Abstract
Glucuronoxylomannan (GXM) is the major Cryptococcus neoformans capsular polysaccharide and represents the main virulence factor of this fungus. In in vitro studies we have demonstrated previously that this acidic and high-molecular-weight polysaccharide suppresses lymphoproliferation, modulates cytokine production and promotes apoptosis in spleen mononuclear (Spm) cells from rats. In this study we demonstrate that these phenomena also occur in vivo after the intracardiac inoculation of GXM into normal Wistar rats. The results of this study show suppression of the proliferative response Spm cells to concanavalin A (Con A) or heat-killed C. neoformans (HKCn) in the first 2 weeks after polysaccharide administration. In addition, increased levels of interleukin (IL)-10 were produced by Con A-stimulated Spm cells, coinciding with immunohistochemical GXM detection in the white pulp of spleen. In particular, high production of IL-10 with diminution of IL-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α synthesis were detected 14 days after GXM administration. In situ cell death detection by TdT-mediated biotin–dUTP nick-end labelling (TUNEL) reaction in sections of spleen, lung and liver demonstrates apoptosis in tissues with deposits of GXM. These data demonstrate the in vivo ability of GXM to modify cytokine synthesis by Spm cells and to promote host cell apoptosis.
Keywords: apoptosis, Cryptococcus neoformans, glucuronoxylomannan, IL-10, immunosuppression
Introduction
The encapsulated fungus, Cryptococcus neoformans, causes life-threatening mycoses in patients immunocompromised by immunosuppressive therapies or AIDS.1,2 This facultative intracellular pathogen3 is also able to depress cell-mediated immunity, and under certain circumstances to cause persistent and latent infections in immunocompetent hosts.4–6 The capability of C. neoformans to mediate disease in individuals with apparently normal immunity is indicative of the expression of microbial characteristics that promote its ability to evade normal host defences.7,8 The capsule represents the main virulence factor of this yeast and its mechanisms of pathogenesis are those involved in modulation of the host immune response.9 88% of the fungal envelope is composed of an acidic and viscous polysaccharide, designated glucuronoxylomannan (GXM), which is released continuously by encapsulated yeasts during their replication.10,11 High levels of GXM in the body fluids of patients with cryptococcosis have a direct relationship with the severity of disease.1,2 In this sense, several studies have indicated that this high-molecular-weight polysaccharide could be responsible for the pathobiological phenomena attributed to the capsule.12–16 Experiments performed in vitro with human or murine mononuclear cells have shown that C. neoformans capsular polysaccharides impair lymphoproliferation,12–14 induce interleukin (IL)-4 and IL-10 by splenic adherent cells from mice15 and GXM, in particular, stimulates IL-10 liberation by human monocytes.16
In accordance with these findings, we have also demonstrated in our laboratory the ability of purified GXM to induce non-specific suppression of T lymphocyte activation and Th2 cytokine profile in cultures of spleen mononuclear (Spm) cells from normal rats.17 Furthermore, our studies have provided strong cytometric, molecular and morphological evidence demonstrating that purified GXM promotes cell death by apoptosis in Spm cells and T lymphocytes in vitro.18 These phenomena were also found in rats infected with C. neoformans, which showed altered function of phagocytic cells, diminution of cell-mediated immunity, secretion of Th2 cytokines (particularly increased levels of IL-10) by Spm cells and induction of programmed cell death in the infected organs.17–24
The in vivo immunomodulatory effects of purified C. neoformans capsular antigens have been investigated mainly in mice. These reports have revealed immunological paralysis or suppression of the B lymphocyte response in mice inoculated with high doses of capsular polysaccharides or GXM.25,26 In addition, Murphy and coworkers27–29 have described a cascade of antigen-specific suppressor T cells that inhibited delayed-type hypersensitivity, and Blackstock et al.30 have observed a macrophage-suppressive lymphokine released by T cells after the injection of solubilized cryptococcal antigens in mice.
Taking previous studies into account, the purpose of this work was to investigate further the effects of purified GXM on a normal host immune response. Further to our previous reports, showing that GXM in vitro suppresses T cell response to a mitogen stimulus, modifies the cytokine profile and induces apoptosis of rat Spm cell,17,18 we focused our research on the analysis of these phenomena in vivo during 30 days after GXM administration in Wistar rats.
