccharide conjugate vaccines into many industrialized countries over the past 15 years has resulted in the virtual elimination of invasive Hib disease. However, despite the success of the vaccine programme several factors may interfere with the effectiveness of the vaccine in the routine programme, as observed in the UK recently. Such factors may include interference with other concomitant vaccines, waning immunity in the absence of booster doses of vaccine, and reduced natural boosting as a result of decreased transmission of the organism. However, the burden of disease remains highest in resource-poor countries and urgent efforts are needed to provide the benefits of this vaccine for children living in regions where it cannot be used for economic and logistical reasons.
Keywords: Haemophilus influenzae, conjugate, vaccine, immunological memory, polysaccharide
Introduction
It is no coincidence that many of the bacteria responsible for invasive bacterial disease in childhood have a polysaccharide capsule. The capsule may provide a survival advantage for these organisms during transmission and colonization but also facilitates survival in the blood in the pathogenesis of invasive disease through resistance to complement mediated killing and phagocytosis.1,2 Antibody against the polysaccharide capsule is central to naturally acquired immunity against many of these organisms. The polysaccharide capsule of Salmonella typhi (St), Haemophilus influenzae type b (Hib), Streptococcus pneumoniae (Sp) and Neisseria meningitidis (Nm) provides both an opportunity and a challenge for vaccine prevention of life threatening bacterial infections in childhood.
Whilst varied in chemical structure bacterial capsular polysaccharides share the common immunological property of T-independent B-cell activation, which is associated with poor or absent immunogenicity in infants and a failure to induce immunological memory at any age. Therefore, purified capsular polysaccharide vaccines are generally not satisfactory in early childhood where the burden of disease is highest. Protein–polysaccharide conjugation has provided a solution to the problems of polysaccharide immunogenicity in childhood by recruiting T cells to the immune response. Protein–polysaccharide conjugate vaccines for Hib were the first to be introduced into routine use within a population, and have been highly effective in reducing the burden of Hib disease.
The initial success of the Hib conjugate vaccines masked the multifaceted and dynamic nature of the events underlying the vaccine's protective efficacy. However the increase in Hib vaccine failures seen in the UK from 1999 onwards has revealed the complexity of interaction between vaccine, immune response and Hib population dynamics.
Polysaccharide immunobiology
Hib disease and natural immunity
Since 1931 it has been known that some strains of Haemophilus influenzae possess a polysaccharide capsule and that there are 6 capsular serotypes (a–f).3 Invasive isolates from patients are predominantly type b organisms which possess a polyribosyl ribitol phosphate (PRP) capsule. Hib is a significant cause of bacterial infections including meningitis, septicaemia, epiglottitis, pneumonia and septic arthritis, especially in young infants. However, in the majority of individuals, Hib is a commensal of the nasopharynx and only a minority of those exposed or who are carriers of the organism suffer invasive disease. It has been suggested that the polysaccharide capsule may confer a survival advantage by allowing evasion of mucosal immune responses or by facilitating transmission between hosts by reducing desiccation.4 In terms of invasive disease the polysaccharide capsule can also be shown to inhibit serum bactericidal activity and complement mediated phagocytosis.5,6 During the 1930s and 1940s it was established that antipolysaccharide antibody was protective against invasive Hib disease.7 Age-specific profiles of anti-PRP antibodies show a characteristic pattern.8 Relatively high levels of transplacentally acquired anti-PRP antibodies fall over the first months of life to very low levels by around 6 months of age. Subsequently, antibody titres rise again during the second year of life, presumably as a result of exposure to Hib in the nasopharynx or other organisms with cross-reactive antigens.9–11 The age-specific incidence of invasive disease is inversely related to the titre of anti-PRP antibodies, the highest incidence of disease in an unvaccinated population occurring in the interval between the loss of maternal antibody and the generation of antibody by the child's own B cells. In the UK, the majority of invasive disease occurred during the first 2 years of life.12 In other populations, particularly in the developing world, the majority of disease occurs even earlier during infancy.13,14
Polysaccharide as a vaccine antigen
The type b polysaccharide capsule is attractive as a vaccine antigen since invasive disease is almost exclusively restricted to type b organisms and antipolysaccharide antibodies are important in natural immunity. The induction of anti-PRP antibodies at an age young enough to protect those most at risk of Hib disease has been the goal of vaccine development.
Early observations with Hib demonstrated the limitations of plain polysaccharide as a vaccine antigen. When given during the first 2 years of life, purified PRP induces relatively low titres of serum antibodies that are usually insufficient to protect against invasive disease.15–17 In terms of immunological memory, antibody induced by PRP wanes quickly and subsequent immunization shows no evidence of immunological priming in any age group.18
The immunological mechanisms underlying these observations are not fully understood. Polysaccharide, although recognized by B-cell receptors, cannot be presented to T cells in conjunction with major histocompatibility complex (MHC) class II molecules. B cells therefore lack the ability to directly recruit cognate T-cell help when stimulated with polysaccharide. The polysaccharide's interaction with the B cell is thus termed T-independent (TI). The lack of specific T-cell interactions in the immune response limits the immunogenicity of PRP, and the development of memory B cells with class-switched antibody and subsequent avidity maturation cannot occur. However, experimental studies with mice are suggestive of an as yet poorly defined requirement for T cells even in TI interactions.19 It is possible that the antigen is presented in other forms to T cells, such as via CD1 presentation by dendritic cells.20
It remains unclear whether the poor antibody responses in the first 2 years of life are caused by infrequent Hib carriage at this age or immunological immaturity. The same vaccine given in later childhood can induce significant antibody responses.16,18,21 There is some evidence for specific inhibitory mechanisms that act in infancy to delay the development of TI responses.22 Another possibility is that there is unlikely to have been a natural selective pressure to develop responses to polysaccharide alone as polysaccharide would not be encountered in an isolated form requiring a protective immune response in the natural world, being invariably associated with protein antigens in a whole organism. Polysaccharide and protein encountered in the context of a whole organism might allow B cells to recruit cognate T-cell help and generate memory. The response to plain polysaccharide in older individuals would then be due to the presence of memory B cells from past carriage of Hib or organisms with cross-reactive epitopes. However, although carriage is associated with high serum anti-PRP antibody titres10,23 evidence for Hib organisms stimulating immunity in a T-cell dependent fashion is indirect.24,25 In addition, disease frequently fails to stimulate an antibody response under 2 years of age and may even suppress the response to further vaccination suggesting that the induction of immunity by Hib organisms is modulated by a variety of factors.26,27
Although many bacterial polysaccharides of Sp, Nm and Hib are TI this is not an invariant feature of all. Both serogroup A meningococcal and serotype 3 pneumococcal polysaccharide produce good responses in infancy.22,28 There are also examples from other species of bacteria of polysaccharide capsules with TD properties.29 Striking as these findings are, with possible implications for vaccine research, these phenomena have not been investigated extensively in humans.
