Abstract
The interaction of the immunoglobulin A (IgA) molecule with its specific cellular receptor is necessary to trigger a variety of effector functions able to clear IgA-opsonized antigens. The human IgA-specific Fc receptor, FcαRI or CD89, is expressed on cells of the myeloid lineage. Recently, CD89 homologues have been identified in rats and cattle. Because non-human primates represent well established models for a variety of human diseases and for the testing of immunotherapeutic strategies, we cloned and sequenced cDNAs corresponding to the CD89 gene from rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Macaque sequences of full-length CD89 consist of five exons of length identical to the corresponding human CD89 exons. The rhesus and cynomolgus macaque derived amino acid sequences are highly homologous to each other (99·3% identity) and exhibit 86·5% and 86·1% identity to the human counterpart, respectively. Transfection of HeLa cells with plasmids containing the cloned macaque cDNAs resulted in the expression of surface molecules recognized by an anti-human CD89 antibody. Five splice variants were identified in rhesus macaques. Three of the five variants are similar to described human CD89 splice variants, whereas two variants have not been described in humans. Three splice variants were identified in cynomolgus macaques. Of the three variants, one is present also in humans and rhesus macaques, whereas the other two are shared with rhesus macaques but not humans. Similarly to the human CD89, macaque CD89 is expressed on myeloid cells from peripheral blood. The characterization of macaque CD89 represents an essential step in establishing a non-human primate model for the testing of immunotherapeutic approaches based on the manipulation of the IgA/CD89 interaction.
Keywords: CD89, IgA receptor, macaques, splice variants, non-human primate models
Introduction
Fc receptors (FcRs) are expressed on a variety of immune effector cells and, by binding the constant region of antibody molecules, provide an essential link between the humoral and cellular arms of the immune system.1 FcαRI (CD89), the Fc receptor specific for immunoglobulin A (IgA) molecules, is a transmembrane glycoprotein that belongs to the immunoglobulin gene superfamily.2 It is expressed on monocytes, eosinophils, neutrophils and macrophages3 and its signalling capacity is dependent on the association with the FcR γ-chain subunit.4 The human CD89 gene is located within the leucocyte receptor cluster on chromosome 195 appears to exist as a single copy2 spans 12 kb and includes five exons.6 Exons S1 and S2 encode the leader peptide, EC1 and EC2 each specify an extracellular immunoglobulin-like domain of 103 amino acids, and TM/C codes for the transmembrane domain (19 amino acids) and cytoplasmic tail (41 amino acids) of the receptor.
The CD89/IgA interaction mediates immune effector responses, including antibody-dependent cell-mediated cytotoxicity, phagocytosis and respiratory burst, as well as release of cytokines and inflammatory mediators.3 Therefore, this interaction is necessary to maintain the integrity of the immune responses in both systemic and mucosal compartments. Indeed, IgA is the most abundantly produced antibody isotype and represents a first line of defence at mucosal surfaces. The IgA system differs in the animal species most extensively studied. In humans, there are two IgA subclasses, designated IgA1 and IgA2. Both subclasses bind FcαRI with a low affinity of approximately Ka = 106 m−1.7 Only one IgA subclass is present in mice8 whereas rabbits possess 13 IgA subclasses.9 In rhesus macaques, IgA molecules are characterized by an extremely high level of intraspecies heterogeneity.10,11 This IgA heterogeneity appears in several non-human primate species.12 Interestingly, no FcαRI homologue has been identified in mice. Recently, FcαRI homologues have been identified in rats13 and cows.14 Here, we describe the rhesus and cynomolgus macaque CD89.
Materials and methods
Amplification, cloning and sequencing of macaque CD89
Heparinized blood samples were collected from one healthy rhesus macaque (Macaca mulatta) and one healthy cynomolgus macaque (Macaca fascicularis). Total RNA was extracted from whole blood using the QIAamp RNA Blood Mini Kit (Qiagen Inc., Valencia, CA), and reverse transcribed into cDNA using oligo d(T)17 primers, followed by primer extension with the AMV reverse transcriptase (Roche Molecular Biochemicals, Indianapolis, IN). Polymerase chain reaction (PCR) amplification of the cDNA was performed with Expand High Fidelity polymerase (Roche Molecular Biochemicals). The nucleotide sequences of the PCR primers used to amplify the complete CD89 transcripts have been previously reported by Pleass and coworkers.15 Forward (RP1) and reverse (RP2) primers specific for the human CD89 located at the start codon of the S1 exon and the stop codon of the TM/C exon, respectively, were employed. The PCR conditions used were those described by Pleass and coworkers15 except that 40cycles of PCR were performed.
