Abstract
Ligation of CD40 by CD4 T cells through CD154 is key both to germinal centre induction and follicular T-dependent Ig class switching, but its requirement for aspects of T cell priming and extrafollicular antibody responses is less clear. Here comparison of the T helper (Th) type 2 response in lymph nodes from wild-type mice and CD154-deficient mice after immunization with alum-precipitated antigen reveals selective effects of this immunodeficiency. The timing and magnitude of the early interleukin (IL)-4 induction and proliferation in T cells of the T zone were unaltered by CD154 deficiency. As expected, germinal centres were not induced. Additionally the T-dependent extrafollicular antibody response, which initially requires T cell help but expands without further T cell involvement, was severely curtailed. The median number of extrafollicular antigen-specific plasma cells was 370-fold lower in CD154-deficient mice. Of these plasma cells the median proportion that had switched to IgG1 was <5%, while in wild-type mice the proportion was 89%. Surprisingly, some CD154-deficient lymph nodes showed substantial switching to IgG1. Commensurately, increases in γ1 germline transcripts and Blimp-1 mRNA were observed, albeit significantly lower than in controls, but activation-induced cytidine deaminase mRNA was undetectable in CD154-deficient mice. These experiments demonstrate that the acquisition of some T cell priming characteristics can be CD154-independent; in contrast, T-dependent extrafollicular responses require CD154. Thus functional CD154 ligation during the first encounter of T cells and B cells in the T zone is critical for follicular and extrafollicular antibody responses.
Keywords: CD154, IL-4, plasma cells, T cell priming, Th2
Introduction
Alum-precipitated protein antigens (Ag) typically induce Th2 responses. Following immunization with such Ag dendritic cells take up and process Ag and mature into Ag-presenting cells as they migrate to the T zones of down-stream secondary lymphoid tissues.1 The activation of naïve T cells is rapid, as some Ag-specific T cells have already undergone up to six rounds of division 3 days after immunization. Furthermore, by 3 days a proportion of the Ag-specific T cells have been induced to produce IL-4 mRNA.2–5 As CD4 T cells are primed to a Th2-inducing Ag they acquire the ability to interact with B cells that have specifically taken up Ag. The primary interaction of primed Th2 cells with B cells in the T zone induces germline transcription of γ1 and ɛ immunoglobulin (Ig) H constant region genes. As a consequence of this interaction with T cells, activated B cells can grow either in follicles to form germinal centres or as plasmablasts in the extrafollicular response.6 The extrafollicular response, in contrast to germinal centre formation, develops in the absence of further interaction with T cells.7 Thus extrafollicular responses are a key element in T-dependent responses and the site of the extrafollicular response in lymph nodes is the medulla. By day 7 of T-dependent antibody responses to alum-precipitated Ag the extrafollicular response, which generates the earliest antibody, has reached its peak and germinal centre formation is well under way. Thus, by studying the response to T-dependent Ag on day 3 and day 7 of the response features of T cell priming, the extrafollicular response and germinal centre development can be examined.
The early induction of IL-4 production during T cell priming was observed first by Kelso and colleagues.8 The importance of this early IL-4 synthesis is manifest in mice that either do not produce or cannot respond to IL-4. These mice produce only small germinal centres in response to alum-precipitated Ag and fail to repress the expression of the Th1 regulator T-bet.3,5 Thus IL-4 induced during priming is vital for the maintenance of Th2 features. In contrast, the development of the extrafollicular response in these mice is unimpaired, with similar numbers of Ag-specific plasma cells being generated, and furthermore a similar proportion of these plasma cells have switched to IgG1.
Among the key molecules identified as playing a role in the induction and maintenance of T-dependent antibody responses is CD40. This can be expressed constitutively both by B cells and mature dendritic cells.9 A key ligand to CD40, CD154,10 is contained within a proportion of primed CD4 T cells and is brought rapidly to the surface on cognate interaction with B cells.11 Deficiency in this ligand is manifest as a syndrome, both in mice and humans, with selective deficiency in affinity maturation of antibody responses and T-dependent Ig class switching.12–14 Germinal centres typically are not induced in CD154-deficient mice and this deficiency is associated with normal or elevated IgM levels. While induction of IgM by T-dependent Ag is impaired in CD154-deficient mice they retain the capacity to mount T cell-independent antibody responses. As such, although CD40 ligation plays a key role in Ig class switching its role in triggering B cells to grow as plasmablasts in T-dependent extrafollicular antibody responses is less clear.