Protein–polysaccharide conjugates
Over 60 years ago it was demonstrated that bacterial polysaccharides conjugated to proteins could enhance the immunogenicity of the polysaccharide and induce protective immunity in an animal model.30,31 Following further studies with a variety of bacterial polysaccharides and in the light of the limitations of plain polysaccharide vaccine antigens, Hib PRP was shown to be more immunogenic when covalently linked to a protein carrier and to show boosted responses characteristic of T-dependent memory.32–35 PRP conjugated to a variety of proteins is more immunogenic than PRP alone and primes for a subsequent response to PRP in both adults, children and infants.21,35–39
The immunology underlying the improved immunogenicity and priming induced by conjugate vaccines is still only partially understood. Covalent linkage of protein and polysaccharide is essential for the enhanced immunogenicity of glycoconjugate vaccines.40 In addition in vitro studies, using peripheral blood mononuclear cells (PBMC) from adults given Hib conjugate vaccine, indicate that maximum antibody production and T-cell proliferation require direct contact between T and B cells.41 These data are consistent with the classical mode of presentation of carrier protein in conjunction with MHC II to the T cell, leading to germinal centre formation with antibody class switching, avidity maturation and memory B-cell production.42 Such cognate T- and B-cell interactions require the involvement of costimulatory molecules such as CD40–CD40L and CD27/CD70.43–45 CD4+ T cells specific for the carrier protein have been detected following glycoconjugate vaccination and secreted both T helper 1 (Th1) and Th2 cytokines.46 Studies of other glycoconjugate vaccines in children have shown an increase in CD40L mRNA expression in PBMC following vaccination, further suggesting the importance of cognate T- and B-cell interactions in the response to conjugate vaccines.47 The importance of other cell types in the initial immune response to conjugate vaccines is highlighted by recent work with Hib-outer membrane protein complex (OMPC) vaccine. In contrast to tetanus and CRM197 Hib conjugates Hib-OMPC conjugate vaccines appear to engage Toll-like receptor 2 on dendritic cells, perhaps altering their regulation of T-cell responses to the vaccine This interaction may be a factor that contributes to the improved immunogenicity of this particular vaccine in infants after a single dose.48
While there still appears to be some variability in the immune response with age, conjugate vaccines bypass the relative unresponsiveness of the infant immune system for plain polysaccharide and provide the basis for the generation of memory B cells and priming of the immune system.
Immunological memory to polysaccharide antigens
The nature of immunological memory elicited by polysaccharide–protein conjugate vaccines has become a key issue in the light of the rise in true vaccine failures in the UK since 1999. The accepted method of demonstrating immunological memory is by the administration of plain Hib polysaccharide to individuals who have had previous Hib vaccine compared to individuals who have not (controls). A more rapid rise and higher final titre of anti-PRP antibody in the group boosted with PRP as compared to the controls is taken as evidence of priming.21 In addition antibody avidity maturation has been suggested as an indicator of immunological memory, the implication being that it should correlate with the existence of primed responses to booster vaccination.49 Although priming is the conventional measure of immunological memory the relationship of resting antibody levels to B-cell memory is an increasingly important consideration.50,51
In practice primary infant immunization with Hib conjugates induces significant initial antibody titres that subsequently wane, in some cases to levels that would not be considered protective.10,23,52,53 Priming can be demonstrated for Hib conjugates even when antibody titres have fallen to low levels.54 In addition, children vaccinated with Hib conjugates show evidence of priming following a booster dose of PRP even if postprimary immunization antibody responses were poor.
It is now recognized that there is a difference between memory responses, as evidenced by priming, and protective immunity.55,56 Children with invasive Hib disease in both vaccinated and unvaccinated populations have been shown to have antibody responses suggestive of immunological memory, indicating that this was not sufficient to protect them.25,57 Hence, in the case of invasive Hib disease, conventional measures of immunological memory are not synonymous with protection.
The accepted means of assessing immune memory, as described above, following immunization with Hib vaccine provides limited data about the nature of memory for the individual or for the population. Indeed, the measurement of an antibody response to a polysaccharide challenge at a single timepoint gives no insight into the quality or kinetics of the antibody produced. Where antibody levels are below a protective threshold, it is known that several days will elapse after exposure to an antigen before antibody levels in serum rise, even in a primed individual. This window allows for the possibility of invasive disease, in an individual with immunological memory if invasion occurs too quickly after nasopharyngeal acquisition. A single measure of antibody concentration following a booster immunization provides only a limited assessment of the degree of protection in a primed individual.
The quality of antibody and the kinetics of the immune response in an individual may not provide the rule for the whole population. In any population there will be a distribution of the quality and quantity of memory responses to a vaccine such that a proportion of the population will still be at risk of infection even if on a population level there appears to be good evidence of protective memory responses.58 Although antibody titre is established as a correlate of protection there is no clear correlation between measures of immunological memory and protection at either an individual or population level. This is clearly of great concern in a population whose antibody titre has waned below the protective threshold.
Current models of B-cell memory suggest that there are populations of long lasting plasma cells secreting resting levels of antibody and populations of B memory cells maintaining this pool of cells and subserving the rapid responses seen with priming.50 Although B memory cells are identified by their class switched somatically hypermutated immunoglobulin and their possession of cell surface markers such as CD27,59 it is less clear how to identify long-lived plasma cells in humans. The production and persistence of memory B cells and long-lived plasma cells in relation to Hib vaccination has not been studied in depth.
T-cell memory produced by Hib conjugate vaccines will be specific for peptide sequences derived from the carrier proteins of these vaccines (N. meningitidis outer membrane proteins, tetanus toxoid, CRM197 mutant diptheria toxoid and diptheria toxoid). The presence of such proteins as vaccine antigens on their own in the routine immunization schedule may contribute to Hib conjugate immunogenicity.60 However, carrier protein specific T cells will be unable to contribute to a secondary response to an invasive Hib bacterium because they are not specific for Hib related peptide sequences. This phenomenon may explain some of the difference between the existence of immunological memory without protective immunity.61 There is evidence to suggest that natural exposure to Hib can generate priming and thus by implication T-cell memory in individuals. Antibody responses typical of a secondary response were seen in some children following invasive Hib disease.25 In these children the only likely source of previous exposure, to account for the existence of a secondary response, would be through carriage of Hib or organisms with cross-reactive antigens. Support for the concept of carriage acting to prime for memory has also been gained from detailed molecular analysis of antibody responses to PRP vaccination in adults who had not previously been vaccinated.24,62 These studies demonstrated a degree of clonality and mutation of Hib specific antibody sequences, in relation to germline sequences, that was strongly suggestive of a secondary response despite the individuals lack of previous vaccination. Individuals who were primed through Hib carriage would have memory T cells specific for Hib peptide sequences. It is possible that the lack of Hib specific T-cell help in a vaccinated individual may confer a reduced ability to respond to Hib than an individual whose immunity was acquired through carriage.
Polysaccharide and carrier protein influence on immunogenicity
Hib conjugate vaccines consist of a length of PRP polysaccharide linked to a protein carrier. Such vaccines differ in the length of polysaccharide, the nature of the protein carrier and the method of linkage of the two. In general, although these various Hib conjugates are immunogenic and efficacious, it is clear that they are not identical in performance63–70(Table 1).
Table 1.
| Vaccine | Polysaccharide | Carrier protein | Linkage | Antibody response in infancy |
|---|---|---|---|---|
| PRP-D | Medium | Diptheria Toxoid | 6-carbon | Moderate, after 2nd dose |
| HbOC | Small | CRM197 mutant C. diptheria toxin protein | None | Good, after 2nd dose |
| PRP-OMP | Medium | N meningitidis protein outer membrane complex | Thioether | Moderate, after 1st dose |
| PRP-T | Large | Tetanus toxoid | 6-carbon | Good, after 2nd dose |
Reproduced from; Kelly and Moxon Is Haemophilus influenzae Type b Disease Finished,70 with the kind permission of Kluwer Academic/Plenum Publishers.
