Abstract
We have previously reported that human neutrophils pretreated with tumour necrosis factor-α (TNF-α) and then exposed to a variety of agents such as immune complexes, zymosan, phorbol 12-myristate 13-acetate (PMA), C5a, fMLP, or granulocyte–macrophage colony-stimulating factor (GM-CSF), undergo a dramatic stimulation of apoptosis, suggesting that TNF-α is able to prime an apoptotic death programme which can be rapidly triggered by different stimuli. We report here that this response involves the participation of Mac-1 (CD11b/CD18), is dependent on caspases 3, 8 and 9, and is associated with both a loss of mitochondrial transmembrane potential and a down-regulation in expression of the anti-apoptotic protein, Mcl-1. Interestingly, we also found that the anti-apoptotic cytokine interleukin-1 (IL-1) improves the ability of TNF-α to promote apoptosis, supporting the notion than TNF-α, acting together with IL-1, may favour the depletion of neutrophils from the inflammatory areas during the course of acute inflammation.
Keywords: caspases, IL-1, Mac-1, survival
Introduction
As a first line of defence against bacterial and fungal infections, neutrophils are rapidly recruited to inflammatory sites, where the expression of their constitutive apoptotic programme can be modified by a number of agents.1,2 It has been described that granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-1, IL-2, leukotriene B4, C5a, lipopolysaccharide (LPS) and corticosteroids inhibit neutrophil apoptosis,3–10 whereas proteolytic enzymes, immune complexes, bacteria and viruses stimulate neutrophil apoptosis.11–14 Controversial results, on the other hand, have been reported regarding the effects of fMLP, granulocyte colony-stimulating factor (G-CSF) and IL-6.3,6–8,10
Tumour necrosis factor (TNF-α) is a pluripotent cytokine (produced by a variety of leucocytes) with the ability to stimulate multiple inflammatory responses mediated by neutrophils. TNF-α has been shown to enhance the expression of CD11b/CD18, increase neutrophil adhesion to endothelium, trigger adherent neutrophils to release large amounts of reactive oxygen intermediates (ROI), and promote neutrophil degranulation, phagocytosis and antibody-dependent cell-mediated cytotoxicity.15–19 These responses appear to be involved not only in host defence, but also in the development of inflammatory diseases such as glomerulonephritis, sepsis and the adult respiratory distress syndrome.20,21 However, TNF-α also appears to play an important role in the resolution of inflammation, as suggested by an enhanced inflammatory response observed in TNF-α−/− mice after bacterial infections.22,23 It has been suggested that this effect involves the stimulation of neutrophil apoptosis by TNF-α.23,24In vitro studies performed in different laboratories, however, have described disparate findings regarding the effects of TNF-α on neutrophil apoptosis. It has been reported to stimulate, have no effect on, and to delay neutrophil apoptosis.4,25–27 As suggested by Murray et al.,28 these discrepant results could be attributed, at least in part, to differences in the time of exposure of neutrophils to TNF-α. There is general agreement that after 2–4 hr of culture, a subpopulation of neutrophils (20–30%) undergo apoptosis as a consequence of TNF-α treatment. By contrast, when apoptosis was evaluated after longer time-periods, i.e. 24–48 hr, TNF-α did not increase apoptosis; in fact, at these time-points, a significant delay of neutrophil apoptosis was observed in TNF-α-treated cells compared with controls.4,25–28
While these results support the notion that neutrophils are relatively resistant to TNF-α, in terms of apoptosis induction, our recent results support another view. We found that neutrophils pretreated with TNF-α and then exposed to a variety of agents, such as immune complexes, zymosan, phorbol 12-myristate 13-acetate (PMA), C5a, the chemotactic peptide fMLP, and GM-CSF, undergo a dramatic stimulation of apoptosis, suggesting that TNF-α is able to prime an apoptotic death programme which can be rapidly triggered by neutrophil activation.29 In the present study, we analyse the mechanisms involved in this response.
Materials and methods
Reagents
The following agents were used: human immunoglobulin G (IgG), zymosan (Z), rhodamine 123 (Rh123), acridine orange, ethidium bromide and propidium iodide (Sigma, St Louis, MO). Zymosan-activated serum (ZAS), used as a source of C5a, was prepared by incubating 15 mg of Z with 1 ml of fresh serum with end-over-end rotation for 1 hr at 37°. Then, serum was heat inactivated for 30 min at 56°. After centrifugation (1000 g, 15 min, 4°), the supernatant was collected and stored at −70°. Immobilized IgG (iIgG) was prepared by incubating microplates (96-well flat-bottom) with IgG (1 mg/ml in saline) for 18 hr at 37°. Before use, the plates were washed six times with saline. Recombinant human TNF-α, IL-2, IL-6, IL-12 and interferon-γ (IFN-γ) were purchased from Sigma, and IL-1, IL-8, GM-CSF and the IL-1 receptor antagonist (IL-1RA) were from R & D Systems (Minneapolis, MN). For blocking studies we used the F(ab′)2 fragment of IgG1 anti-CD18 (from supernatants of hybridoma TS 1/18; ATCC, Rockville, MD) and IgM anti-CD11b (Mo1; Immunotech, Marseille, France). In these studies, neutrophils were preincubated with the corresponding monoclonal antibody (mAb) for 30 min at 4°. Concentrations of mAb three- to fivefold higher than those needed to saturate all binding sites (1–10 µg/ml), as determined by fluorescence-activated cell sorter (FACS) analysis, were used. The inhibitors of caspases 3 (Z-DEVD-FMK), 8 (Z-IETD-FMK) and 9 (Z-LEHD-FMK) were from R & D Systems.
