Abstract
CTLA4 (CD152) is a transmembrane molecule expressed on activated T cells and functions as a negative regulator of T cell activation upon binding to the costimulatory molecules CD80/86. In this study, CTLA4eEGFP constructs were engineered by cloning the extracellular domains of ovine and human CTLA4 (CTLA4e) ‘in frame’ with the enhanced green fluorescent protein (EGFP). Recombinant adenoviral vectors were generated by incorporation of the CTLA4eEGFP sequence into the adenoviral genome using homologous recombination in Esherichia coli. The functional activity of the adenoviral vectors was shown by the secretion of the CTLA4eEGFP upon infection of ovine fibroblasts and the binding of the fusion protein to the target ovine and human dendritic cells expressing CD80/86 receptors by flow cytometry. The EGFP tag facilitated molecular size determinations and quantification of the secreted ovine CTLA4 fusion protein by immunoprecipitation and enzyme-linked immunosorbent assay (ELISA), respectively, using anti-GFP mAbs. Ovine dendritic cells obtained from pseudoafferent lymphatic cannulation of sheep were characterized based on high major histocompatibility complex (MHC) class II expression and cross-reactivity with monoclonal antibodies to the human dendritic cell markers, CD83 and CMRF-56. In addition, ovine dendritic cells (DC) were transfected with the adenoviral CTLA4eEGFP and when used as stimulators in a mixed lymphocyte reaction showed a reduced capacity to induce allogeneic lymphocyte proliferation. This study verifies that the ovine CTLA4eEGFP fusion protein functions similarly to its human homologue and that DC modified with adenoviral CTLA4-EGFP may provide an effective therapeutic approach in targeting alloreactive T cells to prolong allograft acceptance in a preclinical ovine model of renal transplantation.
Keywords: adenovirus, CTLA4, green fluorescent protein, MLR, sheep
Introduction
The interaction of the costimulatory molecule CD28 on T cells with its receptors, CD80/86 on antigen-presenting cells results in T cell activation.1 However, the control of T cell activation is regulated by CTLA4, a structural homologue of CD28 which is expressed on activated T cells.2,3 The binding of CTLA4 to CD80/86 inhibits T cell proliferation by down-regulation of interleukin (IL)-2 production and blockade at the G1 phase of the cell cycle.4,5 In addition, the stronger affinity of CTLA4 to its receptors6 has been exploited experimentally to generate soluble CTLA4-Ig fusion proteins, which serve as costimulatory blockade agents by competing with CD28.4
Dendritic cells (DC) are the major antigen-presenting cells that initiate rejection against organ transplants. Genetic modification of donor DC is a possible means of inducing allospecific tolerance as these cells express donor antigen/major histocompatibility complexes (MHC) and migrate readily to lymphoid tissues. The pretransplant administration of immature DC, with low costimulatory molecule expression of CD80/86, shows only a modest prolongation of allograft survival7 because these dendritic cells mature subsequently in vivo to express high levels of costimulatory molecules. However, attempts to inhibit DC maturation have been assessed by genetic modification with immunomodulatory molecules. The cytokines IL-10 and transforming growth factor (TGF)-β are able to down-regulate the expression of costimulatory molecules including the CD80/86 ligands, arresting DC in an immature state.8,9 There is accruing evidence that genetic manipulation of DC to express TGF-β10 and IL-1011 results in the inhibition of rejection. Moreover, the approach of blocking the CD80/86 ligands using CTLA4-Ig has shown improved allograft survival in an islet transplant model in mice by the use of a DC cell-line modified genetically with an adenoviral CTLA4-Ig vector.12 To date reports on the use of genetically modified DC in large animal models of allotransplantation are not forthcoming.
This study validates the in vitro effects of ovine DC transfected with an adenovirus encoding a novel ovine CTLA4eEGFP fusion protein construct. Ovine DC were obtained by the cannulation of the prefemoral lymphatic vessel of sheep. DC phenotype and function was confirmed by strong expression of the CD83 and MHC class II markers and the potency to stimulate allogeneic ovine lymphocyte proliferation.