Infant vaccination with Hib conjugates usually occurs at a time when there is persistence of passively acquired maternal antibodies. Pre-existing Hib antibody modestly suppresses the antibody response to Hib conjugate vaccines.71 However, this effect is less noticeable when vaccines are given at older ages and does not seem to affect immunological memory. Passively acquired maternal antibody to tetanus increases the immunogenicity of tetanus conjugates in some studies and not in others.72–74 Similarly, although prior active immunization with carrier protein frequently enhances anti-PRP antibodies, diminished responses have also been noted particularly when carrier protein immunization is undertaken in the first month of life.60,75–78 The situation is complex and may vary for different vaccines in different settings.76 In a population with high attack rates in the first weeks of life, a reduced antibody response to the first dose of a vaccine have a significant impact in terms of efficacy even if the peak response following a full vaccination course is unaltered.
The nature of the carrier protein influences priming. A comparison of three different Hib conjugates (HbOC, PRP-OMP and PRP-T) given at 2, 3 and 4 months showed a priming response for all three vaccines when a polysaccharide ‘booster’ was given at 1 year of age. However the magnitude of response to PRP-T at 1 year of age was less than that of the other two vaccines.79 The relevance of this measure of priming to long-term vaccine efficacy is not clear.
A further level of complexity is the development of combination vaccines where there may be interactions between the other component antigens and the conjugate. Physical interactions may occur between the individual components (e.g. precipitation when mixed) or the immune response to one antigen may be altered by the immune response to another (e.g. in a situation of limited antigen presentation capacity, an antigen that is presented more efficiently could reduce the degree to which a second antigen is presented to T cells). A well-documented example of the problems of combination vaccines is afforded by the interaction of Hib when combined with diphtheria, tetanus acellular pertussis (DPT-aP) vaccine. Such combination vaccines result in reduced primary immunogenicity and immunological priming related to the acellular pertussis component.80 Simultaneous administration at separate sites shows no such effect.
Hib conjugates are immunogenic and prime for memory. However, there are occasional cases of an isolated and specific inability to respond to Hib and other protein-polysaccharide conjugate vaccines. The immunological basis of this unresponsiveness is unclear but may be important in revealing mechanisms of conjugate vaccine induce immunity.
Direct and indirect measures of protection
Efficacy trials
The efficacy of PRP conjugated to tetanus or diphtheria toxoids as carrier proteins against invasive Hib disease in childhood was proven in several trials including those in Finland, USA, Africa and the UK.81–84 Estimates of efficacy for invasive disease range from 90 to 100% for up to 1 year following vaccination, across areas with widely differing disease epidemiology. Subsequent experience has shown a highly significant reduction of Hib disease in countries where the vaccine has been introduced as a routine.85–89
There have been notable exceptions to these high levels of efficacy. The choice of conjugate, the number of doses and their timing together with the local epidemiology of invasive Hib disease are all important variables in determining protective effectiveness. A PRP-D conjugate that had shown efficacy in Finland was not associated with protection when introduced into an Alaskan population with high levels of disease early in life. Variation in the kinetics and magnitude of antibody induced by different Hib conjugate vaccines are directly relevant (Table 1). In the same Alaskan population PRP-OMP was introduced as a routine Hib vaccine in 1991. A substantial decline in Hib disease followed. In 1996 this schedule was changed to the use of HbOC, which was available as a combination vaccine with diphtheria, tetanus and pertussis (DPT). The intention behind the change in regimes was to reduce the number of injections at each visit. In the year following this change there were more cases of invasive Hib disease than the total number over the previous 5 years since the introduction of the vaccine. The resurgence of invasive Hib disease was thought to reflect the high incidence of invasive Hib in the early months of life and the relatively later onset of immunity following the two doses of HbOC as compared with the previous regime using PRP-OMP.90 The development of immunity very early in infancy is particularly important in countries and ethnic groups where there is a high attack rate in the first weeks of life and where most episodes of invasive disease occur by the end of the first year.
Serological correlates of protection
Determining reliable correlates of vaccine efficacy is increasingly important where efficacy trials are no longer affordable or ethical, but new preparations, such as combination vaccines, are under development. In addition reliable serological correlates of protection are important for individual clinical advice and the interpretation of population based sero-epidemiology. Inferring efficacy of a vaccine from serological variables is not trivial as conjugate vaccines induce protection via antibody, immunological memory and herd immunity.
Correlates of protection for invasive Hib disease are based on measurements of anti-PRP antibody and are derived from studies of natural immunity, plain-polysaccharide vaccine responses and passive immunization.91 In adult sera values of anti-PRP antibody >0·04 µg/ml92 or >0·15 µg/ml93 were presumed to indicate immunity as the incidence of Hib disease was very low in the adult populations. However such adults would be expected to have a degree of immunological memory suggesting that these values may be an over-estimate. An efficacy study of plain-PRP polysaccharide vaccine in Finland was the basis for concluding that 1 µg/ml post vaccination was sufficient to confer protection for the following 12 months.94 Of note, the same group proposed >0·15 µg/ml to give a good inverse correlation with disease incidence in a non-vaccinated population. This again suggests the influence of immunological memory which might be expected to have a role in natural immunity whereas it is demonstrably absent with the plain-PRP vaccine. Lastly, the administration of immunoglobulin containing anti-PRP antibody to children in a high risk population suggested protective levels of 0·05–0·15 µg/ml. The interpretation of these data are complicated by problems of interassay standardization which are still a significant problem for the Hib enzyme-linked immunosorbent assay.
The above data indicate that protection correlates with serum antibody concentrations of 0·04–1·0 µg/ml. However this provides no information on the role of immunological memory, isotype or avidity of antibody. In addition, it is likely that correlates may vary between populations, either caused by host genetic variability in the immune response to vaccine and pathogen, or because the epidemiology of invasive Hib disease is different. Both Alaskan and Finnish infants made comparable antibody responses to a PRP-D vaccine. The efficacy in Finnish infants under 2 was 90% whereas there was no demonstrable efficacy in the Alaskan population.91 Whilst there are accepted correlates of protection for Hib it is not clear to what extent these are adequate for all individuals or all populations.
Hib conjugate vaccines and hib carriage
The response to routine infant immunization with Hib conjugate vaccines extends beyond the individual immunological response. An effect on Hib carriage is particularly important for a pathogen whose sole host is man. It is now clear that immunization has resulted in profound changes in the dynamics of Hib carriage.
Carriage
Given the relative inadequacy of plain-PRP vaccines in protecting against invasive disease and the absence of any evidence of their effect on carriage, it was surprising that Hib conjugate vaccines dramatically reduced carriage in children within vaccinated populations.88,95–97 In a UK population carriage rates of 8–12% were seen in preschool children prior to the introduction of routine Hib conjugate vaccines.10,95 A study in a fully vaccinated group of preschool Oxfordshire children put the carriage rate at 1·3%.23 It is notable that vaccination does not appear to eliminate concurrent carriage but prevents establishment of further episodes.95 The mechanisms by which carriage is reduced are not clear. Previous studies with plain-polysaccharide vaccines had suggested mucosal immunoglobulin A (IgA) was insufficient to prevent colonization.16,98 In a study using a Hib conjugate, serum IgG correlated with salivary IgG post vaccination suggesting the possibility of transudation of serum IgG across mucosal surfaces.99 A threshold serum IgG concentration of 5 µg/ml was associated with a reduction in carriage in children given Hib conjugates in the Dominican Republic.100 It is likely that Hib IgG antibodies cross mucosal barriers above a minimum threshold value.