Materials and methods
Microorganism and preparation of GXM
C. neoformans var. neoformans serotype A, strain 102/85 from the National University of Cordoba stock culture collection was used in all the experiments. Cultures were grown on Sabouraud glucose agar slants at 37° and maintained by weekly subculture on the same medium, checked periodically for assimilation pattern, urease production and virulence.24 Heat-killed C. neoformans (HKCn) were prepared by incubating the C. neoformans isolate for 1 hr at 60°.31 As reported by Cherniak et al.,11 the GXM was prepared by precipitation with ethanol and hexadecyltrimethyl ammonium bromide (CTAB, Sigma Chemical Co., St Louis, MO) with slight modifications.17 Detection of neutral carbohydrates was performed by the phenol–sulphuric acid method of Dubois et al.32 In order to avoid endotoxin contamination of the experimental results, the whole process of GXM preparation was performed using sterile water, phosphate-buffered saline (PBS), plastic- or glassware. In addition, in vitro experiments were performed previously in the absence or presence of polymixin B (Sigma-Aldrich) in order to exclude lipopolysaccharide (LPS) contamination.17,18
Animals and GXM inoculation
Female inbred Wistar rats, 8–12 weeks old, were used in this study. Animals were housed and cared for in the animal resource facilities of the Department of Clinical Biochemistry, Faculty of Chemical Sciences, National University of Córdoba, in accordance with institutional guidelines. Rats were injected via the intracardiac (i.c.) route with 0·5 ml of GXM preparation in PBS containing 200 µg of carbohydrate. Seven, 14 and 30 days after GXM i.c. inoculation rats were anaesthetized and killed, and spleens, lungs and livers were removed. Half the spleen was fixed in formalin and the remainder was processed to obtain a single cell suspension. Sera were collected and stored at −20°. PBS-treated rats were used as negative controls and three animals from each group were used in the experiments. All experimental protocols were approved by the Animal Experimentation Ethics Committee, Faculty of Chemical Sciences, National University of Cordoba.
Measurement of serum GXM levels
Titres of the cryptococcal antigen in the sera of experimental animals were determined by using a commercially available latex agglutination assay (Immuno-Mycologics, Inc., Norman, OK). Serial twofold dilutions of serum were tested for ability to agglutinate latex particles coated with anti-GXM according to the manufacturer's protocol. The limit of detection of this kit is 0·5 ng/ml and approximately 15 ng/ml of C. neoformans capsular polysaccharide gave a positive reaction up to one-quarter dilution. Results are expressed as mean log2 antigen titre ± SEM.
Tissue immunohistochemistry
Localization of GXM in tissues and cytospin preparations of Spm cells from freshly explanted spleens was observed by immunohistochemistry, as described previously.6,33–35 GXM-specific antibody 2H1 (mouse IgG1) was kindly provided by Dr Arturo Casadevall (Albert Einstein College of Medicine, Bronx, NY) and used as primary antibody.36 Peroxidase-conjugated goat antimouse IgG (Sigma) was used as a secondary antibody and colour was developed with diaminobenzidine (DAB, Sigma). For all immunohistochemical studies, negative controls consisting of the primary antibody omission and staining of tissues from PBS-treated rats were carried out. Cytospin preparations of Spm cells were counterstained with haematoxylin and the percentage of cells with typical apoptosis morphological changes was determined by light microscopy, as was chromatin condensed against the nuclear periphery and the entire nucleus coagulated into small or dense balls, etc.37 At least 200 cells were counted in three different cytospin preparations of each animal.
Spleen cell culture and proliferation assay
Spleen cells were prepared as described previously.24 Briefly, spleens were removed aseptically from PBS- or GXM-inoculated rats, minced and passed through stainless steel mesh to obtain single-cell suspensions in endotoxin-free RPMI-1640 (Sigma) with 1% fetal calf serum (FCS) and Gentamicin (50 mg/l) (Sigma). Spleen mononuclear (Spm) cells were obtained by Ficoll-Hypaque (Sigma) gradient centrifugation, washed, counted and resuspended in the same medium supplemented with 10% heat-inactivated calf fetal serum (FCS) and β-mercaptoethanol (5 × 10−5 m) (Sigma). For immunohistochemistry, cytospin preparations of Spm cells were performed after being washed twice in PBS. Spm cells (1·5 × 106/ml) were cultured in medium alone or in the presence of Con A (10 µg/ml, Sigma) for 96 hr at 37°, and 5% CO2 in humid atmosphere. Antigen-specific response was evaluated by culturing Spm cells (1·5 × 106/ml) in the presence or absence of HKCn (1·5 × 106 yeast/ml) for 7 days.13 Proliferative response was determined in the cultures pulsed for 18 hr with 1 µCi [3H]-thymidine (Comisión Nacional de Energía Atómica, CNEA) by measuring its incorporation into cell DNA using a Beta liquid scintillation counter (Beckman, LS 7000).
Cytokine measurement
Supernatant fluids from cultures performed as described above were collected for cytokine determination. The supernatants were collected after 24 hr of culture for IL-2, 48 hr for interferon (IFN)-γ, and 67 hr for IL-10, tumour necrosis factor (TNF)-α and IL-4.17 Supernatants were frozen at −70° until analysed. Cytokines were measured by sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol, using two cytokine-specific monoclonal antibodies (mAbs) for each cytokine, one of which was biotinylated. Antibodies purchased from Biosource International (CA, USA) were used for IFN-γ determination, and antibodies from BD PharMingen (CA, USA) were used for the remaining cytokines. Dilutions of recombinant rat IL-2, IL-10, TNF-α, IL-4 (BD PharMingen) and IFN-γ (Biosource International) were used as standards. After appropriate washing, the plates were reacted with horseradish peroxidase streptavidin (Sigma) followed by o-phenylenediamine addition (5–20 min), and stopped with 25 µl sulphuric acid. The reaction was read using a Microplate Reader (Biorad, CA). Results are expressed as fold increase of cytokine levels in supernatants of Spm cells from GXM-treated rats over cytokine levels produced by cells from control animals.
