Abstract
Upon Ag uptake and response to maturation stimuli, dendritic cells (DC) are directed through lymphatic or blood vessel endothelium to T cell areas of secondary lymphoid tissues by the constitutively expressed CC chemokines CCL19 and CCL21. We have shown that mature (m) murine CD8α+ DC exhibit poorer migratory ability to these chemokines than classic CD8α– DC by quantifying their in vitro chemotaxis through unmodified Transwell® filters. We hypothesized that lower surface expression (compared to CD8α– mDC) of the adhesion molecule CD11b on CD8α+ DC might limit their ability to adhere to filter pores in vitro and/or endothelium in vitro/in vivo. To test the role of this and/or other adhesion molecules (CD11a, CD31, CD54 and CD62L) in regulating murine DC subset migration, we used specific mAbs to block their function and quantified their migration through resting or tumour necrosis factor (TNF)-α-activated endothelial cell (EC) layered-Transwell® filters. Both CD8α+ and CD8α– subsets migrated through resting EC (albeit less than in the absence of EC) in response to CCL19 and CCL21, and migration through TNF-α-activated EC was enhanced. In contrast to reports concerning human DC, transendothelial migration of the murine DC subsets was not dependent on CD11b, CD31, or CD62L expression by these cells. CD54 and CD11a, however, were at least partly involved in DC/EC interactions. This is the first report to examine adhesion molecules involved in transendothelial migration of murine DC subsets.
Keywords: chemokines, adhesion molecules, endothelial cells, chemotaxis
Introduction
In mice and humans, several dendritic cell (DC) subsets with immunoregulatory properties and therapeutic potential have been identified.1–3 Their trafficking in vivo requires interactions with endothelial cells (EC). Under both steady-state and inflammatory conditions, immature DC leave the bloodstream, aided by interactions between integrins on their surface (reportedly, for human monocyte-derived DC, CD11a/CD11b/CD18 but not CD11c4) and Ig superfamily members on EC (e.g. CD545) that promote rolling and tethering, and also by homophilic engagement of CD31 for transendothelial extravasation.4,6,7 Upon entrance into peripheral tissue, immature DC endo/phagocytose Ag, begin to mature and modify their cell surface chemokine receptor (CR) expression. Concomitant down-regulation of CR for inflammatory chemokines and up-regulation of CR for lymphoid chemokines initiate recruitment of DC to secondary lymphoid tissues. This process requires reverse transmigration of DC through EC into lymphatic vessels, a process that appears to depend on expression of CD29 (β1-integrin), CD49d (VLA-4), CD49e (VLA-5)4 tissue factor and multidrug resistance protein-18–10 on DC.
Rolling and tethering of mouse and human leucocytes have been studied extensively. The intercellular adhesion molecules that regulate these processes have been elucidated for T cells, natural killer (NK) cells and monocytes.11,12 Recent studies have revealed both the physical means by which leucocytes traverse EC (most reports indicate that, with the possible exception of NK cells that may migrate through EC, leucocytes wedge between adjacent EC13) and the adhesion molecules involved.12 Very few reports, however, have identified adhesion molecules that direct DC extravasation and these studies have been limited to human DC populations distinct in origin as well as in stage of maturation.4,14
We have observed15 inferior ability of murine CD8α+ mature (m) DC to migrate in vitro to CC chemokines compared to classic CD8α– mDC. A possible explanation for this discrepancy is differential cell surface expression of intercellular adhesion molecules. CD8α+ DC are characterized frequently by the absence/low expression of the integrin CD11b. By contrast, CD8α– DC are classically identified as ‘myeloid’ DC by their high surface expression of this molecule.15–18 We considered it feasible that comparatively low expression of CD11b on CD8α+ DC might account for their reduced migratory capacity in vitro15 and their inferior trafficking ability in vivo19,20 as CD54, constitutively expressed on resting (albeit at low/moderate levels) and (up-regulated on) activated EC11 is a major ligand of CD11b. Further, it has been reported4,14,21 that human monocyte-derived DC require CD11b for adhesion to EC. However, data concerning murine DC/EC/adhesion molecule interactions currently are lacking. Thus, there is a gap in knowledge of potential targets for manipulation of DC migration (including migration of DC administered systemically or locally) for possible therapeutic application. Basic information concerning which adhesion molecules permit DC subsets to traverse (vascular) endothelium to enter peripheral tissues and which facilitate DC traffic from the periphery (through vascular/lymphatic endothelium) into secondary lymphoid organs is essential for optimizing their therapeutic delivery.
Herein, we have examined the role of specific adhesion molecules (CD11a, CD11b, CD31, CD54 and CD62L) in regulation of CC chemokine-induced migration of murine splenic CD8α+ and CD8α– mDC through resting or activated EC, using Transwell® filters. This system has allowed us evaluate further the ability of CCL19 and CCL21 to regulate mDC subset migration and provided a controlled environment in which specific adhesion molecules thought to be important for transendothelial migration of mDC could be examined.
Materials and methods
Mice
Male C57BL/10 J (B10; H2b), 8–12 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). They were maintained in the specific pathogen-free facility of the University of Pittsburgh Medical Center and fed Purina rodent chow (Ralston Purina, St. Louis, MO, USA) and tap water ad libitum. Experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee.
