Abstract
Allergen-specific immunotherapy is commonly performed with allergen extracts adsorbed to aluminium hydroxide (alum). The undesirable effects associated with the use of alum, including granuloma formation at the site of injection and stimulation of T helper 2 (Th2) cytokine production, has generated interest in alternative allergen carriers, one being carbohydrate-based particles (CBPs). Here, we have investigated the in vitro effects of the recombinant major cat allergen Fel d 1 (rFel d 1) coupled to CBPs (CBP–rFel d 1) on human monocyte-derived dendritic cells (MDDCs) obtained from healthy blood donors. A majority of the CD1a+ MDDCs internalized fluorescein isothiocyanate-labelled CBP–rFel d 1, as demonstrated by flow cytometry and confocal laser-scanning microscopy. Furthermore, an up-regulation of the expression of the costimulatory molecule, CD86, on the MDDCs was induced by CBP–rFel d 1, but not by rFel d 1 or CBPs alone. Finally, three- and fourfold increases in the release of interleukin-8 and tumour necrosis factor-α, respectively, were observed when MDDCs were cultured in the presence of CBP-rFel d 1. Altogether, our results indicate that the use of CBPs as an allergen carrier and adjuvant is a promising candidate for the improvement of allergen-specific immunotherapy.
Keywords: adjuvant, allergen-specific immunotherapy, Fel d 1, microparticles, monocyte-derived dendritic cells
Introduction
Immunoglobulin E (IgE)-mediated allergic diseases1 affect ≈ 25% of the population in industrialized countries and the incidence continues to increase,2 underlining the importance of developing efficient treatment strategies. Although allergen-specific immunotherapy (SIT), the only curative treatment for allergic disease presently available, is often successful,3 such therapy may give rise to unwanted side-effects, such as anaphylactic reactions.4,5 In an attempt to avoid such undesirable side-effects, the allergen extracts employed in SIT are commonly adsorbed to aluminium hydroxide (alum), which delays release of the allergen. However, this approach is associated with the formation of granuloma at the site of injection, as well as other adverse effects.6–9 In addition, alum preferentially stimulates T helper 2 (Th2) responses,10 in direct contrast to the aim of suppressing the Th2-dependent mechanisms underlying allergy.11 Therefore, the development of new adjuvants designed to enhance the efficiency of SIT and with fewer side-effects, is highly desirable. Carbohydrate-based particles (CBPs) have been proposed as an alternative to alum in this context.7 Proteins can be covalently coupled to these particles via cyanogen bromide activation, and the particle size (2 µm in diameter) has been optimized for efficient phagocytosis.12 It has recently been shown that in the case of mice immunized with the timothy grass pollen allergen Phl p 5, a more T helper 1 (Th1)-skewed immune response was elicited when this allergen was bound to CBPs than when adsorbed to alum or delivered together with free CBPs.7 This observation indicates that CBPs influence the nature of the T-cell response to the allergen.
Of crucial significance for the outcome of the T-cell response is the efficiency with which the allergen is presented to allergen-specific T cells by antigen-presenting cells (APCs). The most important APC, the dendritic cell (DC), is capable both of promoting tolerance (by stimulating regulatory mechanisms) and immunity (by inducing effector T-cell responses).13 A key event in the presentation of antigens to T cells by DCs is the maturation of the latter cells, a process which is strongly dependent on stimuli of the innate immune system, e.g. adjuvants. Upon maturation of DCs, major histocompatibility complex (MHC) class II molecules, such as human leucocyte antigen (HLA)-DR, and costimulatory molecules, such as CD80 and CD86, are up-regulated, while at the same time the capacity to internalize antigens decreases. Depending on the nature of the stimulus [e.g. the duration and affinity of MHC antigen–peptide–T-cell receptor (TCR) binding], DCs also produce different cytokines which influence the response and polarization of the T cells.14,15 In light of this central role played by DCs in the regulation of T-cell responses, it is of obvious importance to elucidate how allergens and adjuvants interact with and affect DCs.
In this study, we investigated the effects of allergen coupled to CBPs on human monocyte-derived dendritic cells (MDDCs) from healthy individuals. The major cat allergen, Fel d 1, produced as a recombinant (r) protein with properties similar to those of the native allergen,16 was covalently coupled to CBPs (CBP-rFel d 1). Uptake of the CBP-rFel d 1 and its effects on the expression of costimulatory molecules and maturation markers, as well as on the release of cytokines by human DCs, were analysed. We found that the CBP-rFel d 1 complex exhibits properties that makes it capable of activating human DCs, a finding of considerable importance in connection with evaluation of this novel adjuvant as an alternative to alum in SIT.