The EGFP tag of ovine CTLA4eEGFP allowed the real-time visualization of transfected cells by fluorescent microscopy, while the use of anti-GFP mAbs permitted molecular size determinations of the fusion protein on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) by immunoprecipitation and quantitation by enzyme-linked immunosorbent assay (ELISA) despite the lack of ovine specific mAbs for CTLA4. Moreover, high levels of transfection of DC with adenoviral vectors was achieved by the technique of combining the cationic liposome, lipofecatmine, together with adenoviral particles to optimize gene transduction. Thus genetically modified ovine dendritic cells with alloinhibitory characteristics may be examined in our established preclinical ovine model of renal allograft transplantation.13
Material and methods
Cloning of the extracellular domain of ovine and human CTLA4
Peripheral blood mononuclear cells (PBMC) were isolated from ovine and human peripheral blood by density gradient separation using LymphoprepTM (Nycomed, Norway) and resuspended in complete medium at 1 × 106 cells/ml. Human buffy coats were obtained from healthy blood donors (Australian Red Cross Transfusion Service, Adelaide, Australia). Total RNA was extracted from PBMC stimulated with 1 µg/ml Con A FOR 48 hr by the established method of Chomczynski and Sacchi.14 Reverse transcription was performed with MMLV reverse transcriptase (GibcoBRL, USA) on 1 µg of total RNA. The extracellular domains of human and ovine CTLA4 were polymerase chain reaction (PCR) amplified using Tth polymerase (Fisher Biotech, USA) for 26 cycles of 30 seconds at 94°, 30 seconds at 55° and 30 seconds at 72°, followed by a final extension at 72° for 7 min. OvCTLA4e PCR was performed using forward (5′GGGAGATCTATGGCTTGCTCTGGATTCCAGAGTC-3′) and reverse (5′-GAGGTACCGAATCCGGGCATGGTTCTGGA-3′) primers based on the published sequence15 (GenBank Accession number: AF092740). HuCTLA4e PCR was performed using forward (5′-GGGAGATCTATGGCTTGCCTTGGATTTCAGCGGC-3′) and reverse (5′-GGGGTACCGAATCTGGGCACGGTTCTGGATCA-3′) primers based on the published sequence16 (GenBank Accession number: NM_005214). BglII and KpnI restriction sites (underlined) were engineered into the forward and reverse primers, respectively. PCR products were digested with BglII and KpnI and cloned ‘in frame’ with the EGFP gene in a pEGFP-N1 expression vector (Clontech, USA). The CTLA4 insert in the resulting plasmid vector constructs povCTLA4eEGFP and phuCTLA4eEGFP were confirmed to be authentic by sequencing and GenBank database analysis.
Generation of adenoviral CTLA4eEGFP constructs
Adenoviral constructs containing the ovine and human CTLA4eEGFP (ADVovCTLA4eEGFP and ADVhuCTLA4eEGFP, respectively) and the EGFP-vector blank (ADV-EGFP) were generated using protocols established by He.17 Ovine and human CTLA4eEGFP was excised from povCTLA4eEGFP and phuCTLA4eEGFP, respectively, by BglII/NotI digestion and ligated into the pShuttleCMV vector (Stratagene, USA). Homologous recombination was achieved by coelectroporating PmeI linearized recombinant pShuttleCMV vector with the pAdEasy1 vector into BJ5183 cells. PAdEasy1 vector is a replication-deficient adenovirus containing the genome for the AD 5-strain that was kindly provided by Dr B. Vogelstein, Howard Hughes Medical Institute. In order to generate recombinant adenoviral particles, the adenoviral constructs, ADVovCTLA4eEGFP, ADVhuCTLA4eEGFP and ADV-EGFP were linearized with PacI and used to transfect the HEK-293 packaging cell line with Lipofectamine (GibcoBRL, USA). Infectious adenoviral particles were obtained by freeze–thaw-lysis of the transfected HEK-293 cells and purified by CsCl density gradient ultra-centrifugation.18 Quantification of adenoviral titres was performed by the cytopathic effect assay19 and values expressed as plaque forming units (pfu).
