Abstract
Dendritic cells (DC) are the most potent of antigen-presenting cells. The most important function of DC is to initiate the immune response by presenting antigens to naïve T lymphocytes. Currently, little is known about the basic action of lycopene in murine bone marrow (BM)-derived DC. In the present study, we have revealed that lycopene significantly attenuates the phenotypic and functional maturation of murine BM-DC, especially in lipopolysaccharide-induced DC maturation. We found that lycopene down-regulates the expression of costimulatory molecules (CD80 and CD86) and major histocompatibility complex type II molecules. We also determined that lycopene-treated DC were poor stimulators of naïve allogeneic T-cell proliferation and induced lower levels of interleukin-2 in responding T cells. They also exhibited impaired interleukin-12 production. Additionally, lycopene was able to inhibit mitogen-activated protein kinases, such as ERK1/2, p38 and JNK, and the transcription factor, nuclear factor-κB. Assessment of the in vivo effects of lycopene may reveal an inability to induce a normal cell-mediated immune response, despite the ability of the cells to migrate to the spleen. This data provides new insight into the immunopharmacology of lycopene and suggests a novel approach to the manipulation of DC for therapeutic application.
Keywords: lycopene, dendritic cell, mitogen-activated protein kinases, NF-κB
Introduction
Dendritic cells (DC) are the most potent of antigen-presenting cells (APCs). Immature DC reside in peripheral tissues, effectively capture and process antigen and migrate to peripheral lymphoid tissues. After the uptake of antigen and exposure to inflammatory agents, DC undergo a process of maturation such that they have a greatly diminished capacity for antigen uptake and processing.1,2 The maturing DC migrate to the lymphoid organs, where they stimulate naive T cells through the signals both of major histocompatibility complex (MHC) molecules presenting antigen peptides, and costimulatory molecules.3,4 DC are also highly responsive to inflammatory cytokines, such as tumour necrosis factor-α (TNF-α), or bacterial products, such as lipopolysaccharide (LPS), encountered in peripheral organs, which induces a series of phenotypic and functional changes in DC.5,6 Similar changes, indicative of maturation, have also been reported following infection with mycoplasma, viruses, intracellular bacteria and parasites.7–9 These phenotypic changes parallel the functional transition of DC from antigen-capturing cells to APCs.
Lycopene, the most abundant carotenoid responsible for the red colour of tomatoes, is the predominant circulating carotenoid in Western diets.10 The protective effect of lycopene is usually ascribed to its ability to act as an antioxidant and singlet oxygen quencher, thereby inhibiting the destructive effects of reactive oxygen species (ROS).11,12 In addition, previous studies have indicated that people consuming tomatoes and tomato products are less likely to suffer from cancer, coronary heart disease and other chronic diseases.11,13 To date, many epidemiological studies have shown an intricate relationship between the consumption of tomatoes and the risk of cancer and coronary heart disease.14–17 Protection by lycopene against lipid peroxidation and oxidative DNA damage has also been investigated in cell culture.18 Furthermore, consumption of tomato products has been reported to reduce the susceptibility of lymphocyte DNA to damage.19 It should be mentioned that lycopene was either an antioxidant or a pro-oxidant, depending on the oxidant used.20 However, no information has yet been published about the action of lycopene on phenotypic and functional maturation of murine bone marrow-derived DC.
In this study we investigated whether lycopene affects the phenotypic and functional maturation of murine DC via mitogen-activated protein kinases (MAPKs). We show, for the first time, that lycopene inhibits the phenotypic and functional maturation of DC and suppresses the LPS-induced activation of ERK1/2, p38 and JNK in DC. Also, lycopene was shown to decrease the nuclear translocation of nuclear factor-κB (NF-κB) p65 in LPS-stimulated DC.
Materials and methods
Animals and chemicals
Male 8–12-week-old C57BL/6 (H-2Kb and I-Ab) and BALB/c (H-2Kd and I-Ad) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). They were housed in a specific pathogen-free environment within our animal facility for at least 1 week before use. Lycopene was purchased from Sigma.
Generation of bone marrow (BM)-derived murine myeloid DC
BM-derived murine myeloid DC were generated as described in detail previously,21 with a minor modification. Briefly, bone marrow cells from the femura and tibiae of C57BL/6 mice were flushed and depleted of red blood cells (RBC) by hypotonic lysis using an RBC-lysing buffer (Sigma, St Louis, MO). Cells were grown from precursors at a starting concentration of 1 × 106 cells/ml in RPMI-1640, supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), l-glutamine, non-essential amino acids, sodium pyruvate, penicillin-streptomycin, HEPES and 2-mercaptoethanol (2-ME) (all from Sigma) for 3 hr, and then non-adherent cells were washed out. Approximately 20 ng/ml recombinant mouse (rm) granulocyte–macrophage colony-stimulating factor (GM-CSF) and 20 ng/ml rm interleukin (IL)-4 (R & D Systems, Minneapolis, MN) were added to the culture medium, referred to subsequently as a complete medium. In a parallel experiment, 1, 5 or 10 µm lycopene was added to the culture medium on day 5. On day 6 of culture, non-adherent and loosely adherent cells were collected as BM-DC.