Materials and methods
Production of the allergen
Recombinant Fel d 1, expressed in Escherichia coli, was produced as described previously.16 This protein was subsequently separated from endotoxins on a Detoxi-gelTM (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. The endotoxin concentration in the final rFel d 1 preparation (as determined by the Limulus amoebocyte test; Charles River Laboratories, Kent, UK) was found to be < 0·2 ng/mg of protein, corresponding to < 2 pg/ml of endotoxin in our incubations. This level of endotoxin did not affect the cytokine production or expression of costimulator/maturation markers by MDDCs, as demonstrated by control experiments in which 2 pg/ml of lipopolysaccharide (LPS) (L8274, Escherichia coli serotype 026-B6; Sigma, Steinheim, Germany) was added alone to the MDDCs.
Coupling of rFel d 1 to CBPs
The CBPs (2 µm micro-Sepharose; Pharmacia Diagnostics AB, Uppsala, Sweden) were activated with cyanogen bromide and coupling performed thereafter, as described previously,7,17 by stirring rFel d 1 (0·4 mg) in phosphate-buffered saline (PBS) with 18 mg of activated CBPs on ice. Determination of uncoupled rFel d 1 remaining in the supernatant using the bicinchoninic acid (BCA) Protein Assay (Pierce), indicated covalent binding of 430 µg of rFel d 1/ml of CBP suspension. In addition, rFel d 1 labelled with fluorescein isothiocyanate (FITC; Sigma) was also coupled to CBPs, essentially as described.18 After washing in sterile PBS, 18 mg of the rFel d 1 coupled to CBPs (CBP-rFel d 1), or of CBPs alone, was resuspended in 0·3 ml of RPMI-1640 (Gibco, Invitrogen Corporation, Paisley, UK) supplemented with 25 µg/ml gentamicin (Gibco) and subsequently stored at 4°. This CBP–rFel d 1 preparation contained 0·02 ng of endotoxin (measured as described above) per ml of solution, resulting in 0·2 pg/ml of endotoxin in our incubations. For control experiments, bovine serum albumin (BSA; Sigma) was labelled with FITC and coupled to CBPs using this same procedure.
Preparation of MDDCs
Concentrated peripheral blood cells (buffy coats) from healthy donors were obtained from the blood bank of the Karolinska University Hospital, and the study was approved by the local ethics committee. All donors tested negative with Phadiatop (which detects serum IgE antibodies directed towards the most common aero-allergens in Sweden) and, furthermore, exhibited no serum IgE directed towards cat dander (Pharmacia CAP System; Pharmacia Diagnostics AB). Immature MDDCs (iMDDCs) were differentiated from monocytes as described previously.19,20 In brief, following isolation of peripheral blood mononuclear cells (PBMCs) by Ficoll–Paque (Pharmacia) gradient centrifugation, the CD14+ monocytes were isolated by magnetic antibody cell sorting (MACS) microbead separation (Miltenyi Biotec, Bergisch Gladbach, Germany) in accordance with the manufacturer's instructions. These monocytes were subsequently cultured for 6 days in ‘complete’ RPMI (cRPMI) [consisting of RPMI-1640 (Gibco) supplemented with 25 µg/ml gentamicin (Gibco), 10% (v/v) heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT), 2 mm l-glutamine, 100 IU/ml penicillin (Gibco), 100 µg/ml streptomycin (Gibco) and 50 µmβ-mercaptoethanol (KEBO-lab, Spånga, Sweden)] in the additional presence of 800 U recombinant interleukin-4 (rIL-4; Nordic BioSite, Täby, Sweden) and 10 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Biosource International, Camarillo, CA). Fresh rIL-4 (800 U/ml) and GM-CSF (10 ng/ml) were added after 3 days of culture. After 6 days the MDDCs exhibited a typical immature phenotype, as demonstrated by flow cytometric analysis (Table 1).20–22 The median viability of these iMDDCs, as determined by Trypan blue exclusion, was 95% (range: 91–98%; n = 8).
Table 1.