TdT-mediated biotin–dUTP nick-end labelling (TUNEL) staining for detection of apoptosis
Apoptosis in tissue sections was detected using the TUNEL technique,38 in which reagents from an in situ cell death detection kit (Boehringer Mannheim, Indianapolis, IN) are used to label the cleaved DNA ends that occur in apoptotic cells. Tissue sections from control or GXM-injected rats were processed according to the manufacturer's protocol. The samples were counterstained with methylgreen and photographed.
Statistics and data presentation
Three animals per group were used in all studies, and experiments were performed in triplicate samples of individual animals from each group. Data are expressed as mean ± SEM and analysed statistically using Student's t-test, except from cryptococcal antigen titres in sera which were compared by Mann–Whitney U-test. A value of P < 0·05 was considered to be significant. All experiments were repeated and equivalent results were obtained.
Results
Serum GXM levels
Serum polysaccharide levels were determined by a latex agglutination assay (Fig. 1). One week after GXM inoculation, two of three rats had a GXM titre of 1:64 and the third rat showed a higher positive latex endpoint (1:28). Fourteen days after administration of GXM, rats showed a significant diminution of serum GXM titres (1:32) with respect to those observed on day 7. These titres of GXM persisted 30 days after polysaccharide inoculation.
Figure 1.
Serum GXM concentrations in rats inoculated with 200 µg of GXM. Data presented are means ± standard errors of the means (SEM) for three individual rats at each time period. The graph is representative of two independent experiments. *P < 0·03 (14 days versus 7 days, GXM-treated rats).
Tissue GXM localization in lung, spleen and liver
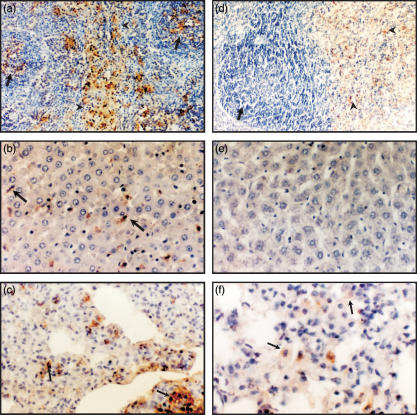
In order to determine the pattern of distribution and persistence of polysaccharide in tissues, we studied the immunohistochemical localization of GXM after i.c. inoculation, 200 µg per rat. Lung, liver and spleen were immunostained 7, 14 and 30 days after GXM injection, using the GXM-specific monoclonal antibody 2H1.36 Rats killed on day 7 revealed strong GXM immunoreactivity in sections from three organs analysed (Fig. 2a,b,c). Spleen showed GXM deposits in white and red pulp (Fig. 2a), and liver revealed GXM immunoreactivity in cells with the morphological features of Kupffer cells (Fig. 2b). In addition, lung from GXM-treated rats presented GXM deposits and tissue parenchyma thickening (Fig. 2c). Fourteen days after GXM administration, tissues revealed a similar GXM distribution to those observed on day 7 (data not shown). Thirty days after polysaccharide inoculation, a weaker GXM immunoreactivity was observed in tissues compared to that observed on previous days of treatment (Fig. 2d,e,f). Fungal polysaccharide was cleared from the white pulp of spleen and detected exclusively in the red pulp (Fig. 2d). GXM storage in liver was almost non-existent (Fig. 2e); however, lung revealed persistent polysaccharide immunoreactivity (Fig. 2f).
Figure 2.
Representative micrograph of immunohistochemistry for GXM in rat tissue after GXM administration (200 µg). GXM-specific mAb 2H1 and peroxidase-conjugated goat antimouse IgG were used as primary and secondary antibodies. Colour was developed with DAB-H2O2 and sections were counterstained with haematoxylin. (a) Spleen sections from GXM-inoculated rats (day 7) reveal immunoreactivity in white (arrow) and red pulp (arrow head). Magnification × 100. (b) GXM immunoreactivity within cells with morphological features of Kupffer cells in liver sections from 7-day-treated rats. Magnification × 200. (c) Lung from 7-day-inoculated rats shows GXM immunoreactivity (arrows). Magnification × 200. (d) Spleen sections from 30-day-treated rats reveal GXM detection in red pulp (arrow head) and GXM clearance from white pulp (arrow). Magnification × 100. (e) Liver sections show scarce GXM immunoreactivity after 30 days post-GXM inoculation. Magnification × 200. (f) Lung sections from 30-day-inoculated rats show intracellular GXM immunoreactivity (arrows). Magnification × 400.