There is a large literature addressing the role of the CD40:CD154 dyad in T cell priming in vivo. The results have shown that the influence of CD154 on T cell priming varies greatly with the way T cells are stimulated, the nature of the antigenic stimulus and whether the response is being studied in its physiological context.15–24 Thus, since IL-4 production can result from T cell priming and affect germinal centre development there is the potential that CD154 ligation may be important for its early induction.
The objective of the present study is to focus on the role of CD154 in the early events during primary lymph node responses to a Th2-inducing Ag. The almost indispensable role of CD154 on germinal centre formation and the resulting T-dependent Ig class switching is amply documented and is not considered in detail in the present study. Rather, the focus is on three early events in the response to a Th2 Ag: (i) the onset and extent of T cell proliferation in the T zone; (ii) the primary induction of IL-4 synthesis and (iii) the induction of extrafollicular B cell growth as plasmablasts in the medullary cords of responding lymph nodes.
Materials and methods
Mice and immunizations
CD154–/– mice25 were obtained from colonies maintained in house. There were at least four animals in each group for each time-point. Matched C57/BL6 mice were used as controls. Alum-precipitated NP-CGG was prepared as described previously.26 Adult mice were injected into both rear footpads with 20 µg alum-precipitated NP-CGG plus 5 × 108 heat-killed Bordetella (B.) pertussis. 2 mg of BrdU was administered intraperitoneally (i.p.) 2 h before sacrifice.26
Immunohistological reagents, staining and analysis
For in situ study of immune responses 5 µm sections were taken from frozen lymph nodes for immunohistology and 2 × 25 µm sections were taken for the preparation of RNA (see below), as described elsewhere.2 The nodes were orientated so that sections were cut through the cortex, T zone and medulla and maximum diameter sections were selected for staining and cDNA preparation.
Immunohistological reagents and staining were as described earlier.3 Cells were triple-stained for CD3, IgD and BrdU or double-stained for NP and either IgG1 or IgM. Binding of rat antimouse CD3 (Serotec, Oxford, UK) was detected with biotinylated rabbit antirat antibodies (Dako, High Wycombe, UK), followed by streptavidin ABComplex–alkaline phosphatase (Dako). The bound alkaline phosphatase activity was then detected using naphthol AS-MX phosphate and Fast Blue salt with levamisole. Similarly, NP-binding cells were detected using NP-conjugated sheep IgG and biotinylated rabbit antigoat/sheep antibodies (Dako). Sheep antimouse IgD (The Binding Site, Birmingham, UK) was labelled with peroxidase-labelled donkey antisheep (The Binding Site). Primary rat antimouse IgG1 or IgM were labelled using rabbit antirat peroxidase (Dako). Horseradish peroxidase was detected using diaminobenzidine tetrahydrochloride solution. BrdU incorporated into DNA in cells was made accessible by treatment for 20 min with 1 m HCl at 60°; this treatment also inactivated any alkaline phosphatase activity from the previous stainings, without affecting the previous colour deposition. BrdU was then detected with a mouse anti-BrdU (Dako) primary antibody followed by a biotinylated goat antimouse antibody and streptavidin ABComplex–alkaline phosphatase (Dako) added. In this case the colour was developed using Tris-buffered saline pH 8·2 and Fast Red TR salt (Sigma). The area of cut surface of lymph nodes was determined using the point counting technique of Weible.27
Reverse transcription of mRNA and its relative quantitation by polymerase chain reaction (PCR)
RNA and cDNA were prepared as described previously.3 Briefly, RNA was purified from tissue sections using RNAzol B (Biogenesis, Poole, UK) according to protocol. The RNA pellet was reverse transcribed by standard methods using Moloney murine leukaemia virus reverse transcriptase (Invitrogen, Paisley, UK).