Blood samples
Blood samples were obtained from healthy donors who had taken no medication for at least 10 days before the day of sampling. Blood was obtained by venepuncture of the forearm vein, and it was drawn directly into heparinized plastic tubes.
Neutrophil isolation
Neutrophils were isolated by Ficoll–Hypaque gradient centrifugation (Ficoll; Pharmacia, Uppsala, Sweden)(Hypaque; Winthrop Products, Buenos Aires, Argentina) and dextran sedimentation, as described previously.30 Cell suspensions contained > 96% neutrophils, as determined by May–Grunwald–Giemsa-stained cytopreps, and the levels of monocyte contamination were always < 0·2%, as evaluated by CD14 staining and flow cytometry. The cells were suspended in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 1% fetal calf serum (FCS) (Invitrogen).
Cell cultures
Aliquots of 0·10 ml of neutrophil suspensions (2·5 × 106/ml) were placed in 96-well flat-bottom microplates. Unless stated otherwise, neutrophils were treated with 10 ng/ml of TNF-α for 1–2 min at 37°. Then, cells were stimulated with different agents. Apoptosis was evaluated after 3 hr of culture at 37° in 5% CO2, as described below.
Quantification of cellular apoptosis and viability by fluorescence microscopy
Quantification was performed, as previously described,31 using the fluorescent DNA-binding dyes acridine orange (100 µg/ml, to determine the percentage of cells that had undergone apoptosis) and ethidium bromide (100 µg/ml; to differentiate between viable and non-viable cells). With this method, non-apoptotic cell nuclei show ‘structure’, i.e. variations in fluorescence intensity that reflect the distribution of euchromatin and heterochromatin. By contrast, apoptotic nuclei exhibit highly condensed chromatin that is uniformly stained by acridine orange. In fact, the entire apoptotic nucleus is present as bright spherical beads. To assess the percentage of cells showing morphological features of apoptosis, at least 200 cells were scored in each experiment. Previous observations have demonstrated that morphological assessment of neutrophil apoptosis closely correlates with results obtained using other methods to assay apoptosis, such as propidium iodide staining and annexin V binding.32
Quantification of neutrophil apoptosis by propidium iodide staining and flow cytometry
The proportion of neutrophils that display a hypodiploid DNA peak, i.e. apoptotic cells, was determined using a modification of the protocol of Nicoletti et al.33 Briefly, cell pellets containing 2·5 × 106 neutrophils were suspended in 400 µl of hypotonic fluorochrome solution (propidium iodide, 50 µg/ml in 0·1% sodium citrate plus 0·1% Triton-X-100) and incubated for 2 hr at 4°. The red fluorescence of propidium iodide in individual nuclei was measured using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Analysis of mitochondrial permeability transition by flow cytometry
Mitochondrial permeability transition was performed by flow cytometry, as previously described, using Rh123, a cell-permeant, cationic, fluorescent dye that is readily sequestered by active mitochondria without inducing cytotoxic effects.34 Loss of mitochondrial transmembrane potential results in a diminished cell ability to accumulate the green fluorochrome Rh123. In all cases, Rh123 (0·1 µg/ml) was added to cultures for 30 min before analysis by flow cytometry.
Analysis of the expression of intracellular Mcl-1 by flow cytometry
Neutrophils (1 × 106) were fixed with 2% formaldehyde for 15 min at 4°, washed with saline and permeabilized with 0·1% saponin for 15 min. Then, cells were resuspended in saline supplemented with glycine (0·1 mg/ml), incubated with anti-Mcl-1 IgG1 (Pharmingen, San Diego, CA, USA) or mouse IgG1 (an isotype-control antibody) (Sigma), and intracellular expression of Mcl-1 was analysed by flow cytometry using fluorescein isothiocyanate (FITC)–rabbit anti-mouse IgG.
Analysis of the expression of intracellular Mcl-1 by Western blotting
Cell were harvested after different treatments and washed twice with cold phosphate-buffered saline (PBS). Whole-cell lysates were prepared using a 3% sodium dodecyl sulphate (SDS) lysis buffer [10 mm HEPES, pH 8·0, 1·5 mm MgCl2, 10 mm KCl, 1 mm dithiothreitol (DTT), 0·5 mm phenylmethylsulphonyl fluoride (PMSF), 0·1% Nonidet P-40 (NP-40), 3% SDS, and complete protease inhibitors] (Boehringer Mannheim, Mannheim, Germany). Lysates were boiled for 5 min and protein was quantified using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). Samples were then frozen at −80° until use. Proteins (25 µg per sample) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on a 12% SDS polyacrylamide gel. After electrophoresis, proteins were transferred to poly(vinylidene difluoride) (PVDF) membranes (Sigma). Membranes were blocked for 1 hr at room temperature in PBS/Tween-20 containing 5% (w/v) bovine serum albumin (BSA). A rabbit polyclonal anti-Mcl-1 immunoglobulin (Pharmingen) was added at a dilution of 1 : 200 in PBS containing 2% BSA and incubated overnight at 4°. Membranes were then washed and incubated for 1 hr with a goat antirabbit-horseradish peroxidase (HRP) secondary antibody at a dilution of 1 : 3500 in PBS/Tween-20 containing 2% non-fat dry milk. Protein bands were visualized by enhanced chemiluminescence (ECL) (Amersham).