Effects of GXM administration on Spm cell proliferative response
Seven days after GXM treatment, Con A stimulation of Spm cells resulted in a similar proliferative response to that observed in Con A-stimulated Spm cells from control animals (Fig. 3). Conversely, Spm cells from rats injected with GXM 14 days earlier showed suppression of blastogenic response to Con A compared to Spm cells from control animals (P < 0·04). On day 30, Spm cells restored the lymphoproliferation to similar levels to those observed in Spm cells from controls.
Figure 3.
Blastogenic response to Con A at 7, 14 and 30 days post-GXM administration. Spm cells (1·5 × 106/ml) from PBS-inoculated (control) and GXM- injected rats were cultured in medium alone or stimulated with 10 µg/ml of Con A for 96 hr in 5% CO2 at 37°. After 18 hr of pulse, [3H]thymidine incorporation was measured. Each column shows the mean ± SEM of three animals in each group and the results are representative of three independent experiments. *P < 0·04 (GXM-inoculated rats versus control animals).
We also evaluated the antigen-specific proliferative response of Spm cells from GXM-inoculated and control rats in response to HKCn (Fig. 4). HKCn stimulation of Spm cells resulted in a diminution of lymphoproliferation 7 and 14 days after GXM injection, compared to lymphoproliferation of Spm cells from control rats (P < 0·05 and P < 0·03, respectively). However, on day 30 the proliferative response of Spm cells to HKCn was similar to controls.
Figure 4.
Blastogenic response to heat-killed yeast (HKCn) at 7, 14 and 30 days post-GXM administration. Spm cells (1·5 × 106/ml) from control and GXM-treated rats were cultured in presence of HKCn (1·5 × 106/ml) for 7 days in 5% CO2 at 37°. After 18 hr of pulse, [3H]thymidine incorporation was measured. Each column shows the mean ± SEM of three animals in each group and the results are representative of three independent experiments. *P < 0·05 and **P < 0·03 (7- and 14-day GXM-inoculated rats versus control animals, respectively).
Effects of GXM administration on cytokine production by spleen cells
To determine whether administration of GXM could modify cytokine production by Spm cells, levels of IL-2, IL-4, IL-10, IFN-γ and TNF-α were measured in cultures of Con A-stimulated splenocytes (Fig. 5). IL-4 was not detected above the background levels using the ELISA method on any of the days analysed (data not shown). On day 7 a statistically significant increase of IFN-γ, IL-2 and IL-10 levels was detected with respect to Spm cells from control rats (P < 0·02, P < 0·002 and P < 0·03, respectively). In addition, no significant difference was observed in TNF-α production between Spm cells from control or inoculated animals. In contrast, 14 days after GXM inoculation, levels of IFN-γ produced by Spm cells from GXM-treated rats were not modified, and IL-2 or TNF-α production diminished with respect to controls (P < 0·01 and P < 0·001). Nevertheless, IL-10 synthesis of splenocytes from 14-day GXM-treated rats was increased significantly with respect to controls (P < 0·05). Finally, on day 30, production of IFN-γ, IL-2, IL-10 and TNF-α by spleen cells was similar to control rats. Cytokine production by Spm cells incubated with medium alone or HKCn was not detected by the ELISA method used.
Figure 5.
Cytokine production by Con A-stimulated Spm cells from rats after GXM administration. Spm cells (1·5 × 106/ml) from PBS-inoculated (control) and GXM-injected rats were cultured in 5% CO2 at 37° in presence of 10 µg/ml of Con A. The supernatants for cytokine measurement were collected at 24 hr for IL-2, 48 hr for IFN-γ and 67 hr for IL-10 and TNF-α. ELISA capture assay was used to determine the cytokine levels. Bars represent SEM values. Results are expressed as fold increase of cytokine levels in cell cultures from GXM treated-rats over cytokine levels in cell cultures from control rats. Each point shows the mean ± SEM of three animals in each group and the results are representative of three independent experiments. *P < 0·002, ·P < 0·03, +P < 0·02, ··P < 0·05, **P < 0·01, &P < 0·001 (GXM-treated rats versus control rats).