DC isolation and maturation
DC were isolated as described.16 Briefly, B10 mice were treated with CHO cell-derived recombinant huFlt3L (FL) (Immunex; now Amgen, Seattle, WA, USA) for 10 days [10 µg/mouse/day intraperitoneally (i.p.)] to allow isolation of a greater number of DC from fewer mice than isolation of DC from untreated animals.22 We have shown previously that in vivo treatment of DC with FL does not affect their CR expression or migration.15 Spleens were excised, macerated gently through wire mesh and red blood cells lysed. No digestive procedures were employed, in order to minimize possible perturbation of cell surface Ag expression. To allow DC to undergo maturation, splenocytes were incubated overnight (18 hr) in RPMI-1640 (Life Technologies Inc, Gaithersburg, MD, USA) supplemented with antibiotics, 10% v/v heat-inactivated fetal bovine serum (FBS) (Life Technologies, Inc.), and r mouse granulocyte-macrophage colony stimulating factor (GM-CSF) (1000 U/ml, Schering Plough, Kenilworth, NJ, USA) at 37°, in 5% CO2 in air. DC were then enriched by centrifugation over a 14·5% w/v nycodenz solution (Sigma-Aldrich, St Louis, MO, USA). Following overnight culture, both CD8α– and CD8α+ DC were both phenotypically and functionally mature, as evidenced by moderate to high expression of surface MHC class II (IAb), CD80, CD86 and CD54 (Fig. 1) and vigorous stimulatory activity for naive allogeneic (C3H/HeJ; H2k) T cells in mixed leucocyte reactions (MLR), as we have described previously16 (data not shown). These DC are referred to subsequently as mature (m) DC.
Figure 1.
Phenotypic maturation of overnight-cultured splenic CD8α– and CD8α+ DC. Bulk spleen cell suspensions were isolated and cultured overnight (18 hr) as described in the Materials and Methods. DC were enriched via centrifugal density separation, washed in fresh media and resuspended in PBS for staining of the cell surface molecules specified. Events were gated for CD11c and CD8α expression by the DC. Open histograms denote appropriate Ig isotype controls; shaded histograms denote specific surface molecule expression. Data are representative of results from at least three separate experiments.
DC subset purification
mDC subsets were purified as described.15 Briefly, overnight-cultured, mDC-enriched splenocytes were incubated with magnetic beads coupled to anti-CD8α mAb (Miltenyi Biotec, Auburn, CA, USA) at 4° for 15 min according to the manufacturer's instructions. Half the bulk mDC population was then passed through a positive selection column (Miltenyi Biotec) to obtain a population of magnetically labelled (CD8α+) mDC (purity: 80–90% CD11c+ CD8α+ 10–20% double negative); the remaining cells were passed through a depletion column (Miltenyi Biotec) to select for a population of unbound (CD8α–) mDC (purity: 85–95% CD11c+CD8α–; 5–15% double negative). mDC were then used for both phenotypic and functional analyses.
Endothelial cells
Endothelial cells (EC) from the Mile Sven 1 (MS1) SV40-transformed, pancreatic (islet of Langerhans) cell line derived from a C57BL/6 (B6; H2b) background23 (ATCC, Manassas, VA, USA) were a gift from Dr Timothy M. Carlos (Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA). The EC were grown to confluence in normal, uncoated cell culture flasks in EC media: RPMI-1640 supplemented with 5% v/v FBS, antibiotics, fungicide® (an antifungal supplement; Sigma) and 1% v/v sodium bicarbonate (as advised by Dr Carlos). The MS1 cell line requires no exogenous growth factors/cytokines. EC were split at confluence (to prevent overgrowth) for phenotypic and chemotaxis assays. Excess EC cultures were cryopreserved as available, to provide back-up cultures: the cells were frozen in 10% dimethyl sulphoxide (DMSO) (one part DMSO: nine parts EC media) in liquid N2. The MS1 EC cell line expresses typical EC markers (Fig. 2 and refs24–26) and are thus morphologically and phenotypically (with the exception of MAdCAM-1, which MS1 EC lack) similar to vascular endothelium. This EC cell line is not susceptible to activation by interleukin (IL)-1β (personal communication, Dr Carlos), but overnight exposure to tumour necrosis factor (TNF)-α (10 ng/ml; R&D Systems, Minneapolis, MN) elicits the expression/up-regulation of typical activated EC markers (Fig. 2;24–26). For chemotaxis assays, EC confluence was determined by Giemsa staining after layering 5–6·5 × 104 EC on uncoated 5 µm Transwell® filters for 18–48 hr. Optimal EC numbers were found to be 6·5 × 104 after overnight (18 hr) or 5 × 104 after 48 hr culture.
Figure 2.
TNF-α induces up-regulation of various integrins and Ig superfamily members on murine EC. EC were propagated as described in the Materials and Methods. They were cultured for 24 hr in the absence (‘resting’ EC; left histograms) or presence (‘activated’ EC; right histograms) of 0, 5, 10 or 20 ng/ml TNF-α (data shown for 10 ng/ml only), washed in PBS, trypsinized, washed in fresh media and then resuspended in PBS for staining of cell surface adhesion molecule expression. Open histograms denote appropriate Ig isotype controls; shaded histograms denote specific adhesion molecule expression. Data are representative of results obtained from three separate experiments.