Statistical analysis
The Student's paired t-test was used to determine the significance of differences between means, and a value of P < 0·05 was taken as indicating statistical significance.
Results
Involvement of Mac-1 in the promotion of neutrophil apoptosis by TNF-α
The leucocyte αMβ2 integrin (also known as Mac-1, complement receptor type 3 and CD11b/CD18) plays a critical role in neutrophil adhesion, migration, phagocytosis and cytotoxicity.35,36 Moreover, Mac-1 has been proposed to act as a ‘signalling partner’ for other leucocyte receptors.37 Controversial data have been reported regarding the role of Mac-1 in the stimulation of neutrophil apoptosis by TNF-α. Walzog and colleagues38 demonstrated that Mac-1 aggregation, induced by antibody cross-linking, potentiates induction of apoptosis by TNF-α, while van den Berg et al.39 and Avdi et al.40 showed that the proapoptotic action of TNF-α is prevented by the blocking of CD11b. On the other hand, Whitlock et al.41 showed that Mac-1 engagement on neutrophils can either inhibit or enhance apoptosis triggered by TNF-α, depending on the activation state of the integrin. Thus, clustering of inactive Mac-1 prevents stimulation of apoptosis by TNF-α, while an increase in the rate of apoptosis was induced by the clustering of activated Mac-1. Finally, Zhang et al.42 have recently reported that TNF-α enhances neutrophil apoptosis after phagocytosis of complement-coated particles through a mechanism dependent on Mac-1 activity.
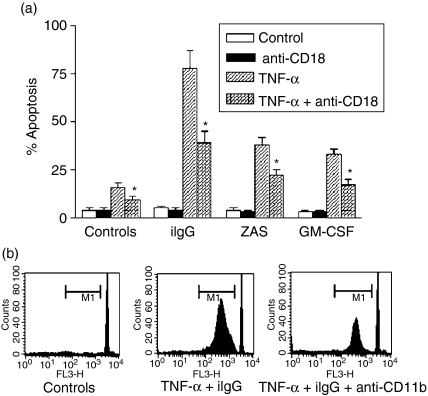
To determine whether the observed increase in the apoptotic rate of neutrophils pretreated with TNF-α and then exposed to conventional agonists was dependent on CD18, neutrophils were preincubated with F(ab′)2 fragments of a blocking antibody directed to CD18, the common β chain of leucocyte adhesion proteins, prior to stimulation. Figure 1(a) shows that blocking of CD18 significantly prevents not only the stimulation of neutrophil apoptosis induced by TNF-α alone, but also the marked increase in the apoptotic rate observed for neutrophils cultured with TNF-α together with iIgG, ZAS (used as a source of C5a) or GM-CSF, indicating that CD18 is involved in the promotion of apoptosis. Similar results were observed using a blocking antibody directed to CD11b (Fig. 1b). As expected, no inhibitory effect was observed using F(ab′)2 fragments of mouse IgG as an isotype-matched antibody (data not shown). Together, these results support that stimulation of apoptosis triggered by conventional agonists in TNF-α-pretreated neutrophils involves, at least in part, a Mac-1-dependent pathway.
Figure 1.
Promotion of neutrophil apoptosis by tumour necrosis factor-α (TNF-α) involves a Mac-1-dependent pathway. Neutrophils (2·5 × 106/ml) were cultured for 30 min at 4° alone or in the presence of saturating concentrations of blocking monoclonal antibodies (mAbs) directed to CD18 [TS 1/18 F(ab′)2] (a) or CD11b (Mo1) (b). Then, cells were cultured in the presence or absence of TNF-α (10 ng/ml) for 1–2 min at 37° and treated with immobilized immunoglobulin G (iIgG), zymosan-activated serum (ZAS) (1/10) or granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml) (a) or iIgG (b). After 3 hr, the percentage of apoptotic cells was determined by fluorescence microscopy (a) or by flow cytometry (b), as described in the Materials and Methods. (a) Results are expressed as the mean ± standard error of the mean (SEM) of five or six experiments. *Statistical significance (P < 0·01) compared to neutrophils cultured with TNF-α in the absence of anti-CD18-blocking antibodies. (b) Histograms of a representative experiment (n = 4) in which M1 represents the fraction of nuclei with hypodiploid DNA content. Sub-M1 events represent cell debris.