An alternate form of CD89, known also as FcαRIb (which lacks exon TM/C but contains nucleotide sequences present in the intron located between exon EC2 and exon TM/C of variant 1) has been described.16 There are two FcαRIb isoforms, known as CD89 transcript variants 9 and 10.16 In order to amplify this alternate form, we designed the FCARB2 primer (5′-TCTAGCGAGGAAGTGAAAGCGG-3′) located at the 1024–1003 nt of the CD89 transcript variant 9 (GenBank NM_133279) that, used with the RP1 primer, allows amplification of the complete FcαRIb transcripts. After reverse transcription of total RNA, the cDNA was amplified with the FCARB2 and RP1 primers using the same conditions described for the RP1 and RP2 primers.15 Fifty microlitres of a PCR reaction was run on a 2% agarose gel. The bands of interest were excised from the gel, purified using the QIAquick Gel Extraction Kit (Qiagen Inc.) and ligated into the pCR2·1 vector (Invitrogen, Carlsbad, CA). After transformation into the appropriate Escherichia coli, colonies from each sample were expanded. Plasmid DNA was screened on a 1% agarose gel after EcoRI digestion, to confirm the presence of the correct fragment size. All DNA sequences were determined using the ABI Prism Dye Termination Cycle Sequencing kit (PerkinElmer, Wellesley, MA) on an ABI model 3100 automated sequencer (PerkinElmer). The forward and reverse M13 primers were used for sequencing. Completed sequences were edited and aligned using the MacVector sequence analysis package (Accelrys, Burlington, MA).
Expression of macaque CD89 cDNAs in HeLa cells
Full length rhesus and cynomolgus CD89 cDNAs ligated into the pCR2·1 vector were subjected to digestion with XhoI and EcoRI. The resulting fragments were ligated into the XhoI and EcoRI digested expression vector pcDNA3.1 (+) (Invitrogen) and amplified in E. coli. Twenty µg of expression vector was then added to HeLa cells suspended in 250 µl of Dulbecco's modified Eagle's minimal essential medium (DMEM) at 14 × 106 cells/ml. After 10 minutes of incubation on ice, cells were electroporated by one or two pulses using the power supply set to 300 V, 25 mA, and 25 W and the Electroporator II (Invitrogen) set to 1000 µF and 8 Ω, according to the manufacturer's recommendations. Cells were then incubated at room temperature for 10 min and grown in 10 ml of DMEM with 10% fetal calf serum in 5% CO2 at 37°. For rhesus macaque CD89, stably transfected cells were established by selection with Geneticin (400 µg/ml) added 72 hr post transfection. After several passages non-transfected and transfected HeLa cells were harvested from cell culture following three phosphate buffered saline (PBS) washes from wells with 90% confluent growth. 1 × 106 cells were stained at 4° with either 20 µl of phycoerythrin-conjugated mouse anti-human CD89 (clone A59) or SimultestTM Control γ1/γ2a (both from BD PharMingen, San Diego, CA) for 15 min, followed by three washes with PBS. A59 binds to the extracellular domain 2 (EC2) of CD89·17 Cells were then fixed with 1% paraformaldehyde and analysed using a FACSCalibur flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose CA). Repeated flow analysis of cells transfected for rhesus CD89 confirmed that cells stably expressed rhesus CD89. Expression of cynomolgus macaque CD89 in HeLa cells was determined as described above, with the exception that only transient transfectants were generated and examined by flow cytometry analysis.
Determination of CD89 expression on blood leucocytes
Blood from four rhesus and seven cynomolgus macaques was collected in ethylenediaminetetraacetic acid (EDTA) Vacutainer® tubes (Becton-Dickinson) by venipuncture under anaesthesia. Leucocyte expression of CD89 was analysed by two-colour flow cytometry analysis using phycoerythrin (PE)-conjugated anti-human CD89 and fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies for CD14, CD20, and CD3 and cy-chrome conjugated anti-CD16 (BD Pharmingen and BD Immunocytometry Systems). Monoclonal antibody clones for CD14, CD20, CD3 and CD16 were M5E2, L27, SP34, and 3G8, respectively. SimultestTM Control γ1/γ2a was used to detect nonspecific binding of mouse IgG to cells. Staining of whole blood was done using a standard procedure. Briefly, 100 µl of blood was incubated with 20 µl of each antibody in the dark at room temperature. Erythrocytes were lysed with 2 ml of BD PharM Lyse (BD Pharmingen), washed three times with PBS and fixed with 1% paraformaldehyde. Five thousand events were counted by flow cytometry.