Relative quantitation of specific cDNA species to β-actin message using multiplex PCR and adjustment for section size was performed as described previously.3 Probes for cytokines and switch transcripts were detected via a 5′ label with FAM (Applied Biosystems, Warrington, UK), while probes for β-actin were 5′ labelled with VIC (Applied Biosystems). Sequences for β-actin, IL-4, γ1 germline transcripts and γ2a germline transcripts have all been described.3 Sequences for B lymphocyte-induced maturation protein 1 (Blimp-1) were forward: TTTGGAGGATCTGACCCGAAT; reverse: CTCCACCATGGAGGTCACATC; probe: TGAAGAAATTGAGAGGTTCGACATCAGCG. Sequences for activation-induced cytidine deaminase (AID) were forward: GTCCGGCTAACCAGACAACTTC; reverse: GCTTTCAAAATCCCAACATACGA; probe: ACGAAGTCGATGACTTGCGAGATGCA. Reaction tubes contained Universal PCR Master Mix (Applied Biosystems), β-actin-specific primers and probe, test gene-specific primers and probe and cDNA template. Reaction conditions were the standard conditions for the TaqMan PCR with 60° annealing temperature but with 45 PCR cycles. The relative signal per cell was quantified by setting thresholds within the logarithmic phase of the PCR for β-actin and the test gene and determining the cycle number at which the threshold was reached (CT). The CT for the target gene was subtracted from the CT for β-actin. The relative amount was calculated as 2ΔCT.
NP-specific antibody enzyme-linked immunosorbent assay (ELISA)
Serum IgM and IgG1 antibodies to NP were detected by ELISA.3 NP conjugated to BSA was coated onto plates at a concentration of 5 µg/ml. Primary antibodies were added and diluted stepwise. Secondary alkaline-phosphatase-linked goat antimouse isotype antibodies were added (Southern Biotechnology Associates, AL, USA). Colour was developed using p-nitrophenylphosphate in diethanolamine pH 9·8 as substrate, and plates read at 405 nm.
Statistical analyses
Statistical analysis was carried out using the Mann–Whitney non-parametric rank-sum test.
Results
The early proliferation of naïve T cells in the T zone and the induction of IL-4 synthesis are unimpaired in CD154-deficient mice
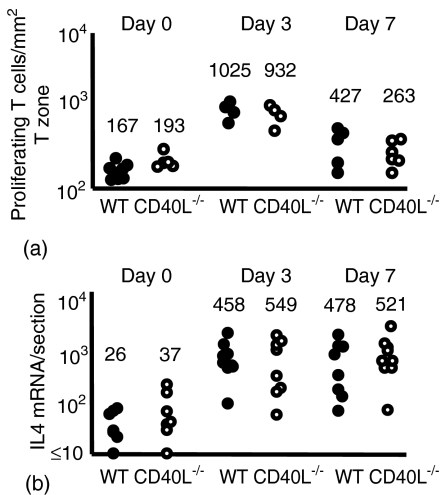
Wild-type and CD154-deficient mice were immunized in the foot with the Th2 Ag alum-precipitated NP-CGG with heat-killed B. pertussis. To study the T cell proliferative response in the draining popliteal lymph node non-immunized mice and mice immunized 3 or 7 days previously were subject to 2-hr pulses with BrdU. The proportion of T cells in S phase in draining lymph nodes was determined by immunohistology. There was a significant proliferative response by day 3 in both the immunized groups compared to the non-immunized control mice (Mann–Whitney P < 0·01). The numbers of CD4 T zone T cells in S phase in the wild-type and CD154-deficient groups were comparable both on day 3 and day 7 (Fig. 1a).
Figure 1.
The induction of T cell proliferation and IL-4 production is not affected by the loss of CD154. Mice were immunized with NP-CGG and killed B. pertussis and responses assessed 3 and 7 days after immunization. Closed and open circles, respectively, represent individual lymph node responses from wild-type and CD154-deficient mice. Mice were immunized in the footpad with alum-precipitated NP-CGG and killed B. pertussis and responses in the draining lymph node assessed. (a) By day 3 after immunization there was a clear increase in T cells in S phase of cell cycle, which returns to basal levels by day 7. (b) The level of IL-4 mRNA induced in CD154-deficient mice is comparable to that of wild-type mice 3 and 7 days after immunization. Median values for each of the groups are included above the data points. Data are representative of at least two repeat experiments.