Flow cytometric analysis
Cell surface phenotypic analysis was performed by flow cytometry using an EPICS Elite ESP analyzer (Beckman Coulter, Hialeah, FL, USA). All staining procedures were performed at 4°. Fc receptors on the DC were first blocked with 10% v/v normal goat serum for 10 min, then stained with mAb for 30 min. DC purity after immunomagnetic bead sorting was assessed by determining the expression of CD11c and CD8α using FITC- and PE-conjugated mAbs, respectively. Phenotypic analysis of additional DC surface markers was performed on cells gated for CD11c (PE-conjugated mAb) and CD8α (Cy-Chrome-conjugated mAb) expression using the following mAbs: FITC-conjugated anti-CD11a (anti-LFA-1), anti-CD11b (Mac-1), -CD31 (PECAM-1), -CD54 (ICAM-1) and -CD62L (L-Selectin) (each 1–2 µg/ml; BD PharMingen, San Diego, CA, USA). Confluent EC were exposed to 0, 5, 10 or 20 ng/ml TNF-α in EC media for 0, 4, 8, 18 or 24 hr in uncoated six-well plates, washed thoroughly with phosphate buffered saline (PBS), trypsinized, washed in PBS and then stained by normal flow cytometric analysis protocol. Fc receptors on EC were first blocked with 10% v/v normal goat serum and then stained using the following mAbs: FITC-conjugated anti-CD31, -CD54, -CD62L or biotinylated-CD62E (E-selectin), -CD102 (ICAM-2) or -CD106 (VCAM-1) (all from BD PharMingen). Biotinylated cells were washed and then stained further with a FITC-conjugated streptavidin mAb (BD PharMingen). Cells stained with the appropriate isotype-matched Ig (BD PharMingen) were used as negative controls. After staining, cells were fixed in 4% v/v paraformaldehyde.
Blocking of specific adhesion molecules on DC subsets
Nycodenz-enriched, immunomagnetic bead-purified, overnight-cultured, CD8α– and CD8α+ mDC were incubated with appropriate functional blocking mAb [CD11a (2D7),27 CD11b (M1/70),28–30 CD31 (390),31,32 CD54 (3E2)27,33 and CD62L (MEL-14),34,35] or isotype control Ig for 30 min at 4°. The DC were then washed and split for staining with FITC-conjugated mAbs to measure the extent of blocking or used in chemotaxis assays. Optimal blocking was determined to be between 10 and 30 µg/ml mAb.
CC chemokines, EC activation and chemotaxis assays
Mouse r CC chemokines CCL19 and CCL21 for chemotaxis assay experiments were acquired from R&D Systems (Minneapolis, MN, USA). The assays were performed as described,15 but with the addition of either a resting or activated EC layer over the Transwell® filter. Five (activated) or 6·5 (resting) × 104 EC were layered over Transwell® filters with 5 µm pores, 24–48 hr before each experiment. In assays with activated EC, 5 × 104 EC were layered 48 hr preceding the migration assay, so that 10 ng/ml TNF-α could be added 24 hr after layering and 24 hr before the chemotaxis assay. DC + EC were incubated for 4 hr for resting EC and 2 hr for activated EC. After the appropriate incubation period, the Transwell® filters were removed and migrated DC from the 24-well plates, collected and enumerated using a Coulter Counter. For accurate comparison between experiments, results were expressed as the percentage of migrated DC. Migration assays were performed in duplicate and each experiment was performed at least three times.
Statistical analyses
Student's unpaired t-test was performed to determine statistical significance using Statview and Microsoft Excel for Macintosh. A P value < 0·05 was considered to be significant.
Results
Phenotypic characterization of resting and activated EC
Primary cultures of mouse EC are difficult to procure and maintain. Therefore, we employed the established MS1 EC cell line. Resting MS1 cells were morphologically normal and, as expected, expressed low surface levels of the adhesion molecules CD31 and CD54 but high levels of CD62E, CD102 and CD106 (Fig. 2), as described previously for normal EC, and phenotypically similar to vascular EC (excepting their lack of expression of MAdCAM-1).24–26 To induce EC activation, we used TNF-α11,36,37 and analysed CD11b (negative control), CD31, CD54 and CD106 expression 0, 4, 8, 16, and 24 hr after exposure to the cytokine. This allowed us determine the minimum period of exposure required for optimal up-regulation of the adhesion molecules (data not shown). Twenty-four-hr exposure to TNF-α (10 ng/ml) resulted in maximal up-regulation of all the molecules analysed, except CD102, which already was expressed by virtually all of the EC at 0 hr (Fig. 2). Although there are no reports currently regarding EC expression of CD11b, there was a modest upregulation of this molecule after 24 hr exposure to TNF-α as well (Fig. 2). Analysis of EC adhesion molecule expression after 24 hr exposure to lower (5 ng/ml) or higher (20 ng/ml) concentrations of TNF-α revealed no significant difference in expression compared to that observed in the presence of 0 or 10 ng/ml, respectively (data not shown).