Immunoprecipitation
Gene transfer was conducted by electroporation of Chinese hamster ovary (CHO) cells with plasmid constructs and infection of fibroblasts with recombinant adenovirus. After overnight incubation, cells were cultured in methionine/cysteine free RPMI (ICN, USA) supplemented with glutamine and 1% fetal calf serum (FCS) and incubated at 37° for 1 hr. Media was replaced with 1·5 ml methionine/cysteine free media containing 100 µCi/ml Tran35S-LabelTM (ICN Biomedicals, CA) and incubated overnight. Conditioned media were treated with 10% (v/v) protease inhibitor and blocked with 5% (v/v) normal rabbit serum. Media was precleared with Staphylococcus aureus in RIPA buffer (1m NaCl, 10% sodium deoxycholate, 10% SDS, 1% NP40, 1m TRIS-HCL) at 11 600 g for 5 min at 4°. Supernatant was treated with 5 µg mouse anti-GFP mAb (Roche, USA) and incubated at 4° rotating overnight. S. aureus supplemented with 1 mg/ml ovalbumin (100 µl) was added for 1 hr and washed twice with RIPA buffer at 3870 g. Pellets were resuspended in Laemmli's buffer with an aliquot reduced with β-mercaptoethanol and after incubation at 100° for 15 min samples were run on 12·5% SDS-PAGE gels.20 Gels were stained with coomassie blue and impregnated with AmplifyTM (Amersham, UK) for 30 min prior to autoradiography on Kodak XAR5 film.
Preparation of ovine and human CTLA4eEGFP fusion protein
Ovine adult skin fibroblasts were infected with adenoviral particles at a multiplicity of infection (MOI) of 50. Adenoviral constructs consisted of either ov- or hu-CTLA4eEGFP and an EGFP vector blank control. Cells were incubated with the appropriate recombinant adenoviral vector for 1 hr to permit viral adsorption followed by the addition of fresh medium and incubation for a further 48 hr to allow for gene transduction. Conditioned medium was obtained by centrifugation of the cultures.
Quantification of EGFP and CTLA4eEGFP fusion protein by ELISA
An ELISA was established to quantify the CTLA4eEGFP secreted from adenoviral infected ovine fibroblasts. Due to the unavailability of ovine CTLA4 specific antibodies, capture and detection antibodies were directed against the EGFP fusion protein. Titertek Immuno Assay Plates (Flow Laboratories, Netherlands) were coated with polyclonal goat anti-GFP antibody (Rockland, USA) at 2 µg/ml in bicarbonate buffer (pH 9·6) for 2 hr at room temperature and then incubated overnight at 4°. Standard curves were generated by titrating recombinant EGFP (Clontech, USA) in the antibody-coated plates at a concentration range of 0·001–10 ng/ml. Serial dilutions of the unknown concentrations of EGFP and CTLA4eEGFP in conditioned medium were also added to the antibody-coated plates. After overnight incubations, and following washes with phosphate buffered saline (PBS) Tween-20, 1 µg/ml of mouse anti-green fluorescent protein (GFP) mAb was used to detect the captured fusion protein and native EGFP for 2 hr at room temperature. Then a 1/1000 dilution of the secondary biotinylated horse anti-mouse IgG conjugate (Vector, USA) in 10% FCS was added to the plates and was detected with a 1/1000 dilution of streptavidin–alkaline–phosphatase (Roche, USA) in PBS/Tween-20. The colorimetric reaction was produced by the addition of Sigma 104 phosphatase substrate (Sigma, USA) in diethanolamine buffer and absorbance at A405 was determined after terminating the reaction with 0·5m EDTA. Because the standard curve was derived with native EGFP the values obtained for CTLA4eEGFP depict equivalent molar concentration.