The induction of IL-4 production was studied by reverse transcription (RT)-PCR on lymph node sections. Previously we have shown that the early IL-4 response in draining lymph nodes of mice immunized in this way is in Ag-specific CD4 T cells.5 Both wild-type and CD154-deficient mice produced a significant IL-4 mRNA response by day 3 (day 0 versus day 3 levels: P < 0·001 for wild-type and P < 0·005 for CD154-deficient mice) and this increase was sustained on day 7 (Fig. 1b). Once more no significant difference was seen in the levels of IL-4 induction between the wild-type and CD154-deficient mice.
Thus, in these responses the markers of T cell priming, the induction to proliferate and induce IL-4 mRNA occur independently of CD154 ligation.
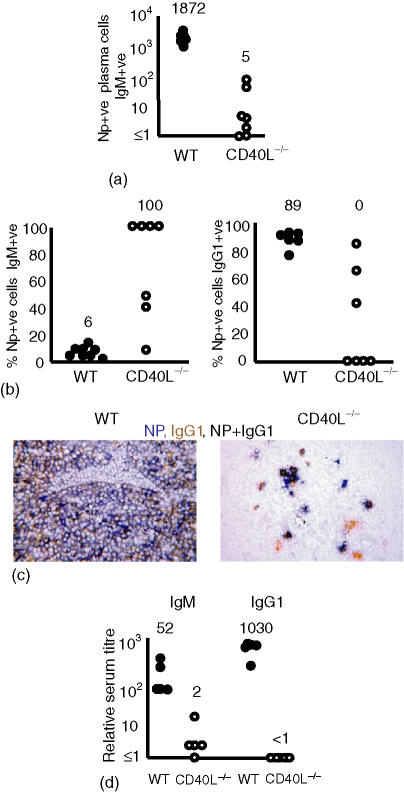
The induction of the extrafollicular antibody response to NP-CGG is markedly impaired in CD154-deficient mice
In the T-dependent response the extrafollicular plasma cell response is at its peak by day 7.6 Thus we examined the extrafollicular response at this point to investigate the role played by CD154 ligation in its establishment. Wild-type mice had generated large numbers of NP-specific plasma cells in the medullary cords (Fig. 2a) and most of these had switched to produce IgG1 (Fig. 2b,c). This was reflected in the titres of NP-specific antibody in the serum at this time (Fig. 2d). By contrast, CD154-deficient mice produced small and erratic extrafollicular NP-specific plasma cell responses (Fig. 2a). The median serum IgM titre on day 7 was one-30th of that seen in wild-type mice and NP-specific IgG1 could not be detected (Fig. 2d). Despite these findings, immunohistology revealed that some CD154-deficient mice produced small extrafollicular responses. Some of the NP-specific plasma cells generated had switched to express IgG1 (Fig. 2b). The proportion of NP-specific plasma cells switched to IgG1 was found to be greatest in the nodes with the highest number of NP-specific cells. The two nodes with the most NP-positive cells also had the highest proportion of switched cells. An example of a CD154-deficient lymph node with a high number of switched plasma cells is shown in Fig. 2(c), right-hand panel. No switched NP-specific IgG2a plasma cells were observed (data not shown). IgG3, an indicator of T-independent Ig class switching,28 was not detected (data not shown). No non-immunized mice had NP-specific cells.
Figure 2.