Results
Cloning and sequencing of macaque CD89 cDNA
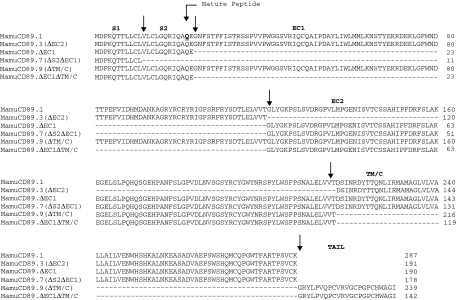
We cloned and sequenced macaque cDNA obtained through reverse transcription of total RNA followed by amplification performed using primers complementary to sequences located in the S1 and TM/C exons. The introduction of errors into the sequence was minimized by using a high fidelity polymerase with proofreading ability. To validate the amplification strategy, we amplified, cloned and sequenced the full length human CD89 as well as two human alternatively spliced variants. All human sequences matched those available in GenBank (accession numbers NM_002000, NM_133271 and NM_133279) (data not shown). We then used the same strategy to amplify, clone and sequence rhesus and cynomolgus macaque CD89. Each sequence was confirmed by cloning and sequencing the products of another independent PCR performed using the same total RNA. A complete transcript including all five exons along with several additional transcripts representing alternatively spliced forms of the CD89 mRNA were identified. Figure 1 shows the deduced amino acid sequences of the complete cDNA from rhesus and cynomolgus macaques along with the corresponding human sequences. All five macaque exons were of length identical to the corresponding human CD89 exons. The rhesus macaque and the cynomolgus macaque CD89 amino acid sequences exhibit 86·5% and 86·1% identity to the human counterpart, respectively. The rhesus macaque CD89 amino acid sequence shows 99·3% identity to the corresponding cynomolgus macaque sequence. Therefore, the CD89 sequences from these two non-human primate species are highly homologous to each other, differing for only two amino acids (a methionine/isoleucine substitution at position 40 and a phenylalanine/leucine substitution at position 85). We did not identify amino acid differences in the S1 and S2 exons between human and macaque sequences. The majority of human/macaque substitutions are clustered in the EC1 exon.
Figure 1.
Alignment of CD89 derived amino acid sequences obtained by cloning and sequencing rhesus macaque (GenBank accession number AY386684) and cynomolgus macaque (GenBank accession number AY386690) cDNA from whole blood and comparison with the published human sequence (GenBank NM_002000). Amino acid differences are underlined. The first amino acid of the preprotein is numbered as residue 1. The mature peptide starts at residue 22. Arrows indicate distinct domains. The first two amino acids for EC1 are encoded at the end of the S2 exon. The signal peptide is encoded by both S1 and S2 sequences. Potential N-glycosylation sites, cysteines involved in disulfide bonds and arginine 209 critical for association with the FcRγ chain are bolded. Hu: Homo sapiens; Mamu: Macaca mulatta; Mafa: Macaca fascicularis.
We cloned and sequenced macaque cDNA obtained through reverse transcription of total RNA followed by amplification performed using primers complementary to sequences located in the S1 and TM/C exons. The introduction of errors into the sequence was minimized by using a high fidelity polymerase with proofreading ability. To validate the amplification strategy, we amplified, cloned and sequenced the full length human CD89 as well as two human alternatively spliced variants. All human sequences matched those available in GenBank (accession numbers NM_002000, NM_133271 and NM_133279) (data not shown). We then used the same strategy to amplify, clone and sequence rhesus and cynomolgus macaque CD89. Each sequence was confirmed by cloning and sequencing the products of another independent PCR performed using the same total RNA. A complete transcript including all five exons along with several additional transcripts representing alternatively spliced forms of the CD89 mRNA were identified. Figure 1 shows the deduced amino acid sequences of the complete cDNA from rhesus and cynomolgus macaques along with the corresponding human sequences. All five macaque exons were of length identical to the corresponding human CD89 exons. The rhesus macaque and the cynomolgus macaque CD89 amino acid sequences exhibit 86·5% and 86·1% identity to the human counterpart, respectively. The rhesus macaque CD89 amino acid sequence shows 99·3% identity to the corresponding cynomolgus macaque sequence. Therefore, the CD89 sequences from these two non-human primate species are highly homologous to each other, differing for only two amino acids (a methionine/isoleucine substitution at position 40 and a phenylalanine/leucine substitution at position 85). We did not identify amino acid differences in the S1 and S2 exons between human and macaque sequences. The majority of human/macaque substitutions are clustered in the EC1 exon.
Expression of full-length macaque CD89 cDNA in HeLa cells
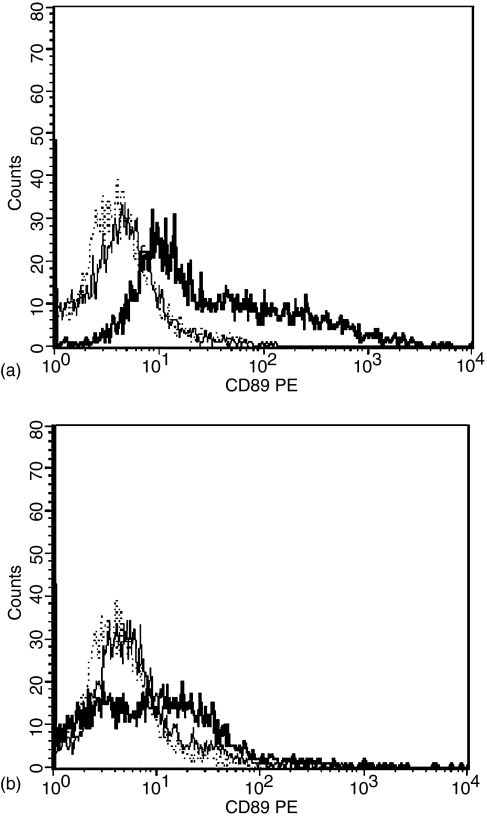
To ascertain whether or not expression of the isolated full-length CD89 cDNA resulted in the production of a cell surface product, we generated HeLa cell transfectants using plasmids containing either rhesus or cynomolgus macaque full-length CD89 cDNA. Transfected cells were stained with a PE-conjugated anti-human CD89 and a mouse isotype control and analysed by flow cytometry. Transfected cells exhibited increased fluorescence intensity when stained for CD89 as compared to staining with a control mouse antibody (Fig. 2). Staining with anti-human CD89 PE did not result in detectable fluorescence of untransfected HeLa cells indicating that CD89 was not expressed in these cells prior to introduction of the expression vectors. The lower fluorescence intensity levels observed in cynomolgus macaques as compared to rhesus macaques are likely to reflect the transient transfection of Hela cells with the cynomolgus macaque cDNA (as mentioned above, stable transfectants were used to detect cell surface expression of rhesus macaque CD89).