Apoptosis induction by GXM in vivo
We have demonstrated recently that GXM in vitro is able to induce apoptosis of rat splenocytes.18 In this study we investigated whether this phenomenon occurs in vivo, after the injection of GXM into normal rats. Apoptosis was studied by the TUNEL reaction in the organs described above, which showed GXM retention. On day 7, detection of apoptosis in situ revealed scattered TUNEL-positive cells only in sections of lung (Fig. 6a). Apoptotic cells were found in association with metachromasia of methylgreen, suggesting the presence of polysaccharide39 (Fig. 6a,b). Moreover, 7 days after GXM administration spleen and liver sections did not show differences in the TUNEL reaction with respect to control rats (data not shown). In contrast, 14 days after GXM administration, TUNEL staining demonstrated numerous apoptotic cells in all three organs analysed (Fig. 6b,c,d). Lung and spleen showed TUNEL-positive cells next to metachromatic areas, and liver revealed apoptotic cells that resembled Kupffer cells. In addition, GXM-specific immunohistochemical staining was performed in cytospin preparations of Spm cells from control and GXM-treated rats (Fig. 6e). Analysis of nuclei with typical apoptosis morphology showed that 7 days after GXM treatment there was no difference between inoculated and controls rats (data not shown); however, a significant increase in the percentage of apoptotic cells was observed in Spm cells from 14-day-treated rats compared to controls (Fig. 6b). These cytospin preparations showed that the majority of cells with cytoplasmic polysaccharide reactivity presented typical apoptotic nuclei (Fig. 6e). Finally, 30 days after GXM administration apoptotic cells were not detected in sections from liver, but some TUNEL-positive cells were still observed in lung and spleen (data not shown). Moreover, on day 30 no difference was found in the percentage of apoptotic cells in cytospin preparations of Spm cell from GXM-treated or control rats.
Figure 6.
Representative micrograph of DNA end-labelling (TUNEL) of tissue sections from rats after 7 and 14 days of GXM administration, and immunohistochemistry for GXM in cytospin preparations from rat Spm cells (14 days post-inoculation). (a) TUNEL labelling of lung sections from 7-day GXM-treated rats reveals positive staining (arrow) next to metachromatic areas of methylgreen (arrow heads). Magnification × 400. (b) TUNEL positive cells (arrows) and metachromatic zones (arrow heads) in lung sections from 14-day GXM-treated rats. Magnification × 400. (c, d) TUNEL-positive cells (arrows) in liver and spleen sections from 14-day GXM-treated rats. Magnification × 400. (e) GXM immunoreactivity within Spm cells with nuclei with morphological features of apoptosis (14 days post-inoculation). Magnification (1000×). Cytospin preparations were counterstained with haematoxylin. (f) Percentage of cells with morphological features of apoptosis in cytospin preparations of Spm cell from control and 14-day GXM-injected rats. Three animals per group were used and three slides from each animal were prepared. At least 200 cells were counted by light microscopy in each cytospin preparations. Each column shows the mean ± SEM and the graph is representative of two independent experiments. *P < 0·05.
Discussion
C. neoformans capsular polysaccharide, GXM, is a Type 2 T cell-independent (TI-2) antigen that represents a major virulence factor of this fungus.40 No mammalian enzymes are known to digest GXM in vivo41 and individuals with chronic cryptococcosis and meningoencephalitis present extensive GXM storage in infected organs.42 Furthermore, it is known that complex polysaccharides, which are not digested by the lysosomal enzymes, can be retained by macrophages for long periods of time with immunopathological implications.43 It is well established that high levels of cryptococcal antigens in fluid and tissues from both human and experimental animals result in humoral and cellular immunosuppression.44 The mechanisms underlying immunosuppression induced by GXM have been studied mainly in in vitro systems, whereas these phenomena have been rarely explored in vivo. The results presented in this study contribute further to the knowledge of the in vivo effects of GXM on the immune response from an immunocompetent host, showing that the presence of purified GXM in tissues modulates cytokine synthesis and induces apoptosis of host cells.
We analysed first the clearance and organ distribution of GXM after i.c. inoculation of 200 µg into normal rats. Our results agree with previous reports33,45–51, showing that GXM persists in low titres in serum and accumulates in tissues for an extended time. In this study, GXM was detected in serum over the 30 days analysed, with a significant diminution of titres between 7 and 14 days after inoculation, indicating a slow serum antigen clearance. Furthermore, retention of capsular polysaccharides has been reported in organs that are rich in cells of the mononuclear phagocyte system, such as spleen and liver, and macrophages in particular serve as a reservoir for GXM in the body.33,48,51 In this study, during the first 2 weeks after polysaccharide administration, the immunohistochemical detection of GXM in rat tissues revealed a strong positive reaction in all three organs analysed. In particular, spleen sections showed prominent staining of white and red pulp (Fig. 2) and liver revealed detection of GXM in kupffer cells. We also extended the analysis to GXM distribution in lung tissue by using the i.c. route to inoculate animals. In this sense, minimal GXM detection has been reported by using intravenous inoculation of GXM into experimental animals.33,51 Taking into account that we focused on exploring the effects of GXM deposits in tissues, and lung represents a target organ during cryptococcal infections, the i.c. route was chosen with the hypothesis that extensive polysaccharide storage would be found in this organ. The results show strong immunohistochemical detection of GXM in lung, which persists at least 30 days after i.c. inoculation of 200 µg. Finally, clearance of GXM from tissues was seen on day 30, as a low positive immunohistochemical reaction was observed in lung and red pulp of spleen, with almost complete polysaccharide elimination from liver (Fig. 2). Spleen has been described as the main organ involved in long-term storage of GXM45 and retention in lung has been described in persistent C. neoformans pulmonary infection, where GXM accumulated in pulmonary macrophages.6