Monocyte-derived dendritic cell (MDDC) surface antigen expression
| Surface antigen | Immature MDDCs, Day 6 (n = 8) | LPS-matured MDDCs, Day 8 (n = 8) | ||
|---|---|---|---|---|
| %* | MFI† | %* | MFI† | |
| CD1a | 91 (85–99) | 224 (129–539) | – | – |
| CD11b | 99 (97–100) | 104 (64–134) | – | – |
| CD11c | 99 (96–100) | 42 (31–61) | – | – |
| CD14 | 1 (1–1) | 18 (13–52) | – | – |
| CD40 | 97 (93–99) | 37 (32–50) | – | – |
| CD80 | 68 (42–80) | 18 (15–22) | 97 (88–99) | 50 (43–66) |
| CD83 | 8 (2–13) | 13 (11–24) | 79 (63–94) | 13 (9–18) |
| CD86 | 44 (11–57) | 51 (36–61) | 99 (97–100) | 174 (141–236) |
| HLA-DR | 98 (95–100) | 98 (81–150) | 100 (100–100)‡ | 156 (93–185)‡ |
Percentage of positively stained MDDCs as analysed by flow cytometry. Results are given as median percentage (range).
Mean fluorescence intensity (MFI) values, presented as median (range).
n = 3.
HLA, human leucocyte antigen; LPS, lipopolysaccharide.
Uptake of CBP-rFel d 1 by iMDDCs
Initial experiments with FITC-labelled BSA coupled to CBPs, designed to determine optimal conditions for the uptake by iMDDCs, revealed that this uptake increased, with time, up to 24 hr (data not shown), and this length of incubation was thus chosen for characterization of the uptake of CBP-rFel d 1 and rFel d 1. For this purpose, iMDDCs were suspended at a cell density of 0·5 × 106 cells/ml in cRPMI. FITC-labelled rFel d 1 (10 µg/ml), or FITC-labelled CBP-rFel d 1 (25 µl/ml of suspension), was added to the iMDDCs and then cultured in a 0·4-ml volume in 48-well Falcon Multiwell flat-bottom tissue culture plates (Becton Dickinson, San José, CA) for 24 hr at 37° under humidified 6% CO2 in air. After this incubation, the cells were transferred to 5-ml round-bottom polystyrene tubes (Becton Dickinson) for analysis by flow cytometry and confocal laser-scanning microscopy (CLSM).
Stimulation of MDDC maturation
Immature MDDCs, harvested on day 6, were resuspended at a cell density of 0·4 × 106 cells/ml in cRPMI supplemented with rIL-4 (800 U/ml) and GM-CSF (10 ng/ml), and subsequently cultured in a 1-ml volume in 24-well Falcon Multiwell flat-bottom tissue culture plates (Becton Dickinson). These incubations were carried out either in the presence of CBP-rFel d 1 (10 µl/ml of suspension, corresponding to ≈ 5 µg/ml of rFel d 1), CBP alone (10 µl/ml of suspension), rFel d 1 alone (10 µg/ml) or LPS (0·1 µg/ml; L8274, Escherichia coli serotype 026-B6; Sigma), or with medium only, for 48 hr at 37° under humidified 6% CO2 in air. The concentration of free rFel d 1 employed was twice as high as the bound concentration in order to ensure that any effects attributed to the CBP-rFel d 1 were not simply caused by the presence of an amount of the allergen that was higher than believed, based on the indirect protein determinations described above. After the incubation, the MDDCs were transferred to 5-ml, round-bottom polystyrene tubes (Becton Dickinson) for flow cytometric analysis.
Flow cytometric analysis
Flow cytometric analysis of the expression of surface markers was performed using a FACSCalibur flow cytometer and cellquest software (Becton Dickinson). The following FITC- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) were employed for phenotyping the iMDDCs: anti-CD11b–PE (ICRF44), anti-CD11c–PE (B-ly6), anti-HLA-DR–FITC (L243), anti-CD14–FITC (Leu-M3), anti-CD40–FITC (5C3), anti-CD80–FITC (L307.4), anti-CD83–FITC (HB15e) and anti-CD86–FITC (2331 FUN-1), all from Pharmingen/Becton Dickinson; anti-CD1a–PE (T6-RD1; Coulter, Beckman-Coulter, Miami, FL); and anti-ICOS-L–PE (MIH12), anti-PD-L1–PE (MIH1) and mouse IgG1–PE-conjugated isotype control (eBioscience, San Diego, CA). Isotype-matched mAbs, mouse FITC- or PE-conjugated IgG1, and mouse FITC-conjugated IgG2a (Pharmingen/Becton Dickinson and eBiosciences) were used as controls. An iMDDC gate was set according to light scatter properties, which generally included 90% of the total cell population. Only cells contained within these gates were considered in calculating the percentage of positive cells.