Flow cytometric analysis
On day 6, BM-DC were harvested, washed with phosphate-buffered saline (PBS) and resuspended in fluorescence-activated cell sorter (FACS) washing buffer (2% fetal bovine serum and 0·1% sodium azide in PBS). Cells were first blocked with 10% (v/v) normal goat serum for 15 min at 4° and stained with phycoerythrin (PE)-conjugated anti-H-2Kb[major histocompatibility complex (MHC) class I], anti-I-Ab (MHC class II), anti-CD80, and anti-CD86 with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c (PharMingen, San Diego, CA) for 30 min at 4°. The stained cells were analysed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Cytokine assay
Cells were first blocked with 10% (v/v) normal goat serum for 15 min at 4° and then stained with FITC-conjugated CD11c antibody for 30 min at 4°. The cells stained with the appropriate isotype-matched immunoglobulin were used as negative controls. The cells were fixed and permeated using the Cytofix/Cytoperm kit (PharMingen), according to the manufacturer's instructions. Intracellular IL-12p40/p70 was stained with fluorescein R-PE-conjugated antibodies (PharMingen) in a permeation buffer. The cells were analysed on a FACSCalibur flow cytometer using the cellquest program. In addition, murine IL-12p70 from DC was measured using an enzyme-linked immunosorbent assay (ELISA) kit (PharMingen), according to the manufacturer's instructions. The detection limit for IL-12p70 was 7·8 pg/ml.
Endocytosis assay
To analyse the endocytosis of DC, 1 × 105 cells were incubated at 37° for 1 hr with 1 mg/ml dextran–FITC (42 000 molecular weight; Sigma). After incubation, the cells were washed twice with cold Hanks' balanced salt solution (HBSS), and stained using PE-conjugated anti-CD11c immunoglobulin (PharMingen). The double-stained DC were analysed using a FACSCalibur flow cytometer. In addition, parallel experiments were performed at 4° to show that the uptake of dextran by BM-derived murine DC is inhibited at low temperatures.
Mixed lymphocyte reaction (MLR) induced by DC
Responder T cells used for the allogeneic T-cell reaction were isolated on a magnetic antibody cell sorting (MACS) column (Miltenyi Biotec, Bergisch Gladbach, Germany) passing through mononuclear cells from BALB/c mice. They were determined to be composed mainly of CD3+ cells (> 93%) when examined by staining with FITC-conjugated anti-CD3 immunoglobulin (PharMingen). BM-derived murine DC were treated with 50 µg/ml mitomycin C (Sigma) for 1 hr and added in graded doses to 1 × 105 allogeneic T cells in U-bottom 96-well microtitre culture plates. Cell proliferation during the last 18 hr of the 72-hr culture was quantified by the [3H]thymidine (NEN-DuPont, Boston, MA) uptake of cells incubated with 0·5 µCi of [3H]thymidine (NEN-DuPont). The cells were harvested onto glass fibre filters (Inotech Biosystems, Zurich, Switzerland) and the radioactivity was measured in a scintillation counter. The results are presented as the mean counts per minute (c.p.m.) of triplicate cultures.
Assessment of ERK, p38 kinase and JNK by Western blotting
The cells were exposed to LPS (100 ng/ml) in the presence or absence of 10 µm lycopene pretreatment, and, following 15 and 30 min of incubation at 37°, the cells were washed twice with cold PBS and lysed with modified RIPA buffer [1·0% Nonidet P-40, 1·0% sodium deoxycholate, 150 nm NaCl, 10 mm Tris–HCl (pH 7·5), 5·0 mm sodium pyrophosphate, 1·0 mm NaVO4, 5·0 mm NaF, 1·0 µg/ml leupeptin, and 0·1 mm phenylmethylsulfonyl fluoride) for 15 min at 4°. Lysates were cleared by centrifugation (14 000 g, 20 min, 4°). The protein content of cell lysates was determined using the Micro bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL). Equivalent amounts of proteins were separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and analysed by Western blotting using an anti-phospho-ERK (p-ERK; Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-JNK (p-JNK; Santa Cruz Biotechnology) or anti-phospho-p38 (p-p38; Santa Cruz Biotechnology) MAPK monoclonal antibody (mAb) for 1 hr, as described by the manufacturer of the antibodies. Following washing three times with PBS containing 0·1% Tween-20 (TBST), membranes were incubated with secondary horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) for 1 hr. After washing four times, total for 10 min, the blots were developed using the enhanced chemiluminescence (ECL) system (Amersham, Buckinghamshire, UK) by following the manufacturer's instructions.