DC subset migration is affected by the state of activation of EC
As reported previously,15 in vitro chemotaxis experiments with freshly isolated splenic DC revealed that they were unable to migrate significantly in response to any CC chemokine tested. Furthermore, they did not exhibit a change in intracellular Ca++ levels in response to these chemokines or migrate in significant numbers to T cell areas of secondary lymphoid tissues following their in vivo administration.15 Thus, in the present experiments, we chose not to pursue analysis of the in vitro migration of freshly isolated DC through EC. Rather, we focused attention on overnight-cultured DC subsets (CD8α– and CD8α+) as these mDC (Fig. 1) both migrate to secondary lymphoid chemokines in vitro and migrate to secondary lymphoid tissue following their i.v. administration.15,20
Maximal mDC chemotactic migration occurred between 2 and 2·5 hr after addition of the DC to the upper wells of Transwell® filters (after 2·5 hr random DC movement in wells without chemokine increases, giving false positive migratory responses) (data not shown). Because the EC layer added an additional barrier for the DC to traverse, we performed time–course assays to determine the optimal period for assessment of mDC migration. As expected, maximal DC migration through resting EC required an extended incubation period (approximately 4–4·5 hr) compared with migration through uncoated filters (2 hr) (data not shown). Somewhat unexpectedly, maximal mDC migration through activated EC occurred faster than through unmodified filters (approximately 1·5–2 hr) (data not shown).
As expected from previous reports of their reactivity to the CC chemokines tested,4,14,15,21,38 overnight-matured DC migrated through unmodified Transwell® filters only in response to CCL19 (Fig. 3a) and CCL21 (data not shown). Both mDC subsets retained this ability in the presence of resting EC (Fig. 3b,c). Interestingly, despite the prolonged incubation period (from 2 hr to 4 hr) allowed for DC migration through EC-layered filters, both mDC subsets showed a decreased ability to migrate through an EC barrier compared to migration through unmodified filters (compare Fig. 3a to 3b and 3c). mDC migration through activated EC (Fig. 3d,e) in response to CCL19 and CCL21 was greater than that through resting EC (Fig. 3b,c), and equal or superior to that through unmodified filters (Fig. 3a). This was due probably to the up-regulated expression of intercellular adhesion molecules on the surface of activated EC (Fig. 2), which could facilitate migration through interaction with counter-receptors on the DC.
Figure 3.
mDC subset migration through resting EC displays similar kinetics to mDC migration through unmodified Transwells® filters, but with an overall decrease in the number of migrating DC. Immunobead-sorted CD8α– and CD8α+ DC were placed in the upper wells of unmodified (a) or resting EC-layered (b, c) Transwell® filters over graded concentrations of CC chemokines. Immunobead-sorted CD8α– and CD8α+ DC were placed in the upper wells of TNF-α-activated EC-layered Transwell® filters over graded concentrations of CCL19 (d) or CCL21 (e). DC migration through unmodified filters solely to 10 nm chemokine was analysed in parallel, to ensure that the decreased percentage of migrating DC was due to the presence of EC and not to a decreased ability of the DC to migrate in general (data not shown). Results are means ± 1 SD. Data are from a single experiment representative of at least three performed. *, P < 0·05, between CD8α– and CD8α+ DC.
Transendothelial migration of DC subsets does not require the same adhesion molecules as reported for human monocyte-derived and blood DC
CD31 and CD54 were expressed at low levels on resting EC and up-regulated upon EC activation (Fig. 2). Both LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) (on CD8α– DC) expressed on murine and human DC are ligands of CD54, whereas CD31, its own homophilic ligand, is expressed on some murine and human DC (Fig. 1;4,14). There is also evidence that CD11a, CD11b and CD31 are involved in human monocyte–DC/EC binding (CD11a/CD11b/CD18) and extravasation (CD31)4,8 to/through EC. Human CD123+ plasmacytoid DC reportedly utilize CD54 in their transendothelial extravasation.14 CD62L is involved in rolling and tethering of activated and memory T cells on EC, and facilitates T cell exit from blood into lymph39,40 through ligation of MAdCAM-1 and CD34. It has also been reported recently to play a role in human plasmacytoid DC exodus from the blood.41 Therefore we investigated whether blocking interactions between these adhesion molecules and their counter-receptors would affect transendothelial migration of murine splenic mDC subsets. Because the expression of adhesion molecules on EC is dependent on the state of activation of the EC barrier,4,14 we chose to analyse both resting and activated EC in these experiments.
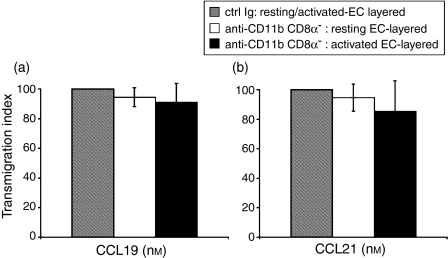
Splenic CD8α+ mDC express lower levels of CD11b than do CD8α– mDC (Fig. 1;15,16), offering a possible explanation for the difference in mDC subset migration through EC. To determine whether blocking CD11b would reduce CD8α– mDC migration to the level exhibited by CD8α+ mDC, we incubated CD8α– mDC with anti-CD11b mAb before evaluating their migratory responses to CCL19 and 21 (Fig. 4). Despite reports that blocking CD11b or CD18 on human DC impairs their adhesion to/transmigration through EC,4,14,21 blocking of CD11b on CD8α– mDC did not interfere significantly with their migration through either resting or activated EC (Fig. 5).