TI-2 antigens are capable of inducing regulatory cytokines in the spleens of mice.52,53 For instance, the high-molecular-weight polysaccharide antigen of Echinococcus multilocularis acellular laminated layer induces a Th2 shift in vivo.54 In addition, injection of cryptococcal polysaccharide in mice resulted in immunosuppression with generation of T suppressor cells and release of an unidentified lymphokine that inhibited the microbicide function of macrophages.27–30 It has also been demonstrated in mice that GXM in vivo inhibits leucocyte migration into sites of acute inflammation.55 The results presented here demonstrate that the presence of GXM in the white pulp of spleen from normal rats impairs non-specific and specific lymphoproliferation with modification of normal cytokine synthesis. In particular this polysaccharide induces, in vivo, increased levels of IL-10 production by spleen cells. After 14 days of GXM administration, increased IL-10 production by splenocytes, with suppression of non-specific lymphoproliferation, IL-2 and TNF-α production was observed (Figs 3 and 5). To our knowledge, this is the first demonstration in which GXM inoculation alters cytokine production by splenocytes from an immunocompetent host, and these data confirm previous observations from experiments performed in vitro by our laboratory and others.13,15–18 In addition, we have previously published IL-10 involvement in the immunosuppressive phenomenon that occurred 14 days after intraperitoneal C. neoformans infection in rats.17 The cellular population and mechanisms involved in the in vivo modification of cytokine profile by GXM remains unknown. Macrophages could be involved in this effect due to their ability to secrete IL-10 in presence of GXM in vitro.16
On the other hand, in situ detection of programmed cell death in tissue sections of GXM-treated rats performed in this study demonstrates that purified GXM is also able to induce apoptosis in vivo. Several studies have demonstrated that pathogen-derived molecules can promote host cell apoptosis, representing one of the key factors in microorganism survival strategy.56–58 Recently, Ibata-Ombetta et al.59 have demonstrated that a surface Candida albicans phospholipomannan promotes survival of phagocytosed yeast through macrophage apoptosis. In cryptococcosis, we have previously shown apoptosis induction during the immunosuppressor period of experimental infection in rats and the ability of purified GXM to induce apoptosis of splenocytes in vitro.18 In this study, TUNEL reaction revealed numerous apoptotic cells in lung, liver and spleen 14 days after GXM inoculation (Fig. 6b,c,d) which coincided with blastogenic splenocytes unresponsiveness and cytokine production modulation. Apoptosis was associated with the presence of polysaccharide in these organs because apoptotic cells were detected next to metachromatic zones of the methylgreen; however, no detection of apoptotic cells in liver coincided with clearance of GXM. In addition, some Kupffer cells were positive for TUNEL reaction (Fig. 6c) which coincided with immunohistochemical GXM localization. Association between GXM and apoptosis was also observed in Spm cells from 14-day-treated rats. In cytospin preparations of splenocytes, cells with a positive cytoplasmic GXM reaction showed nuclei with morphological features of apoptosis (Fig. 6e). We have observed previously intracellular GXM in macrophages undergoing apoptosis during disseminated cryptococcosis in rats.17 Thus, these findings suggest that intracellular retention of polysaccharide in phagocytic cells could promote programmed cell death. In this regard, Feldmesser et al.3 have demonstrated intracellular polysaccharide production associated with latency and cellular cytotoxity during pulmonary cryptococcosis in mice. Therefore, the results obtained in this work demonstrate for the first time that GXM promotes programmed cell death in vivo, and support previous evidence from our group to show that GXM causes apoptosis of host cells. Further investigations are currently under way in order to elucidate the cellular population and mechanisms involved in apoptosis induced by the capsular polysaccharide of C. neoformans.
In conclusion, immunomodulation of host defences observed during cryptococcal infections could be a consequence of polysaccharide storage in body tissues. This study demonstrates that GXM deposits in rat tissues coincide with down-regulation of lymphoproliferation, induction of IL-10 production and apoptosis of host cells. These phenomena could represent strategies for the persistence of C. neoformans in the infected host.
Acknowledgments
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECyT-UNC). L. S. Chiapello and J. Baronetti are recipients of fellowships from CONICET. D. T. Masih is Member of the Research Career from CONICET, Argentina. We thank Dr Roxana Cano for her help with experiments.