Preparation of nuclear extracts and assessment of NF-κB activation by Western blotting
DC nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce), according to the manufacturer's instructions. The existence of an NF-κB p65 subunit in the nuclear extracts was determined by Western blot analysis with anti-NF-κB p65 subunit immunoglobulin as a probe (Santa Cruz Biotechnology).
Generation of DC from spleen and culture
Mice were injected intraperitoneally with lycopene (50 nmol) every 3 days before the administration of 20 µg of LPS in a lateral vein of the tail. Twenty-four hours after LPS challenge, they were scarified, their spleens were disrupted and the cells were centrifuged at 400 g for 5 min, resuspended in RPMI-1640 supplemented with 10% heat-inactivated FBS, l-glutamine, non-essential amino acids, sodium pyruvate, penicillin-streptomycin, HEPES and 2-ME (all from Sigma) for 2 hr, and then non-adherent cells were washed out. The residual adherent cells were maintained in the culture medium and incubated overnight at 37° in a 5% CO2 atmosphere. After incubation, DC (which exhibit adherence capacity in the first hours of culture) become non-adherent and float in the medium. The cells were gated on CD11c+ for DC.
Statistics
The results were expressed as the mean ± standard deviation (SD) of the indicated number of experiments. The statistical significance was estimated using a Student's t-test for unpaired observations. A P-value of < 0·05 was considered to be significant.
Results
Lycopene impairs the phenotypic maturation of DC
BM cells were cultured for 6 days in RPMI supplemented with GM-CSF (20 ng/ml) and IL-4 (20 ng/ml). Different concentrations of lycopene were added to culture on day 6 in the presence or absence of LPS (100 ng/ml). Because lycopene at > 25 µm was found to be cytotoxic to BM cells, a concentration of ≈ 10 µm was used.
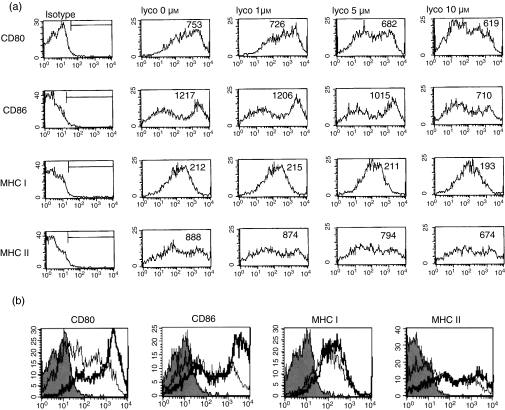
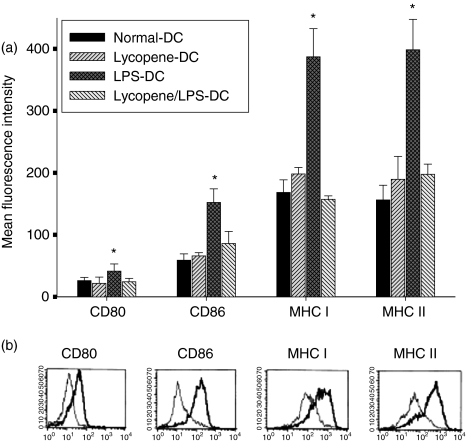
In the first set of experiments, we investigated the effect of different concentrations of lycopene on DC maturation. BM-derived cells were cultured for 24 hr in the presence of 0–10 µm lycopene, as described in the Materials and methods. As shown in Fig. 1(a), 10 µm lycopene was sufficient to inhibit the expression of CD80, CD86 and MHC class I and class II in CD11c+ cells on day 6. The inhibitory effect of lycopene was dose-dependent and targeted primarily the expression of CD80, CD86 and MHC class II, which were down-regulated markedly at 10 µm (Fig. 1a). The stimulation of cells with LPS from day 5 onwards resulted in the strong up-regulation of CD80, CD86 and MHC class I and II expression within 24 hr (Fig. 1b). Treatment with 10 µm lycopene in the presence of LPS impaired the expression of the costimulatory molecules, CD80 and CD86, and, to a lesser extent, the expression of MHC class I and II molecules (Table 1).
Figure 1.
Lycopene suppresses the expression of costimulatory molecules, CD80 and CD86, and major histocompatibility complex (MHC) class I and II in a dose-dependent manner during dendritic cell (DC) maturation. DC were generated as described in the Materials and methods. On day 6, the cells were harvested and analysed by two-colour flow cytometry. The cells were gated on CD11c+. Lycopene was added to DC at concentrations of 1, 5 or 10 µm for 24 hr and the expression of surface molecules was analysed (a). DC were either untreated (isotype control) or were stimulated for 24 hr with 100 ng/ml lipopolysaccharide (LPS), in the absence (thick lines) or presence (thin lines) of 10 µm lycopene on day 6 (b). The histogram is from one representative experiment out of three performed.