The extrafollicular antibody response is severely impaired but not completely ablated in the absence of CD154. Mice were immunized with NP-CGG and killed B. pertussis and responses assessed 7 days after immunization. Closed and open circles, respectively, represent individual lymph node responses from wild-type and CD154-deficient mice. (a) Numbers of NP-specific plasma cells per section recruited into the extrafollicular response. In five of eight non-immunized CD154-deficient lymph node sections no IgM or IgG1 plasma cells were observed and in all lymph node sections no NP-specific cells were detected. The other three lymph node sections contained 1, 2 and 5 IgG1 plasma cells (data not shown). (b) The proportion of the NP-specific plasma cells shown in (a) that were non-switched or switched to IgG1 7 days after immunization. In CD154-deficient mice, the majority of NP-specific extrafollicular cells are unswitched (left hand panel). In contrast, most plasma cells in wild-type mice have switched to IgG1 (right hand panel); some NP-specific IgG1 cells are detectable. (c) Immunohistology showing the clear IgG1 dominance of the NP-response in wild-type mice (left hand panel) and the clear detection of reduced numbers of IgG1-producing NP-specific cells in some CD154-deficient mice (right-hand panel). (d) NP-specific serum titres, non-immunized mice had antibody titres < 1 (data not shown). Median values for each of the groups are included above the data points. Data are representative of at least two repeat experiments.
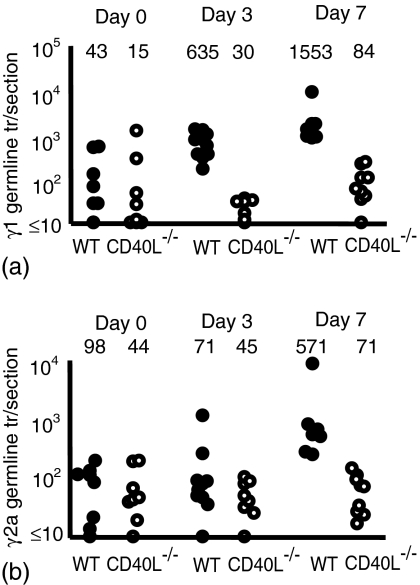
The production of γ1 germline transcripts in response to immunization with alum-precipitated proteins is induced when B cells first encounter primed Ag-specific T cells,7 and thus offers an approach to study when T cells first interact with B cells. In this model their induction can be detected from day 3 onwards. Deficiency in CD40 ligation was found to profoundly affect the induction of γ1 and γ2a germline transcripts (Fig. 3). There was no significant induction of transcripts at day 3 although by day 7 a small but significant increase in γ1 germline transcripts was seen (P < 0·01). Thus, although primed T cells have retained some characteristics of Th2 priming they have lost the capacity to drive B cell maturation from the time of their earliest interaction.
Figure 3.
The induction of T-dependent germline transcript production is affected strongly by the absence of CD154. Mice were immunized with NP-CGG and killed B. pertussis and responses assessed 3 and 7 days after immunization. Closed and open circles, respectively, represent individual lymph node responses from wild-type and CD154-deficient mice. (a) Levels of γ1 germline transcript production and (b) levels of γ2a germline transcript production. Median values for each of the groups are included above the data points. Data are representative of at least two repeat experiments.
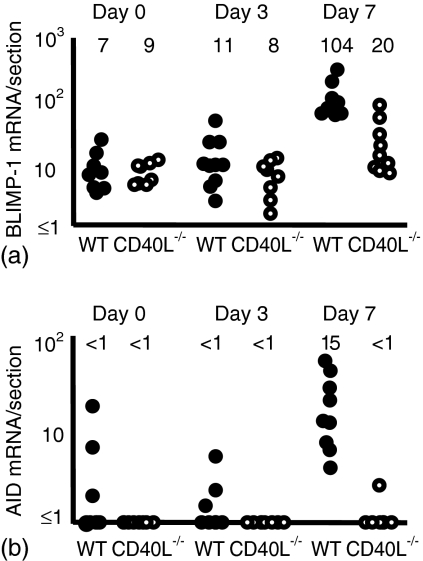
CD154-deficient mice can induce BLIMP-1, but fail to express activation-induced cytidine deaminase (AID) in response to immunization
The identification of Ag-specific IgG1 cells suggested that some B cell maturation occurs in CD154-deficient mice after immunization. Because some plasma cells were induced in the CD154-deficient mice, markers of B cell development were examined. Figure 4 shows that neither wild-type nor CD154-deficient mice induced significant levels of the plasma cell maturation marker B lymphocyte-induced maturation protein 1 (Blimp-1)29 by day 3, but by day 7 there had been a significant induction of Blimp-1 (day 7 > non-immunized: wild-type mice P < 0·001 and CD154-deficient mice P < 0·05). The induction of Blimp-1 was greater in the wild-type mice compared to CD154-deficient mice (P < 0·002).