Following incubation for 24 hr with FITC-labelled CBP-rFel d 1 or FITC-labelled rFel d 1, iMDDCs were labelled with anti-CD1a or isotype-matched mAbs, fixed in 2% paraformaldehyde (Sigma) for 15 min and thereafter subjected to flow cytometric analysis. The gates were set on the basis of the fluorescence intensities of iMDDCs incubated with FITC-labelled rFel d 1 or FITC-labelled CBP-rFel d 1 alone, and then stained with an anti-CD1a–PE isotype-matched mAb, as well as the fluorescence intensity of iMDDCs stained with this mAb only in the absence of FITC-labelled components.
At least 104 cells per sample were analysed and the flow cytometer was calibrated at regular intervals according to the manufacturer's instructions.
Analysis by CLSM
Following incubation for 24 hr with FITC-labelled CBP-rFel d 1 or FITC-labelled rFel d 1, MDDCs were fixed in 2% paraformaldehyde for 15 min and then air-dried onto three-well microscope glass-slides (≈104 cells per well; Novakemi, Enskede, Sweden). Slides were stored at −80° until required for immunocytochemical staining. This staining was carried out using anti-CD1a mAb (Immunotech, Marseille, France) diluted 1 : 20 in 4% BSA, followed by washing with PBS. A secondary goat anti-mouse mAb conjugated with Alexa Fluor 546 (Molecular Probes, Eugene, OR) was used for detection and the slides were mounted with paraphenylenediamine (PPD; Sigma).
The stained MDDCs were examined using a CLSM (TCS SP2; Leica Microsystems, Mannheim, Germany) equipped with one argon and two HeNe lasers. In order to avoid potential cross-talk between the detectors, the two fluorophores were examined by sequential scanning. FITC was excited with the 488-nm laser line, and the light emitted in the wavelength region of 500–650 nm was detected. Alexa 546 was excited by the 543-nm laser line with detection of emission in the wavelength region of 560–700 nm. Z-series' of multiple images were obtained by scanning the cells in sections (at Z-steps of 0·5 µm).
Analysis of cytokine production
In connection with incubation of the iMDDCs (0·4 × 106 cells/ml) with CBP-rFel d 1, rFel d 1, CBP, LPS or medium alone (see above), 100-µl samples of the supernatants were collected after 6 and 48 hr for cytokine analysis. These samples were centrifuged in order to remove cells and then maintained frozen at −20° until analysis. The CBA human inflammation kit (Becton Dickinson) was utilized to assay IL-1β, IL-6, IL-8, IL-10, IL-12p70 and tumour necrosis factor-α (TNF-α), in accordance with the manufacturer's instructions. The lower limits of detection were 20 pg/ml.
Statistical analysis
The non-parametric Friedman two-way analysis of variance was applied in connection with the statistica software (StatSoft Inc, Tulsa, OK). Friedman multiple comparison tests were performed as described previously.23 A P-value of < 0·05 was considered to be statistically significant. In cases where the concentration of a cytokine was below the limit of detection, the value of this limit was used in the statistical calculations.
Results
iMDDCs are capable of ingesting CBP-rFel d 1 particles
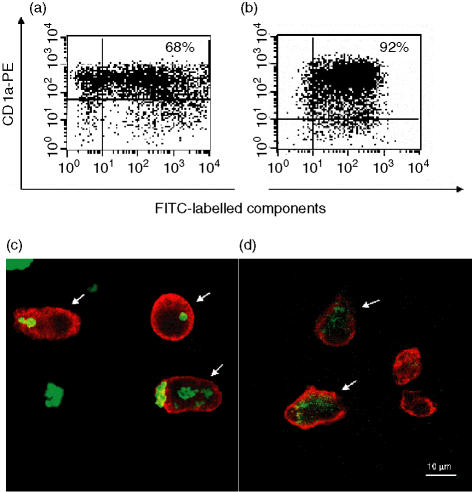
Flow cytometry revealed that after 24 hr of incubation, a majority of the CD1a+ MDDCs (median 68%, range 54–75%; n = 5) were positive for CBP-rFel d 1 (Fig. 1a), and almost all (median 90%, range 81–95%; n = 5) of the CD1a+ iMDDCs were rFel d 1 positive (Fig. 1b). The uptake was studied further with CLSM. By scanning the iMDDCs and generating optical sections of multiple images, it was demonstrated that both CBP-rFel d 1 (Fig. 1c) and rFel d 1 (Fig. 1d) had been internalized by the CD1a+ iMDDCs. The patterns of uptake differed in these two cases: MDDCs incubated with CBP-rFel d 1 showed great variation, internalizing several, only a few or no particles (Fig. 1c), whereas rFel d 1 was evenly distributed in the cytoplasm of all double-positive cells (Fig. 1d).