Table 1.
Lycopene significantly inhibits the expression of costimulatory and major histocompatibility complex (MHC) molecules on CD11c+ dendritic cells (DC)* in the presence of lipopolysaccharide (LPS)
| % Positive cells (MFI†) | ||||
|---|---|---|---|---|
| Surface antigen | Normal-DC | Lyco-DC | LPS-DC | Lyco/LPS-DC |
| CD80 | 91 ± 6 (793 ± 56) | 89 ± 3 (629 ± 34) | 93 ± 2 (965 ± 126) | 90 ± 2 (751 ± 61) |
| CD86 | 65 ± 4 (1156 ± 123) | 64 ± 5 (753 ± 46) | 83 ± 4 (1380 ± 99) | 67 ± 8 (897 ± 78) |
| MHC class I | 90 ± 3 (225 ± 13) | 87 ± 4 (198 ± 17) | 93 ± 6 (229 ± 34) | 90 ± 5 (203 ± 9) |
| MHC class II | 79 ± 11 (898 ± 36) | 75 ± 7 (682 ± 21) | 87 ± 6 (1126 ± 85) | 80 ± 3 (858 ± 65) |
Bone marrow (BM)-derived DC were generated as described in the Materials and methods (± 20 µm). On day 5, maturation was induced by stimulation with LPS (100 ng/ml) for 24 hr. Two-colour flow cytometry was used to determine the level of antigen expression on CD11c+ DC.
MFI, mean fluorescence intensity. Data are from one experiment representative of three performed.
Lycopene inhibits LPS-induced IL-12 production by DC
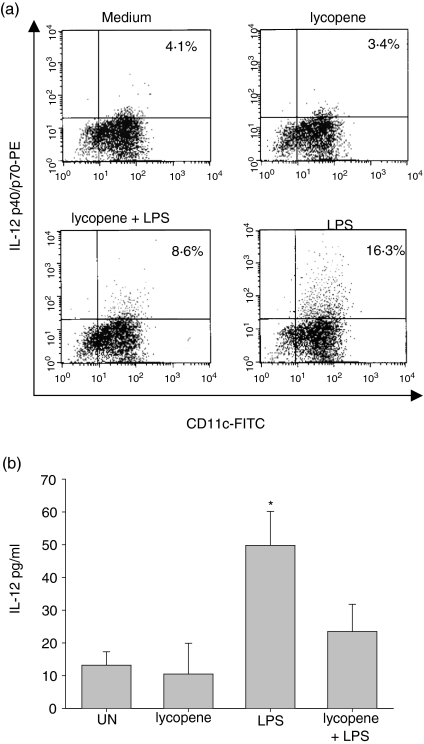
We next examined the ability of BM-DC to produce proinflammatory cytokines, because DC, like monocytes and macrophages, are thought to be a source of proinflammatory molecules.22,23 It has been reported that IL-12 is a specific marker of functionally activated DC.24,25 To study the affect of lycopene on IL-12 production, we analysed both intracellular IL-12p40/p70 and bioactive IL-12p70 production in the presence of lycopene. As shown in Fig. 2(a), lycopene significantly inhibited the production of IL-12p40/p70 compared to the sample without lycopene treatment (Fig. 2a), whereas IL-10 was not detectable (data not shown). IL-10 was also not detectable after stimulation with LPS (100 ng/ml) (data not shown). The inhibitory effect of lycopene on IL-12p70 production was confirmed using an ELISA. Untreated DC or lycopene-treated DC secreted a low concentration of IL-12p70 (14·2 ±4·1 pg/ml and 10·5 ± 9·3 pg/ml, respectively). LPS-treated DC secreted higher concentrations of IL-12 than untreated DC (49·8 ± 9·5 pg/ml). As shown in Fig. 2(b), lycopene impaired the production of bioactive IL-12p70 in the presence of LPS stimulation (24·1 ± 6·3 pg/ml), indicating that exposure to lycopene impaired the capability of DC to produce bioactive IL-12p70 (Fig. 2b).
Figure 2.
Lycopene-impaired interleukin-12 (IL-12) production by murine dendritic cells (DC). Murine DC were stimulated with lycopene (10 µm) for 24 hr in the absence or presence of lipopolysaccharide (LPS). The CD11c+ DC subset was subsequently detected by intracellular cytokine staining (a). DC (5 × 105 cells/ml) were cultured for 24 hr and bioactive IL-12 p70 production was analysed using enzyme-linked immunosorbent assay (ELISA) from culture supernatants (b). The data represent the means (± standard deviation) of three separate experiments.
