Abstract
Oligodeoxynucleotides (ODN) with unmethylated CpG dinucleotides mimic the immune stimulatory activity of bacterial DNA in vertebrates and are recognized by Toll-like receptor 9 (TLR9). It is also possible to detect immune activation with certain phosphorothioate sequences that lack CpG motifs. These ODN are less potent than CpG ODN and the mechanism by which they stimulate mammalian leucocytes is not understood. We here provide several lines of evidence demonstrating that the effects induced by non-CpG ODN are mediated by TLR9. First, non-CpG ODN could not stimulate cytokine secretion from the splenocytes of TLR9-deficient (TLR9–/–) mice. Second, immunization of TLR9+/+ but not TLR9–/– mice with non-CpG ODN enhanced antigen-specific antibody responses, although these were T helper type 2 (Th2)-biased. Third, reactivity to non-CpG ODN could be reconstituted by transfection of human TLR9 into non-responsive cells. In addition, we define a new efficient immune stimulatory motif aside from the CpG dinucleotide that consists of a 5′-TC dinucleotide in a thymidine-rich background. Non-CpG ODN containing this motif induced activation of human B cells, but lacked stimulation of Th1-like cytokines and chemokines. Our study indicates that TLR9 can mediate either efficient Th1- or Th2-dominated effects depending on whether it is stimulated by CpG or certain non-CpG ODN.
Keywords: B cells, dendritic cells, CpG DNA, Toll-like receptors
Introduction
Small synthetic oligodeoxynucleotides (ODN) containing unmethylated deoxycytidine-deoxyguanosine (CpG) dinucleotides are able to mimic the immune stimulatory activity of bacterial DNA.1 Certain sequence motifs with central CpG dinucleotides are responsible for this stimulation. We have recently shown that the optimal CpG motif for activating human cells is 5′-GTCGTT-3′, whereas the optimal motif for activating mouse cells is 5′-GACGTT-3′.2,3 The strength of the immune response is determined by the unmethylated CpG motif itself, as well as the number and position of CpG dinucleotides in the ODN and the spacing between such motifs.2 Modifications at the 5-position of cytosine are usually not very well tolerated.1 Phosphorothioate modification of CpG ODN enhances their in vitro activity usually by about 10–100-fold.2–6
Studies using mice deficient in Toll-like receptor 9 (TLR9) demonstrated that this TLR subtype is essential for the effects mediated by bacterial or viral DNA and CpG ODN.7 The responsiveness to CpG ODN stimulation in vitro could be reconstituted by transfection of unresponsive cells with human TLR9.8,9 In addition, recent publications strongly suggest that CpG DNA can directly bind to TLR9.10,11 TLR9 is a member of a family of at least 12 different receptors recognizing certain molecular structures that are present in pathogens.12–14 Recently, TLRs were also shown to be responsible for the recognition of double-stranded RNA (TLR3), small antiviral compounds, guanosine nucleoside analogues, or viral single-stranded RNA (TLR7 and TLR8).15–19
So far only a few types of human immune cells have been demonstrated to express TLR9. B cells and plasmacytoid dendritic cells (pDC) express intracellular TLR9 and can be directly stimulated by CpG ODN.10,20 The pDC are the main producers of interferon-α (IFN-α), and these cells are unique in their ability to respond to CpG ODN by producing type I interferons.20–22 In contrast, mouse cells of the myeloid lineage, such as mDC, monocytes, or macrophages, all express TLR9.23 Other immune cells, such as human αβ- or γδ-T cells, or natural killer (NK) cells, appear to be negative for TLR9 and seem to be only indirectly stimulated by CpG ODN.12
Aside from the CpG-specific effects, there are also CpG-independent immune stimulatory effects of phosphorothioate ODN.24 Many of these non-sequence-specific effects may be the result of the increased affinity of phosphorothioate ODN for binding a wide variety of proteins.25 Phosphorothioate thymidine homopolymers, in contrast to other homopolymers, can induce the stimulation of human B cells or murine splenocytes.26,27 We previously demonstrated that the number of thymidine nucleobases in non-CpG phosphorothioate ODN, as well as the length of such ODN, directly relate to the magnitude of their stimulatory effects, especially on human B cells.28 Nevertheless, the mechanism of immune activation by non-CpG ODN and the existence of specific stimulatory motifs besides the CpG motifs still remain to be elucidated. TLR9 was reported to be responsive to CpG but not to non-CpG ODN stimulation.8,29,30 Therefore, it was possible that non-CpG ODN acted via another pathway, which might also explain the relatively small effects observed with non-CpG ODN when compared to CpG ODN.
Here, we extended our previous studies and investigated the dependency of non-CpG ODN-mediated immune stimulation on the expression of TLR9 in vivo as well as in vitro. Surprisingly, TLR9 is required for mediating immune modulatory effects, not only by CpG ODN, but also by non-CpG ODN. In addition, efficient TLR9-mediated activation by such ODN depended strongly on a stable phosphorothioate backbone. Non-CpG ODN in vitro induced efficient B-cell stimulation but lacked the stimulation of T helper type 1 (Th1) -like chemokines or cytokines from human peripheral blood mononuclear cells (PBMC) or pDC. In addition, we describe a non-CpG motif, a TC dinucleotide placed at the 5′ end of thymidine-containing ODN, that tremendously enhances the stimulatory capacity of non-CpG ODN without mediating Th1-like effects. Our data suggest that the TLR9 receptor can induce different profiles of immune activation in response to different ligands, and provide a mechanistic explanation for previous observations that a Th2-biased immune stimulatory effect is associated with certain non-CpG ODN.31–33
Materials and methods
Oligodeoxynucleotides
All ODN were purchased from Biospring (Frankfurt, Germany), controlled for identity (MALDI-TOF MS) and purity (capillary gel electrophoresis) by Coley Pharmaceutical Group (Langenfeld, Germany) and had undetectable endotoxin levels (< 0·1 EU/ml), as measured by the Limulus assay (BioWhittaker, Verviers, Belgium). ODN were suspended in sterile, endotoxin-free Tris–ethylenediaminetetraacetic acid (Sigma, Deisenhofen, Germany), and stored and handled under aseptic conditions to prevent both microbial and endotoxin contamination. All dilutions were carried out using pyrogen-free phosphate-buffered saline (Life Technologies, Eggenstein, Germany).
TLR assays
Stably transfected HEK293 cells expressing human TLR9 have been described previously.8,17 Briefly, HEK293 cells were transfected by electroporation with vectors expressing human TLR9 and a 6xNFκB-luciferase reporter plasmid. Stable transfectants (3 × 104 cells/well) were incubated with ODN for 16 hr at 37° in a humidified incubator. Each data point was performed in triplicate. Cells were lysed and assayed for luciferase gene activity (using the BriteLite kit from Perkin-Elmer, Zaventem, Belgium). Stimulation indices were calculated in reference to the reporter gene activity of medium without addition of stimulus.