Promotion of neutrophil apoptosis by TNF-α involves caspases 3, 8 and 9
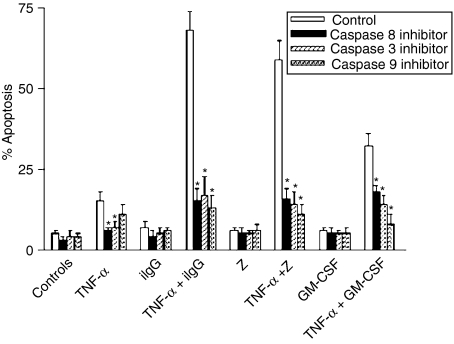
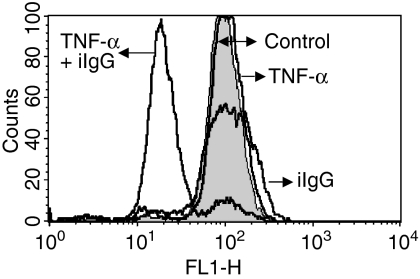
Because recent studies have shown that TNF-α could stimulate neutrophil apoptosis through both caspase-dependent and -independent mechanisms,43,44 we examined whether inhibition of caspases could prevent the promotion of neutrophil apoptosis by TNF-α. In agreement with previous reports showing that stimulation of neutrophil apoptosis by TNF-α involves caspases 8 and 3,24,43 we observed that specific inhibitors of caspase 8 (Z-IETD-FMK) and 3 (Z-DEVD-FMK) significantly delayed the apoptosis of neutrophils cultured with TNF-α alone (Fig. 2). The inhibitor of caspase 9 (Z-LEHD-FMK) also delayed the apoptosis of neutrophils treated with TNF-α alone, although this inhibition was not statistically significant. Of note, stimulation of apoptosis of TNF-α-pretreated neutrophils triggered by iIgG, Z, or GM-CSF was markedly prevented, not only by the inhibitors of caspases 3 and 8, but also by the inhibitor of caspase 9, suggesting that apoptosis involves activation of the mitochondrial-dependent death pathway. The possible participation of this pathway was further examined using Rh123, a positively charged probe that accumulates in mitochondria, depending on its transmembrane potential.34,45,46 Figure 3 shows that TNF-α-pretreated neutrophils incubated with iIgG, but not with TNF-α or IgG alone, undergo an early and dramatic reduction in the uptake of Rh123, supporting that the induction of apoptosis is associated with a loss of the mitochondrial transmembrane potential. Of note, the uptake of Rh123 was assessed after 120 min of culture, a time-point at which neutrophils treated with TNF-α plus iIgG showed < 30% apoptosis, supporting that the ΔΨm collapse is induced prior to DNA fragmentation.
Figure 2.
Inhibitors of caspases 8, 3 and 9 suppress the promotion of neutrophil apoptosis by tumour necrosis factor-α (TNF-α). Neutrophils (2·5 × 106/ml) were cultured for 30 min at 37° with the inhibitors of caspases 8 (Z-IETD-FMK), 3 (Z-DEVD-FMK) and 9 (Z-LEHD-FMK) (25 µm). Then, TNF-α (10 ng/ml) was added, and cells were treated with immobilized immunoglobulin G (iIgG), zymosan (Z) (50 µg/ml) or granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml). After 3 hr at 37°, the percentage of apoptotic cells was determined by fluorescence microscopy. Results are expressed as the mean ± standard error of the mean (SEM) of five or six experiments. *Statistical significance (P < 0·01) of neutrophils cultured in the presence of caspase inhibitors versus neutrophils cultured in the absence of caspase inhibitors.
Figure 3.
Promotion of neutrophil apoptosis by tumour necrosis factor-α (TNF-α) is associated with the loss of mitochondrial transmembrane potential, which was measured using rhodamine 123 (Rh123), as described in the Materials and methods. Neutrophils (2·5 × 106/ml) were treated for 1–2 min at 37°, with or without TNF-α (10 ng/ml), and then cultured in the presence or absence of immobilized immunoglobulin G (iIgG) for 2 hr at 37°. Rh123 (0·1 µg/ml) was then added to cell cultures for 30 min before analysis by flow cytometry. Loss of mitochondrial transmembrane potential results in a diminished ability of the cells to accumulate the green fluorochrome Rh123. Histograms of a representative experiment are shown (n = 4).
Promotion of neutrophil apoptosis by TNF-α is associated with a down-regulation in expression of the anti-apoptotic protein Mcl-1
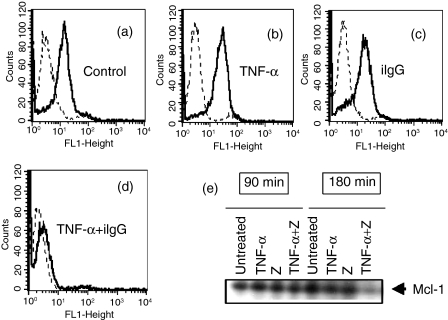
Apoptosis is regulated by the Bcl-2 family of proteins, with certain members of this family acting to protect from apoptosis (i.e. Bcl-2, Bcl-XL and Mcl-1), whereas others act to promote apoptosis (i.e. Bax, Bcl-XS and Bad).47,48 Previous work has shown that the cellular levels of Mcl-1 (an anti-apoptotic protein with a very short half life) decline as neutrophils undergo apoptosis and are enhanced by agents, such as GM-CSF, which promote neutrophil survival.47,49–51 Taking this into account, we then analysed whether promotion of apoptosis by TNF-α could be related to a decreased expression of Mcl-1. The results of flow cytometry (Fig. 4a) show a dramatic down-regulation of Mcl-1 expression in neutropils cultured with TNF-α plus iIgG. As expected, similar results were observed by Western blot analysis in neutrophils cultured with TNF-α plus Z (Fig. 4e).
Figure 4.