References
- 1.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficency syndrome. N Engl J Med. 1989;321:794–9. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 2.Eng RHK, Bishburg E, Smith SM. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986;81:19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- 3.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–37. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monari C, Retini C, Pietrella D, Pallazzetti D, Pitzurra L, Casadevall A. Cryptococcus neoformans differently regulates B7–1 (CD80) and B7–2 (CD86) expression on human monocytes. Eur J Immunol. 1998;28:114–21. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 5.Huffnagle G, Strieter RM, Standiford T, McDonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocytes chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol. 1995;155:4790–7. [PubMed] [Google Scholar]
- 6.Goldman D, Lee S, Mednick A, Montella L, Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68:832–8. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A, Pirofski LA. Host–pathogen interactions: redefining the basic concept of virulence and pathogenicity. Infect Immun. 1999;67:3703–13. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozel TR. Virulence factors of Cryptococcus neoformans. Trends Microbiol. 1995;3:295–9. doi: 10.1016/s0966-842x(00)88957-x. [DOI] [PubMed] [Google Scholar]
- 9.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–17. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 10.Cherniak R, Reiss E, Turner SH. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:139–250. [Google Scholar]
- 11.Cherniak R, Morris LC, Anderson BC, Meyer SA. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect Immun. 1991;59:59–64. doi: 10.1128/iai.59.1.59-64.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syme RM, Bruno TF, Kozel TR, Mody CH. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun. 1999;67:4620–7. doi: 10.1128/iai.67.9.4620-4627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mody CH, Syme RM. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–9. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664–9. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida GM, Andrade RM, Bento CAM. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4+T cells in a dominant Th2 pattern. J Immunol. 2001;167:5845–51. doi: 10.4049/jimmunol.167.10.5845. [DOI] [PubMed] [Google Scholar]
- 16.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel TR. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–9. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiapello L, Iribarren P, Cervi L, Rubinstein H, Masih DT. Mechanisms for induction of immunosuppression during experimental cryptococcosis. Role of glucuronoxylomannan. Clin Immunol. 2001;100:96–106. doi: 10.1006/clim.2001.5046. [DOI] [PubMed] [Google Scholar]
- 18.Chiapello LS, Aoki MP, Rubinstein HR, Masih DT. Apoptosis induction by glucuronoxylomannan of Cryptococcus neoformans. Med Mycol. 2003;41:347–53. doi: 10.1080/1369378031000137260. [DOI] [PubMed] [Google Scholar]
- 19.Masih DT, Rubinstein HR, Sotomayor CE, Ferro ME, Riera CM. Non-specific immunosuppression in experimental cryptococcosis in rats. Mycopathology. 1986;94:79–84. doi: 10.1007/BF00437371. [DOI] [PubMed] [Google Scholar]
- 20.Sotomayor CE, Rubinstein HR, Riera CM, Masih DT. Immunosuppression in experimental cryptococcosis in rats. Induction of afferent T suppressor cells to a non-related antigen. J Med Vet Mycol. 1987;65:67–75. doi: 10.1080/02681218780000111. [DOI] [PubMed] [Google Scholar]
- 21.Masih DT, Sotomayor CE, Rubinstein HR, Riera CM. Immunosuppression in experimental cryptococcosis in rats. Induction of efferent T suppressor cells to a non-related antigen. Mycopathology. 1991;144:179–86. doi: 10.1007/BF00437212. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein HR, Sotomayor CE, Cervi LA, Riera CM, Masih DT. Immunosuppression in experimental cryptococcosis in rats: modification of macrophage functions by T suppressor cells. Mycopathology. 1989;108:11–9. doi: 10.1007/BF00436778. [DOI] [PubMed] [Google Scholar]
- 23.Rossi GR, Sastre DA, Rubinstein HR, Masih DT. Biochemical basis for the killing of Cryptococcus neoformans by rat peritoneal cells. J Med Vet Mycol. 1994;32:405–14. [PubMed] [Google Scholar]
- 24.Rossi GR, Cervi LA, Sastre DA, Masih DT. Lack of involvement of nitric oxide in the macrophage-mediated inhibition of spleen cells proliferation during experimental cryptococcosis. Clin Immunol Immunopathol. 1998;86:16–26. doi: 10.1006/clin.1997.4459. [DOI] [PubMed] [Google Scholar]
- 25.Gadebush HH. Active immunization against Cryptococcus neoformans. J Infect Dis. 1958;102:219–26. doi: 10.1093/infdis/102.3.219. [DOI] [PubMed] [Google Scholar]
- 26.Sundstrom JB, Cherniak R. T-cell dependent and T-cell independent mechanism of tolerance to glucuronoxylomannan of Cryptococcus neoformans serotype A. Infect Immun. 1993;61:1340–5. doi: 10.1128/iai.61.4.1340-1345.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JW, Cozad GC. Immunological unresponsiveness induced by Cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy JW, Mosley RL, Moorhead JW. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells and induction of second-order suppressor cells. J Immunol. 1983;130:2876–81. [PubMed] [Google Scholar]
- 29.Murphy JW, Mosley RL. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second order T suppressor cells (TS2) J Immunol. 1985;134:577–84. [PubMed] [Google Scholar]