The reduction in Hib carriage directly facilitates herd immunity and significantly contributes to the protection of the vaccinated population.101,102 However, in a non-vaccinated population, Hib encountered in the course of childhood may contribute to immunity by repeated stimulation of antibody production thereby inducing both individual and herd immunity. Thus in a vaccinated population reduction in carriage results in a decrease in natural boosting and, in the absence of further doses of vaccine, serum antibody titres wane. Initial efficacy trials, involving only a subset of the population may have underestimated this effect. Since 2000, lower antibody titres have been observed in children aged 3 years as compared to titres from 3 years old children vaccinated in the early 1990s (D. Kelly, unpublished observation). These lower titres may reflect decreased boosting through carriage. The adult population in most countries has never had Hib conjugate vaccines and may be very dependent on the ‘natural boosting’ of Hib carriage to maintain Hib immunity. In the UK there has been an increase in disease in the over-15s since 1999, although the levels of disease are not dissimilar to those prior to the introduction of Hib conjugates in infancy.103 This may reflect waning immunity in the adult population as a result of reduced carriage rates in the vaccinated population and herd immunity.
An effect on carriage appears to be a feature of all the licensed conjugate vaccines against the encapsulated bacterial pathogens (Hib, Nm and Sp) that are commonly found as nasopharyngeal commensals. However, the epidemiology of carriage varies among these species and the effect of conjugate vaccines on carriage dynamics is not amenable to universal predictions.
Natural selection in Hib
Carriage of several capsular variants of H. influenzae in the nasopharynx raises the possibility that vaccine pressure against carriage of type b strains could increase the prevalence of other capsule types (a,c–f) in carriage and disease. Previous work has shown that some aspects of virulence are shared by other capsule types5 but it is unclear what differentiates Hib from other serotypes in its potential to cause invasive disease.
The virulence potential of type b organisms may be related to properties other than the capsule and it is possible that capsule switching could enable the emergence of an organism with the genome of a type b clone bearing a non-b capsule. Evidence for capsule switching has been documented, although it is thought to be a very rare event.104 Bacterial strains (clones) which have undergone capsule switching can have significant epidemiological consequences even without the imposition of vaccine related selective pressures. The relatively low rates of carriage and disease caused by H. influenzae strains other than type b make it difficult to rule out the impact of selective pressures with any statistical rigour because the numbers involved are too small. Nonetheless, more than 11 years of UK surveillance since the introduction of routine immunization with Hib conjugates has not shown any trend towards an increase in disease caused by nontype b encapsulated strains of H. influenzae.105
Capsule switching has been clearly described for N. meningitidis. In Oregon, USA, a rise in meningococcal disease cause by a serogroup B ST-32/ET-5 clonal complex was noted starting in 1993. In 1994 serogroup CST-32/ET-5 clones were first isolated and shown to have arisen by allelic exchange of a capsule biosynthesis gene from a serogroup B organisms.106
Insights from vaccine failure
As a result of the routine use of Hib conjugates in infancy in the UK the incidence of invasive Hib disease fell from 22·9/100 000 in 1990 to 0·65/100 000 in 1998 in those less than 5 years old.107 Initial estimates of efficacy indicated the adequacy of the UK schedule where immunization is given at 2, 3 and 4 months and there is no booster despite low antibody titres in preschool children.53,108 Although Hib conjugate vaccines are highly protective, there have continued to be small numbers of true vaccine failures.109–111 Detailed studies of UK children with invasive Hib disease found that true vaccine failures (Hib disease after three doses of vaccine) accounted for about half the incidence of invasive Hib disease in the late 1990s. The median age of children presenting with vaccine failure was 23 months and 61% presented with meningitis. In only 44% was there a clinical risk factor (e.g. prematurity, malignancy) or immunoglobulin subclass deficiency.109
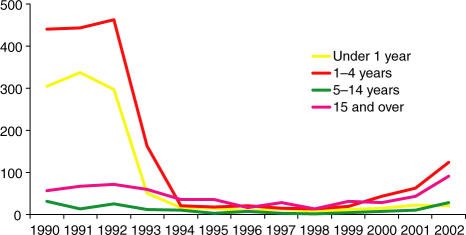
Between 1999 and 2003 there was a steady rise in cases of invasive Hib disease in fully immunized children under 5 in the UK to 4·6/100 000 in 2002 (see Fig. 1).
Figure 1.
Cases of invasive Hib disease by age, England and Wales, 1990–2002. Data from Trotter et al. (2003).105 Reproduced by permission of the Health Protection Agency.
During this period there was no significant change in vaccine coverage102 and it seems unlikely that demographic and social variables could have altered fast enough to account for the speed of increase in cases. The UK is almost unique in the rise in vaccine failures observed since 1999. Specific features of the UK vaccination programme (lack of a booster dose, the initial ‘catch-up’ campaign and use of acellular pertussis combination vaccines) appear to have been factors contributing to the increase in vaccine failures at this time.
UK population-based data confirms what has been observed during immunogenicity studies, that following primary immunization antibody levels wane rapidly over the following years to a point where, if not boosted, significant proportions of a population of children have titres below conventional measures of protection.52 Within the UK there was an initial optimism regarding the lack of need for a booster vaccination53 supported by demonstration of primed responses even in the face of low antibody titres.54 However, in countries where routine boosting with Hib conjugates at 18 months of age is undertaken there has been no increase in vaccine failures and evidence of significant levels of antibody up to 10 years of age.112 The low titres observed in the UK may have been exacerbated by the loss of ‘natural boosting’ associated with a reduction in carriage. A comparison of vaccinated and unvaccinated children in the UK presenting with Hib meningitis suggests the presence of immunological memory in the vaccinated children. This was judged by their greater antibody response in comparison to those unvaccinated57 showing that priming is not necessarily protective. In addition a recent re-evaluation of efficacy data has demonstrated that UK Hib conjugate vaccine effectiveness falls at 2 years post vaccination, correlating with the time of lowest anti-PRP titres.102
A further factor in the timing of the appearance of vaccine failures has been the original ‘catch-up’ campaign associated with the introduction of routine infant Hib conjugate immunization in the UK in 1992. All those aged 6 months to 4 years received a single dose of vaccine. This boosted the levels of antibody in these age groups beyond that expected had these age groups received only primary immunization at 2, 3 and 4 months of age.52 The ‘catch-up’ campaign increased the apparent effectiveness of vaccination by boosting immunity in older age groups with a concomitant effect on herd immunity. Predictably, with increasing time since the ‘catch-up campaign’, the mean antibody titres in 1–5-year-old age groups have fallen so as to reflect those expected in a steady state following immunization at 2, 3 and 4 months without a booster. From 1998 onwards all those under 4 years would have had vaccination as per the infant immunization schedule.
Perhaps of prinicipal importance, a final factor appears to have contributed to the rapid increase in vaccine failures seen from 1999 onwards. Preceding the increase in vaccine failures there was a shortage of combined Diphtheria/Tetanus/whole-cell Pertussis/Hib (DTwP-Hib) conjugate vaccine. As a result there was widespread use of a DTaP-Hib vaccine from late 1999 onwards. Reduced antibody responses to Hib conjugates have been well documented using acellular pertussis/Hib combinations.80 This effect is increased by accelerated immunization schedules such as that in the UK113 and more marked with increasing doses of acellular pertussis combinations.114 However, immunogenicity had been noted to be within the range of immunogenicity obtained for the use of Hib vaccines administered as a separate vaccine and found to be highly efficacious. A case–control study of true Hib vaccine failures in UK children showed a significantly increased incidence of disease among those who received DTaP-Hib combination vaccines.107 The odds ratio for vaccine failures in those who received 3 doses of DTaP-Hib versus controls was 6·4 (95% CI 3·1–13·2). The precise immunological mechanism responsible for the excess of vaccine failures following the combination vaccine is not known.