Promotion of neutrophil apoptosis by tumour necrosis factor-α (TNF-α) is associated with down-regulation of the expression of the anti-apoptotic protein Mcl-1. Neutrophils (2·5 × 106/ml) were incubated for 1–2 min at 37°, with or without TNF-α (10 ng/ml), and then cultured in the presence or absence of immobilized immunoglobulin G (iIgG) for 3 hr at 37° (a–d) or zymosan (Z) (50 µg/ml) for 90 or 180 min at 37° (e). The expression of Mcl-1 was then evaluated by flow cytometry (a–d) or immunoblotting (e), as described in the Materials and methods. Representative experiments are shown (n = 4).
Promotion of neutrophil apoptosis by TNF-α is increased by IL-1
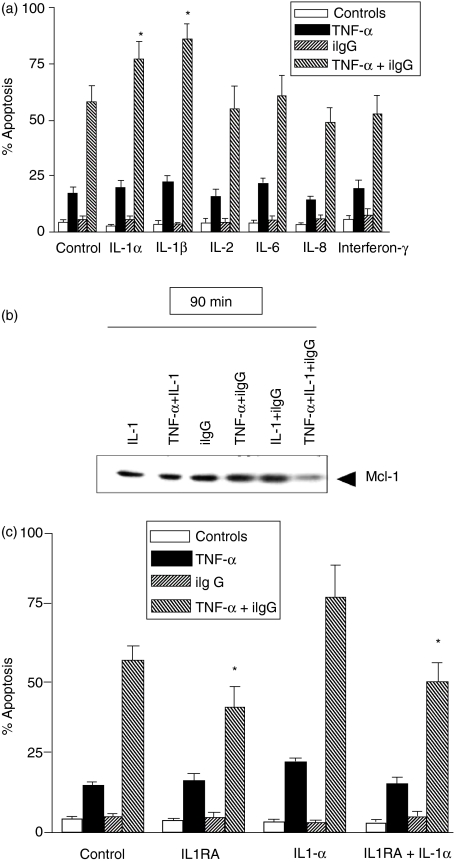
Various inflammatory cytokines are able to prolong the survival of neutrophils by preventing apoptosis.4–7,9 While GM-CSF seems to be the most potent anti-apoptotic agent for neutrophils, we have shown that, in contrast to the anti-apoptotic action exerted by GM-CSF on resting neutrophils, it stimulated apoptosis in TNF-α-pretreated cells.29 Similar results have been published by Daigle et al.52 We next investigated whether other anti-apoptotic cytokines could modulate the stimulation of apoptosis induced by TNF-α. Cells were treated with or without TNF-α and/or iIgG, in the presence or absence of the anti-apoptotic cytokines IL-1, IL-2, IL-6, IL-8 and IFN-γ4,7,9,53–55 and apoptosis was evaluated after 3 hr of culture. Figure 5(a) shows that none of these cytokines was able to modulate the rate of apoptosis of neutrophils treated with TNF-α alone, but IL-1α and IL-1β significantly increased the rate of apoptosis of neutrophils pretreated with TNF-α + iIgG. Moreover, in agreement with the results of Fig. 1 (showing that promotion of apoptosis by TNF-α involves a Mac-1-dependent pathway), we observed that blocking of CD18 partially prevented the stimulation of apoptosis in neutrophils cultured with IL-1α/TNF-α together with iIgG [% apoptosis=78 ± 9 versus 46 ± 6 for neutrophils cultured in the absence or presence of F(ab′)2 fragments of a blocking antibody directed to CD18, respectively, and then exposed to IL-1α/TNF-α and iIgG (n = 5, P ≤ 0·01)]. We then analysed whether the stimulation of apoptosis induced by IL-1 was also associated with a loss of Mcl-1 expression. Western blot analysis showed that cells cultured with IL-1α/TNF-α together with iIgG undergo a marked down-regulation of Mcl-1 expression as early as 90 min after treatment (Fig. 5b).
Figure 5.
Promotion of neutrophil apoptosis by tumour necrosis factor-α (TNF-α) is increased by interleukin (IL)-1. (a) Neutrophils (2·5 × 106/ml) were cultured for 1–2 min at 37°, with or without TNF-α (10 ng/ml), in the presence or absence of IL-1α, IL-1β, IL-2, IL-6, IL-8, or interferon-γ (IFN-γ) (50 ng/ml). Then, the cells were treated with immobilized immunoglobulin G (iIgG) for 3 hr and apoptosis was revealed by fluorescence microscopy. (b) Neutrophils (2·5 × 106/ml) were cultured for 1–2 min at 37°, with or without TNF-α (10 ng/ml), in the presence or absence of IL-1α (50 ng/ml), and then treated with iIgG for 90 min. Then, the expression of Mcl-1 was assessed by Western blotting. A representative experiment is shown (n = 4). (c) Neutrophils (2·5 × 106/ml) were cultured for 1–2 min at 37°, with or without TNF-α (10 ng/ml), in the presence or absence of the IL-1 receptor antagonist (IL-1RA) (1 µm) and/or IL-1α (50 ng/ml). Cells were then treated with iIgG for 3 hr and apoptosis was revealed by fluorescence microscopy. Results are expressed as the mean ± standard error of the mean (SEM) of four to six experiments. *Statistical significance (P < 0·05): IL-1α and IL-1β versus control (a); IL-1RA versus control and IL-1RA + IL-1 versus IL-1α (c).