Cell purification
Peripheral blood buffy coat preparations from healthy human donors were obtained from the Blood Bank of the University of Düsseldorf (Germany) and PBMC were purified by centrifugation over Ficoll–Hypaque (Sigma). Cells were cultured in a humidified incubator at 37° in RPMI-1640 medium supplemented with 5% (v/v) heat-inactivated human AB serum (BioWhittaker) or 10% (v/v) heat inactivated fetal calf serum, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Sigma).
Cytokine detection and flow cytometric analysis
PBMC were resuspended at a concentration of 3 × 106 cells/ml and added to 48-well flat-bottomed plates (1 ml/well) or 96-well round-bottomed plates (250 μl/well). PBMC were incubated with various ODN concentrations and culture supernatants were collected after the indicated time points. If not used immediately, supernatants were stored at −20° until required.
Amounts of cytokines in the supernatants were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) Kits [interleukin-6 (IL-6), IFN-inducible protein 10 (IP-10), IFN-γ or IL-10; from Diaclone, Besançon, France] or an in-house ELISA for IFN-α developed using commercially available antibodies (from PBL, New Brunswick, NJ).
All monoclonal antibodies (mAb) for cell surface staining were purchased from Becton-Dickinson (Heidelberg, Germany), except mAb to CD11c which were obtained from DAKO (Hamburg, Germany). Flow cytometric data for CD86-expression on CD11c–, HLA-DRhigh and CD123+ pDC were acquired on a FACSCalibur and were analysed using the computer program cellquest (both from Becton-Dickinson).
For B-cell proliferation assays, CD19+ B cells were identified after culturing 5-[and6-]carboxyfluorescein diacitate succinmidyl ester (CFSE)-labelled PBMC with CpG ODN for 5 days by flow cytometry as described before.9 Decreased CFSE content indicated proliferating B cells.
Isolation of human B cells or pDC
Human B cells were isolated from whole PBMC with the CD19 B-cell isolation kit as described by the manufacturer (Miltenyi, Bergisch-Gladbach, Germany). To determine purity, cells were stained with mAb to CD20 and CD14 and identified by flow cytometry. In all the experiments B cells were more than 95% pure. Purified B cells (2 × 105−5 × 105 cells/ml) were incubated with increasing concentrations of ODN for 24 hr and IL-6 was measured as described above. Purity was also confirmed using lipopolysaccharide as a stimulus that should not induce IL-6 secretion from human B cells.
The pDC were enriched with the BDCA-4 pDC isolation kit following the manufacturer's (Miltenyi) instructions. This enrichment was confirmed by staining with mAb to CD123, CD11c and HLA-DR and cells (5 × 105 cells/ml) were cultured in the presence of IL-3 (10 ng/ml; R & D Systems GmbH, Wiesbaden, Germany) for 48 hr with 0·5 μm ODN. IFN-α in the supernatant was measured as described above.
Animals
Female BALB/c mice (6–8 weeks of age) were used for all experiments. They were purchased from Charles River Canada (Quebec, Canada) and housed in micro-isolators at the animal care facility of the Ottawa Hospital Research Institute. TLR9-deficient mice have been described before7 and were obtained from Dr Shizuo Akira (Osaka, Japan). The animals were back-crossed to BALB/c for several generations.
In vitro murine assays
BALB/c or TLR9–/– mouse splenocytes were used for all in vitro assays. Spleens were removed from anaesthetized animals under aseptic conditions and were homogenized. Splenocytes were re-suspended in RPMI-1640 medium (Life Technologies) supplemented with 2% normal mouse serum (Cedarlane Laboratories, Ontario, Canada), 2 mm l-glutamine, penicillin–streptomycin solution (final concentration of 1000 U/ml and 1 mg/ml, respectively), and 5 × 10−5 mβ-mercaptoethanol (all from Sigma). Splenocytes were plated in 96-well, round-bottomed plates (5 × 106 cells/ml). Each splenocyte sample was plated in quadruplicate and the cells were incubated in a humidified 5% CO2 incubator at 37° for 48 hr. Supernatants were harvested and a commercially available assay kit for IL-6 (mouse OptEIA kit; PharMingen, Mississauga, ON, Canada) was used, according to the manufacturer's instructions, to assay cytokine levels.
Immunization of mice
For systemic immunization naïve BALB/c or TLR9–/– mice (n=5/group) were immunized at 0 and 4 weeks by intramuscular injection with 1 μg hepatitis B surface antigen (HBsAg) subtype ad (International Enzymes, CA, USA) alone or in combination with 50 μg CpG or non-CpG ODN in saline, 25 μg Al3+ (alum; Alhydrogel ‘85′-Superfos Biosector, Vedbaek, Denmark) or complete Freund's adjuvant (CFA; Sigma, 1 : 1 v/v). Animals were bled at 4 and 8 weeks, and plasma was recovered and stored at −20° until assay.
For mucosal vaccine delivery, mice (n=5 to n=10/group) were immunized on days 0, 1 and 2 with 10 μg formalin-inactivated tetanus toxoid (TT, Aventis Pasteur, Swiftwater, PA), alone or combined with 10 μg ODN in saline. Mucosal formulations were administered using a 1-cm3 tuberculin syringe (Becton-Dickinson, Franklin Lakes, NJ) attached to a 20-gauge olive tip steel feeding tube (Fine Science Tools Inc., North Vancouver, BC, Canada) which was passed into the stomach. The titre of TT-specific antibodies in plasma was taken at 8 weeks, which was 4 weeks post-boost.
Antibodies against HBsAg (anti-HBs) or anti-TT antibodies [total immunoglobulin G (IgG), IgG1 or IgG2a] were detected and quantified by end-point dilution ELISA assay as described previously31 which was performed in duplicate on samples from individual animals.
Statistical analysis
Statistical analysis was performed using the instat program (Graph PAD Software, San Diego, CA). The statistical difference between groups was determined by Student's t-test (for two groups) or by one-factor analysis of variance (anova) followed by Tukey's test (for three or more groups) on raw data or transformed data (log10, for heteroscedastic populations).
Results
Induction of B-cell proliferation and B-cell IL-6 or IL-10 secretion by non-CpG ODN
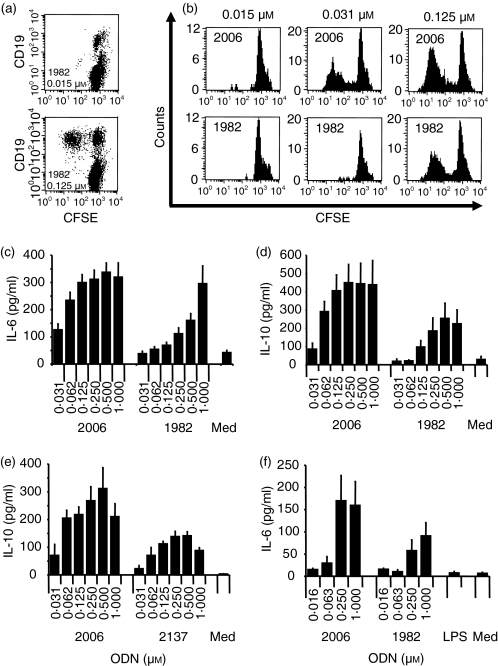
B-cell proliferation was measured by decreasing CFSE staining of freshly isolated PBMC. Cells staining low with CFSE were found to be mainly CD19-positive B cells for CpG or non-CpG ODN (for non-CpG ODN 1982 see Fig. 1a). The commonly used control non-CpG ODN, 1982 (for sequences see Table 1), induced human B-cell proliferation, although higher concentrations were needed to yield activation comparable to CpG ODN 2006 (Fig. 1b). In addition, proliferating B cells stimulated with ODN 1982 showed a larger cell size and higher granularity as described previously for CpG ODN (data not shown).34 Furthermore, ODN 1982 induced B cells to secrete IL-6 and IL-10 (Figs 1c,d), as has been reported with CpG ODN.1 However, higher concentrations were required and levels of cytokine production were usually higher with CpG ODN 2006. Other non-CpG ODN such as the GC control (ODN 2137) of CpG ODN 2006 behaved similarly, as shown for the secretion of IL-10 from human PBMC in Fig. 1(e). Similar results for IL-6 secretion were obtained with non-CpG ODN 1982 compared to CpG ODN 2006 when stimulation was carried out on purified human B cells (Fig. 1f).