Figure 2.
Expression of recombinant macaque CD89 on HeLa cells. Cells were transfected with a plasmid containing full-length (a) rhesus macaque CD89 cDNA or (b) cynomolgus macaque CD89 cDNA and stained with anti-human CD89: thick line (rhesus MFI = 120·70, SD = 343·04; cynomolgus 30·35, SD = 345·04) or with isotype control mouse IgG: thin line (rhesus MFI = 8·65, SD = 7·40; cynomolgus MFI = 9·27, SD = 34·47). Untransfected HeLa cells stained with anti-human CD89: dotted line (MFI = 5·26, SD = 28·69). MFI = Mean fluorescence intensity, SD = standard deviation. Stable transfectants were used for detection of rhesus macaque CD89 and transient transfectants were used for detection of cynomolgus macaque CD89. 5000 events were counted per sample.
Identification of macaque CD89 alternatively spliced transcripts
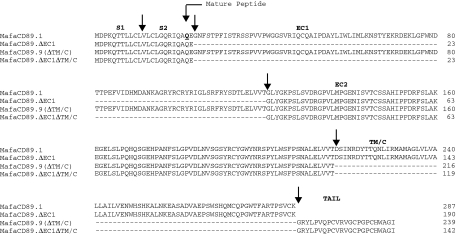
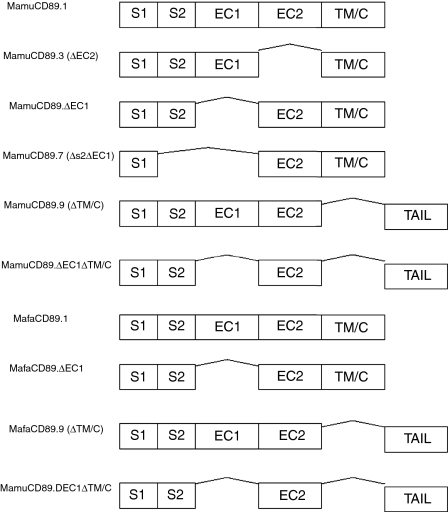
Several distinct mRNA isoforms of the human CD89 have been identified. These isoforms are generated through deletion of exons or parts of exons via alternative RNA splicing, resulting in the expression of closely related but functionally different receptor variants.15,18–20 Therefore, alternative splicing may represent the mechanism underlying the diversification of CD89 functions.15 Ten human variants are listed in the GenBank data base. In addition to the complete CD89 transcript, we have identified several alternatively spliced transcripts in macaques. Specifically, five splice variants were present in the rhesus macaque blood sample analysed and three splice variants in the cynomolgus macaque sample. Figure 3 depicts the derived amino acid sequences of the rhesus macaque variants. Three of the five variants (MamuCD89.3, MamuCD89.7 and MamuCD89.9) are similar to described human CD89 isoforms3 whereas two variants (MamuCD89.ΔEC1 and MamuCD89.ΔEC1ΔTM/C) have not been described in humans. The derived amino acid sequences of the splice variants from cynomolgus macaques are shown in Fig. 4. Of the three isoforms, one (MafaCD89.9) is present also in humans and rhesus macaques, whereas the other two (MafaCD89.ΔEC1 and MafaCD89.ΔEC1ΔTM/C) are shared with rhesus macaques but not humans. Figure 5 shows the schematic representation of the complete CD89 transcript and corresponding splice variants identified in both macaque species. The two variants identified in macaques and not described in humans are characterized by a deletion of the EC1 exon or by a deletion of the EC1 and TM/C exons. The latter variant maintains the tail sequences, as found in a human isoform with deletion of the TM/C exon. This human isoform is designated FcαRIb and results from an alternate splicing that, by skipping the 3′ splice site located at the end of the exon EC2, introduces a tail of 23 new amino acids before reaching the stop codon.16
Figure 3.
Alignment of the five rhesus macaque CD89 splice variants (GenBank accession number AY386684-AY386689) with full-length CD89 from the same species. Arrows indicate distinct domains. Mamu: Macaca mulatta.
Figure 4.
Alignment of the three cynomolgus macaque CD89 splice variants (GenBank accession number AY386690-AY386693) with full-length CD89 from the same species. Arrows indicate distinct domains. Mafa: Macaca fascicularis.
Figure 5.