- 30.Blackstock R, McCormack JM, Hall NK. Induction of a macrophage-suppressive lymphokine by soluble cryptococcal antigens and its association with models of immunologic tolerance. Infect Immun. 1987;55:233–9. doi: 10.1128/iai.55.1.233-239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauman SK, Nichols KL, Murphy JW. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J Immunol. 2000;165:158–67. doi: 10.4049/jimmunol.165.1.158. [DOI] [PubMed] [Google Scholar]
- 32.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 33.Goldman D, Lee S, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–53. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman D, Casadevall A, Cho Y, Lee S. Cryptococcus neoformans meningitis in the rat. Lab Invest. 1996;75:759–70. [PubMed] [Google Scholar]
- 35.Goldman D, Lee S, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62:4755–61. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–41. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337–47. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 38.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barka T, Anderson PJ, editors. Histoquímica. 1st edn. Madrid: Atika; 1967. [Google Scholar]
- 40.Sundstrom JB, Cherniak R. The glucuronoxylomannan of Cryptococcus neoformans Serotype A is a type 2 T-cell-independent antigen. Infect Immun. 1992;60:4080–7. doi: 10.1128/iai.60.10.4080-4087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doering TL. A unique α-1,3 mannosyltransferase of the pathogenic fungus Cryptococcus neoformans. J Bacteriol. 1999;181:5482–8. doi: 10.1128/jb.181.17.5482-5488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SC, Casadevall A, Dickson DW. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol. 1996;148:1267–74. [PMC free article] [PubMed] [Google Scholar]
- 43.Leyva-Cobian F, Outschoorn IM, Carrasco-Marin E, Alvarez-Dominguez C. The consequences of the intracellular retention of pathogen-derived T-cell-independent antigens on protein presentation to T cells. Clin Immunol Immunopathol. 1997;85:1–15. doi: 10.1006/clin.1997.4426. [DOI] [PubMed] [Google Scholar]
- 44.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis: a study in 111 cases. Ann Intern Med. 1974;80:176–81. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 45.Muchmore HG, Nan SE, Felton FG, Fromtling RA. Cryptococcal capsular polysaccharide clearance in non immune mice. Mycopathology. 1982;78:41–5. doi: 10.1007/BF00436580. [DOI] [PubMed] [Google Scholar]
- 46.Scott EN, Muchmore HG, Felton FG. Enzyme-linked immunosorbent assays in murine cryptococcosis. Sabouraudia. 1981;19:257–65. doi: 10.1080/00362178185380431. [DOI] [PubMed] [Google Scholar]
- 47.Chapin-Robertson K, Bechtel C, Waycott S, Kontnick C, Edberg SC. Cryptococcal antigen detection from the urine of AIDS patients. Diagn Microbiol Infect Dis. 1993;17:197–201. doi: 10.1016/0732-8893(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 48.Lendvai N, Casadevall A, Liang Z, Goldman DL, Mukherjee J, Zuckier L. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J Infect Dis. 1998;177:1647–59. doi: 10.1086/515329. [DOI] [PubMed] [Google Scholar]
- 49.Humphrey JW. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981;11:212–20. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- 50.Humphrey JH, Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981;11:221–8. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- 51.Grinsell M, Weinhold LC, Cutler JE, Han Y, Kozel TR. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J Infect Dis. 2001;184:479–87. doi: 10.1086/322787. [DOI] [PubMed] [Google Scholar]
- 52.Liew FW. Th1 and Th2 cells: a historical perspective. Nature Rev Immunol. 2002;2:55–60. doi: 10.1038/nri705. [DOI] [PubMed] [Google Scholar]
- 53.Leiva LE, Butler B, Hempe J, Ortigas AP, Sorensen RU. Up-regulation of CD40 Ligand and induction of a Th2 response in children immunized with pneumococcal polysaccharide vaccine. Clin Diagn Lab Immunol. 2001;8:233–40. doi: 10.1128/CDLI.8.2.233-240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai WJ, Hemphill W, Waldvogel A, Ingold K, Deplazes P, Mossmann H, Gottstein B. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of CD4+ T cells. Infect Immun. 2001;69:6074–83. doi: 10.1128/IAI.69.10.6074-6083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong ZM, Murphy JW. Intravascular cryptococcal culture filtrate CneF and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect Immun. 1995;63:770–8. doi: 10.1128/iai.63.3.770-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lüder CGK, Gross U, Lopes MF. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite–host interactions. Trends Parasitol. 2001;17:480–6. doi: 10.1016/s1471-4922(01)02016-5. [DOI] [PubMed] [Google Scholar]
- 57.Korostoff J, Wang JF, Kieba I, Miller M, Shenker BJ, Lally ET. A Actinobacillus actinomycetemcomitans leucotoxin induces apoptosois in HL-60 cells. Infect Immun. 1998;66:4474–83. doi: 10.1128/iai.66.9.4474-4483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080–7. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibata-Ombetta S, Idziorek T, Trinel PA, Poulain D, Jouault T. Candida albicans phospholipomannan promotes survival of phagocytosed yeast through modulation of Bad phosphorylation and macrophage apoptosis. J Biol Chem. 2003;278:13086–93. doi: 10.1074/jbc.M210680200. [DOI] [PubMed] [Google Scholar]