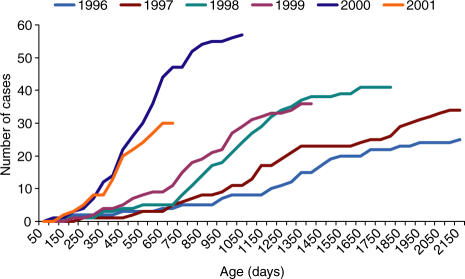
Confirmatory evidence for the idea that multiple factors have coincided to result in vaccine failure is found from a graphical analysis of the cumulative number of Hib cases by age for annual birth cohorts since 1996 (see Fig. 2). A trend to increased amounts of invasive Hib disease at earlier ages is seen between 1996 and 1999 prior to the use of acellular pertussis containing vaccine in the UK. This trend is compatible with the effects of falling carriage rates and the gradual waning of herd immunity amongst those immunized in the catch-up campaign. The more marked increase in incidence between 1999 and 2000 coincides with the use of acellular pertussis combination vaccine. It is unclear whether the trend noted prior to 1999 would have become significant in public health terms without the subsequent introduction of acellular pertussis combinations. Of note in this regard is recent experience in the Netherlands where there was no catch-up campaign when the vaccine was introduced in the Netherlands and a whole cell pertussis vaccine is used.115 In 1999 the schedule was changed from 3, 4, and 5 months with a Hib booster at 11 months to 2, 3 and 4 months with the booster retained. A large increase in the number of Hib cases was seen in 2002.
Figure 2.
Cumulative reports of Hib vaccine failures by age, England and Wales, by birth cohort (cases to December 2002). From Trotter et al. (2003).105 Reproduced by permission of the Health Protection Agency.
In response to the rise in Hib cases in the UK a further ‘catch-up’ campaign has been undertaken. By giving an additional single dose of Hib conjugate to each child between 6 months and 4 years of age116 the aim is to boost anti-PRP antibodies in all those who are immunized and increase herd immunity. Finding a long-term solution may be more complex, particularly with the current public debate on vaccine safety and the ever increasing number of antigens added to the routine vaccination schedule, each with varying effects on immunogenicity.
Just as syndromes of immune deficiency have provided invaluable insight into the function of different components of the immune system, the study of vaccine failures provide the potential for novel insight into vaccine immunology. A systematic approach to the documentation of immunological and genetic information from children with vaccine failure requires a sustained surveillance infrastructure.
Conclusion
The increased use of acellular pertussis combination vaccines in the UK from late 1999 onwards precipitated a large number of Hib vaccine failures. This occurred in the context of waning population immunity in part a result of the relatively short period of protection offered by the use of Hib conjugate vaccines at 2, 3 and 4 months without a booster. A more comprehensive understanding of the interaction between vaccine and natural immune responses, herd immunity and disease is gradually emerging. Despite many similarities in epidemiology and the immunobiology of protein-polysaccharide conjugate vaccines for Hib, Sp and Nm the emergent epidemiology for disease caused by each organism in a vaccinated population may well be different. For both Sp and Nm there are significant periods of risk later in life (in old age and in teenagers, respectively) so that there may be even more problems associated with waning immunity than have been seen so far with Hib. Furthermore, there are already capsular variants of Nm and Sp that will not initially be covered by current vaccines raising significant concerns that strain replacement will occur.
The introduction of new vaccines is always a public health experiment. Experience with Hib conjugate vaccines emphasizes the prolonged duration of time over which surveillance must be undertaken to monitor the results of such experiments. However, such surveillance can provide valuable insights into vaccine immunobiology and reassurance about the ability of vaccines to reduce the burden of these serious diseases. Most resource-poor countries will not have access to conjugate vaccines despite a significant disease burden in many areas.117 The provision of Hib is a WHO priority,118 but cost and logistics remain significant hurdles in delivery.119 In addition, basic epidemiological information is missing for some regions120 and significant steps could be taken today by defining burdens of disease and expanding implementation of these highly efficacious vaccines.
References
- 1.Anderson P, Johnston RB, Jr, Smith DH. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972;51:31. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller PF, Smith AL, Smith DH, Anderson P. Role of immunity in the clearance of bacteremia due to Haemophilus influenzae. J Infect Dis. 1978;138:427. doi: 10.1093/infdis/138.4.427. [DOI] [PubMed] [Google Scholar]
- 3.Pittman M. Variation and type specificity in the bacterial species Haemophilus influenzae. J Exp Med. 1931;53:471. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moxon ER, Kroll JS. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 5.Swift AJ, Moxon ER, Zwahlen A, Winkelstein JA. Complement-mediated serum activities against genetically defined capsular transformants of Haemophilus influenzae. Microb Pathog. 1991;10:261. doi: 10.1016/0882-4010(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 6.Winkelstein JA, Moxon ER. The role of complement in the host's defense against Haemophilus influenzae. J Infect Dis. 1992;165(Suppl. 1):S62. doi: 10.1093/infdis/165-supplement_1-s62. [DOI] [PubMed] [Google Scholar]
- 7.Alexander H, Heidleberger M, Leidy G. The protective or curative element in type B H. influenzae rabbit serum. Yale J Biol Med. 1944;16:425. [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson P, Smith DH, Ingram DL, Wilkins J, Wehrle PF, Howie VM. Antibody of polyribophate of Haemophilus influenzae type b in infants and children: effect of immunization with polyribophosphate. J Infect Dis. 1977;136(Suppl.):S57. doi: 10.1093/infdis/136.supplement.s57. [DOI] [PubMed] [Google Scholar]
- 9.Schneerson R, Robbins JB. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075: K100: H5. N Engl J Med. 1975;292:1093. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- 10.Barbour ML, Booy R, Crook DW, Griffiths H, Chapel HM, Moxon ER, Mayon-White D. Haemophilus influenzae type b carriage and immunity four years after receiving the Haemophilus influenzae oligosaccharide-CRM197 (HbOC) conjugate vaccine. Pediatr Infect Dis J. 1993;12:478. doi: 10.1097/00006454-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Hall DB, Lum MK, Knutson LR, Heyward WL, Ward JI. Pharyngeal carriage and acquisition of anticapsular antibody to Haemophilus influenzae type b in a high-risk population in southwestern Alaska. Am J Epidemiol. 1987;126:1190. doi: 10.1093/oxfordjournals.aje.a114758. [DOI] [PubMed] [Google Scholar]
- 12.Booy R, Hodgson SA, Slack MP, Anderson EC, Mayon-White RT, Moxon ER. Invasive Haemophilus influenzae type b disease in the Oxford region (1985–91) Arch Dis Child. 1993;69:225. doi: 10.1136/adc.69.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennet JV, Platonov AE, Slack MPE, Mala P, Burton AH, Robertson SE. Vaccines and Biologicals. Geneva: WHO; 2002. Haemophilus influenzae type b (Hib) meningitis in the pre-vaccine era. a global review of incidence, age distributions, and case-fatality rates. [Google Scholar]