Figure 1.
Uptake of carbohydrate-based particle (CBP)–rFel d 1 complexes, or rFel d 1 alone, by immature monocyte-derived dendritic cells (iMDDCs). MDDCs were incubated for 24 hr with fluorescein isothiocyanate (FITC)-labelled CBP–rFel d 1 or rFel d 1, then stained with anti-CD1a–phycoerythrin (PE) and analysed by flow cytometry (a and b) and confocal laser-scanning microscopy (CLSM) (c and d). More than 60% of the CD1a+ MDDCs were positive for FITC-labelled CBP–rFel d 1 (a), and more than 90% of the CD1a+ MDDCs were positive for FITC-labelled rFel d 1 (b). The percentage of double-positive cells is indicated in the upper right-hand corner. The results presented here are from one of five independent experiments. Multiple Z-series' images obtained by CLSM revealed that the CBP–rFel d 1 (c) and rFel d 1 (d) had been internalized by the MDDCs. Cells sectioned through their centre are indicated with arrows. These images have been optically merged, with red representing Alexa 546-conjugated antibodies detecting CD1a, and green representing FITC-labelled CBP–rFel d 1 or rFel d 1. The scale bar in (d) is also valid for (c). Here, the results from one of four experiments are shown.
CBP-rFel d 1 induces up-regulation of CD86 expression on MDDCs
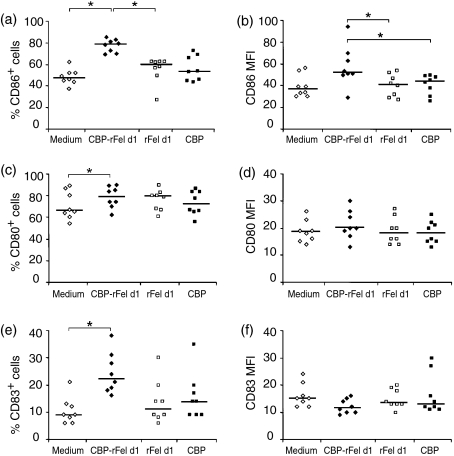
Incubation of iMDDCs with CBP-rFel d 1 for 48 hr resulted in a significant increase in the percentage of cells expressing CD86 (P < 0·05, n = 8), as compared to cells incubated with medium or rFel d 1 only (Fig. 2a). In addition, the level of CD86 expression on the surface of individual cells, as indicated by the mean fluorescence intensity (MFI), also increased after incubation with CBP-rFel d 1 (P < 0·05) in comparison to exposure to rFel d 1 or CBP alone (Fig. 2b), although in comparison with unstimulated cells, this increase did not reach statistical significance (P < 0·075). Furthermore, CBP-rFel d 1 significantly enhanced the percentage of cells expressing CD80 and CD83, as compared with unstimulated cells (Fig. 2c,e). No significant change in the expression of HLA-DR (n = 3) or of the two other markers of costimulation analysed, i.e. ICOS-L and PD-L1 (n = 3), was observed (data not shown). The positive control, i.e. stimulation with LPS, induced up-regulation of CD80, CD83 and CD86 (Table 1). Furthermore, twofold higher expression of HLA-DR and PD-L1 compared with cells cultured in medium only was demonstrated (MFI values, percentage of cells expressing PD-L1, increased fivefold). LPS did not stimulate any up-regulation of ICOS-L, in agreement with previous findings.24
Figure 2.