Schematic representation of the complete CD89 transcript and corresponding splice variants identified in rhesus and cynomolgus macaques. Mamu: Macaca mulatta; Mafa: Macaca fascicularis.
CD89 expression on the cell surface of macaque leucocytes
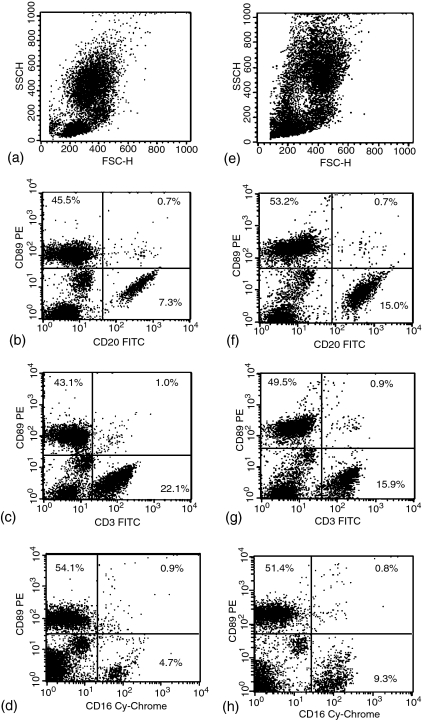
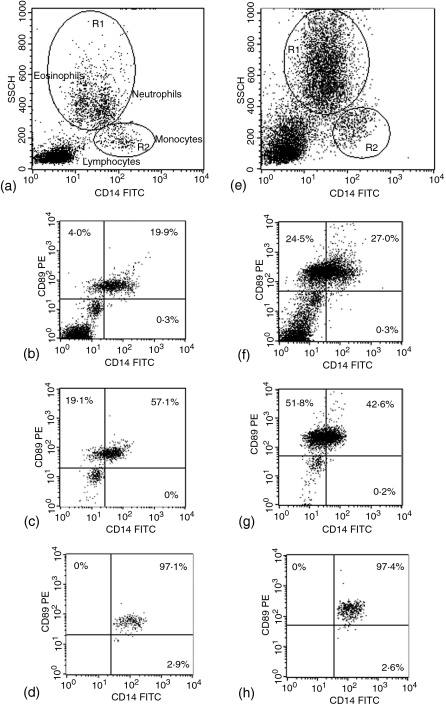
Human CD89 is expressed only in selected cells of the myeloid lineage and not in lymphocytes. To determine whether or not macaque CD89 is similarly expressed, whole blood from four rhesus and seven cynomolgus macaques was stained with the anti-human CD89 PE as well as various FITC-conjugated antibodies against markers of leucocyte populations and then analysed by flow cytometry. Both macaque species expressed CD89 on granulocytes and monocytes, but not on lymphocyte populations. Cells positive for markers of B cells, T cells and natural killer cells (CD20, CD3 and CD16, respectively) were all CD89 negative in both species (Fig. 6). In humans, CD16 is present on neutrophils as well as on natural killer cells.21 However, our results indicate that either macaque granulocytes do not express CD16 or granulocyte CD16 is present in a form not recognized by the antibody clone used in our study. Rhesus macaque leucocytes stained for CD14 can be plotted along with their side scatter properties to distinguish the lymphocyte, eosinophil, neutrophil, and monocyte populations.22 Taking advantage of this, we stained whole blood for CD89 and CD14, and gated cell populations using a sidescatter versus CD14 dot plot (Fig. 7). Without gating, CD14/CD89 staining identified three clusters of cells: a double negative population in the bottom left-hand corner corresponding to lymphocytes, a population with intermediate fluorescence on both axes, and a cluster of cells positive for CD89 with a broad range of expression for CD14. Gating of the granulocyte population revealed that the latter two populations contained granulocytes. The cluster of cells with intermediate fluorescence likely corresponds to a portion of eosinophils, as eosinophils are known to exhibit greater autofluorescence than other cell populations.22 A percentage of the eosinophil population was always found in the CD89 high fluorescence cluster of cells, with the remaining CD89 positive cells representing neutrophils and monocytes.
Figure 6.
Two-colour dot-plots of whole blood leucocytes from a representative rhesus (a–d) and cynomolgus macaque (e–h). Forward scatter (FSC) versus side scatter (SSC) (a and e); CD89 versus CD20 (b and f); CD3 (c and g); and CD16 (d and h).
Figure 7.
Two-colour dot-plots of whole-blood leucocytes from a representative rhesus (a–d) and cynomolgus macaque (e–h). (a and e) CD14 versus side scatter. CD14 versus CD89: (b and f) total leucocytes; (c and g) granulocytes; (d and h) monocytes. Gates used for granulocyte (R1) and monocyte (R2) populations of rhesus (a) and cynomolgus macaque (e) are shown.