Figure 4.
In the absence of CD154, the plasma cell marker BLIMP-1 is induced, but AID, which is required for Ig class switching and somatic hypermutation in Ig V-region genes is not. Mice were immunized with NP-CGG and killed B. pertussis and responses assessed 3 and 7 days after immunization. Closed and open circles, respectively, represent individual lymph node responses from wild-type and CD154-deficient mice. (a) The induction of BLIMP-1 mRNA is significantly higher by 7 days after immunization than in non-immunized controls. (b) Levels of AID mRNA expression are below the level of detection in the absence of CD154. Median values for each of the groups are included above the data points. Data are representative of at least two repeat experiments.
Wild-type mice had well-developed germinal centres by day 7, but as expected no germinal centres were detected in CD154-deficient mice (data not shown). Both germinal centre B cells and plasmablasts undergoing switch recombination express AID.30 Not surprisingly, AID was strongly induced in the wild-type mice by day 7, but essentially no detectable AID mRNA was found before or after immunization in the CD154-deficient mice (Fig. 4b). In non-immunized CD154-deficient mice the median level of AID expression was more than fivefold lower than in wild-type non-immunized mice. Thus the capacity to induce Blimp-1 is retained, but an absolute requirement for CD154 is required for AID expression.
Discussion
This study has focused on two aspects of the early T-dependent response, the early induction of T cells to proliferate and produce IL-4 mRNA, and their ability to interact with B cells and drive the extrafollicular antibody response. The results show different effects of CD154 loss at different points of the response. The features of T cell proliferation and IL-4 induction were retained, but the capacity to drive plasmablast expansion was lost.
Proliferation of naïve transgenic CD4 T cells in response to Ag can be seen in the T zone by the second day after immunization.31 Howland et al.32 reported that CD154 deficiency in naïve T cells carrying an ovalbumin-specific transgenic T cell receptor had only a small impairment in T cell proliferation 3 days after immunization with ovalbumin, while absence of CD28 profoundly affected this response. Deficiency in signalling through OX40 on T cells has been associated paradoxically with increased proliferation during priming in vivo.33 An earlier study16 reported that naïve CD154-deficient T cells were able to proliferate to non-specific stimuli to a comparable degree as wild-type T cells ex vivo. In contrast, the level of proliferation seen in Ag-experienced T cells induced to proliferate ex vivo in the presence of Ag was severely impaired. Here, we have looked at T cell proliferation in the T zone where it occurs physiologically in the period immediately following cognate interaction with dendritic cells. Thus, although some T cells may require CD154 to proliferate ex vivo, there are other mechanisms that compensate for its loss in the host. One alternative pathway potentially involved may rely upon the interaction between tumour necrosis factor (TNF)-related activation-induced cytokine (TRANCE) and its ligand receptor activator of nuclear factor kappa B (RANK). This pathway has been shown to be important during priming of T cells in the response of CD40-deficient mice to lymphocytic choriomeningitis virus,34 which are able to mount effective CD4-mediated responses in the absence of either CD40 or CD154.35,36 It has been reported that CD154 is up-regulated after T cell activation37,38 suggesting that CD154 may be important after the induction of T cell activation but not necessarily initiating it, possibly by potentiating the Ag-priming capacity of dendritic cells.39 More severe defects in priming in CD40-deficient mice than in mice lacking CD154 have been described, and this has been attributed to alternative CD40 ligands not expressed by T cells.40–42
The present study shows that induction of IL-4 during Th2 priming is independent of CD154 signalling. CD40 ligation in combination with IL-4 can induce IgG1-switched plasma cells in vitro.43 This IL-4 is critical for the maintenance of the Th2 response by suppressing Th1 characteristics and promoting germinal centre development.3,5 This may be the reason for it being a major factor in late primary and memory responses in promoting IgG1-secreting plasma cell expansion.44,45 In contrast, IL-4 is not essential for the early extrafollicular plasma cell response that also involves IgG1 switching.5,7 As molecules such as IL-4 can exhibit such selective effects it was possible that CD154 may also have a relatively small role in the induction of the extrafollicular response. The present study was designed to test this and shows clearly that CD154 is important. The impairment of induction of B cells to grow as plasmablasts and switch Ig class is due most probably to loss of T cell-mediated ligation of B cell CD40 during cognate interaction of primed T cells with B cells in the T zone during their first encounter. This conclusion is supported by the observed strong impairment in γ1 germline transcript production seen on day 3. Furthermore, the interaction between T cells and B cells that enter the extrafollicular response are restricted anatomically to the T zone because T cells are not observed in the medullary cords when the plasmablast population is expanding.6