Figure 1.
Non-CpG ODN 1982 stimulates B-cell proliferation and induces IL-6 and IL-10 secretion. Human PBMC (a–e) were cultured with ODN 2006 (CpG B-Class), 1982 (non-CpG) or 2137 (GC of 2006) for 5 days (a, b), 24 hr (c) or 48 hr (d, e). (a, b) CFSE-stained human PBMC were harvested and B-cell proliferation was measured by decreased CFSE brightness of CD19+ B cells by flow cytometry. The result shown is for two different concentrations of ODN 1982 for one representative donor (a). In (b) are the histograms for CD19+ proliferating B cells upon incubation with different concentrations of ODN 2006 or 1982 for one representative out of three different donors. (c–e) Supernatants were harvested and IL-6 or IL-10 secretion was measured by ELISA. The mean ± SEM of six (c), eight (d) or three (e) donors are shown. (f) Purified human B cells were cultured for 24 hr with the indicated ODN concentrations. IL-6 was measured in the supernatant. The mean ± SEM of five donors is shown.
Table 1.
ODN sequences used in this study
| ODN | Sequence 5′→3′ |
|---|---|
| 1826 | T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*C*G*T*T |
| 1982 | T*C*C*A*G*G*A*C*T*T*C*T*C*T*C*A*G*G*T*T |
| 2006 | T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T*T*T*G*T*C*G*T*T |
| 2137 | T*G*C*T*G*C*T*T*T*T*G*T*G*C*T*T*T*T*G*T*G*C*T*T |
| 2138 | T*C*C*A*T*G*A*G*C*T*T*C*C*T*G*A*G*C*T*T |
| 2178 | C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*C*CC*C*C |
| 2183 | T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 2351 | T-C-C-A-T-G-A-G-C-T-T-C-C-T-G-A-G-C-T-T |
| 2352 | T*C*C-A-T-G-A-G-C-T-T-C-C-T-G*A*G*C*T*T |
| 5314 | T-C-C-A-G-G-A-C-T-T-C-T-C-T-C-A-G-G-T-T |
| 5529 | T*C*G*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 6056 | T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 20201 | T*C*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 21113 | A*A*A*A*A*A*A*A*A*A*A*A*A*A*A*A*A*A*A*A |
| 21114 | C*G*C*G*C*G*C*G*C*G*C*G*C*G*C*G*C*G*C*G*C*G*C*G |
| 21115 | G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G*G |
| 21570 | T*C*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 21571 | T*A*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 21572 | T*G*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 21573 | T*Z*T*T*T*T*T*T*T*T*T*T*T*T*T*T*T |
| 21698 | A*C*C*A*G*G*A*C*A*A*C*A*C*A*C*A*G*G*A*A |
| 21699 | T**C*C*A*G*G*A*C*A*A*C*A*C*A*C*A*G*G*A*A |
phosphorothioate linkage; -: phosphodiester linkage; Z: 5-methyl-cytosine.
Non-CpG ODN do not stimulate the production of type I and type II interferons or CXCL-10
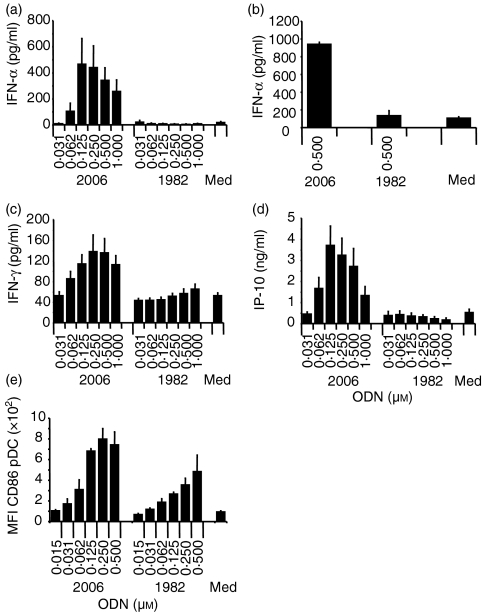
Given the ability of phosphorothioate non-CpG ODN such as 1982 to activate human B cells, albeit with weaker effects and at higher concentrations than required for CpG ODN, we hypothesized that non-CpG ODN might have the same full range of immune effects as induced by CpG ODN. One of the most striking attributes of CpG ODN is their ability to induce strong Th1 immune responses, mediated in part by the induction of IFN-α production from pDC. However, the results of the present study indicate that CpG ODN 2006, but not ODN 1982, can stimulate significant production of IFN-α from human PBMC (Fig. 2a), even at concentrations of up to 16 μm or at various culture periods (6 hr, 16 hr, 24 hr, or 48 hr) (data not shown). Similar results were obtained using pDC-enriched cell cultures (Fig. 2b). The Th1 cytokine IFN-γ, which was efficiently induced by CpG ODN 2006, appeared not to be considerably enhanced by non-CpG ODN 1982 (Fig. 2c). Furthermore, the Th1-related chemokine CXCL-10 (IP-10) was not produced above background in the presence of ODN 1982 (Fig. 2d; and up to 2 μm, not shown). Similar results were obtained with ODN 2137 (data not shown). To investigate further whether pDC respond at all to ODN lacking CpG dinucleotides, human PBMC were cultured with CpG ODN 2006 or ODN 1982 and up-regulation of CD86 was measured on CD123+ CD11c– pDC by flow cytometry (Fig. 2e). Although not as efficient as CpG ODN 2006, ODN 1982 did induce enhanced expression of CD86.
Figure 2.
Non-CpG ODN do not stimulate Th1-like effects in human PBMC. Human PBMC (a, c, d) were incubated with the indicated concentrations of ODN 2006 or 1982 for 48 hr. Supernatants were harvested and secretion of IFN-α, IFN-γ, or IP-10 was measured by ELISA. Mean ± SEM of six (a, c) or nine (d) donors is shown. (b) PDC-enriched cell cultures were incubated with 0·5 μm ODN 2006 or 1982 for 48 hr. Cells were harvested and IFN-α in the supernatant was measured by ELISA. The mean ± SEM of two donors is shown. (e) Up-regulation of CD86 on CD11c–, HLA-DRhigh and CD123+ pDC was measured upon culture of human PBMC with ODN 1982 or 2006 for 48 hr by flow cytometry and the mean ± SEM of the mean fluorescence intensity of three donors is shown.