Discussion
Macaques are widely used in biomedical research as models for pathogenesis studies, vaccine development and testing of immunotherapeutic approaches, including experimental strategies to prevent transplant rejection.23–31 It is well recognized that macaques infected with simian immunodeficiency virus or simian–human immunodeficiency viruses are the best animal model currently available to study acquired immune deficiency syndrome pathogenesis and vaccine development.32 Given the importance of CD89 in the immune response, we identified and characterized CD89 cDNA in two different macaque species. Results from our experiments show that HeLa cells transfected with plasmids containing rhesus macaque or cynomolgus macaque CD89 cDNA express the CD89 molecule on their cell surface, and this molecule is recognized by an anti-human CD89 antibody. In addition, our results indicate that, similarly to the human counterpart, macaque CD89 is expressed on blood leucocytes of the myeloid lineage.
The rhesus and cynomolgus macaque CD89 amino acid sequences exhibit 86·5% and 86·1% identity the human counterpart, respectively, and are highly homologous to each other (99·3% identity). The human CD89 cDNA encodes a protein containing six potential N-glycosylation sites, four of which are located in the extracellular domains.2 Presence of ordered carbohydrates at these four sites (N44, N58, N120, and N156) has been recently demonstrated in the crystal structure of the human CD89·33 The other two sites are located at position 165 and 177. Differentially glycosylated CD89 species are expressed on monocyte/macrophages and granulocytes.34 As shown in Fig. 1, the six glycosylation sites are also present in the CD89 sequences from both macaque species. However, an additional potential glycosylation site is present in the EC1 domain of the macaque CD89 (asparagine at position 4). In the human CD89 molecule, cysteines involved in disulphide bonds are located at position 28 and 79 of the EC1 domain and at position 125 and 172 of the EC2 domain. As expected, these four cysteines are conserved in the CD89 sequences from both macaque species. Additionally, arginine 209, critical for CD89 association with the signalling molecule FcR γ chain35 is conserved in macaques.
Human CD89 interacts with human IgA molecules through residues located on the EC1 domain. These residues, which include Y35, Y81 and R82 (along with R52 and to a lesser extent H85 and Y86), appear to form a single face at the N-terminus of the molecule and have been identified by scanning mutagenesis.7,36 While Y35 and Y81 are conserved in both macaque species, the arginine at position 52 and the tyrosine at position 86 are substituted by a glutamic acid and a serine, respectively, in both macaque species. In addition, the histidine located at position 85 of the human sequence is replaced by a phenylalanine in rhesus macaques and by a leucine in cynomolgus macaques. Recently, additional human CD89 residues involved in IgA binding (along with those previously described) have been identified by analysing the crystal structure of the molecule.33 Of these amino acids, L54, F56, W57 and G84 are conserved in the human and macaque CD89 molecules, whereas the arginine at position 53 and the lysine at position 55 of the human molecule are substituted by a lysine and by a glycine, respectively, in both macaque species. To date, the only CD89 homologue model for IgA binding is the recently described bovine CD89 (bCD89), which was found to bind human and bovine IgA similarly.14 Binding of macaque IgA to macaque CD89 has not yet been shown. Interestingly, two of the human CD89 residues identified in the crystal structure as contributing to IgA binding (R53 and K55) are lysine and glycine, respectively, in both bovine and macaque CD89. This and the observation that bCD89 is more dissimilar than macaque CD89 to human CD89 indicates that, although some of the amino acids involved in the human IgA/CD89 interaction are not conserved in macaques, macaque CD89 is likely to bind IgA from the same species.
Lack of identification in the macaque samples of additional splice variants similar to human transcripts may be due to different factors. Macaques may express only a limited number of the isoforms described in humans or, most likely, the identified macaque isoforms are a reflection of the samples used for RNA purification and therefore of transcripts that are present in cells found in peripheral blood. Human CD89 splice variants are indeed cell-type specific. The CD89.2 isoform is found only in alveolar macrophages.19 Similarly, expression of the CD89.3 variant (ΔEC2) is cell-type specific.37 The presence of variants found only in macaques may indicate that such variants are specific to these species or that the corresponding human isoforms have not been identified yet. In addition, it has been demonstrated that the ratio of variants to wild type CD89 is regulated by inflammatory cytokines and can be differentially modulated by diseases.37,38 Indeed, we have observed differences in the relative intensity of bands for cDNA of the various splice variants different individuals (data not shown). Therefore, CD89 isoform expression may be dependent on the particular individual analysed and on the concomitant presence of inflammatory conditions or other disorders. Clearly, additional studies are necessary to fully characterize the entire set of macaque CD89 splice variants.
The IgA Fc receptor has been characterized only in a few species. The rat CD89 gene homologue shares 53% amino acid identity with the human CD89.13 Only full-length CD89 and a single splice variant (characterized by a deletion of the S2 exon) have been identified in rat spleen.13 The cattle CD89 homologue shares 56·2% amino acid identity with the human CD89. However, no splice variants have been reported in this species.14 The identification of several variants in macaques indicates that CD89 transcript processing may vary considerably between species.
There is currently increased interest in the manipulation of the IgA/CD89 interaction for immunotherapeutic purposes.39–41 CD89 represents an effective target molecule for immunotherapy mediated by bispecific antibodies.42 Results from a more recent study show that a chimeric surfactant protein D/anti-CD89 protein may effectively target pathogens to neutrophils.43 Like humans, macaques express CD89 on granulocytes and monocytes. Because of the potential use of macaques as models for the development of IgA-based therapies, the characterization of CD89 and corresponding isoforms in these species represents an essential step to define a valuable system for the testing of therapeutic antibodies.