(i) Human monocyte-derived DC.
Human monocytes were obtained by adherence of 5 × 107 PBMC in 75 cm2 plastic tissue culture flasks (Corning, USA). After the removal of non-adherent cells, the monocytes were first differentiated into immature DC in complete medium supplemented with 400 U/ml IL-4 (Peprotech, USA) and 800 U/ml granulocyte macrophage-colony stimulating factor (GM-CSF) (Kenilworth, USA) for 5 days and then matured by the addition of 10 ng/ml tumour necrosis factor (TNF)-α (Genzyme Corporation, USA) for a further 2 days.21
(ii) Ovine lymphatic DC.
Ovine lymphatic cells were collected by cannulation of the pseudoafferent lymphatic vessels from 2-year-old ewes as described previously by Dandie.22 The prefemoral lymph nodes were excised surgically and after a period of 6–8 weeks of agistment the lymphatic vessels were identified by subcutaneous administration of 15 mg methylene blue (Pharmalab, Australia) and cannulated with a CBASTM heparin-coated catheter (Carmeda, USA). The cannula was externalized and drained into a 100-ml sterile bag containing 2500 IU heparin and 40 mg gentamycin. Approval for these surgical procedures was obtained from the TQEH institutional animal ethics committee. DC were enriched on a 14% (w/v) metrizamide (Sigma, USA) density gradient and characterized by flow cytometry.
(a) Ovine DC phenotypic analysis.
Cell-surface phenotype of the human monocyte-derived DC and the metrizamide-enriched ovine lymphatic DC was analysed by flow cytometry. Monoclonal antibodies (mAbs) were either against ovine cell-surface antigens or were mAbs known to cross-react with their ovine homologue. Briefly, cells were washed in PBS containing 1% FCS and 0·1% sodium azide in FACS wash buffer and incubated for 30 min on ice with 50 µl of either saturated supernatant or 1–2 µg/ml purified primary antibody. After two washes in FACS wash buffer, cells were incubated for 30 min on ice with isotype-specific, fluorescein isothiocyanate (FITC)-conjugated, anti-mouse antibodies and fixed in FACS lysing solution (BD Biosciences, USA). Isotype-control antibodies were used to determine background staining. MAbs used were: αCD1a (SBUT6), αCD4 (44·38), αCD8 (38·65), αCD25 (9·14), αCD44 (25·32), Class I (41·19), Class II DP (28·1) obtained from the Centre of Animal Biotechnology, School of Veterinary Science, University of Melbourne. Other antibodies included αCD14 (VPM65), αCD83 (HB15A17·11), αCD31 (CO.3E1D4) and αCD21 (CC21) from Serotec, USA. CMRF56 antibody was obtained from Dr D. Hart, Mater Medical Research Institute, Brisbane, and 1D4·5 from Dr P. Hart, Flinders University of South Australia and X63 (P3 X63Ag8) from ATCC, USA.
(b) CTLA4eEGFP binding activity.
Ovine and human DC were used as target cells to establish the binding activity of ovCTLA4eEGFP. Primary mAbs directed against the CD80 (Immunotech, France) and CD86 (Serotech, UK) ligands were used to confirm the expression of these molecules on the human DC. The CTLA4eEGFP binding assay involved three steps. DC were incubated first with 200 µl of ovCTLA4eEGFP at a concentration of 20 µg/ml. Secondly, cells were washed and incubated with a primary anti-GFP (IgG1) mAb (Roche, USA). X63 was used as an IgG1-isotype-matched negative control mAb. Finally, a polyclonal anti-mouse Ig Fab2 FITC-conjugated antibody (Silenus, Australia) was added to the cells to detect the primary antibody. Human CTLA4Ig (4 µg, R&D Systems, USA) was added to both human and ovine DC prior to the addition of ovCTLA4eEGFP in the binding assay to establish the specificity of binding to CD80/86. Flow cytometric analysis was performed using a Becton Dickinson FACScan.