Requirement for TLR9 to mediate immune stimulation by non-CpG ODN
It is possible that the immune stimulatory effects of non-CpG ODN could be mediated through TLR9, which is known to be the receptor for CpG ODN, or through a different immune pathway. To determine the TLR9-dependency of immune stimulation induced by non-CpG ODN, we first examined the ability of these ODN to activate IL-6 production in vitro from the spleen cells of wild-type and TLR9–/– mice. Non-CpG ODN 2137 induced low levels of IL-6 secretion from wild-type cells (Table 2), that were approximately one-fifth to one-tenth of those induced by CpG ODN 2006. However, neither CpG ODN 2006 nor non-CpG ODN 2137 induced detectable IL-6 secretion in spleen cells from TLR9–/– mice (Table 2).
Table 2.
IL-6 secretion induced by CpG and non-CpG ODN in murine TLR9–/– splenocytes
| IL-6 secretion (pg/ml) | |||
|---|---|---|---|
| ODN | Conc. (μg/ml) | TLR9+ / + | TLR9–/– |
| None | 98·3 ± 4·9 | 54·4 ± 36·4 | |
| 2006 | 1 | 923·8 ± 20·2 | 79·5 ± 73·9 |
| 3 | 1977·6 ± 191·5 | 80·8 ± 50·4 | |
| 10 | 3449·2 ± 225·7 | 113·1 ± 30 | |
| 2137 | 1 | 190 ± 5·6 | 80 ± 54·1 |
| 3 | 258·3 ± 17·6 | 97·1 ± 39·9 | |
| 10 | 335 ± 21·7 | 95·4 ± 25·5 | |
TLR9+/+ or TLR9–/– BALB/c splenocytes were cultured with the indicated ODN for 48 hr. Supernatants were harvested and IL-6 was measured by ELISA. The mean ± SD of two independent ELISA of one out of two experiments is shown.
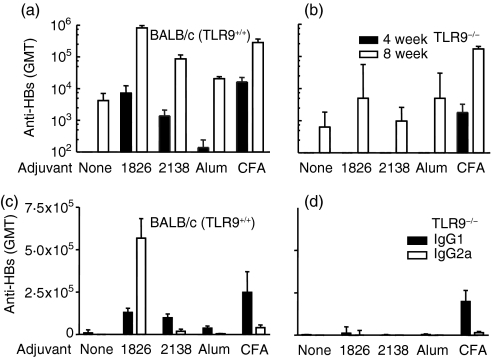
The optimized human CpG ODN 2006 cross-reacts with the murine TLR9, although maximal murine TLR9 stimulation is only achieved with the optimized mouse CpG ODN 1826.8,9 Therefore, we further evaluated in vivo CpG ODN 1826 and its GC control ODN 2138 (for sequences see Table 1) as vaccine adjuvants to HBsAg in TLR9+/+ or TLR9–/– mice, and compared them to alum and CFA (Fig. 3). Mice immunized with HBsAg plus ODN 1826 had higher antibody titres compared to animals immunized with antigen alone (Fig. 3a). The anti-HBs titres were also higher in mice immunized with HBsAg plus non-CpG ODN 2138 compared to animals immunized with antigen alone at 4 weeks post-boost (P=0·0056) (Fig. 3a). However, there was no difference in anti-HBs titres in TLR9–/– mice immunized with HBsAg alone and antigen plus CpG ODN 1826 or non-CpG ODN 2138 (Fig. 3b). Antibody titres in wild-type mice with non-CpG ODN 2138 were higher than those with alum (P=0·01) (Fig. 3a). CFA, which presumably can also activate immunity through other TLRs, had an equal adjuvant effect in TLR9–/– and TLR9+/+ mice (P=0·03 for both BALB/c and TLR9–/– mice) (Fig. 3a, b).
Figure 3.
Non-CpG ODN have Th2 adjuvant activity that is absent in TLR9–/– mice. Normal or TLR9-deficient BALB/c mice (n=5) were immunized at 0 and 4 weeks with 1 μg HBsAg alone (no adjuvant=none), with 50 μg of CpG ODN (1826), control ODN (2138), Al3+ (25 μg; alum) or CFA (1 : 1 v/v) by intramuscular injection. IgG levels were measured at 4 weeks post-prime or 4 weeks post-boost (8 weeks). Total IgG is shown in (a) and (b) (black bars at 4 weeks and open bars 4 weeks post-boost), IgG1 or IgG2a titres post-boost are given in (c) and (d) for normal and TLR9–/– animals, respectively.
In mice, IgG isotype distribution can be used as an indication of the Th bias of the immune response, with high IgG2a/IgG1 ratios indicating a Th1-biased immune response. According to the results of this study, CpG ODN 1826 induced a Th1-biased antibody response with three- and four-fold increases in IgG2a over IgG1 titres at 4 weeks (IgG2a/IgG1 ratio, 2·6) and 8 weeks (IgG2a/IgG1 ratio, 4·4) post primary immunization, respectively (Fig. 3c). Animals immunized with HBsAg using alum, CFA, or non-CpG ODN as adjuvant showed more Th2-biased antibody responses with higher IgG1 over IgG2a titres (IgG2a/IgG1 ratios with non-CpG ODN 2138, 0·1 and 0·2 at 4 and 8 weeks post-immunization, respectively) (Fig. 3c). CFA induced a Th2-biased immune response even in TLR9–/– mice (Fig. 3d). There was no adjuvant effect of CpG or non-CpG ODN in TLR9–/– mice and they had similar titres and ratios of IgG subtypes with CpG ODN, non-CpG ODN and HBsAg alone (Fig. 3d).
TLR9 is sufficient to restore NFκB activation by ODN lacking CpG dinucleotides
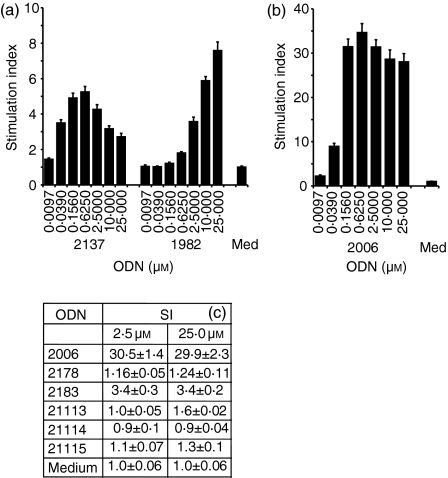
Even though the results in the TLR9-deficient mice strongly suggested that TLR9 was required for non-CpG ODN-mediated in vitro cytokine secretion or in vivo adjuvant effects, it remained possible that TLR9 alone may not be sufficient to mediate non-CpG ODN stimulation. To distinguish between these possibilities, we tested the ability of non-CpG ODN 1982 and 2137 to induce transcription from a human TLR9-transfected HEK293 NFκB reporter cell line.9 Non-CpG ODN 2138 (GC control of CpG ODN 1826) induced similar TLR9-dependent NFκB activation in experiments not shown. Incubation with increasing ODN concentrations (Fig. 4a) resulted in considerable NFκB signalling, although the non-CpG ODN were strongly inferior to CpG ODN 2006 (Fig. 4b).