Acknowledgments
The authors thank Dr Harold McClure (Yerkes National Primate Research Center) for providing rhesus macaque blood samples and Dr Jerilyn Pecotte (Southwest National Primate Research Center) for providing cynomolgus macaque blood samples. This work was supported in part by NIH grants RR10755 and RR00165, by the Research Program Enhancement from the GSU Office of Research and Sponsored Programs and by the Georgia Research Alliance.
References
- 1.Ravetch JV, Kinet J. Fc receptors. Annu Rev Immunol. 1991;9:457–92. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–72. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro RC, van de Winkel JGJ. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 4.Pfefferkorn LC, Yeaman GR. Association of IgA-Fc receptors (Fc alpha R) with Fc epsilon RI gamma 2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of gamma 2. J Immunol. 1994;153:3228–36. [PubMed] [Google Scholar]
- 5.Kremer EJ, Kalatzis V, Baker E, Callen DF, Sutherland GR, Maliszewski CR. The gene for the human IgA Fc receptor maps to 19q13.4. Hum Genet. 1992;89:107–8. doi: 10.1007/BF00207054. [DOI] [PubMed] [Google Scholar]
- 6.de Wit TP, Morton HC, Capel PJ, van de Winkel JG. Structure of the gene for the human myeloid IgA Fc receptor (CD89) J Immunol. 1995;155:1203–9. [PubMed] [Google Scholar]
- 7.Wines BD, Hulett MD, Jamieson GP, Trist HM, Spratt JM, Hogarth PM. Identification of residues in the first domain of human Fc alpha receptor essential for interaction with IgA. J Immunol. 1999;162:2146–53. [PubMed] [Google Scholar]
- 8.Shimizu A, Takahashi N, Yaoita Y, Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982;28:499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- 9.Burnett RC, Hanly WC, Zhai SK, Knight KL. The IgA heavy-chain gene family in rabbit. cloning and sequencing analysis of 13 C alpha genes. EMBO J. 1989;8:4041–7. doi: 10.1002/j.1460-2075.1989.tb08587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scinicariello F, Attanasio R. Intraspecies heterogeneity of immunoglobulin alpha-chain constant region genes in rhesus macaques. Immunology. 2001;103:441–8. doi: 10.1046/j.1365-2567.2001.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules. sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2003.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumiyama K, Saitou N, Ueda S. Adaptive evolution of the IgA hinge region in primates. Mol Biol Evol. 2002;19:1093–9. doi: 10.1093/oxfordjournals.molbev.a004167. [DOI] [PubMed] [Google Scholar]
- 13.Maruoka T, Nagata T, Kasahara M. Identification of the rat IgA Fc receptor encoded in the leukocyte receptor complex. Immunogenetics. 2004;55:712–6. doi: 10.1007/s00251-003-0626-1. [DOI] [PubMed] [Google Scholar]
- 14.Morton HC, Pleass RJ, Storset AK, Dissen E, Williams JL, Brandtzaeg P, Woof JM. Cloning and characterization of an immunoglobulin A Fc receptor from cattle. Immunology. 2004;111:204–11. doi: 10.1111/j.0019-2805.2003.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pleass RJ, Andrews PD, Kerr MA, Woof JM. Alternative splicing of the human IgA Fc receptor CD89 in neutrophils and eosinophils. Biochem J. 1996;318:771–7. doi: 10.1042/bj3180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijk TB, Bracke M, Caldenhoven E, Raaijmakers JA, Lammers JW, Koenderman L, de Groot RP. Cloning and characterization of Fc alpha Rb, a novel Fc alpha receptor (CD89) isoform expressed in eosinophils and neutrophils. Blood. 1996;88:4229–38. [PubMed] [Google Scholar]
- 17.Morton HC, van Zandbergen G, van Kooten C, Howard CJ, van de Winkel JGJ, Brandtzaeg P. Immunoglobulin-binding sites of human FcaRI (CD89) and bovine Fcγ2R are located in their membrane-distal extracellular domains. J Exp Med. 1999;189:1715–22. doi: 10.1084/jem.189.11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton HC, Schiel AE, Janssen SW, van de Winkel JG. Alternatively spliced forms of the human myeloid Fc alpha receptor (CD89) in neutrophils. Immunogenetics. 1996;43:246–7. doi: 10.1007/BF00587311. [DOI] [PubMed] [Google Scholar]
- 19.Patry C, Sibille Y, Lehuen A, Monteiro RC. Identification of Fc alpha receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol. 1996;156:4442–8. [PubMed] [Google Scholar]
- 20.Reterink TJ, Verweij CL, van Es LA, Daha MR. Alternative splicing of IgA Fc receptor (CD89) transcripts. Gene. 1996;175:279–80. doi: 10.1016/0378-1119(96)00152-7. [DOI] [PubMed] [Google Scholar]