Lycopene increases the endocytosis of dextran–FITC in LPS-treated DC
The expression of surface molecules and IL-12 production indicated that lycopene profoundly inhibited the phenotypic and functional maturation of in vitro-generated murine DC. However, these results did not exclude the possibility that lycopene caused a general inhibition of DC physiological functions. Therefore, we investigated the ability of lycopene-treated DC to endocytose dextran–FITC. After incubation of murine DC with lycopene in the presence or absence of LPS, dextran–FITC was added to the culture medium. The percentage of double-positive cells (CD11c ± PE × dextran–FITC) did not differ from the percentage of lycopene-treated DC to untreated DC. The percentage of LPS-stimulated DC was lower than that of untreated DC. Moreover, the lycopene-treated DC showed a higher endocytic capacity for dextran–FITC than LPS-stimulated DC (Fig. 3), again indicating that they were phenotypically and functionally immature DC. The same experiments were also performed at 4° to show that the uptake of dextran by DC is inhibited at low temperatures. At 4°, dextran was internalized by < 10% of DC.
Figure 3.
Dendritic cells (DC) stimulated with lycopene increase antigen uptake. DC (1 × 105 cells) were treated with 10 µm lycopene in the absence or presence of lipopolysaccharide (LPS) (100 ng/ml) for 24 hr. The endocytic activity of the DC was determined by flow cytometry after treatment with dextran–fluorescein isothiocyanate (FITC). Thereafter, the cells were washed twice with cold Hanks' balanced salt solution (HBSS) and stained using phycoerythrin (PE)-conjugated anti-CD11c. The control endocytic activity was determined after treatment with dextran–FITC at 4°. The numbers represent the percentages of cells. To confirm the results, these experiments were repeated three times.
Lycopene decreases the allostimulatory capacity of DC
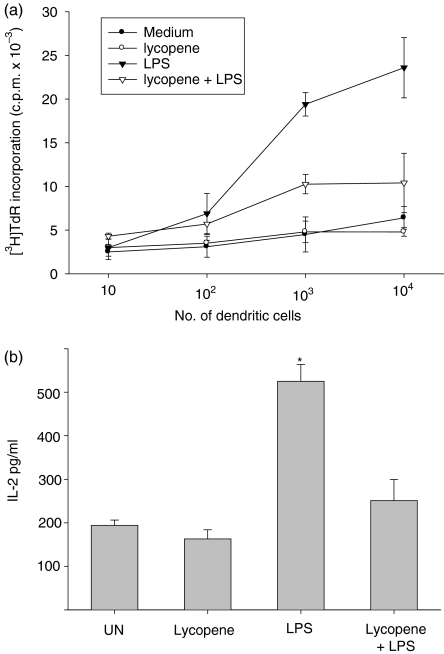
To test whether lycopene impairs the maturation of LPS-stimulated DC, DC from day 5 cultures that had been incubated with lycopene for 24 hr were tested for their capacity to stimulate allogeneic T cells. As shown in Fig. 4, LPS-treated DC stimulated proliferative responses more effectively than did untreated DC, while lycopene-treated DC impaired proliferative responses derived from the LPS stimulation. Also, untreated DC were approximately twofold more efficient in T-cell stimulation. Additionally, T-cell activation was assessed by IL-2 release. Lycopene-treated DC strongly inhibited IL-2 secretion from allogeneic T cells compared with that from LPS-stimulated DC (Fig. 4b). Importantly, maturation induced by LPS stimulation (24 hr, 100 ng/ml) strongly promoted the allostimulatory capacity of the untreated DC, whereas exposure to lycopene only marginally impaired the allostimulatory capacity. This observation indicates that the lycopene-treated DC were, at least partially, maturation resistant.
Figure 4.
Lycopene decreases the proliferation of allogeneic T cells and interleukin-2 (IL-2) production through the maturation of dendritic cells (DC). DC were cultured in medium, with or without 10 µm lycopene, for 45 min, followed by incubation with lipopolysaccharide (LPS) (100 ng/ml) for 24 hr. The treated DC were harvested and washed extensively to remove lycopene. A mixed lymphocyte/leucocyte reaction (MLR) was conducted for 5 days, as described in the Materials and methods. A background level of [3H]thymidine ([3H]TdR) uptake was determined by measuring reactions without stimulators (a). IL-2 levels in 48 hr-MLR supernatants (stimulator/responder ratio: 0·1) were analysed by enzyme-linked immunosorbent assay (ELISA) (b).Similar results were obtained in three separate experiments.