Figure 4.
Phosphorothioate ODN lacking CpG dinucleotides stimulate human TLR9-mediated NFκB signalling. HEK293 cells stably transfected with vectors expressing human TLR9 and an NFκB-luciferase construct were incubated for 16 hr with increasing concentrations of ODN. NFκB stimulation was measured through luciferase activity. (a) shows a dose–response comparing ODN 2137 (GC inversion of ODN 2006) and 1982 (non-CpG). (b) shows the TLR9-mediated NFκB stimulation induced by CpG ODN 2006. (c) compares the NFκB activation induced by ODN 2006 (CpG ODN), 2178 [poly (C)], 2183 [poly (T)], 21113 [poly (A)], 21114 [poly (CG)] and 21115 [poly (G)]. One representative experiment out of two similar experiments is shown.
As with other immune stimulatory effects of non-CpG ODN, the thymidine content was critical for TLR9-dependent signalling. Phosphorothioate poly (C) (ODN 2178), poly (A) (ODN 21113), poly (CG) (ODN 21114) or poly (G) (ODN 21115) ODN did not induce considerable TLR9-mediated NFκB signalling (Fig. 4c). In contrast, a poly (T) ODN appeared to stimulate TLR9-dependent signalling (Fig. 4c).
Influence of the non-CpG phosphorothioate backbone on TLR9-mediated immune stimulation
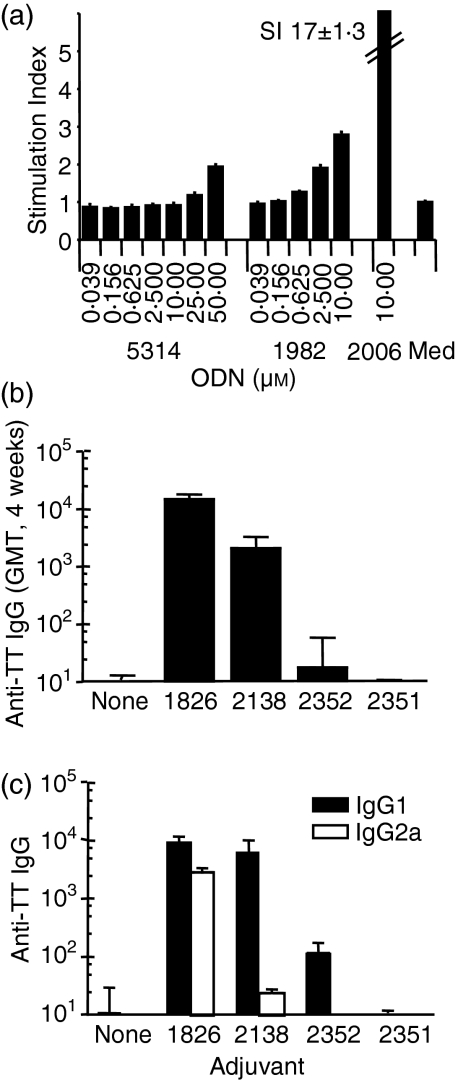
To determine the effect of the phosphorothioate backbone on the TLR9-dependent NFκB activation by ODN such as ODN 1982 or ODN 2138, we incubated human TLR9 transfectants with increasing concentrations of phosphorothioate ODN 1982 and the same sequence except containing only phosphodiester linkages (ODN 5314) (Fig. 5a). ODN 5314 gave little NFκB stimulation, and only at concentrations greater than 25 μm, whereas ODN 1982 showed stimulation at lower concentrations.
Figure 5.
TLR9-mediated immune stimulation by non-CpG ODN in vitro or in vivo is dependent on the phosphorothioate backbone. (a) TLR9 transfectants were incubated as described in Figure 4 with non-CpG phosphorothioate ODN 1982, phosphodiester ODN 5314 or CpG ODN 2006 (10 μm). NFκB stimulation was measured through luciferase activity. (b) BALB/c mice (n=5–10) were immunized by oral delivery of 10 μg TT alone (no adjuvant=none), or combined with 10 μg CpG ODN 1826, non-CpG control ODN 2138, the same sequence as chimeric phosphodiester/phosphorothioate ODN (ODN 2352) or phosphodiester ODN (ODN 2351). TT-specific IgG titres in plasma were measured 4 weeks after the final immunization. (c) shows IgG1 and IgG2a titres.
To determine the role of the phosphorothioate backbone on the adjuvant effect by non-CpG ODN 2138, we evaluated responses to oral immunization with TT using the same sequence with a chimeric phosphorothioate/phosphodiester backbone (ODN 2352; for sequences see Table 1) or with a phosphodiester backbone (ODN 2351). TT alone did not induce measurable plasma anti-TT antibody titres, and only with the addition of CpG ODN 1826 or non-CpG ODN 2138 were significant levels of IgG detected (P < 0·001 and P < 0·05 for CpG ODN 1826 and non-CpG ODN 2138, respectively), although those with ODN 2138 were inferior to those with the same dose of CpG ODN 1826 (P < 0·01) (Fig. 5b). Neither the chimeric nor the phosphodiester versions of ODN 2138 had a considerable adjuvant effect at the dose used. Ratios of IgG subtypes with CpG ODN, non-CpG ODN, or TT alone indicate that only the CpG ODN induced a strong IgG2a response. The non-CpG ODN induced a Th2-biased response (Fig. 5c).