Small numbers of NP-specific plasmablasts were generated during the response in CD154-deficient mice. Where this was observed at least some level of directional switching to IgG1 occurred. The absence of concomitant switching to IgG2a or IgG3 argues against the limited bypass of the role of CD40 ligation being mediated by type 1 interferon,46 or BAFF.47 These trigger pathways common to those activated by CD40 ligation, but they are associated with indiscriminate switching as opposed to selective switching to IgG1. It is unclear why some CD154-deficient nodes had higher numbers of IgG1 switched cells than others. As the number of plasma cells observed in CD154-deficient lymph nodes was extremely small, random variations in the location of plasma cells in the medulla may have become a factor, and cells may have been missed due to the orientation of the section through the lymph node. Another possibility is bystander activation of cells during the response to environmental antigens.
A formal in vivo requirement of CD154 for the normal expression of AID was demonstrated. AID is expressed in germinal centre B cells and is involved in the class switch recombination events that occur in these sites.30,48 In the small numbers of switched plasmablasts seen in CD154-deficient lymph nodes in this study it was possible that AID was expressed but that its expression was too low to be detected by RT-PCR of lymph node sections.
In conclusion, this report confirms that T cell-mediated ligation of CD40 on dendritic cells is not critical for the induction of IL-4 production and has little overall effect on the primary T zone CD4 T cell proliferative response. It has a profound effect on the ability of CD4 T cells to induce B cells that have taken up Ag to proliferate, either as plasmablasts in medullary cords or in follicles to form germinal centres. It is still unclear if these defects in cognate interaction between T and B cells reflects solely the absence of T cell-mediated ligation of CD40 on B cells or in addition influences the way T cells are induced to find those B cells that have bound Ag.
Acknowledgments
This work was funded by the British Medical Research Council. Mice were kindly provided by Peter Lane.
Abbreviations
- B.
Bordetella
- BrdU
5-bromo-2-deoxyuridine
- NP
(4-hydroxy-3-nitrophenyl) acetyl
- CGG
chicken gamma globulin
- Blimp-1
B lymphocyte-induced maturation protein 1
- AID
activation-induced cytidine deaminase
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan ICM, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham AF, Fallon PG, Khan M, Vacheron S, Acha-Orbea H, MacLennan IC, McKenzie AN, Toellner KM. Th2 activities induced during virgin T cell priming in the absence of IL-4, IL-13, and B cells. J Immunol. 2002;169:2900–6. doi: 10.4049/jimmunol.169.6.2900. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham AF, Toellner KM. Rapid development of Th2 activity during T cell priming. Clin Dev Immunol. 2003;10:1–6. doi: 10.1080/10446670310001598537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham AF, Serre K, Toellner KM, Khan M, Alexander J, Brombacher F, MacLennan ICM. Pinpointing IL-4-independent and IL-4-influenced acquisition and maintenance of Th2 activity by CD4 T cells. Eur J Immunol. 2004;34:686–94. doi: 10.1002/eji.200324510. [DOI] [PubMed] [Google Scholar]
- 6.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 7.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–12. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelso A, Groves P, Troutt AB, Pech MH. Rapid establishment of a stable IL-4/IFN-gamma production profile in the antigen-specific CD4+ T cell response to protein immunization. Int Immunol. 1994;6:1515–23. doi: 10.1093/intimm/6.10.1515. [DOI] [PubMed] [Google Scholar]
- 9.Ling NR, MacLennan ICM, Mason DY. B-cell and plasma cell antigens. new and previously defined clusters. In: McMichael AJ, editor. Leucocyte Typing III. Oxford: Oxford University Press; 1986. pp. 302–35. [Google Scholar]