- 14.Bijlmer HA, van Alphen L, Greenwood BM, et al. The epidemiology of Haemophilus influenzae meningitis in children under five years of age in The Gambia, West Africa. J Infect Dis. 1990;161:1210. doi: 10.1093/infdis/161.6.1210. [DOI] [PubMed] [Google Scholar]
- 15.Parke JC, Jr, Schneerson R, Robbins JB, Schlesselman JJ. Interim report of a controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mecklenburg county, North Carolina (March 1974–March 1976) J Infect Dis. 1977;136(Suppl.):S51. doi: 10.1093/infdis/136.supplement.s51. [DOI] [PubMed] [Google Scholar]
- 16.Peltola H, Kayhty H, Sivonen A, Makela H. Pediatrics. 1977;60:730. Haemophilus influenzae type b capsular polysaccharide vaccine in children. a double-blind field study of 100 000 vaccinees 3 months to 5 years of age in Finland. [PubMed] [Google Scholar]
- 17.Smith DH, Peter G, Ingram DL, Harding AL, Anderson P. Responses of children immunized with the capsular polysaccharide of Haemophilus influenzae, type b. Pediatrics. 1973;52:637. [PubMed] [Google Scholar]
- 18.Kayhty H, Karanko V, Peltola H, Makela PH. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984;74:857. [PubMed] [Google Scholar]
- 19.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 20.Fairhurst RM, Wang CX, Sieling PA, Modlin RL, Braun J. CD1-restricted T cells and resistance to polysaccharide-encapsulated bacteria. Immunol Today. 1998;19:257. doi: 10.1016/s0167-5699(97)01235-8. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg GA, Einhorn MS, Lenoir AA, Granoff PD, Granoff DM. Immunologic priming to capsular polysaccharide in infants immunized with Haemophilus influenzae type b polysaccharide-Neisseria meningitidis outer membrane protein conjugate vaccine. J Pediatr. 1987;111:22. doi: 10.1016/s0022-3476(87)80336-0. [DOI] [PubMed] [Google Scholar]
- 22.Rijkers GT, Sanders EA, Breukels MA, Zegers BJ. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16:1396. doi: 10.1016/s0264-410x(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 23.Heath PT, Bowen-Morris J, Griffiths D, Griffiths H, Crook DW, Moxon ER. Antibody persistence and Haemophilus influenzae type b carriage after infant immunisation with PRP-T. Arch Dis Child. 1997;77:488. doi: 10.1136/adc.77.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hougs L, Juul L, Ditzel HJ, Heilmann C, Svejgaard A, Barington T. The first dose of a Haemophilus influenzae type b conjugate vaccine reactivates memory B cells: evidence for extensive clonal selection, intraclonal affinity maturation, and multiple isotype switches to IgA2. J Immunol. 1999;162:224. [PubMed] [Google Scholar]
- 25.Anderson P, Ingram DL, Pichichero ME, Peter G. A high degree of natural immunologic priming to the capsular polysaccharide may not prevent Haemophilus influenzae type b meningitis. Pediatr Infect Dis J. 2000;19:589. doi: 10.1097/00006454-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Norden CW, Michaels RH, Melish M. Effect of previous infection on antibody response of children to vaccination with capsular polysaccharide of Haemophilus influenzae Type b. J Infect Dis. 1975;132:69. doi: 10.1093/infdis/132.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Trollfors B, Lagergard T, Claesson BA, Thornberg E, Martinell J, Schneerson R. Characterization of the serum antibody response to the capsular polysaccharide of Haemophilus influenzae type b in children with invasive infections. J Infect Dis. 1992;166:1335. doi: 10.1093/infdis/166.6.1335. [DOI] [PubMed] [Google Scholar]
- 28.Gold R, Lepow ML, Goldschneider I, Draper TF, Gotshlich EC. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979;140:690. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- 29.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]
- 30.Goebel WF. Studies on antibacterial immunity induced by artificial antigens. II. Immunity to experimental pneumococcal infection with antigens containing saccharides of synthetic origin. J Exp Med. 1940;72:33. doi: 10.1084/jem.72.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avery O, Goebel WT. Chemo-immunological studies on conjugated carbohydrate-proteins. II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:522. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu C, Schneerson R, Robbins JB, Rastogi SC. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983;40:245. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson P. Antibody responses to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of the type b capsule with the nontoxic protein CRM197. Infect Immun. 1983;39:233. doi: 10.1128/iai.39.1.233-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson P, Pichichero ME, Insel RA. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Invest. 1985;76:52. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granoff DM, Boies EG, Munson RS., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide – diphtheria toxoid conjugate vaccine in adults. J Pediatr. 1984;105:22. doi: 10.1016/s0022-3476(84)80350-9. [DOI] [PubMed] [Google Scholar]
- 37.Anderson P, Pichichero ME, Insel RA. Immunization of 2-month-old infants with protein-coupled oligosaccharides derived from the capsule of Haemophilus influenzae type b. J Pediatr. 1985;107:346. doi: 10.1016/s0022-3476(85)80504-7. [DOI] [PubMed] [Google Scholar]
- 38.Anderson P, Pichichero M, Edwards K, Porch CR, Insel R. Priming and induction of Haemophilus influenzae type b capsular antibodies in early infancy by Dpo20, an oligosaccharide-protein conjugate vaccine. J Pediatr. 1987;111:644. doi: 10.1016/s0022-3476(87)80237-8. [DOI] [PubMed] [Google Scholar]
- 39.Kayhty H, Eskola J, Peltola H, Saarinen L, Makela PH. High antibody responses to booster doses of either Haemophilus influenzae capsular polysaccharide or conjugate vaccine after primary immunization with conjugate vaccines. J Infect Dis. 1992;165(Suppl. 1):S165. doi: 10.1093/infdis/165-supplement_1-s165. [DOI] [PubMed] [Google Scholar]
- 40.Anderson P, Pichichero M, Insel R, Farsad P, Santosham M. Capsular antigens noncovalently or covalently associated with protein as vaccines to Haemophilus influenzae type b: comparison in two-year-old children. J Infect Dis. 1985;152:634. doi: 10.1093/infdis/152.3.634. [DOI] [PubMed] [Google Scholar]
- 41.Breukels MA, Rijkers GT, Voorhorst-Ogink MM, Zegers BJ. Regulatory T cells in the antibody response to Haemophilus influenzae type b polysaccharide. Infect Immun. 1999;67:789. doi: 10.1128/iai.67.2.789-793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siber GR. Pneumococcal disease. prospects for a new generation of vaccines. Science. 1994;265:1385. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 43.Arpin C, Dechanet J, Van Kooten C, et al. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 44.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652. [PubMed] [Google Scholar]
- 45.Symonds J, Bone M, Turner A, Javaid A. Penetration of ofloxacin into bronchial secretions. Drugs. 1987;34(Suppl. 1):33. doi: 10.2165/00003495-198700341-00008. [DOI] [PubMed] [Google Scholar]
- 46.Kamboj KK, King CL, Greenspan NS, Kirchner HL, Schreiber JR. Immunization with Haemophilus influenzae type b-CRM (197) conjugate vaccine elicits a mixed Th1 and Th2 CD (4+) T cell cytokine response that correlates with the isotype of antipolysaccharide antibody. J Infect Dis. 2001;184:931. doi: 10.1086/323342. [DOI] [PubMed] [Google Scholar]