Lycopene directly suppresses the MAPKs and NF-κB pathways in LPS-induced DC maturation
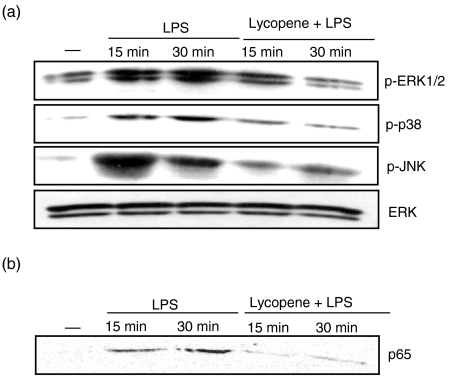
LPS stimulation has been shown to activate the MAPK and NF-κB signal pathways in DC.26,27 As known data, LPS activated p-ERK, p-p38 kinase and p-JNK (Fig. 5a). To evaluate the effect of p-ERK, p-p38 kinase and p-JNK inhibition on DC maturation, immature DC were treated with lycopene before stimulation with LPS. Pretreatment with 10 µm lycopene markedly inhibited the LPS-induced up-regulation of p-ERK, p-p38 and p-JNK. Even though p-MAPK was inhibited, the ERK proteins were constitutively expressed (Fig. 5a). In addition, LPS signal transduction has been shown to activate a variety of signal pathways, including the NF-κB pathway,28 which plays a critical role in the regulation of gene expression. These results indicate that the inhibition of MAPK is involved in the regulation of LPS-induced DC maturation. To study the role of NF-κB activation, immature DC were stimulated with LPS after the pretreatment of lycopene. To determine whether lycopene blocked LPS-induced activation of NF-κB, a nuclear extract was prepared from DC treated with LPS and lycopene, and nuclear translocation of the NF-κB p65 subunit was detected by Western blotting. LPS induced the nuclear translocation of the NF-κB p65 subunit within 30 min. Pretreatment with 10 µm lycopene suppressed the NF-κB p65 nuclear translocation induced by LPS stimulation (Fig. 5b). These results suggest that NF-κB inhibition plays an important role in the suppression of LPS-induced DC maturation.
Figure 5.
Lycopene decreases the activation of mitogen-activated protein kinases (MAPKs) in lipopolysaccharide (LPS)-stimulated dendritic cells (DC). DC were pretreated with 10 µm lycopene for 1 hr before stimulation with LPS (100 ng/ml). Cell lysates were prepared and blotted with anti-ERK1/2 (ERK), anti-phospho-ERK1/2 (p-ERK), anti-phospho-p38 (p-p38), and anti-phospho-JNK (pJNK) (a). The LPS-induced nuclear translocation of the nuclear factor-kappaB (NF-κB) p65 subunit was inhibited by lycopene (b). DC were pretreated with lycopene for 1 hr and then stimulated with 100 ng/ml LPS for the indicated time. Nuclear extracts were blotted with anti-p65 immunoglobulin. The bound antibody was visualized by incubation with biotinylated goat anti-rabbit immunoglobulin G (IgG). The result shown was representative of three independent experiments. The symbol (–) represents the chemically untreated control group.
Intraperitoneal administration of lycopene inhibits LPS-induced DC maturation
To analyse whether the apparent inhibitory effect of lycopene on splenic DC maturation in vivo was mediated simply by drug toxicity or by interfering with the production of DC, we analysed the effects of lycopene on the phenotypic characteristics in LPS-stimulated mice. We isolated spleen-derived DC from all groups and analysed the phenotypic characteristics using flow cytometry, and found that 80–90% of these DC expressed CD11c molecules (Fig. 6). Representative FACS histograms show that splenic DC plus LPS only express significantly detectable costimulatory and MHC molecules. However, after pretreatment with lycopene for 3 days, CD86 and MHC class II molecules were markedly down-regulated 24 hr after LPS challenge. These in vivo data show that lycopene pretreatment inhibits the phenotypic maturation of DC after LPS stimulation, whereas DC may reduce the interaction with T cells to stimulate an antigen-specific immune response.
Figure 6.
In vivo administration of lycopene suppresses the phenotypic maturation of splenic dendritic cells (DC) challenged with lipopolysaccharide (LPS). Mice were injected intraperitoneally with lycopene (50 nmol) every 3 days. One hour after the last injection, the mice were injected with 20 µg of LPS in a lateral vein of the tail. Twenty-four hours later, they were scarified and splenic DC were generated as described in the Materials and methods. The cells were harvested and analysed by two-colour flow cytometry. The cells were gated on CD11c+ for mean fluorescence intensity (MFI) (a) and positive populations (b). The histogram is from one representative experiment out of three performed. MHC, major histocompatibility complex.
Discussion
Epidemiological studies have revealed the chemopreventive effect of lycopene and lycopene-rich tomatoes against various epithelial cancers, including lung cancer.14,29–32 Lycopene reduces the incidence of lung adenocarcinomas in mice treated with chemical carcinogens.33 In animal studies, intraperitoneally or intravenously injected lycopene has been shown to prolong the survival time of bacterially infected mice.34 Also, it was reported that lycopene is associated with the reduction of cyclin D levels and the retention of p27Kip1 in cyclin E-cdk2 complexes in inhibiting cell cycle progression in breast and endometrial cancer cells.35 Although lycopene has an anticancer mechanism, no such mechanism has been known to exist in normal cells, especially DCs.