Sequence-dependence of immune stimulation mediated by non-CpG ODN
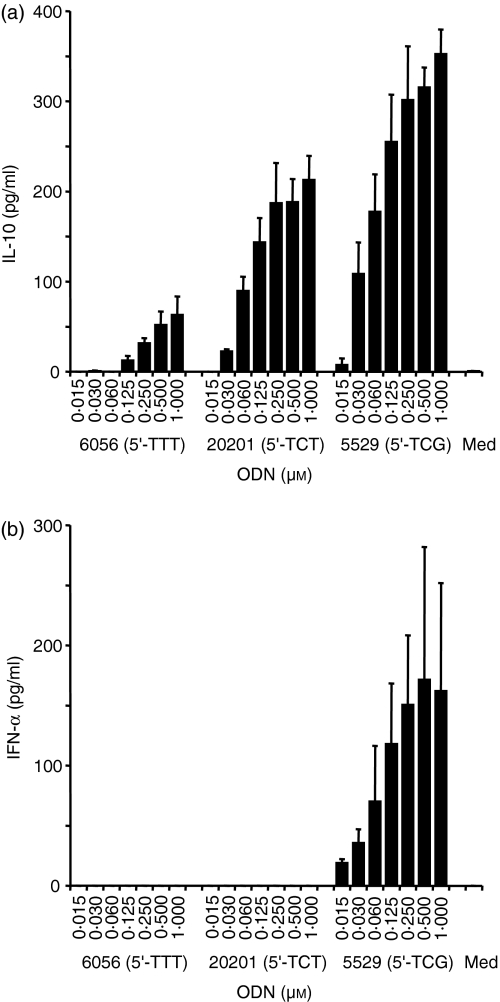
Recently, we demonstrated that the thymidine content as well as the length of an ODN lacking CpG dinucleotides strongly determines its immune stimulatory potential.28 A recent publication suggested that the presence of a sequence such as 5′-CATTTTGT-3′ in a non-CpG ODN would be sufficient to stimulate human B-cell activation.35 Indeed, non-CpG ODN 2137 is thymidine-rich and contains at least in part the described potential non-CpG motif.35 Nevertheless, other non-CpG ODN, such as ODN 1982 or 2138 used in this study, have a lower thymidine content (35% compared to 58% for ODN 2137) and do not have long thymidine stretches as well as having no similarities to the above proposed non-CpG motif. ODN 2138 and 1982 have sequence similarities and both contain a 5′-TC. Therefore, we asked the question if the 5′-TC would be sufficient to induce enhanced TLR9-dependent stimulation. We compared the effects of a phosphorothioate 17-mer poly (T) ODN (ODN 6056 with 5′-TTT) with the same 17-mer sequence containing a 5′-TCT (ODN 20201) or a 5′-TCG (ODN 5529) (for sequences see Table 1). Surprisingly, the secretion of IL-10 from human PBMC was increased at least 300% by modifying only one nucleotide from a thymidine to a cytosine in ODN 20201 (Fig. 6a). A further increase was achieved by introducing a 5′-TCG (ODN 5529). Similar results were obtained for IL-6 production (data not shown). ODN 20201 also increased TLR9-mediated NFκB signalling compared to poly (T) ODN 6056 after incubation of TLR9-transfected HEK293 cells [stimulation index (SI) at 25 μm ODN 6056: 1·4 ± 0·2; ODN 20201: 2·9 ± 0·2]. Nevertheless, only ODN 5529 with a 5′-TCG was able to induce IFN-α production from human PBMC (Fig. 6b). Although stimulating considerable IL-10 secretion, both non-CpG ODN, 6056 and 20201, failed completely to produce IFN-α above background levels (Fig. 6b). This is in agreement with our results obtained with ODN 1982.
Figure 6.
5′-TC stimulates efficient IL-10 production from human PBMC but lacks induction of IFN-α secretion. Human PBMC were stimulated with increasing concentrations of poly (T) ODN 6056 (5′-TTT), 5′-TCT ODN 20201 or 5′-TCG ODN 5529 for 48 hr. Supernatants were harvested and IL-10 (a) or IFN-α (b) secretion was measured by ELISA. The mean ± SEM of three donors are shown.
5′-TC and thymidine content determine the immune stimulatory activity of non-CpG ODN
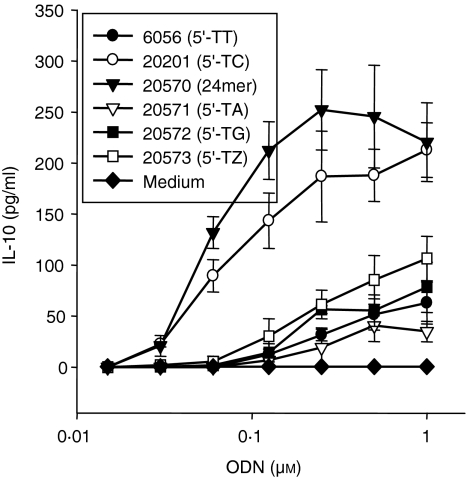
We wanted to investigate further the role of the 5′-TC for human PBMC stimulation. It was possible that nucleotides other than cytosine would have a similar effect. Therefore, we stimulated human PBMC with phosphorothioate ODN with a 5′-TT (ODN 6056), 5′-TC (ODN 20201), 5′-TA (ODN 21571) or 5′-TG (ODN 21572) (Fig. 7). All ODN induced at least some IL-10 secretion from human PBMC. Nevertheless, ODN 20201 with a 5′-TC was clearly superior in its activity to the other non-CpG ODN. In addition, methylation of the cytosine as in ODN 21573 (5′-TZ, Z: 5-methylated cytosine) reduced IL-10 to the same levels as those obtained with the poly (T) ODN 6056. Elongation of ODN 20201 (to 24 nucleotides) further increased IL-10 production, as observed previously for CpG ODN.28,36 None of these described ODN stimulated the production of IFN-α from human PBMC (data not shown).
Figure 7.
Only 5′-TC is capable of enhancing IL-10 production. Human PBMC were cultured with 17-mer ODN containing either a 5′-TT (ODN 6056), 5′-TC (ODN 20201), 5′-TA (ODN 21571), 5′-TG (ODN 21572), or 5′-TZ (Z: 5-methylated C; ODN 21573) or a 24-mer 5′-TC ODN (ODN 21570) for 48 hr. Supernatants were harvested and IL-10 was measured by ELISA. The mean ± SEM of three donors is shown.
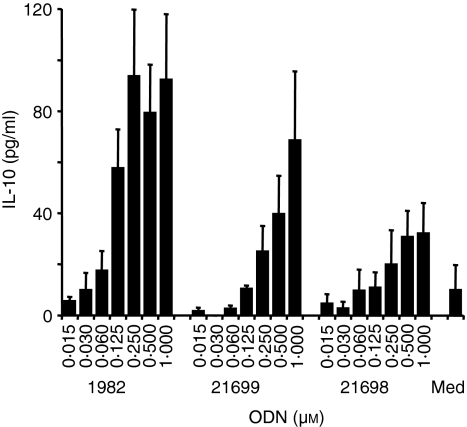
To determine if the thymidine content and the 5′-TC indeed contribute to the stimulatory activity of ODN 1982 we generated ODN with the same sequence that lacked all except the 5′-T (ODN 21699) or all thymidines (ODN 21698) after exchange of thymidine to adenosine. Indeed, reducing the thymidine content, as in ODN 21699, considerably decreased IL-10 production from human PBMC and modification of the 5′-TC appeared to decrease IL-10 secretion further (Fig. 8).
Figure 8.
Thymidine content and 5′-TC are responsible for the immune stimulation mediated by non-CpG ODN 1982. Human PBMC were cultured with non-CpG ODN 1982, 21699 (lacking all thymidines except the 5′-T) or 21698 (lacking all thymidines) for 48 hr. Supernatants were harvested and IL-10 was measured by ELISA. The mean ± SEM of three donors is shown.
Discussion
In this study, we have extended our previous observations on the immune modulatory activity of phosphorothioate ODN that lack unmethylated CpG dinucleotides, and we have revealed a surprising and hitherto unsuspected diversity in the immune effects mediated by TLR9 stimulation. The mechanism of immune modulation induced by such ODN was heretofore not understood. Previous work suggested that CpG ODN, but not non-CpG ODN, could mediate TLR9-mediated signalling.8,29,30 Phosphorothioate ODN can bind to a variety of different cellular proteins25 and therefore it was possible that proteins or receptors other than TLR9 might be involved in the recognition of such ODN.