Expression of the cell-surface antigens CD86 (a, b), CD80 (c, d) and CD83 (e, f), following 48 hr of incubation of monocyte-derived dendritic cells (MDDCs) with carbohydrate-based particle (CBP)–rFel d 1 complexes, rFel d 1, CBP or medium alone. For each marker examined, the results of the flow cytometric analysis are expressed both as the percentage of positive cells (a, c and e) and as mean fluorescence intensity (MFI) values (b, d and f). The data presented originate from experiments with MDDCs derived from eight different healthy blood donors. The horizontal lines indicate median values. *P < 0·05.
MDDCs incubated with CBP-rFel d 1 release TNF-α and IL-8
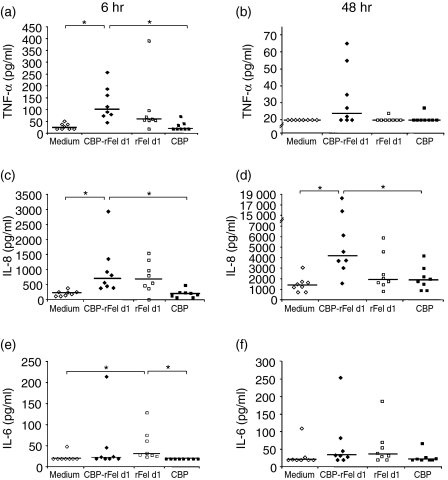
Significant increases in the release of TNF-α and IL-8 were exhibited by MDDCs cultured in the presence of CBP-rFel d 1 for 6 hr, in comparison to cells incubated with CBP or medium alone for the same period of time (Fig. 3a, 3c). The secretion of IL-8 was further increased after 48 hr and the differences between incubations with CBP-rFel d 1 and medium or CBPs alone remained highly significant (Fig. 3d). In contrast, release of TNF-α had diminished considerably at this later time-point (Fig. 3b).
Figure 3.
Release of cytokines by monocyte-derived dendritic cells (MDDCs) incubated in the presence of carbohydrate-based particle (CBP)–rFel d 1 complexes, rFel d 1, CBP or medium alone. Secretion of tumour necrosis factor-α (TNF-α) (a, b), interleukin (IL)-8 (c, d) and IL-6 (e, f) into the supernatant following 6 hr (a, c and e) and 48 hr (b, d and f) of incubation was measured employing the cytometric bead array (CBA) human inflammatory kit (lower limit of detection=20 pg/ml). The data from experiments with MDDCs originating from eight different healthy blood donors are presented. The horizontal lines indicate median values. *P < 0·05.
Furthermore, incubation with rFel d 1 resulted in a significantly higher release of IL-6 after 6 hr than did incubation with medium or CBPs only (Fig. 3e), although, in general, only low levels of IL-6 were detected. In no case was there any significant difference between cytokine release from MDDCs incubated with CBP-rFel d 1 or with rFel d 1 alone. Secretion of the other cytokines analysed, i.e. IL-1β, IL-10 and IL-12p70, by MDDCs was unaffected upon incubation with CBP-rFel d 1, rFel d 1 or CBP (data not shown). In the case of the positive control, i.e. incubation with LPS, release of all the cytokines analysed, with the exception of IL-1β, was elevated by two- to > 250-fold (data not shown).