Unexpectedly, when the effect of the IL-1RA was assessed, using concentrations able to block the binding of IL-1 to neutrophil IL-1R,56 we observed not only that the stimulation of apoptosis induced by exogenous IL-1 was abrogated, but also that IL-1RA significantly decreased the apoptosis of neutrophils cultured with TNF-α plus iIgG in the absence of exogenous IL-1 (Fig. 5c). These results support the notion that IL-1 produced by neutrophils themselves is involved in the stimulation of neutrophil apoptosis by TNF-α plus iIgG.
Discussion
In spite of the large number of studies carried out to analyse neutrophil apoptosis, little is known about how it is regulated in vivo during the course of acute inflammation. However, two lines of evidence support an important role for TNF-α. First, observations made in mice lacking the 55-000 molecular weight type 1 TNF receptor (TNFR1) and exposed to aerosolized Pseudomonas aeruginosa showed an enhanced acute inflammatory response, which was characterized by higher accumulation of neutrophils in the lungs compared with control mice.23 Interestingly, the concentration of chemotactic factors in the bronchoalveolar lavage fluid was found to be lower in TNFR1-deficient mice than in controls, supporting that the higher number of neutrophils in the lungs of TNFR1-deficient mice could be caused by an enhanced survival of neutrophils in the air spaces rather than by an increased rate of neutrophil recruitment.23 The second line of evidence arises from a number of recent in vitro studies performed at different laboratories. While all agree that neutrophils are relatively resistant to TNF-α, in terms of induction of apoptosis in vitro, they support the notion that TNF-α could play an important role in the regulation of neutrophil apoptosis by crosstalk with signalling cascades triggered by a variety of stimuli. Experiments performed at Henson laboratory showed that the activation of β2-integrins stimulates the serine-threonine kinase, Akt, and thus inhibits neutrophil apoptosis.41 By contrast, when activation of β2-integrins occurs in the presence of TNF-α, it leads to an enhancement of neutrophil apoptosis via SH2-containing inositol 5-phosphatase (SHIP) recruitment and inhibition of Akt activity.41,57 Previous studies performed by us have shown that neutrophil treatment with TNF-α enables not only pro-apoptotic agents, such as immune complexes, Z and PMA, but also anti-apoptotic agents, such as C5a and GM-CSF, to trigger a marked stimulation of apoptosis, suggesting that TNF-α is able to prime an apoptotic death programme which can be rapidly triggered by a variety of inflammatory mediators.29 Finally, Simon and co-workers have reported that TNF-α disrupts anti-apoptosis pathways triggered by survival factors, such as GM-CSF and G-CSF, via Sac homology domain 2 containing tyrosine phosphatase (SHP) recruitment and inhibition of the tyrosine kinase Lyn.52,58 Together, these observations support the notion that, during the course of inflammatory processes, TNF-α might promote neutrophil apoptosis by virtue of its ability to subvert the signalling cascades triggered by a variety of stimuli.
In agreement with previous reports, we found that stimulation of apoptosis in neutrophils cultured only with TNF-α involves a Mac-1-dependent pathway.38–41 Moreover, we observed that stimulation of apoptosis, triggered by a variety of stimuli in TNF-α-pretreated neutrophils, also depends, at least in part, on a Mac-1-dependent pathway.
Two very recent studies have shown that stimulation of neutrophil apoptosis by TNF-α involves not only caspase-dependent mechanisms, but also caspase-independent mechanisms. Maiansky et al.43 reported that neutrophil death induced by TNF-α was also observed when caspases were completely inhibited. This type of cell death lacks nuclear features of apoptosis, such as DNA laddering, and demonstrated no Bax redistribution, but it showed mitochondria clustering and plasma membrane phosphatidyl serine (PS) exposure. On the other hand, Liu et al.44 reported that broad-spectrum caspase inhibition suppresses exacerbation of apoptosis triggered by cycloheximide in TNF-α-treated cells but, paradoxically, augments the death of neutrophils cultured with TNF-α alone, which was associated with both apoptotic-like and necrotic-like features. Our present results showed that inhibition of caspases 3 and 8, but not caspase 9, significantly inhibit the stimulation of apoptosis of neutrophils cultured with TNF-α alone. Interestingly, the exacerbation of apoptosis triggered by different inflammatory stimuli in TNF-α-pretreated neutrophils was dramatically suppressed, not only by inhibition of caspases 3 and 8, but also by inhibition of caspase 9, supporting a critical role for caspases in the promotion of cell death. Moreover, the strong inhibition induced by the caspase 9 inhibitor supports the notion that exacerbation of apoptosis involves the participation of the mitochondrial-death pathway, which was confirmed by the loss of the mitochondrial transmembrane potential, evaluated by using the Rh123 method. Of note, the collapse of the mitochondrial transmembrane potential, as well as the marked decrease in the levels of Mcl-1 found in neutrophils pretreated with TNF-α and then exposed to triggering stimuli, were not found in neutrophils cultured with TNF-α alone, thus supporting the idea that both phenomena may be responsible for the exacerbation of apoptosis.