- 47.Leiva LE, Butler B, Hempe J, Ortigas AP, Sorensen RU. Up-regulation of CD40 ligand and induction of a Th2 response in children immunized with pneumococcal polysaccharide vaccines. Clin Diagn Lab Immunol. 2001;8:233. doi: 10.1128/CDLI.8.2.233-240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172:2431. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 49.Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 50.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 51.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 52.Trotter CL, McVernon J, Andrews NJ, Burrage M, Ramsay ME. Antibody to Haemophilus influenzae type b after routine and catch-up vaccination. Lancet. 2003;361:1523. doi: 10.1016/s0140-6736(03)13172-8. [DOI] [PubMed] [Google Scholar]
- 53.Heath PT, Booy R, Azzopardi HJ, et al. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA. 2000;284:2334. doi: 10.1001/jama.284.18.2334. [DOI] [PubMed] [Google Scholar]
- 54.Goldblatt D, Miller E, McCloskey N, Cartwright K. Immunological response to conjugate vaccines in infants: follow up study. Br Med J. 1998;316:1570. doi: 10.1136/bmj.316.7144.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campos M, Godson DL. The effectiveness and limitations of immune memory: understanding protective immune responses. Int J Parasitol. 2003;33:655. doi: 10.1016/s0020-7519(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 56.Zinkernagel RM. On differences between immunity and immunological memory. Curr Opin Immunol. 2002;14:523. doi: 10.1016/s0952-7915(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 57.McVernon J, Johnson PD, Pollard AJ, Slack MP, Moxon ER. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch Dis Child. 2003;88:379. doi: 10.1136/adc.88.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas AH, Granoff DM. Imperfect memory and the development of Haemophilus influenzae type B disease. Pediatr Infect Dis J. 2001;20:235. doi: 10.1097/00006454-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+ IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurikka S. Priming with diphtheria-tetanus-pertussis vaccine enhances the response to the Haemophilus influenzae type b tetanus conjugate vaccine in infancy. Vaccine. 1996;14:1239. doi: 10.1016/s0264-410x(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 61.McVernon J, Mitchison NA, Moxon ER. T helper cells and efficacy of Haemophilus influenzae type b conjugate vaccination. Lancet Infect Dis. 2004;4:40. doi: 10.1016/s1473-3099(03)00859-4. [DOI] [PubMed] [Google Scholar]
- 62.Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur J Immunol. 2000;30:1214. doi: 10.1002/(SICI)1521-4141(200004)30:4<1214::AID-IMMU1214>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 63.Granoff DM, Anderson EL, Osterholm MT, et al. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992;121:187. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- 64.Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992;120:184. doi: 10.1016/s0022-3476(05)80424-x. [DOI] [PubMed] [Google Scholar]
- 65.Kayhty H, Eskola J, Peltola H, Ronnberg PR, Kela E, Karanko V, Saarinen L. Antibody responses to four Haemophilus influenzae type b conjugate vaccines. Am J Dis Child. 1991;145:223. doi: 10.1001/archpedi.1991.02160020117030. [DOI] [PubMed] [Google Scholar]
- 66.Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992;267:1489. [PubMed] [Google Scholar]
- 67.Capeding MR, Nohynek H, Pascual LG, Kayhty H, Sombrero LT, Eskola J, Ruutu P. The immunogenicity of three Haemophilus influenzae type B conjugate vaccines after a primary vaccination series in Philippine infants. Am J Trop Med Hyg. 1996;55:516. doi: 10.4269/ajtmh.1996.55.516. [DOI] [PubMed] [Google Scholar]
- 68.Wenger JD, Booy R, Heath P, Moxon ER. Epidemiological impact of conjugate vaccines on invasive disease caused by Haemophilus influenzae type b. In: Levine MM, Woodrow GC, Kaper JB, Cobon GS, editors. New Generation Vaccines. 2. New York: Marcel Dekker, Inc; 1997. p. 489. [Google Scholar]
- 69.Makela PH, Kayhty H. Evolution of conjugate vaccines. Expert Rev Vaccines. 2002;1:399. doi: 10.1586/14760584.1.3.399. [DOI] [PubMed] [Google Scholar]
- 70.Kelly DF, Moxon ER. Is Haemophilus Influenzae Type b Disease Finished. In: Pollard AJ, McCracken GH, Finn A, editors. Hot Topics in Infection and Immunity in Children. New York: Kluwer Academic/Plenum Publishers; 2004. p. 221. [Google Scholar]
- 71.Englund JA, Glezen WP. Maternal immunization with Haemophilus influenzae type b vaccines in different populations. Vaccine. 2003;21:3455. doi: 10.1016/s0264-410x(03)00350-5. [DOI] [PubMed] [Google Scholar]
- 72.Kurikka S, Olander RM, Eskola J, Kayhty H. Passively acquired anti-tetanus and anti-Haemophilus antibodies and the response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infancy. Pediatr Infect Dis J. 1996;15:530. doi: 10.1097/00006454-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Nohynek H, Gustafsson L, Capeding MR, Kayhty H, Olander RM, Pascualk L, Ruutu P. Effect of transplacentally acquired tetanus antibodies on the antibody responses to Haemophilus influenzae type b-tetanus toxoid conjugate and tetanus toxoid vaccines in Filipino infants. Pediatr Infect Dis J. 1999;18:25. doi: 10.1097/00006454-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Panpitpat C, Thisyakorn U, Chotpitayasunondh T, Furer E, Que JU, Hasler T, Cryz SJ., Jr Elevated levels of maternal anti-tetanus toxin antibodies do not suppress the immune response to a Haemophilus influenzae type b polyribosylphosphate-tetanus toxoid conjugate vaccine. Bull World Health Organ. 2000;78:364. [PMC free article] [PubMed] [Google Scholar]
- 75.Granoff DM, Holmes SJ, Belshe RB, Osterholm MT, McHugh JE, Anderson EL. Effect of carrier protein priming on antibody responses to Haemophilus influenzae type b conjugate vaccines in infants. JAMA. 1994;272:1116. [PubMed] [Google Scholar]
- 76.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 77.Granoff DM, Rathore MH, Holmes SJ, Granoff PD, Lucas AH. Effect of immunity to the carrier protein on antibody responses to Haemophilus influenzae type b conjugate vaccines. Vaccine. 1993;11(Suppl. 1):S46. doi: 10.1016/0264-410x(93)90160-y. [DOI] [PubMed] [Google Scholar]
- 78.Lieberman JM, Greenberg DP, Wong VK, Partridge S, Chang SJ, Chiu CY, Ward JI. Effect of neonatal immunization with diphtheria and tetanus toxoids on antibody responses to Haemophilus influenzae type b conjugate vaccines. J Pediatr. 1995;126:198. doi: 10.1016/s0022-3476(95)70545-7. [DOI] [PubMed] [Google Scholar]
- 79.Granoff DM, Holmes SJ, Osterholm MT, et al. Induction of immunologic memory in infants primed with Haemophilus influenzae type b conjugate vaccines. J Infect Dis. 1993;168:663. doi: 10.1093/infdis/168.3.663. [DOI] [PubMed] [Google Scholar]
- 80.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999;354:2063. doi: 10.1016/S0140-6736(99)04377-9. [DOI] [PubMed] [Google Scholar]
- 81.Eskola J, Peltola H, Takala AK, et al. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987;317:717. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- 82.Mulholland K, Hilton S, Adegbola R, et al. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 83.Booy R, Moxon ER, MacFarlane JA, Mayon-White RT, Slack MP. Efficacy of Haemophilus influenzae type B conjugate vaccine in Oxford region. Lancet. 1992;340:847. doi: 10.1016/0140-6736(92)92719-v. [DOI] [PubMed] [Google Scholar]