Two-way mixed lymphocyte reaction
Conditioned media from recombinant adenoviral infected fibroblasts were quantitated by ELISA and tested for inhibitory activity in a two-way mixed lymphocyte/ leucocyte reaction (MLR) as described below. Either sheep or human PBMC from two unrelated donors were cocultured in 96-well round-bottomed plates to a final cell concentration of 2 × 105 cells per well. The MLR was performed in complete medium in a volume of 200 µl. The mixed cultures were incubated at 37° for 4 days and then pulsed for 20 hr with 1 µCi of [3H]thymidine. The cells were harvested onto filters and the incorporated radioactivity was determined by liquid scintillation counting in a Wallac Microbeta Counter (Turku, Finland). All determinations were expressed as counts per minute (cpm) and performed in triplicate.
Genetically modified DC as stimulators in the MLR
Unlike gene transfer by infection of fibroblasts, DC were genetically modified by combining the cells with the specific recombinant adenovirus (1 × 109 pfu) and 2 µg of lipofectamine (Gibco BRL, USA) in 100 µl RPMI.23 Briefly, after 15 min of incubation at room temperature, DC (1 × 106 cells) were mixed with the adenoviral–lipofectamine complex to a final MOI of 1000 and incubated for a further 2 hr to allow viral adsorption. The transfected DC were incubated for a further 48 hr to establish gene transduction prior to use in the MLR as stimulators.
Results
Expression and secretion of CTLA4eEGFP by genetically modified cells
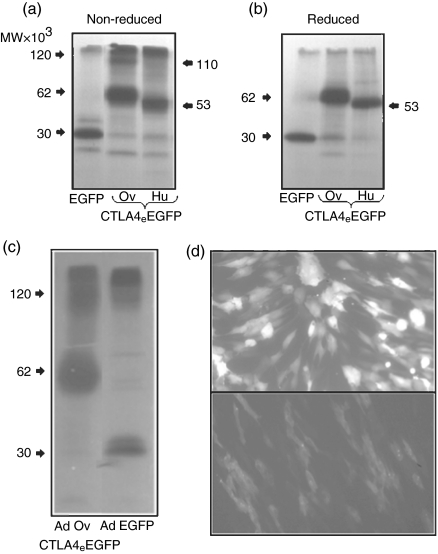
The expression of the transferred CTLA4eEGFP gene into CHO cells (data not shown) and fibroblasts (Fig. 1) was illustrated by detection of green fluorescent cells under UV illumination. Of particular note was that the ADV-EGFP transduced fibroblasts demonstrated stronger nuclear and cytoplasmic localization of the EGFP native protein in contrast to the diffuse cytoplasmic staining observed with those cells modified genetically with ADVovCTLA4eEGFP (Fig. 1d). The differential fluorescent intensity between these cells was also marked by a higher mean-fluorescent intensity reading for the ADV-EGFP-infected fibroblasts (data not shown). These genetically modified cells were shown to secrete ovine and human CTLA4eEGFP fusion proteins by immunoprecipitation of the conditioned media with anti-GFP mAbs (Fig. 1). The conditioned medium from CHO cells transfected with pEGFP-N1 and fibroblasts infected with ADV-EGFP were used as controls for EGFP synthesis. The autoradiograms of the immunoprecipitates obtained from cells treated with both control vectors demonstrated a distinct 30 kDa band corresponding to EGFP, while cells transfected with povCTLA4eEGFP and phuCTLA4eEGFP illustrated bands corresponding to molecular sizes of 62 kDa and 53 kDa, respectively (Fig. 1a, b, c). Higher molecular size bands of 120 kDa and 110 kDa for ovine and human CTLA4eEGFP, respectively, were observed only in nonreduced SDS-PAGE gels indicating the formation of homodimers (Fig. 1a). The immunoprecipitation of conditioned medium derived from fibroblasts infected with ADVovCTLA4eEGFP confirmed the secretion of the ovine molecule by the detection of bands corresponding to both molecular species (62 kDa and 120 kDa) on non-reduced gels (Fig. 1c).