Non-CpG ODN as a vaccine adjuvant with either HBs or TT as an antigen in mice induced enhanced Th2-biased antigen-specific antibody responses compared to the strong Th1-dominated responses observed with CpG ODN. Both these responses appeared to be TLR9-dependent because they were abrogated in TLR9–/– mice using HBsAg. In contrast, CFA which can presumably also activate through other TLRs had equal adjuvant effects in both TLR9+/+ and TLR9–/– mice. In addition, splenocytes of TLR9–/– mice did not respond by IL-6 production to in vitro culture with non-CpG ODN in contrast to wild-type cells. TLR9 was further demonstrated to be sufficient to mediate signalling by non-CpG ODN, although the magnitude of TLR9-mediated stimulation was always inferior to that with CpG ODN. Interestingly, the thymidine content of phosphorothioate non-CpG ODN determined the extent of NFκB stimulation observed with TLR9-transfected cells as reported for human immune cells.26,28,37 Only a phosphorothioate thymidine homopolymer was able to induce TLR9-dependent stimulation. Collectively, these in vitro and in vivo results show that TLR9 is required and sufficient for the NFκB-mediated stimulation induced by ODN lacking CpG dinucleotides. Nevertheless, with these data we cannot completely rule out the involvement of other receptors or adaptor proteins in generating additional co-stimulatory or co-mitogenic signals beside those mediated by NFκB required for immune modulation.
Without a phosphorothioate backbone, the presence of CpG dinucleotides becomes more critical for immune stimulation.28,34 Only a few reports describe immune stimulation mediated by phosphodiester non-CpG ODN, and they had usually to be added at extremely high concentrations and on several occasions.28,35,38 Phosphorothioate ODN, in contrast to phosphodiester ODN, are not only more stable but also show better cellular uptake.4–6,23 When we added a phosphodiester non-CpG ODN to cultures of human TLR9-transfected cells, NFκB activation to a similar level as that with the same phosphorothioate sequence was observed, but only with 10–20 times higher concentrations. In addition, Th2-dominated anti-TT responses observed in mice using non-CpG ODN as an adjuvant to TT required the entire molecule to contain stabilizing phosphorothioate linkages.
The stimulatory capacities of phosphorothioate non-CpG ODN on human immune cells had not previously been investigated in much detail. We observed efficient stimulation of human B cells by non-CpG ODN to proliferate and secrete IL-6, even when purified, although a CpG ODN was superior. Non-CpG ODN lacked completely stimulation of Th1-like cytokines or chemokines such as IFN-α, IFN-γ, or IP-10. This was also true when using cultures enriched for pDC, the main producers of IFN-α upon CpG-mediated stimulation.20 In contrast, reproducible up-regulation of pDC cell surface co-stimulatory molecules by non-CpG ODN was observed. As a vaccine adjuvant in mice, non-CpG ODN induce Th2-dominated immune responses (refs 31–33 and this study), in contrast to the Th1-biased effects seen with CpG ODN. Our in vitro results might provide an explanation for these in vivo observations as non-CpG ODN appear to lack one of the most important features of CpG ODN, the efficient stimulation of Th1-like cytokines, including type I interferons. The pDC type I interferons have been demonstrated to be responsible for the indirect activation of a variety of immune cells such as monocytes, Th1 cells, or NK cells that do not express TLR9.1,23 Antigen-presenting cells that had become competent to present antigen to T cells in the presence of non-CpG ODN (by up-regulation of co-stimulatory molecules) might skew T-cell responses towards Th2 in the absence of these Th1 influences. Therefore, the lack of a Th1-like pattern of cytokine production would result in the default Th2-biased antigen-specific responses.
Recently, it was suggested that thymidine content or the presence of a sequence such as 5′-CATTTTGT-3′ in a non-CpG ODN would be sufficient to stimulate human B-cell activation.28,35 Nevertheless, this does not explain the relatively strong stimulation observed with ODN 1982 or 2138 with relatively low thymidine content and no putative non-CpG motif. We therefore investigated in more detail the possible sequence requirements, beside the thymidine content, to induce immune stimulation by non-CpG ODN. In a previous study we described that the addition of a 5′-TC to a CpG ODN enhances stimulatory activities.2 As both non-CpG ODN contain a 5′-TC but lack a CpG motif, we asked whether the 5′-TC itself would be sufficient to increase immune stimulation. Indeed, the addition of a 5′-TC to a poly (T) ODN greatly enhanced its capability to induce cytokine secretion from human PBMC, although no production of type I interferon above background levels could be observed. In addition, none of the other tested nucleotides, adenosine or guanosine (as a 5′-TA or 5′-TG) or a 5-methylated cytosine (as a 5′-TZ), were able to replace cytosine to enhance cytokine secretion. Increasing the length of a 5′-TC ODN resulted in enhanced IL-10 production, comparable to our previous results with CpG ODN.28,36 The observation that modifying the sequence of ODN 1982 by deleting all thymidines including the 5′-T adjacent to the cytosine considerably decreases immune stimulation confirms that both thymidine content as well as 5′-TC appear to be responsible for the stimulation mediated by this class of ODN. The remaining low stimulation upon deletion of these motifs may be attributed to the guanosines in the sequence or the phosphorothioate backbone itself.1
It was previously described that a 5′-TCG can enhance the stimulatory activity of different CpG ODN classes.2,9,36,39 In addition, we demonstrated that the CpG guanosine position is more flexible to induce human TLR9-dependent immune stimulatory effects than the cytosine position.40 Therefore, the 5′-TCT could also be seen as a modification of a 5′-TCG with the guanosine substituted by a thymidine. Although thymine and guanine have different structures, TLR9 may be more forgiving at the guanosine position and accept more substantial modifications than can be tolerated at the cytosine position. Nevertheless, although such 5′-TCT ODN lacking a guanosine induce some immune stimulation, such as B-cell proliferation, they completely lack the stimulation of Th1-like effects in vitro and in vivo. This may give rise to a completely new class of immune stimulatory ODN that may be useful for promoting Th2 immune responses and demonstrates that an unmodified CpG dinucleotide is essential for the strong Th1 stimulation induced by immune modulatory ODN.
One of the surprising aspects of our work is the demonstration that different TLR9 stimuli can induce Th1 or Th2 patterns of immune activation, depending on the presence or absence of CpG motifs. ODN induction of type I and type II interferon expression, and the secretion of the IFN-inducible protein, IP-10, shows a near absolute requirement for the presence of CpG motifs. The explanation for this finding is unlikely to be a simple matter of affinity, because for every readout except IFN secretion, increased concentrations of the non-CpG ODN could at least partially restore TLR9-dependent responses, including B-cell proliferation, co-stimulatory molecule expression, IL-10 and IL-6 secretion, and in vivo adjuvant activity. To date, only a single signalling pathway downstream from TLR9 has been identified, and this is mediated by MyD88.23 Our data lead us to hypothesize that CpG ODN interact with the TLR9 receptor in a qualitatively different way from non-CpG ODN, and that although either interaction can induce most of the immune effects of TLR9 activation, the induction of the IFN promoter uniquely may be dependent on the presence of a CpG motif. Further studies will be required to elucidate the molecular signalling pathways responsible for this differential signalling through TLR9.