Discussion
In the present investigation we found that in vitro human CD1a+ iMDDCs readily take up the novel allergen–adjuvant complex CBP-rFel d 1 and, furthermore, that this uptake is associated with an up-regulation of the expression of costimulatory molecules, especially CD86 (B7-2), on the surface of these cells. In contrast, exposure to free rFel d 1, which is also efficiently taken up by the iMDDCs, does not give rise to up-regulation of any of the costimulators examined here. Thus, the effect of rFel d 1 on the expression of costimulatory molecules is dependent on the association with CBPs, which may be explained by the different mechanisms for uptake of free rFel d 1 and large CBP-rFel d 1 particles, i.e. by fluid-phase pinocytosis and phagocytosis, respectively.25,26
It is well established that costimulation mediated via CD80/CD86 and CD28 is crucial for the induction of T-cell responses.27 Therefore, the observed up-regulation of CD86 expression by MDDCs in response to CBP-rFel d 1 may be of significance with respect to the application of CBP-coupled allergens in connection with SIT, where the goal is to alter the immune response to the allergen in order to counteract the allergic response. CD86 has been reported to be involved in the development of Th2 responses to allergens,28,29 but there is evidence that CD80 may also promote such responses.30 However, our knowledge regarding the different functional roles of the costimulatory molecules CD80 and CD86 is still limited and it appears probable that a subtle balance between signals from these and other costimulators regulates the final outcome of the T-cell response.31
We observed a negligible effect of CBP-rFel d 1 on MDDC maturation, as reflected in up-regulation of the maturation markers CD83 and HLA-DR. Furthermore, free rFel d 1 and CBP-rFel d 1 induced similar increases in the release of TNF-α and IL-8 by MDDCs. No significantly elevated levels of any of the additional cytokines analysed here were detected after culture of the MDDCs, either with CBP-rFel d 1 or free rFel d 1. Thus, this lack of maturation and low level of cytokine release, in combination with up-regulation of CD86 expression, suggest that the CBP-coupled allergen induces a semimature state of the DCs.32 Such a state will probably be associated with a regulatory or, possibly, a mixed Th1/Th2 response, rather than a potent Th1 response.15 Although numerous adjuvants, recently proposed for application to SIT, are designed to elicit allergen-specific Th1 responses,33–35 it is far from certain that this is the most desirable goal for such therapy.36 The mechanisms by which SIT alleviates allergic symptoms are still poorly understood and, indeed, it has been demonstrated that induction of a Th1 response is not capable of counteracting an established Th2 response.15,37 Furthermore, Th1 responses in mice have actually been reported to be associated with increased susceptibility to allergen sensitization and allergic inflammation.38 Interestingly, high exposure to cat allergen has in fact been proposed to be coupled to a modified Th2 response characterized by allergen-specific IgG4 production in individuals that are asymptomatic to cat.39
The potential T-cell response to CBP-rFel d1 in humans in vivo was not examined here and remains to be elucidated. However, Grönlund et al.7 reported that administration to mice of the timothy grass pollen allergen, Phl p 5, coupled to CBPs elicited a mixed Th1/Th2 response to timothy grass pollen extract, thus demonstrating that allergens coupled to CBPs are also capable of influencing the allergen-specific immune responses in vivo. The adjuvant effect of CBP-coupled allergen might simply be a consequence of the particulate nature of the CBPs per se, as has been shown to be the case for other particulate adjuvants, including alum.40 However, CBPs alone did not have the same effects as when coupled to an allergen, either on MDDCs in vitro, as shown in the present study, or in connection with immunization of mice.7 Alternatively, the mere fact that a large amount of allergen is delivered to the APC in association with the CBPs may explain the altered immune response. In this context, an oligomeric form of the model allergen ovalbumin (OVA) has been found to elicit a different profile of T-cell cytokines, characterized by a much higher interferon-γ (IFN-γ)/IL-4 ratio, than that induced by OVA in its natural, monomeric form, indicating that a high ‘density’ of allergen can, indeed, modify the T-cell response.41 Thus, even if CBPs are in themselves not immunostimulatory, when large amounts of allergen are linked to these particles, various mechanisms for antigen uptake, processing and presentation may alter signalling by the APCs.
In conclusion, we have demonstrated here that CBP-coupled allergen is readily taken up by CD1a+ human dendritic cells and subsequently elicits MDDC responses, which differ from those observed with free allergen. Together with previous findings on mice,7 our study thus indicates that this novel adjuvant and allergen-carrier may be highly useful in connection with the development of allergen-specific immunotherapy.
Acknowledgments
This study was supported by grants from the Swedish Research Council (projects 74x-7924, 74EF-15193 and 74x-13497), the Swedish Foundation for Health Care Sciences and Allergy Research, the Swedish Asthma and Allergy Association's Research Foundation, the Hesselman Foundation, the Swedish Cancer and Allergy Fund, the King Gustaf V 80th Birthday Foundation, Lars Hierta's Foundation, the Konsul Th C Bergh Foundation, the Åke Wiberg Foundation, the Magnus Bergvall Foundation and Karolinska Institutet.