Figure 1.
CTLA4eEGFP expression by genetically modified CHO-cells and fibroblasts. Autoradiographs showing immunoprecipitation with an anti-GFP mAb of 35S-methionine/cysteine-labelled proteins from CHO cells (a and b) transfected with povCTLA4eEGFP, phuCTLA4eEGFP or pEGFP-N1 plasmid DNA and ovine dermal fibroblasts (c) infected with recombinant adenoviral particles containing the ADVovCTLA4eEGFP or ADV-EGFP gene constructs. Immunoprecipitates were subject to electrophoresis in 12·5% SDS-PAGE gels under non-reduced (a) and (c) or reduced (b) conditions. (d) Expression of green fluorescence by adenoviral-transduced fibroblasts under UV-microscopy (magnification × 20). Upper panel (d) shows transduction with ADV-EGFP and lower panel (d) with ADVovCTLA4eEGFP. The autofluorescence of untransfected cells (not shown) was negligible.
Characterization of ovine DC derived from pseudo-afferent lymphatic cannulation
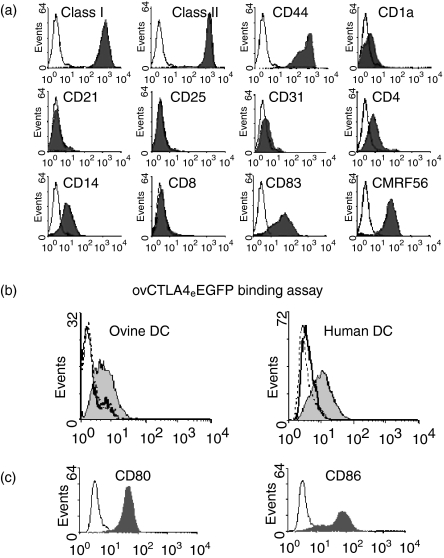
Lymphatic cells obtained from the cannulation of the prefemoral lymph duct consisted of 70–80% of cells with typical dendritic cell morphology upon enrichment with metrizamide density centrifugation. The profiles of DC phenotypic markers were examined on cells that were obtained from the lymphatic drainage for up to 14 days postcannulation. Figure 2(a) shows the profile of DC obtained 8 days postcannulation with high levels of MHC class I, II, CD83, CD44 and CMRF-56 expression. However, while these cells showed low expression of CD1a, CD4 and CD14, the markers CD8, CD21, CD25 and CD31 were undetected. The observations of these DC profiles were typically observed in three different lymphatic cannulations and showed stability in the pattern of the expressed markers with the exception of CMRF-56, which showed a variable profile between animals.
Figure 2.
Phenotypic analyses of ovine lymphatic dendritic cells and the binding activity ovCTLA4eEGFP by flow cytometry (a) Ovine DC obtained by pseudo-afferent cannulation of the prefemoral lymph node were stained with control isotype antibodies (open histograms) and a panel of cell-surface antigen markers (filled histograms). Each histogram is representative of at least three different experiments. (b) Human or ovine DC were incubated with ovCTLA4eEGFP derived from recombinant adenovirus modified fibroblasts. The bound ovCTLA4eEGFP was detected with an anti-GFP mAb as shown in the shaded histogram and the negative-isotype matched mAb (X63) represented by the solid line. The dotted line represents blockade of ovCTLA4eEGFP binding with CTLA4-Ig. (c)Human DC were stained with anti-CD80 and -CD86 mAbs to confirm the presence of the CTLA-4 receptors. Flow cytometric analysis of CD80 and CD86 staining is represented by the shaded histogram with X63 represented by the unshaded histogram.