Figure 4.
Blocking of specific adhesion molecules on mDC subsets. Nycodenz-enriched, bead-sorted CD8α– and CD8α+ mDC were incubated with appropriate (purified or biotinylated) blocking antibody or isotype control Ig for 30 min at 4°. Excess antibody was removed and cells were then stained with FITC-labeled CD11a, CD11b, CD31, CD54 or CD62L to confirm blocking. Events were gated for CD11c+CD8α– (top row) or CD11c+CD8α+ cells (bottom row). Shaded histograms: isotype control Ig-treated DC; open histograms, heavy line: isotype control; open histograms; dotted line: blocking antibody-treated DC. Data are from one experiment representative of at least three experiments performed.
Figure 5.
Blocking of CD11b expression does not influence CD8α– DC migration in vitro. Nycodenz-enriched, bead-sorted CD8α– mDC were treated with either blocking anti-CD11b or isotype control Ig for 30 min at 4°. After washing to remove excess mAb, DC were placed in the upper wells of resting or activated EC-layered Transwells® and allowed to migrate for either 4 hr (resting EC) or 2 hr (activated EC) in response to CCL19 (a) or CCL21 (b). Results are expressed as percentage of mAb-blocked DC migration/control Ig-blocked migration (± 1 SD) to denote the degree to which transmigration was inhibited in the presence of blocking antibody. Data are from one experiment representative of at least two performed.
CD31 has also been implicated in transendothelial migration of human DC4,14 and monocytes.8 Despite evidence of low-moderate expression of this adhesion molecule on mDC (Fig. 1), pretreatment with anti-CD31 mAb (Fig. 4) did not affect the migration of either CD8α– or CD8α+ mDC through resting or activated EC (data not shown).
Blocking of CD54 expression inhibits transendothelial migration of DC subsets in response to CCL19
Although blocking of CD11b had no apparent influence on the migration of CD8α– mDC through resting or activated EC (Fig. 5), blocking of CD54 (Fig. 4) significantly reduced the migration of both mDC subsets through resting EC in response to CCL19 (Fig. 6a) and CCL21 (Fig. 6b). CD8α+ but not CD8α– mDC migration through activated EC was impaired significantly in response to CCL19 (Fig. 6a). Interestingly, whereas DC migration through either resting or activated EC in response to CCL19 was impaired after blocking CD54 (Fig. 6a), CD54 blocking appeared to affect mDC subset migration to CCL21 only through resting EC (Fig. 6b).
Figure 6.
Blocking of CD54 expression reduces murine spleen CD8α– and CD8α+ DC subset chemotaxis through resting or activated EC. mDC were incubated with control isotype Ig or anti-CD54 mAb, washed, resuspended in migration media and then incuated for 4 hr (resting EC) or 2 hr (activated EC). Both subsets exhibited reduced migration through resting EC to CCL19 (a) and CCL21 (b). Only CD8α+ migration was impaired through activated EC and only to CCL19 (a and b). Results are expressed as percentage of mAb-blocked DC migration/control Ig-blocked migration (± 1 SD) to denote the degree to which transmigration was inhibited in the presence of blocking antibody. Data are from one experiment representative of at least two performed. *P < 0·05 and **P < 0·02, compared with control Ig.
Blocking of CD11a expression reduces transendothelial migration of DC subsets in response to CCL19 and CCL21
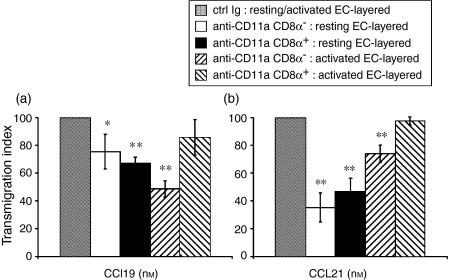
CD11a is expressed highly on both mature splenic DC subsets (Fig. 1). Both CD11a and CD11b have been reported previously to be important in facilitating human monocyte-derived DC adhesion to EC4 although we saw no effect on CD8α– mDC transendothelial migration in the presence of blocking CD11b mAb in our experiments (Fig. 5). However, as murine splenic mDC at least partially require CD54 for their transendothelial migration, we analysed further whether the blocking of another important CD54 ligand, CD11a (Fig. 4), would have a negative effect on DC trafficking. Blocking of CD11a also significantly impaired mDC subset migration through both resting and activated EC-layered Transwells® to both CCL19 and CCL21, but as with anti-CD54 the effect was at most partial. Interestingly, unlike after blocking with anti-CD54 mAb, CD8α– mDC migration was impaired through both resting and activated EC in response to both chemokines tested. By contrast, mCD8α+ transmigration through activated EC in response to either CCL19 or CCL21 was not affected by CD11a blockade.