The most important function of DC is initiating the immune response by presenting antigens to naïve T lymphocytes.1,2,22,36,37 Moreover, DC are known to sustain some chronic inflammatory diseases, such as allergy, hypersensitivity and collagen diseases.38–41 These lines of evidence suggest that the antigen-presenting DC may be an appropriate target for the control of such chronic inflammatory diseases. To date, little is known about the basic action of lycopene in murine BM-DC.
In the present study, we have revealed that lycopene significantly attenuates the phenotypic and functional maturation of murine BM-DC, especially in LPS-induced DC maturation. We found that lycopene strongly down-regulates the expression of costimulatory molecules (CD80 and CD86). Costimulatory molecules in DC bind to CD28 molecules and provide a costimulatory signal to T lymphocytes.22 It has been reported that the expression of IL-12 is a more specific marker of functionally activated DC.24,25 Lycopene also has inhibitory effects on the ability of DC to produce the proinflammatory cytokine, IL-12. This finding may provide a clue to the anti-inflammatory mechanism of lycopene, because DC are also known to produce proinflammatory cytokines in response to some stimuli, and these cytokines might support the immune reaction at the inflammatory lesion.22,23 In the present study, DC produced large amounts of IL-12, but not IL-10, after LPS stimulation, while lycopene-treated DC produced markedly less IL-12 after LPS stimulation. Also, we isolated the antigen-presenting capacity of DC using an MLR assay because the measurement of T-cell proliferation in the absence or presence of DC is a good method of evaluating DC function.42 We also determined that lycopene-treated DC were poor stimulators of naïve allogeneic T-cell proliferation and induced lower levels of IL-2 in respondingT cells. On the basis of our in vitro observation, we also suggest that the antigen-presenting function of DC is directly suppressed by lycopene. Also, administration of lycopene did not induce a significant expression of surface molecules from splenic DC pulsed with LPS. These results indicate novel immunosuppressive properties of lycopene, which may be therapeutically useful in controlling chronic immune and/or inflammatory diseases through the down-regulation of DC maturation.
To gain insight into the mechanisms responsible for altered surface-molecule expression by DC exposed to lycopene, we examined MAPKs. MAPKs, which include the ERK, p38 and JNK subfamilies, are activated in response to stimuli such as treatment with DNA-damaging agents, growth factors and cytokines.43–46 Activation of JNK and p38 kinase is related to stress response, growth arrest and apoptosis,44–46 where ERK is important in mitogenesis and differentiation.47 However, reports exist that JNK activation occurs independently of cell death,48 and, moreover, that JNK activation actually promotes proliferation and cellular transformation.49,50 In addition to the physiological response and activation pattern, MAPK activation is dependent upon the type(s) of MAPKs and cell type. However, in the present study, we have provided evidence that LPS-induced DC maturation is related to MAPKs (ERK, p38 and JNK) and NF-κB, and lycopene suppresses MAPKs and NK-κB in LPS-stimulated DC.
This study provides the first insight into the mechanisms involved in the lycopene-induced suppression of murine BM-DC maturation. At a concentration of 10 µm, lycopene proved to be a potent inhibitor of DC maturation. The inhibitory effect of lycopene on DC maturation is the inhibition of MAPKs and is associated with the suppression of NF-κB p65 translocation. An additional novel aspect of our findings is that the T-cell stimulatory capacity of DC pretreated with lycopene is markedly diminished. Our experimental data also provides new insight into the immunopharmacology of lycopene and suggests a novel approach to the manipulation of DC for therapeutic application.