Binding of ovCTLA4eEGFP to ovine and human DC
The ovCTLA4eEGFP fusion protein obtained from fibroblast infected with ADVovCTLA4eEGFP was quantitated by ELISA and used at a concentration equivalent to 20 µg/ml of EGFP to assess the binding activity to both human and ovine DC. While the human monocyte-derived DC were confirmed by flow cytometry to express the CTLA4 receptors CD80/86 (Fig. 2c) this was not established for the ovine cells due to the unavailability of ovine specific mAbs. Detection of ovCTLA4eEGFP with anti-GFP mAb demonstrated binding to both ovine and human DC as shown by an increased shift in fluorescence compared to the isotype-matched X63 control (Fig. 2b). In contrast the EGFP native protein, as a negative control, showed no reactivity (data not shown). Furthermore, the specificity of the ovCTLA4eEGFP binding to CD80/86 on both human and ovine DC was confirmed by blockade with recombinant human CTLA4-Ig. Interestingly, the binding pattern of ovCTLA4eEGFP mirrored the expression of CD86 in the human DC.
ovCTLA4eEGFP inhibits the two-way MLR
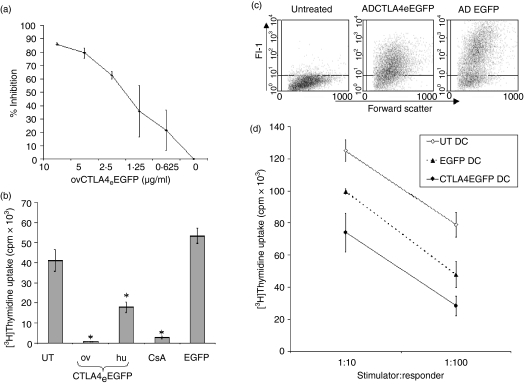
Ovine CTLA4eEGFP and EGFP derived from adenoviral-vector infected fibroblasts were quantified by ELISA and titrated for alloinhibitory activity in a two-way MLR in a concentration range of 0·625–10 µg/ml (Fig. 3a). Optimal inhibition (80–90%) compared to the untreated MLR was demonstrated in a concentration range of 5–10 µg/ml equivalence to native EGFP.
Figure 3.
Alloinhibitory properties of ovCTLA4eEGFP. (a) Dose–response inhibition of a two-way ovine MLR by ovCTLA4eEGFP. Responses are expressed as the percentage of inhibition relative to the untreated MLR. (b) Comparative alloinhibitory properties of ovCTLA4eEGFP, huCTLA4eEGFP and native EGFP. Fusion proteins were normalized to 5 µg/ml of EGFP and added to an ovine MLR. CsA was added at 100 ng/ml as an established inhibitory control. (c) Ovine DC were transfected with ADVovCTLA4eEGFP or ADV-EGFP. Cell transduction efficiency is demonstrated as a dot-plot representation (FL-1 versus forward scatter). (d) DC modified with ADVovCTLA4eEGFP were used as stimulators of ovine PBMC at ratios of 1:10 and 1:100. Unmodified DC and DC modified with ADV-EGFP were used as controls. Each treatment group consisted of triplicates and each graph is representative of three different experiments. *P < 0·05, unpaired Student's t-test.
The comparative inhibitory effects of ovCTLA4eEGFP and huCTLA4eEGFP were also tested in the ovine MLR. The histogram in Fig. 3(b) demonstrated a 98% (P = 0·012) and 65% (P = 0·033) inhibition of the ovine MLR, by the ovine and human fusion proteins, respectively, at a concentration of 5 µg/ml. The observed inhibition of the ovine MLR by the ovCTLA4eEGFP was comparable to the effects of CsA (100 ng/ml), which demonstrated 93% (P = 0·0076) inhibition. The EGFP derived from ADV-EGFP modified cells at a concentration of 2·5–5 µg/ml demonstrated a modest allostimulatory effect compared to the untreated MLR.