Blocking of CD62L expression does not impair transendothelial migration of DC subsets
Although CD62L blocking studies have been performed primarily on lymphocytes,39,40,42–44 impaired in vivo migration of human blood CD123+ DC has been reported after granulocyte-colony-stimulating factor (G-CSF)-induced down-regulation of this selectin.41 CD62L is expressed in moderate levels on murine spleen mDC subsets (Fig. 1). Thus, we investigated whether blocking of this selectin on DC affected their chemotaxis through resting or activated EC. Despite blocking of CD62L on both DC subsets with anti-CD62L mAb (Fig. 4), neither their migration through resting nor activated EC was impaired consistently (data not shown).
Discussion
To undertake these studies, we established an in vitro test environment in which to examine the role of specific cell surface molecules likely to regulate murine mDC subset transendothelial migration in vivo, as these DC, in particular CD8α+ DC, are potential therapeutic candidates.3,16 We first established that splenic mDC subsets were indeed capable of extravasation through resting murine EC in response to CCL19 and CCL21. This migratory ability was reduced compared to their movement through unmodified Transwell® filters or those coated with activated EC, despite a more prolonged (doubled) incubation period (Fig. 3). Due probably to the up-regulation of adhesion molecules on the EC (Fig. 2), mDC trafficked with little to no impairment through activated EC layers, and did so within the same time-frame as DC migrating in the absence of EC (Fig. 3).
Evidence that blocking CD11b inhibits human monocyte-derived DC adhesion to EC by > 60%4 coupled with differential expression of the integrin CD11b by the two subsets (Fig. 1;15–18), suggested an explanation as to why the CD8α+ DC showed a decreased migratory response to CCL19 and CCL21 compared to their CD8α– mDC counterparts (Figs 4 and 5;15). However, despite lower expression of CD11b by CD8α+ mDC, it did not appear that this integrin played a critical role in regulating murine splenic mDC migration: blocking of CD11b on CD8α– mDC had no significant inhibitory effect on their transendothelial migration through either resting or activated EC in response to either CCL19 or CCL21 (Fig. 5a,b). Thus, the differential pattern of migration seen for CD8α+ and CD8α– mDC (Fig. 3;15) may indeed be related to other, as yet unknown, factors.
In contrast to these findings, blocking of either a major ligand of CD11b (CD54) or a ‘partner integrin’ CD11a (CD11a and CD11b both complex with CD18 to bind to CD54) considerably reduced transendothelial mDC subset migration. Anti-CD54 mAb impaired CD8α– mDC migration (through resting EC; Fig. 6a) and reduced further an already weak migratory response of CD8α+ mDC through resting and activated EC to CCL19 (Fig. 6a). Both subsets also exhibited impaired migration to CCL21 through resting EC after blocking of CD54 (Fig. 6b). Only CD8α+ mDC showed impaired transmigration through activated EC after blocking with anti-CD54 mAb (Fig. 6a), and only in response to CCL19. In contrast, CD8α–, but not CD8α+, mDC exhibited decreased migratory ability in response to CCL19 and CCL21 after CD11a blockade (Fig. 7).
Figure 7.
Anti-CD11a mAb reduces mDC subset transmigration through resting and activated EC. mDC were incubated with control isotype Ig or anti-CD11a mAb, washed, resuspended in migration media and then incuated for 4 hr (resting EC) or 2 hr (activated EC). Both subsets exhibited reduced migration through resting EC to CCL19 (a) and CCL21 (b). Only CD8α– migration was impaired through activated EC (a and b). Results are expressed as percentage of mAb-blocked DC migration/control Ig-blocked migration (± 1 SD) to denote the degree to which transmigration was inhibited in the presence of blocking antibody. Data are from one experiment representative of at least two performed. *P < 0·05 and **P < 0·02, compared with control Ig.
The differences between CD8α– and CD8α+ DC migration through activated EC in response to CCL21 are not as marked as in response to CCL19 (Fig. 3b,a, respectively). Both CCL19 and CCL21 have been reported to induce arrest of (human) lymphocytes on endothelium under flow conditions.45 It is interesting to speculate whether the affinity/avidity of the adhesion molecule interactions between DC and EC (not tested in the report by Campbell et al.45) were enhanced due to their 2–4-hr exposure to CCL21. This may potentially explain the apparent increased ability of CD8α+ DC to transmigrate through activated EC (as seen in Fig. 3b) and the ability of both subsets to overcome the migration inhibiting effects of blocking CD54 on DC migration through activated EC (Fig. 6b). It is also possible that the up-regulation of adhesion molecules on the TNF-α-activated EC may have compensated for the loss of functional CD54 and/or CD11a on the DC (Figs 6b and 7).