- 84.Vadheim CM, Greenberg DP, Partridge S, Jing J, Ward JI. Effectiveness and safety of an Haemophilus influenzae type b conjugate vaccine (PRP-T) in young infants. Kaiser-UCLA Vaccine Study Group. Pediatrics. 1993;92:272. [PubMed] [Google Scholar]
- 85.Eskola J, Takala A, Kayhty H, Peltola H, Makela PH. Experience in Finland with Haemophilus influenzae type b vaccines. Vaccine. 1991;9(Suppl.):S14. doi: 10.1016/0264-410x(91)90174-5. [DOI] [PubMed] [Google Scholar]
- 86.Heath PT, McVernon J. The UK Hib vaccine experience. Arch Dis Child. 2002;86:396. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Talan DA, Moran GJ, Pinner RW. Progress toward eliminating Haemophilus influenzae type b disease among infants and children – United States 1987–97. Ann Emerg Med. 1999;34:109. doi: 10.1016/s0196-0644(99)70282-9. [DOI] [PubMed] [Google Scholar]
- 88.Takala AK, Eskola J, Leinonen M, Kayhty H, Nissinen A, Pekkanen E, Makela PH. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 89.Murphy TV, White KE, Pastor P, Gabriel L, Medley F, Granoff DM, Osterholm MT. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA. 1993;269:246. [PubMed] [Google Scholar]
- 90.Galil K, Singleton R, Levine OS, et al. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J Infect Dis. 1999;179:101. doi: 10.1086/314569. [DOI] [PubMed] [Google Scholar]
- 91.Kayhty H. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals. 1994;22:397. doi: 10.1006/biol.1994.1062. [DOI] [PubMed] [Google Scholar]
- 92.Robbins JB, Parke JC, Jr, Schneerson R, Whisnant JK. Quantitative measurement of ‘natural’ and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973;7:103. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Makela PH, Peltola H, Kayhty H, et al. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977;136(Suppl.):S43. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- 94.Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 95.Barbour ML, Mayon-White RT, Coles C, Crook DW, Moxon ER. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis. 1995;171:93. doi: 10.1093/infdis/171.1.93. [DOI] [PubMed] [Google Scholar]
- 96.Murphy TV, Pastor P, Medley F, Osterholm MT, Granoff DM. Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate vaccine. J Pediatr. 1993;122:517. doi: 10.1016/s0022-3476(05)83529-2. [DOI] [PubMed] [Google Scholar]
- 97.Adegbola RA, Mulholland EK, Secka O, Jaffar S, Greenwood BM. Vaccination with a Haemophilus influenzae type b conjugate vaccine reduces oropharyngeal carriage of H. influenzae type b among Gambian children. J Infect Dis. 1998;177:1758. doi: 10.1086/517440. [DOI] [PubMed] [Google Scholar]
- 98.Pichichero ME, Insel RA. Mucosal antibody response to parenteral vaccination with Haemophilus influenzae type b capsule. J Allergy Clin Immunol. 1983;72:481. doi: 10.1016/0091-6749(83)90585-7. [DOI] [PubMed] [Google Scholar]
- 99.Kauppi M, Eskola J, Kayhty H. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J. 1995;14:286. doi: 10.1097/00006454-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Fernandez J, Levine OS, Sanchez J, Balter S, LaClaire L, Feris J, Romero-Steiner S. Prevention of Haemophilus influenzae type b colonization by vaccination: correlation with serum anti-capsular IgG concentration. J Infect Dis. 2000;182:1553. doi: 10.1086/315870. [DOI] [PubMed] [Google Scholar]
- 101.Rushdy A, Ramsay M, Heath PT, Azzopardi HJ, Slack MP. Infant Hib vaccination and herd immunity. J Pediatr. 1999;134:253. doi: 10.1016/s0022-3476(99)70438-5. [DOI] [PubMed] [Google Scholar]
- 102.Ramsay ME, McVernon J, Andrews NJ, Heath PT, Slack MP. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J Infect Dis. 2003;188:481. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 103.HPA. 2003. Laboratory reports of Haemophilus influenzae type B infection by age group and quarter England and Wales 1990–2003. http://www.hpa.org.uk/infections/topics_az/haemophilus_influenzae/data.htm.
- 104.Kroll JS, Moxon ER. Capsulation in distantly related strains of Haemophilus influenzae type b: genetic drift and gene transfer at the capsulation locus. J Bacteriol. 1990;172:1374. doi: 10.1128/jb.172.3.1374-1379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trotter CL, Ramsay ME, Slack MP. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Commun Dis Public Health. 2003;6:55. [PubMed] [Google Scholar]
- 106.Swartley JS, Marfin AA, Edupuganti S, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McVernon J, Andrews N, Slack MP, Ramsay ME. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet. 2003;361:1521. doi: 10.1016/s0140-6736(03)13171-6. [DOI] [PubMed] [Google Scholar]
- 108.Booy R, Heath PT, Slack MP, Begg N, Moxon ER. Vaccine failures after primary immunisation with Haemophilus influenzae type-b conjugate vaccine without booster. Lancet. 1997;349:1197. doi: 10.1016/s0140-6736(96)06392-1. [DOI] [PubMed] [Google Scholar]
- 109.Heath PT, Booy R, Griffiths H, et al. Clinical and immunological risk factors associated with Haemophilus influenzae type b conjugate vaccine failure in childhood. Clin Infect Dis. 2000;31:973. doi: 10.1086/318132. [DOI] [PubMed] [Google Scholar]
- 110.Breukels MA, Spanjaard L, Sanders LA, Rijkers GT. Immunological characterization of conjugated Haemophilus influenzae type b vaccine failure in infants. 2001. p. 32. Clin Infect Dis 1700. [DOI] [PubMed]
- 111.Holmes SJ, Granoff DM. The biology of Haemophilus influenzae type b vaccination failure. J Infect Dis. 1992;165(Suppl. 1):S121. doi: 10.1093/infdis/165-supplement_1-s121. [DOI] [PubMed] [Google Scholar]
- 112.Makela PH, Kayhty H, Leino T, Auranen K, Peltola H, Ekstrom N, Eskola J. Long-term persistence of immunity after immunisation with Haemophilus influenzae type b conjugate vaccine. Vaccine. 2003;22:287. doi: 10.1016/s0264-410x(03)00524-3. [DOI] [PubMed] [Google Scholar]
- 113.Vidor E, Hoffenbach A, Fletcher MA. Haemophilus influenzae type b vaccine: reconstitution of lyophilised PRP-T vaccine with a pertussis-containing paediatric combination vaccine, or a change in the primary series immunisation schedule, may modify the serum anti-PRP antibody responses. Curr Med Res Opin. 2001;17:197. doi: 10.1185/0300799039117063. [DOI] [PubMed] [Google Scholar]
- 114.Daum RS, Zenko CE, Given GZ, Ballanco GA, Parikh H, Germino K. Magnitude of interference after diphtheria-tetanus toxoids-acellular pertussis/Haemophilus influenzae type b capsular polysaccharide-tetanus vaccination is related to the number of doses administered. J Infect Dis. 2001;184:1293. doi: 10.1086/324007. [DOI] [PubMed] [Google Scholar]
- 115.Rijkers GT, Vermeer-de Bondt PE, Spanjaard L, Breukels MA, Sanders EA. Return of Haemophilus influenzae type b infections. Lancet. 2003;361:1563. doi: 10.1016/S0140-6736(03)13201-1. [DOI] [PubMed] [Google Scholar]
- 116.Heath PT, Ramsay ME. Haemophilus influenzae type b vaccine – booster campaign. Br Med J. 2003;326:1158. doi: 10.1136/bmj.326.7400.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.WHO. The Global Programme for Vaccines and Immunisation (GPV) The WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 1998;73:64. [PubMed] [Google Scholar]
- 119.Booy R. Getting Hib vaccine to those who need it. Lancet. 1998;351:1446. doi: 10.1016/S0140-6736(98)22020-4. [DOI] [PubMed] [Google Scholar]
- 120.Lee JW. Haemophilus influenzae in Asia. Pediatr Infect Dis J. 1998;17:S92. doi: 10.1097/00006454-199809001-00001. [DOI] [PubMed] [Google Scholar]