We also examined whether a number of anti-apoptotic cytokines were able to modulate the apoptosis of neutrophils pretreated with TNF-α and then incubated with iIgG. Unexpectedly, we found that the addition of IL-1α and IL-1β did not reduce apoptosis, but rather significantly increased it, without modifying the apoptotic rate of neutrophils cultured with TNF-α or iIgG alone. Interestingly, IL-1RA not only abrogated the proapoptotic effect induced by the addition of exogenous IL-1, but significantly decreased the apoptosis of neutrophils cultured with TNF-α plus iIgG in the absence of exogenous IL-1 (Fig. 5), thus supporting that the production of IL-1 by neutrophils themselves is required to allow maximal levels of neutrophil apoptosis after treatment with TNF-α plus iIgG.
Subversion of the anti-apoptotic signals induced by cytokines and other inflammatory agents appears to be the most important pathway through which TNF-α promotes neutrophil apoptosis. Does this mechanism play a role in the resolution of acute inflammatory processes? Further studies are required to answer this question. Special attention should be given to the temporal sequence at which the different inflammatory mediators appear during the course of inflammation. This point seems to be relevant in view of our previous results, which showed, in contrast to the observations made in resting neutrophils pretreated with TNF-α and then exposed to conventional agonists, no stimulation of apoptosis by TNF-α when it was added to previously activated neutrophils,29 an effect that could be explained, at least in part, by the dramatic shedding of TNF-α receptors that occurs after neutrophil activation.59
Acknowledgments
We thank Selma Tolosa and Federico Ramirez for their technical assistance and María Rita Furnkorn for her secretarial assistance. This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires University School of Medicine, Agencia Nacional de Promoción Científica y Tecnológica (FONCyT), and Fundación Antorchas, Argentina.
References
- 1.Savill JS, Henson PM, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel ‘charge-sensitive’ recognition mechanism. J Clin Invest. 1990;84:1518–27. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci. 1992;83:639–48. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 3.Colotta F, Polentarutti N, Sozzani S, Montovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20. [PubMed] [Google Scholar]
- 4.Brach MA, de Vos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed death. Blood. 1992;80:2920–4. [PubMed] [Google Scholar]
- 5.Yamamoto C, Yoshida S, Taniguchi H, Qin MH, Miyamoto H, Mizuguchi Y. Lipopolysacharide and granulocyte colony-stimulating factor delay neutrophil apoptosis and ingestion by guinea pig macrophages. Infect Immun. 1993;61:1972–9. doi: 10.1128/iai.61.5.1972-1979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pericle F, Liu JH, Diaz JI, Blanchard DK, Wei S, Forni G, Djeu JY. Interleukin-2 prevention of apoptosis in human neutrophils. Eur J Immunol. 1994;24:440–4. doi: 10.1002/eji.1830240226. [DOI] [PubMed] [Google Scholar]
- 7.Liles WC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. [PubMed] [Google Scholar]
- 8.Biffl WL, Moore EE, Moore A, Barnett CC. Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent. J Leukoc Biol. 1995;58:582–4. doi: 10.1002/jlb.58.5.582. [DOI] [PubMed] [Google Scholar]
- 9.Hebert MJ, Takano T, Holthofer H, Brady HR. Sequential morphologic events during apoptosis of human neutrophils: modulation by lipoxygenase-derived eicosanoids. J Immunol. 1996;157:3105–15. [PubMed] [Google Scholar]
- 10.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 2002;61:45663. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 11.Trevani AS, Andonegui G, Giordano M, Nociari M, Fontan P, Dran G, Geffner JR. Neutrophil apoptosis induced by proteolytic enzymes. Lab Invest. 1996;74:711–21. [PubMed] [Google Scholar]
- 12.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 13.Gamberale R, Giordano M, Trevani AS, Andonegui G, Geffner JR. Modulation of human neutrophil apoptosis by immune complexes. J Immunol. 1998;161:3666–74. [PubMed] [Google Scholar]
- 14.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–403. [PubMed] [Google Scholar]
- 15.Gamble JR, Harlan JM, Klebanoff SJ, Vadas MA. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci USA. 1985;82:8667–71. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalaby MR, Aggarwal BB, Rinderknetch E, Svedersky LP, Finkle BS, Jr, Palladino MA. Activation of human polymorphonuclear neutrophil function by interferon-γ and tumor necrosis factor. J Immunol. 1985;135:2069–73. [PubMed] [Google Scholar]
- 17.Klebanoff SJ, Vadas MA, Harlam JM, Sparks LM, Gamble JR, Agosti JR, Waltersdorph AM. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–5. [PubMed] [Google Scholar]
- 18.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–60. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright SD. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–9. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 21.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–43. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 22.Hodge-Dufour J, Marino MW, Horton MR, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–11. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276:L715–27. doi: 10.1152/ajplung.1999.276.5.L715. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita K, Takahashi S, Kobayashi S, et al. Caspases mediate tumor necrosis factor-α induced neutrophil apoptosis and downregulation of reactive oxygen production. Blood. 1999;93:674–85. [PubMed] [Google Scholar]