Acknowledgments
This work was supported by the Korea Science & Engineering Foundation (KOSEF) through the Medical Research Center for Cancer Molecular Therapy at Dong-A University and by Pusan National University in the program, Post-Doc. 2004.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 4.Austyn JM. Dendritic cells. Curr Opin Hematol. 1998;5:3–15. doi: 10.1097/00062752-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 5.De Smedt T, Pajak B, Muraille E, et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184:1413–24. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 7.Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by Mycoplasma infection but not necrotic cells. Eur J Immunol. 2000;30:705–8. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Kolb-Maurer A, Gentschev I, Fries HW, et al. Listeria monocytogenes-infected human dendritic cells: uptake and host cell response. Infect Immun. 2000;68:3680–8. doi: 10.1128/iai.68.6.3680-3688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marovich MA, Mcdowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164:5858–65. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 10.Gerster H. The potential role of lycopene for human health. J Am Coll Nur. 1997;16:109–26. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 11.Rao AV, Agarwal S. Bioavailability and in vivo antioxidant properties of lycopene from tomato products and their possible role in the prevention of cancer. Nutr Cancer. 1998;31:199–203. doi: 10.1080/01635589809514703. [DOI] [PubMed] [Google Scholar]
- 12.Stahl W, Junghans A, Deboer B, et al. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: synergistic effect of lycopene and lutein. FEBS Lett. 1998;427:305–8. doi: 10.1016/s0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Rao AV. Carotenoids and chronic diseases. Drug Metabol Drug Interact. 2000;17:189–210. doi: 10.1515/dmdi.2000.17.1-4.189. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Ascherio A, Rimm EB, et al. Intake of carotenoids and retinal in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 15.Dorgan JF, Sowell A, Swanson CA, et al. Relationship of serum carotenoids, retinal, α-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States) Cancer Causes Control. 1998;9:89–97. doi: 10.1023/a:1008857521992. [DOI] [PubMed] [Google Scholar]
- 16.Kristenson M, Zieden B, Kucinskiene Z, et al. Antioxidant state and mortality from coronary heart diseases in Lithuanian and Swedish men: concomitant cross sectional study of men ages 50. BMJ. 1997;314:629–33. doi: 10.1136/bmj.314.7081.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribaya-Mercado JD, Garmyn M, Gilchrest BA, Russell RM. Skin lycopene is destroyed preferentially over α-carotene during ultraviolet irradiation in humans. J Nutr. 1995;125:1854–9. doi: 10.1093/jn/125.7.1854. [DOI] [PubMed] [Google Scholar]
- 18.Matos HR, Di Mascio P, Medeiros MGH. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch Biochem Biophys. 2000;383:56–9. doi: 10.1006/abbi.2000.2035. [DOI] [PubMed] [Google Scholar]
- 19.Riso P, Pinder A, Santangelo A, Porrini M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. 1999;69:712–8. doi: 10.1093/ajcn/69.4.712. [DOI] [PubMed] [Google Scholar]
- 20.Yeh S, Hu M. Antioxidant and pro-oxidant effects of lycopene in comparison with α-carotene on oxidant-induced damage in Hs68 cells. J Nutr Biochem. 2000;11:548–54. doi: 10.1016/s0955-2863(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down-regulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart DN. Dendritic cells: unique leukocyte populations with control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 23.de Saint-Vis B, Fugier-Viver I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 24.Mosca PJ, Hobeika AC, Clay TM, et al. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–504. [PubMed] [Google Scholar]
- 25.Lapointe R, Toso JF, Butts C, et al. Human dendritic cells require multiple activation signals for the efficient generation of tumor antigen-specific T lymphocytes. Eur J Immunol. 2000;30:3291–8. doi: 10.1002/1521-4141(200011)30:11<3291::AID-IMMU3291>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Arrighi JF, Rebsamen M, Rousset F, et al. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 27.An H, Yu Y, Zhang M, et al. Involvement of ERK, p38 and NF-kB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joyce D, Albanese C, Steer J, et al. NF-kB and cell cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 29.Clinton SK, Emenhiser C, Schwartz SJ, et al. Cis-trans lycopene isomers, carotenoids, and retinal in the human prostate. Cancer Epidermal Biomark Prev. 1996;5:823–33. [PubMed] [Google Scholar]
- 30.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 31.Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30. [PubMed] [Google Scholar]
- 32.Kucuk O, Sarkar FH, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomark Prev. 2001;10:861–8. [PubMed] [Google Scholar]
- 33.Kim DJ, Takasuka N, Kim JM, et al. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MUN, and DMH. Cancer Lett. 1997;120:15–22. doi: 10.1016/s0304-3835(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 34.Lingen C, Ernster L, Lindberg O. The promoting effect of lycopene on the non-specific resistance of animals. Exp Cell Res. 1959;16:384–93. doi: 10.1016/0014-4827(59)90267-8. [DOI] [PubMed] [Google Scholar]
- 35.Nahum A, Hirsch K, Danilenko M, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27Kip1 in the cyclin E–cdk2 complexes. Oncogene. 2001;20:3428–36. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 36.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–40. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu LM, Macpherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naïve T cells in vivo. J Exp Med. 1993;177:1299–307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knight SC, Farrant J, Chan J, et al. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1998;48:277–89. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- 39.Guery JC, Adorini L. Dendritic cells are the most efficient in resenting endogenous naturally processed self-epitopes to class II-restricted T cells. J Immunol. 1995;154:536–44. [PubMed] [Google Scholar]
- 40.Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis. role of CD86 in the synovium. J Immunol. 1996;156:3074–86. [PubMed] [Google Scholar]
- 41.Poulter LW, Janossy G. The involvement of dendritic cells in chronic inflammatory disease. Scand J Immunol. 1985;21:401–11. doi: 10.1111/j.1365-3083.1985.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Voorhis WC, Valinsky J, Hoffman E, et al. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983;158:174–91. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:175–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 44.Whitmarsh AJ, Davis RJ. Transcription AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–77. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 46.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK) – from inflammation to development. Curr Opin Cell Biol. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 47.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 48.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–76. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X, Heidenreich O, Kitajima I, et al. Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene. 1996;13:135–42. [PubMed] [Google Scholar]
- 50.Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;10:2634–45. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]