In summary, this is the first study to show that TLR9 is responsible for the recognition of immune stimulatory non-CpG ODN. Such ODN in vitro and in vivo induce a Th2-like pattern of immune modulatory effects and these effects are dependent on the thymidine content as well as the presence of a 5′-TC sequence. Th2 responses may have therapeutic applications for a number of diseases such as Crohn's disease or organ-specific autoimmune disorders. Thus, there may be a use for such Th2-biased ODN as vaccine adjuvants or in the development of effective Th2-dominated prophylactic or therapeutic strategies.
Acknowledgments
We are grateful to Sybille Tluk, Hanna Hartmann, Tanja Wader, Bettina Schulte and Ming Liu for excellent technical assistance. We thank Dr S. Bauer for providing TLR9 transfected HEK293 cells and Dr S. Akira for providing TLR9–/– mice.
Abbreviations
- CpG
unmethylated deoxycytidine-deoxyguanosine dinucleotide
- HBsAg
hepatitis B surface antigen
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid dendritic cell
- TLR
Toll-like receptor
- TT
tetanus toxoid antigen
References
- 1.Uhlmann E, Vollmer J. Recent advances in the development of immunostimulatory oligonucleotides. Curr Opin Drug Discov Devel. 2003;6:204–17. [PubMed] [Google Scholar]
- 2.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 3.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, Matson S, Herrera CJ, Fisher E, Yu H, Krieg AM. Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphosphonate oligonucleotides. Antisense Res Dev. 1993;3:53–66. doi: 10.1089/ard.1993.3.53. [DOI] [PubMed] [Google Scholar]
- 5.Krieg AM, Matson S, Fisher E. Oligodeoxynucleotide modifications determine the magnitude of B cell stimulation by CpG motifs. Antisense Nucl Acid Drug Dev. 1996;6:133–9. doi: 10.1089/oli.1.1996.6.133. [DOI] [PubMed] [Google Scholar]
- 6.Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J Immunol. 2000;165:4165–73. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- 7.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 8.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer J, Weeratna R, Payette P, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–62. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 10.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 11.Cornelie S, Hoebeke J, Schacht AM, Bertin B, Vicogne J, Capron M, Riveau G. Direct evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific CpG motifs recognition. J Biol Chem. 2004 doi: 10.1074/jbc.M313406200. e-published. [DOI] [PubMed] [Google Scholar]
- 12.Schetter C, Vollmer J. Toll-like receptors involved in the response to microbial pathogens: development of agonists for Toll-like receptor 9. Current Opinion Drug Discovery Development. 2004;7:204–10. [PubMed] [Google Scholar]
- 13.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 14.Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heil F, Ahmad-Nejad P, Hemmi H, et al. The Toll-like receptor 7 (TLR7) -specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–97. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 17.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, Lipford G, Bauer S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 18.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;8:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 19.Diebold SS, Kaisho T, Hemmi H, Akira S Reis e Sousa. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 20.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 22.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 24.Monteith DK, Henry SP, Howard RB, Flournoy S, Levin AA, Bennett CF, Crooke ST. Immune stimulation – a class effect of phosphorothioate oligodeoxynucleotides in rodents. Anticancer Drug Des. 1997;12:421–32. [PubMed] [Google Scholar]
- 25.Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents – is the bullet really magical? Science. 1993;261:1004–12. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Nishioka Y, Reich CF, Pisetsky DS, Lipsky PE. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J Clin Invest. 1996;98:1119–29. doi: 10.1172/JCI118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisetsky DS, Reich CF., III Influence of backbone chemistry on immune activation by synthetic oligonucleotides. Biochem Pharmacol. 1999;58:1981–8. doi: 10.1016/s0006-2952(99)00294-4. [DOI] [PubMed] [Google Scholar]
- 28.Vollmer J, Janosch A, Laucht M, Ballas ZK, Schetter C, Krieg AM. Highly immunostimulatory CpG-free oligodeoxynucleotides for activation of human leukocytes. Antisense Nucl Acid Drug Dev. 2002;12:165–75. doi: 10.1089/108729002760220761. [DOI] [PubMed] [Google Scholar]
- 29.Takeshita F, Leifer CA, Gursel I, Ishii KJ, Takeshita S, Gursel M, Klinman DM. Cutting edge. Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–8. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 30.Chuang TH, Lee J, Kline L, Mathison JC, Ulevitch RJ. Toll-like receptor 9 mediates CpG-DNA signaling. J Leukoc Biol. 2002;71:538–44. [PubMed] [Google Scholar]
- 31.McCluskie MJ, Weeratna RD, Davis HL. The potential of oligodeoxynucleotides as mucosal and parenteral adjuvants. Vaccine. 2001;19:2657–60. doi: 10.1016/s0264-410x(00)00496-5. [DOI] [PubMed] [Google Scholar]
- 32.McCluskie MJ, Davis HL. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine. 2000;19:413–22. doi: 10.1016/s0264-410x(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 33.Sano K, Shirota H, Terui T, Hattori T, Tamura G. Oligodeoxynucleotides without CpG motifs work as adjuvant for the induction of th2 differentiation in a sequence-independent manner. J Immunol. 2003;170:2367–73. doi: 10.4049/jimmunol.170.5.2367. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 35.Elias F, Flo J, Lopez RA, Zorzopulos J, Montaner A, Rodriguez JM. Strong cytosine-guanosine-independent immunostimulation in humans and other primates by synthetic oligodeoxynucleotides with PyNTTTTGT motifs. J Immunol. 2003;171:3697–704. doi: 10.4049/jimmunol.171.7.3697. [DOI] [PubMed] [Google Scholar]
- 36.Jurk M, Schulte B, Kritzler A, et al. C-Class CpG ODN. Sequence requirements and characterization of immunostimulatory activities on mRNA level. Immunobiology. 2004 doi: 10.1016/j.imbio.2004.02.006. in press. [DOI] [PubMed] [Google Scholar]
- 37.Mannon RB, Nataraj C, Pisetsky DS. Stimulation of thymocyte proliferation by phosphorothioate DNA oligonucleotides. Cell Immunol. 2000;201:14–21. doi: 10.1006/cimm.2000.1635. [DOI] [PubMed] [Google Scholar]
- 38.Pisetsky DS, Reich CF., III The influence of base sequence on the immunological properties of defined oligonucleotides. Immunopharmacology. 1998;40:199–208. doi: 10.1016/s0162-3109(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 39.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J Immunol. 2001;166:2372–7. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 40.Vollmer J, Jepsen JS, Uhlmann E, Schetter C, Jurk M, Wader T, Wüllner M, Krieg AM. Modulation of CpG oligodeoxynucleotide-mediated immune stimulation by locked nucleic acid (LNA) Oligonucleotides. 2004;14:23–31. doi: 10.1089/154545704322988021. [DOI] [PubMed] [Google Scholar]