- 10.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 11.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foy TM, Shepherd DM, Durie FH, Aruffo A, Ledbetter JA, Noelle RJ. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–75. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 14.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 15.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 17.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo FM, Scholl PR, Fuleihan RL. CD40 ligand-deficient T cells from X-linked hyper-IgM syndrome carriers have intrinsic priming capability. J Immunol. 2002;168:1473–8. doi: 10.4049/jimmunol.168.3.1473. [DOI] [PubMed] [Google Scholar]
- 19.Gorbachev AV, Heeger PS, Fairchild RL. CD4+ and CD8+ T cell priming for contact hypersensitivity occurs independently of CD40–CD154 interactions. J Immunol. 2001;166:2323–32. doi: 10.4049/jimmunol.166.4.2323. [DOI] [PubMed] [Google Scholar]
- 20.Eshima K, Choi Y, Flavell RA. CD154–CD40-independent up-regulation of B7-2 on splenic antigen-presenting cells and efficient T cell priming by staphylococcal enterotoxin A. Int Immunol. 2003;15:817–26. doi: 10.1093/intimm/dxg080. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki ME, Coren BA, Huynh TN, Redondo DJ, Kikutani H, Webb SR. CD4+ T cell responses to CD40-deficient APCs: defects in proliferation and negative selection apply only with B cells as APCs. J Immunol. 1999;163:5250–6. [PubMed] [Google Scholar]
- 22.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, Janeway CA, Jr, Flavell RA. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–7. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 23.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–3. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 24.Moodycliffe AM, Shreedhar V, Ullrich SE, Walterscheid J, Bucana C, Kripke ML, Flores-Romo L. CD40–CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J Exp Med. 2000;191:2011–20. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Foy TM, Laman JD, et al. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–31. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 26.Luther SA, Gulbranson-Judge A, Acha-Orbea H, MacLennan IC. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J Exp Med. 1997;185:551–62. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weible ER. Principle and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–5. [PubMed] [Google Scholar]
- 28.Mongini PK, Paul WE, Metcalf ES. T cell regulation of immunoglobulin class expression in the antibody response to trinitrophenyl–ficoll. Evidence for T cell enhancement of the immunoglobulin class switch. J Exp Med. 1982;155:884–902. doi: 10.1084/jem.155.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 31.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 32.Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–70. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Orozco N, Chen Z, Poirot L, et al. Paradoxical dampening of anti-islet self-reactivity but promotion of diabetes by OX40 ligand. J Immunol. 2003;171:6954–60. doi: 10.4049/jimmunol.171.12.6954. [DOI] [PubMed] [Google Scholar]
- 34.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–31. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–81. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oxenius A, Campbell KA, Maliszewski CR, Kishimoto T, Kikutani H, Hengartner H, Zinkernagel RM, Bachmann MF. CD40–CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+ T cell functions. J Exp Med. 1996;183:2209–18. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 38.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 39.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brodeur SR, Angelini F, Bacharier LB, et al. C4b-binding protein (C4 BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837–48. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 41.Lazarevic V, Myers AJ, Scanga CA, Flynn JL. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity. 2003;19:823–35. doi: 10.1016/s1074-7613(03)00324-8. [DOI] [PubMed] [Google Scholar]
- 42.Lee BO, Moyron-Quiroz J, Rangel-Moreno J, Kusser KL, Hartson L, Sprague F, Lund FE, Randall TD. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J Immunol. 2003;171:5707–17. doi: 10.4049/jimmunol.171.11.5707. [DOI] [PubMed] [Google Scholar]
- 43.Maliszewski CR, Grabstein K, Fanslow WC, Armitage R, Spriggs MK, Sato TA. Recombinant CD40 ligand stimulation of murine B cell growth and differentiation: cooperative effects of cytokines. Eur J Immunol. 1993;23:1044–9. doi: 10.1002/eji.1830230510. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 45.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 46.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–70. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 47.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, Honjo T. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–6. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]