Abbreviations
- CBP
carbohydrate-based particles
- CLSM
confocal laser-scanning microscopy
- FITC
fluorescein isothiocyanate
- mAb
monoclonal antibody
- IL
interleukin
- MDDC
monocyte-derived dendritic cells
- PE
phycoerythrin
- SIT
allergen-specific immunotherapy
References
- 1.Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–24. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Allergy and Allergic Diseases. Oxford: Blackwell Science; 1997. [Google Scholar]
- 3.Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998;102:157–64. doi: 10.1016/s0091-6749(98)70079-x. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 5.Cox JC, Coulter AR. Adjuvants – a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 6.Gherardi RK, Coquet M, Cherin P, et al. Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived aluminium hydroxide in muscle. Brain. 2001;124:1821–31. doi: 10.1093/brain/124.9.1821. [DOI] [PubMed] [Google Scholar]
- 7.Gronlund H, Vrtala S, Wiedermann U, Dekan G, Kraft D, Valenta R, Van Hage-Hamsten M. Carbohydrate-based particles: a new adjuvant for allergen-specific immunotherapy. Immunology. 2002;107:523–9. doi: 10.1046/j.1365-2567.2002.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterballe O. Side effects during immunotherapy with purified grass pollen extracts. Allergy. 1982;37:553–62. doi: 10.1111/j.1398-9995.1982.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 9.Vogelbruch M, Nuss B, Korner M, Kapp A, Kiehl P, Bohm W. Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy. 2000;55:883–7. [PubMed] [Google Scholar]
- 10.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 11.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–53. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 12.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM. Some interfaces of dendritic cell biology. Apmis. 2003;111:675–97. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4(Suppl. 3):S127–32. doi: 10.1186/ar567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–12. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 16.Gronlund H, Bergman T, Sandstrom K, et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J Biol Chem. 2003;278:40144–51. doi: 10.1074/jbc.M301416200. [DOI] [PubMed] [Google Scholar]
- 17.Axen R, Porath J, Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967;214:1302–4. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- 18.Buentke E, Zargari A, Heffler LC, Avila-Carino J, Savolainen J, Scheynius A. Uptake of the yeast Malassezia furfur and its allergenic components by human immature CD1a+ dendritic cells. Clin Exp Allergy. 2000;30:1759–70. doi: 10.1046/j.1365-2222.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 19.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buentke E, Heffler LC, Wallin RP, Lofman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31:1583–93. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 21.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2. New York: McGraw-Hill; 1988. [Google Scholar]
- 24.Witsch EJ, Peiser M, Hutloff A, Buchner K, Dorner BG, Jonuleit H, Mages HW, Kroczek RA. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol. 2002;32:2680–6. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noirey N, Rougier N, Andre C, Schmitt D, Vincent C. Langerhans'-like dendritic cells generated from cord blood progenitors internalize pollen allergens by macropinocytosis, and part of the molecules are processed and can activate autologous naive T lymphocytes. J Allergy Clin Immunol. 2000;105:1194–201. doi: 10.1067/mai.2000.106545. [DOI] [PubMed] [Google Scholar]
- 27.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 28.Larche M, Till SJ, Haselden BM, North J, Barkans J, Corrigan CJ, Kay AB, Robinson DS. Costimulation through CD86 is involved in airway antigen-presenting cell and T cell responses to allergen in atopic asthmatics. J Immunol. 1998;161:6375–82. [PubMed] [Google Scholar]
- 29.Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, Pestel J. Th2 polarization by Der p 1-pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood. 2001;98:1135–41. doi: 10.1182/blood.v98.4.1135. [DOI] [PubMed] [Google Scholar]
- 30.Straw AD, MacDonald AS, Denkers EY, Pearce EJ. CD154 plays a central role in regulating dendritic cell activation during infections that induce Th1 or Th2 responses. J Immunol. 2003;170:727–34. doi: 10.4049/jimmunol.170.2.727. [DOI] [PubMed] [Google Scholar]
- 31.Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol. 2003;24:314–9. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 32.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 33.Bohle B. CpG motifs as possible adjuvants for the treatment of allergic diseases. Int Arch Allergy Immunol. 2002;129:198–203. doi: 10.1159/000066771. [DOI] [PubMed] [Google Scholar]
- 34.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan – DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–91. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 35.Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DB. Allergy immunotherapy and inhibition of Th2 immune responses: a sufficient strategy? Curr Opin Immunol. 2002;14:644–51. doi: 10.1016/s0952-7915(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 37.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–43. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 39.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JA, Byrne R, Whitty G. Particulate adjuvants can induce macrophage survival, DNA synthesis, and a synergistic proliferative response to GM-CSF and CSF-1. J Leukoc Biol. 2000;67:226–32. [PubMed] [Google Scholar]
- 41.Yang X, Gieni RS, Mosmann TR, HayGlass KT. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993;178:349–53. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]