- 25.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-α. Int Immunol. 1993;5:691–4. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 26.Kettritz R, Gaido ML, Haller H, Luft FC, Jennete CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-α mediated apoptosis of human neutrophils. Kidney Int. 1998;53:84–91. doi: 10.1046/j.1523-1755.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 27.Kettritz R, Scheumann J, Xu Y, Luft FC, Haller H. TNF-alpha-accelerated apoptosis abrogates ANCA-mediated neutrophil respiratory burst by a caspase-dependent mechanism. Kidney Int. 2002;61:502–15. doi: 10.1046/j.1523-1755.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- 28.Murray J, Barbara JAJ, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-α: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–83. [PubMed] [Google Scholar]
- 29.Salamone G, Giordano M, Trevani A, Gamberale R, Vermeulen M, Schetinni J, Geffner J. Promotion of neutrophil apoptosis by TNF-α. J Immunol. 2001;166:3476–83. doi: 10.4049/jimmunol.166.5.3476. [DOI] [PubMed] [Google Scholar]
- 30.Boyum A. Separation of leukocytes from blood and bone narrow. J Lab Invest. 1968;22(Suppl. 97):77–89. [PubMed] [Google Scholar]
- 31.Coligan JE, Kruibeek AM, Margulies DH, Shevach EM, Strober W. Morphological and biochemical assays of apoptosis. In: Coico R, editor. Current Protocols in Immunology. Vol. 3. New York: Wiley; 1994. p. 17. [Google Scholar]
- 32.Coxon A, Tang T, Mayadas TN. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo: a role for granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:923–34. doi: 10.1084/jem.190.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Ricciardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 34.Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–65. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–50. [PubMed] [Google Scholar]
- 36.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–86. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 37.Todd RF, Petty HR. Beta 2 (CD11/CD18) integrins can serve as signaling partners for other leukocyte receptors. J Lab Clin Med. 1997;129:492–8. doi: 10.1016/s0022-2143(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 38.Walzog B, Jeblonsky F, Zakrzewicz A, Gaehtgens P. β2 integrins (CD11b/CD18) promote apoptosis of human neutrophils. FASEB J. 1997;11:1177–86. doi: 10.1096/fasebj.11.13.9367353. [DOI] [PubMed] [Google Scholar]
- 39.Van den Berg JM, Séller S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–73. [PubMed] [Google Scholar]
- 40.Avdi NJ, Nick JA, Whitlock BB, Billstrom MA, Henson PM, Johnson GL, Worthen GS. Tumor necrosis factor-α activation of the c-Jun N-terminal kinase pathway in human neutrophils. J Biol Chem. 2001;276:2189–99. doi: 10.1074/jbc.M007527200. [DOI] [PubMed] [Google Scholar]
- 41.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson P. Differential roles for αMβ2 integrin clustering or activation in the control of apoptosis via regulation of Akt and ERK survival mechanisms. J Cell Biol. 2000;151:1305–20. doi: 10.1083/jcb.151.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–54. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 43.Maiansky NA, Roos D, Kuijpers TW. Tumor necrosis factor alpha induces a caspase-independent death pathway in human neutrophils. Blood. 2003;101:1987–95. doi: 10.1182/blood-2002-02-0522. [DOI] [PubMed] [Google Scholar]
- 44.Liu CY, Takemasa A, Liles WC, et al. Broad-spectrum caspase inhibition paradoxically augments cell death in TNF-alpha-stimulated neutrophils. Blood. 2003;101:295–304. doi: 10.1182/blood-2001-12-0266. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with Rhodamine 123. Proc Natl Acad Sci USA. 1980;77:990–4. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pithon-Curi TC, Schumacher RI, Freitas JJS, Lagranha C, Newsholme P, Palanch AC, Doi SQ, Curi R. Glutamine delays spontaneous apoptosis in neutrophils. Am J Physiol Cell Physiol. 2003;284:C1355–61. doi: 10.1152/ajpcell.00224.2002. [DOI] [PubMed] [Google Scholar]
- 47.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. Bcl-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70:783–92. [PubMed] [Google Scholar]
- 48.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–5. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 49.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–502. [PubMed] [Google Scholar]
- 50.Leuenroth SJ, Grutkoski PS, Ayala A, Simms HH. The loss of Mcl-1 expression in human polymorphonuclear leukocytes promotes apoptosis. J Leukoc Biol. 2000;68:158–66. [PubMed] [Google Scholar]
- 51.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–22. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 52.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat Med. 2002;8:61–7. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 53.Watson RW, Rotstein OD, Parodo J, Bitar R, Marshall JC, William R, Watson G. The IL-1 beta-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1 beta. J Immunol. 1998;161:957–62. [PubMed] [Google Scholar]
- 54.Grutkoski PS, D'Amico R, Ayala A, Simms HH. IL-1beta stimulation induces paracrine regulation of PMN function and apoptosis. Shock. 1999;12:373–81. doi: 10.1097/00024382-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Grutkoski PS, Graeber CT, Ayala A, Simms HH. Paracrine suppression of apoptosis by cytokine-stimulated neutrophils involves divergent regulation of NF-kappa B, Bcl-X (L), and Bak. Shock. 2002;17:47–54. doi: 10.1097/00024382-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Granowitz EV, Clark BD, Mancilla J, Dinarello CA. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991;266:14147–50. [PubMed] [Google Scholar]
- 57.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadock V, Bratton D, Henson P. Activation of SHIP by NADPH oxidase-stimulated Lyn leads to enhanced apoptosis in neutrophils. J Biol Chem. 2002;277:5236–46. doi: 10.1074/jbc.M110005200. [DOI] [PubMed] [Google Scholar]
- 58.Yousefi S, Simon HU. SHP-1: a regulator of neutrophil apoptosis. Semin Immunol. 2003;15:195–9. doi: 10.1016/s1044-5323(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 59.Porteu F, Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]