While CD11b and CD54 are probably involved almost exclusively in leucocyte rolling and tethering on EC (blocking of which would prevent further attachment to and extravasation through EC), CD31 has been reported to play a significant role in leucocyte (T cell, B cell, NK cell, monocyte and human DC) migration through EC.5–7,14,46 Therefore it was surprising that CD31 appeared to have no involvement in transendothelial migration of murine spleen mDC subsets. However, CD31 was expressed at only very low levels on the mDC subsets examined (∼10%; Fig. 1), and is thus an unlikely candidate for involvement in their interactions with EC. The low expression of CD31 on murine spleen DC may be related to their tissue of origin rather than species differences, as in our laboratory, mouse BM-derived DC express moderate levels of CD31 (unpublished observations).
Between 50 and 90% of human blood-isolated DC express CD62L14,41 but there is little information on expression of CD62L by murine DC. It has been shown recently that administration of G-CSF induces up-regulation of CCR7 on human blood plasmacytoid pre-DC with concomitant down-regulation of CD62L, effectively preventing the exodus of these DC from the blood into lymphatic vessels.41 Prior to the latter study, it was believed that G-CSF enhanced selectively the generation of CD11c–CD123+ plasmacytoid pre-DC over classic CD11c+CD123– monocytoid DC.47 It now appears that the cytokine impairs their emigration selectively, resulting in accrual of plasmacytoid pre-DC in the blood, while CD11c+ DC continue to exhibit a normal trafficking pattern (not affecting their numbers in blood). Thus we felt it appropriate to investigate the expression of CD62L on murine spleen DC. Our findings indicate that it is expressed on approximately 50% of spleen mDC (∼25% CD8α– and ∼25% CD8α+ mDC; Fig. 1), and that its expression is not subset-specific (as in human blood DC). Similar to CD11b and CD31, that appear to be important in human4,14 but not murine DC transendothelial migration (this study), blocking of CD62L on either mDC subset did not impair their migration through resting or activated EC (data not shown).
CD62L also binds MAdCAM-1.48,49 Although MAdCAM-1 is not expressed/up-regulated by the MS1 EC cell line, it seems unlikely this vascular endothelium marker is required for murine spleen DC transendothelial migration, as blocking of CD62L:CD34 interactions had a negligible effect on their chemotaxis (data not shown).
Upon first assessment, the results from our blocking antibody experiments appear to be in conflict with those reported for human DC. However, as for freshly isolated spleen and immature BM-derived murine DC, it appears the factors that regulate DC migration may reflect the source/subset/state of activation of DC and the activation status of the EC being analysed. Blocking of CD31 on resting EC before their interaction with human monocyte-derived DC (mature DC, as indicated by their strong induction of naive T cell proliferation in MLR) has been reported to reduce transmigration by 40%.4 However, incubation of CD31-blocked human blood-isolated immature DC subsets (CD11c+ and CD123+) with EC showed significant reduction only in transendothelial migration through activated EC; their migration through resting EC was unaffected after blocking of CD31 on their cell surface.14 Moreover, whereas immature human CD11c+ DC transmigrate normally through resting and activated EC after CD54 is blocked on their cell surfaces, immature CD123+ DC show impaired migration through activated but not resting EC.14 Blood-isolated human immature CD11c+ and CD123+ DC also apparently need a chemotactic agent to elicit significant migration through EC,14 while monocyte-derived DC migrate through EC even in the absence of exogenous chemoattractants.4 Thus, while some of our data may not match those reported for human DC from different sources, it appears likely that, as more DC/EC interactions for each species are examined, patterns will emerge regarding DC tissue source, subset and stage of maturation, as well as EC state of activation, as we have shown previously for the responses of bone marrow (BM)-derived and spleen-isolated immature DC to CC chemokines.15
CD11b expression does not appear to be the factor that regulates the differential migration of CD8α– and CD8α+ DC in vitro (Fig. 3;15). However, as we have reported,15 murine splenic mDC subsets migrate with equal efficiency in vivo. Thus, it is possible that CD8α+ mDC migration is facilitated in vivo by an as yet unknown factor. Further understanding of the influence of these variables will prove invaluable in optimizing appropriate means by which to administer/target DC subsets therapeutically. In conclusion, we demonstrate herein for the first time adhesion molecules that appear to be important in regulating transendothelial migration of murine splenic DC.