- 21.Fleit HB, Wright SD, Unkeless JC. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982;79:3275–9. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafont BAP, Gloeckler L, D'Hautcourt JL, Gut JP, Aubertin AM. One-round determination of seven leukocyte subsets in rhesus macaque blood by flow cytometry. Cytometry. 2000;41:193–202. doi: 10.1002/1097-0320(20001101)41:3<193::aid-cyto6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Solnick JV, Canfield DR, Hansen LM, Torabian SZ. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta) Infect Immun. 2000;68:2560–5. doi: 10.1128/iai.68.5.2560-2565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attanasio R, Pehler K, McClure HM. Immunogenicity and safety of Mycobacterium tuberculosis culture filtrate proteins in non-human primates. Clin Exp Immunol. 2000;119:84–91. doi: 10.1046/j.1365-2249.2000.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guirakhoo F, Weltzin R, Chambers TJ, et al. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol. 2000;74:5477–85. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn CS, French OG, Foley P, Martin EN, Taylor RP. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J Immunol. 2001;166:1057–65. doi: 10.4049/jimmunol.166.2.1057. [DOI] [PubMed] [Google Scholar]
- 27.Custer DM, Thompson E, Schmaljohn CS, Ksiazek TG, Hooper JW. Active and passive vaccination against hantavirus pulmonary syndrome with Andes virus M genome segment-based DNA vaccine. J Virol. 2003;77:9894–905. doi: 10.1128/JVI.77.18.9894-9905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vugmeyster Y, Howell K, McKeever K, Combs D, Canova-Davis E. Differential in vivo effects of rituximab on two B-cell subsets in cynomolgus monkeys. Int Immunopharmacol. 2003;3:1477–81. doi: 10.1016/S1567-5769(03)00147-4. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Montgomery SP, Preston EH, Tadaki DK, Hale DA, Harlan DM, Kirk AD. Studies investigating pretransplant donor-specific blood transfusion, rapamycin, and the CD154-specific antibody IDEC-131 in a nonhuman primate model of skin allotransplantation. J Immunol. 2003;170:2776–82. doi: 10.4049/jimmunol.170.5.2776. [DOI] [PubMed] [Google Scholar]
- 31.Asiedu CK, Dong SS, Lobashevsky A, Jenkins SM, Thomas JM. Tolerance induced by anti-CD3 immunotoxin plus 15-deoxyspergualin associates with donor-specific indirect pathway unresponsiveness. Cell Immunol. 2003;223:103–12. doi: 10.1016/s0008-8749(03)00157-6. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–77. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 33.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003;423:614–20. doi: 10.1038/nature01685. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992;148:1764–70. [PubMed] [Google Scholar]
- 35.Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJA, van de Winkel JGJ. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR gamma Chain. J Biol Chem. 1995;270:29781–7. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]
- 36.Wines BD, Sardjono CT, Trist HM, Lay CS, Hogarth PM. The interaction of Fc alpha RI with IgA and its implications for ligand binding by immunoreceptors of the leukocyte receptor cluster. J Immunol. 2001;166:1781–9. doi: 10.4049/jimmunol.166.3.1781. [DOI] [PubMed] [Google Scholar]
- 37.Togo S, Shimokawa T, Fukuchi Y, Ra C. Alternative splicing of myeloid IgA Fc receptor (Fc alpha R, CD89) transcripts in inflammatory responses. FEBS Lett. 2003;535:205–9. doi: 10.1016/s0014-5793(02)03891-7. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro RC, Moura IC, Launay P, Tsuge T, Haddad E, Benhamou M, Cooper MD, Arcos-Fajardo M. Pathogenic significance of IgA receptor interactions in IgA nephropathy. Trends Mol Med. 2002;8:464–8. doi: 10.1016/s1471-4914(02)02405-x. [DOI] [PubMed] [Google Scholar]
- 39.Dechant M, Valerius T. IgA antibodies for cancer therapy. Crit Rev Oncol Hematol. 2001;39:69–77. doi: 10.1016/s1040-8428(01)00105-6. [DOI] [PubMed] [Google Scholar]
- 40.Corthesy B. Recombinant immunoglobulin A. powerful tools for fundamental and applied research. Trends Biotechnol. 2002;20:65–71. doi: 10.1016/s0167-7799(01)01874-1. [DOI] [PubMed] [Google Scholar]
- 41.Presta LG. Engineering antibodies for therapy. Curr Pharm Biotechnol. 2002;3:237–56. doi: 10.2174/1389201023378256. [DOI] [PubMed] [Google Scholar]
- 42.Valerius T, Stockmeyer B, van Spriel AB, et al. FcalphaRI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood. 1997;90:4485–92. [PubMed] [Google Scholar]
- 43.Tacken PJ, Hartshorn KL, White MR, van Kooten C, van de Winkel JG, Reid KB, Batenburg JJ. Effective targeting of pathogens to neutrophils via chimeric surfactant protein D/anti-CD89 protein. J Immunol. 2004;172:4934–40. doi: 10.4049/jimmunol.172.8.4934. [DOI] [PubMed] [Google Scholar]