Acknowledgments
We thank the Immunex Corporation (now Amgen) for providing Flt3L, Ms Alison Logar for skilled assistance with flow cytometry and Ms Miriam Meade for proficient manuscript preparation. We also thank both Dr Timothy M. Carlos and Ms Darcy Franciola (Department of Medicine, University of Pittsburgh) for their generous provision of the MS1 EC line. The work was supported by National Institutes of Health grants DK 49745, AI41011 and AI/DK 51698.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G, Bianchi G, Bernasconi S, Bersani L, Piemonti L, Sozzani S, Mantovani A, Allavena P. Adhesion, transendothelial migration, and reverse transmigration of in vitro cultured dendritic cells. Blood. 1998;92:207–14. [PubMed] [Google Scholar]
- 5.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 6.Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–8. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- 7.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–60. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 9.Randolph GJ, Luther T, Albrecht S, Magdolen V, Muller WA. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 1998;92:4167–77. [PubMed] [Google Scholar]
- 10.Randolph GJ, Beaulieu S, Pope M, Sugawara I, Hoffman L, Steinman RM, Muller WA. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci USA. 1998;95:6924–9. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 12.Muller WA. Leukocyte–endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–34. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 13.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–15. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Rosa G, Longo N, Rodriguez-Fernandez JL, Puig-Kroger A, Pineda A, Corbi AL, Sanchez-Mateos P. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol. 2003;73:639–49. doi: 10.1189/jlb.1002516. [DOI] [PubMed] [Google Scholar]
- 15.Colvin BL, Morelli AE, Logar AJ, Lau AH, Thomson AW. Comparative evaluation of CC chemokine-induced migration of murine CD8α+ and CD8α– dendritic cells and their in vivo trafficking. J Leukoc Biol. 2004;75:275–85. doi: 10.1189/jlb.1202613. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8α+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002;168:143–54. doi: 10.4049/jimmunol.168.1.143. [DOI] [PubMed] [Google Scholar]
- 17.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8α+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 18.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–73. [PubMed] [Google Scholar]
- 19.Smith AL, de St Groth BF. Antigen-pulsed CD8α+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–8. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruedl C, Bachmann MF. CTL priming by CD8(+) and CD8(−) dendritic cells in vivo. Eur J Immunol. 1999;29:3762–7. doi: 10.1002/(SICI)1521-4141(199911)29:11<3762::AID-IMMU3762>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi G, D'Amico G, Varone L, Sozzani S, Mantovani A, Allavena P. In vitro studies on the trafficking of dendritic cells through endothelial cells and extra-cellular matrix. Dev Immunol. 2000;7:143–53. doi: 10.1155/2000/39893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbiser J, Moses M, Fernandez C, et al. Oncogenic H-Ras stimulates tumor angiogenesis by distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–66. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garlanda C, Dejana E. Heterogeneity of endothelial cells specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1993–202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 25.Pober JS. Immunobiology of human vascular endothelium. Immunol Res. 1999;19:225–32. doi: 10.1007/BF02786490. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 27.Masten BJ, Yates JL, Pollard Koga AM, Lipscomb MF. Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am J Respir Cell Mol Biol. 1997;16:335–42. doi: 10.1165/ajrcmb.16.3.9070619. [DOI] [PubMed] [Google Scholar]
- 28.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982;156:1000–9. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springer TA, Davignon D, Ho MK, Kurzinger K, Martz E, Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–95. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 30.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin HS, Shen HM, Yan HC, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–53. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 32.Rosenblum WI, Nelson GH, Wormley B, Werner P, Wang J, Shih CC. Role of platelet-endothelial cell adhesion molecule (PECAM) in platelet adhesion/aggregation over injured but not denuded endothelium in vivo and ex vivo. Stroke. 1996;27:709–11. doi: 10.1161/01.str.27.4.709. [DOI] [PubMed] [Google Scholar]
- 33.Scheynius A, Camp RL, Pure E. Reduced contact sensitivity reactions in mice treated with monoclonal antibodies to leukocyte function-associated molecule-1 and intercellular adhesion molecule-1. J Immunol. 1993;150:655–63. [PubMed] [Google Scholar]
- 34.Pizcueta P, Luscinskas FW. Monoclonal antibody blockade of 1-selectin inhibits mononuclear leukocyte recruitment to inflammatory sites in vivo. Am J Pathol. 1994;145:461–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–75. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intercellular adhesion molecule 1 and the CD11/CD18 integrins. J Exp Med. 1996;183:451–62. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McColl SR. Chemokines and dendritic cells: a crucial alliance. Immunol Cell Biol. 2002;80:489–96. doi: 10.1046/j.1440-1711.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- 39.Symon FA, McNulty CA, Wardlaw AJ. P- and 1-selectin mediate binding of T cells to chronically inflamed human airway endothelium. Eur J Immunol. 1999;29:1324–33. doi: 10.1002/(SICI)1521-4141(199904)29:04<1324::AID-IMMU1324>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–64. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuckovic S, Kim M, Khalil D, et al. Granulocyte-colony stimulating factor increases CD123hi blood dendritic cells with altered CD62L and CCR7 expression. Blood. 2003;101:2314–7. doi: 10.1182/blood-2002-03-0973. [DOI] [PubMed] [Google Scholar]
- 42.Ng-Sikorski J, Linden L, Eierman D, Franzen L, Molony L, Andersson T. Engagement of 1-selectin impairs the actin polymerizing capacity of β2-integrins on neutrophils. J Cell Sci. 1996;109:2361–9. doi: 10.1242/jcs.109.9.2361. [DOI] [PubMed] [Google Scholar]
- 43.Bosse R, Vestweber D. Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur J Immunol. 1994;24:3019–24. doi: 10.1002/eji.1830241215. [DOI] [PubMed] [Google Scholar]
- 44.Venturi GM, Tu L, Kadono T, et al. Leukocyte migration is regulated by 1-selectin endoproteolytic release. Immunity. 2003;19:713–24. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 45.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–4. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 46.Bird IN, Spragg JH, Ager A, Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–60. [PMC free article] [PubMed] [Google Scholar]
- 47.Pulendran B, Banchereau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–72. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 48.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–8. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 49.Elangbam CS, Qualls CW, Jr, Dahlgren RR. Cell adhesion molecules – update. Vet Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]