ADVovCTLA4eEGFP transfected DC inhibit allostimulation
In this set of experiments ovine DC were transfected with ADVovCTLA4eEGFP and compared against cells transfected with ADV-EGFP as a control. Fluorescence resulting from the EGFP-tag in transfected DC was evaluated for both the native and fusion protein by flow cytometry (Fig. 3c). While both transfectants showed an equivalent level of transfection (70%), the ADV-EGFP transfected DC displayed a higher EGFP fluorescence as revealed by an MFI of 200 in contrast to an MFI of 50 for ADVovCTLA4eEGFP transfectants.
In the DC-MLR at stimulator:responder ratios of 1:10 and 1:100 the ADVovCTLA4eEGFP transfected DC demonstrated an inhibition of 41% (P = 0·003) and 64% (P = 0·003) compared to the unmodified DC, respectively. Surprisingly, in contrast to the two-way MLR, ADV-EGFP transfected DC also inhibited the MLR but to a lesser extent than ADVovCTLA4eEGFP transfected DC (Fig. 3d). Similar results were obtained with human transfected DC (data not shown). To clarify the observed immunomodulation by ADVovCTLA4eEGFP, phenotypic analysis was confined to human DC due to the lack of availability of ovine reactive mAbs (data not shown). These analyses revealed that ADVovCTLA4eEGFP transfection showed a modest up-regulation of CD40 expression on DC whereas ADV-EGFP transfection resulted in a 15–20% down-regulation. However, both constructs produced only modest increases in the expression of MHC class II, CD86 and CD83, which is consistent with previously reported effects mediated by adenoviral transfection.8
Discussion
This study reports the generation and characterization of a fusion protein consisting of CTLA4 uniquely tagged with EGFP. Of important relevance to this study is the preparation of an ovine CTLA4 construct in a recombinant adenoviral vector to be used in a gene therapy strategy in a large animal model of allograft transplantation. The sheep model of renal transplantation established in this laboratory13 provides an inexpensive approach to study the intervention of allograft rejection compared to primate models and is clinically more relevant than murine and rodent models.
The proposed strategy for use in the ovine model to improve allograft outcome will utilize genetically modified dendritic cells. With respect to this approach we have obtained dendritic cells by the cannulation of the prefemoral lymph nodes of sheep. In contrast to the human or murine situation the feasibility of generating ovine DC from peripheral blood monocyte precursors was restricted by the availability of ovine-specific GM-CSF and IL-4. The study by Blacklaws24 has attempted to generate ovine DC from monocytes, although the efficiency of obtaining large numbers for an experimental therapeutic strategy is potentially limited by poor yields. Based on our experience with lymphatic cannulation, approximately 5 million DC/day was obtained routinely with a purity of 70–80% following metrizamide gradient enrichment. The daily collection of DC and cryopreservation of cells provides a means for building up a cache for subsequent cellular therapy in a transplant setting. In addition to the earlier studies by Dandie25 on ovine DC phenotypes our study provides a more definitive characterization of these cells by the cross-reactivity of mAbs to the human DC markers, CMRF-5626 and CD83. Indeed, these isolated DC demonstrated other typical features, which included high MHC class II expression and the ability to provoke a strong proliferative stimulus when challenged with allogeneic lymphocytes. Additional phenotypic characteristics were revealed by the binding of ovCTLA4eEGFP fusion protein to ovine DC, which recognizes the expression of CD80/86 on these cells.
Prior to the studies on the genetic modification of ovine DC with ADVovCTLA4eEGFP, the structural and functional characteristics of ovCTLA4eEGFP were confirmed by comparisons with its human homologue. Following transfection of CHO cells, immunoprecipitation of conditioned media from transduced cells indicated that CTLA4eEGFP is secreted predominantly in a monomeric form, although there is a minor proportion of high molecular species identified only in non-reducing SDS-PAGE, which is indicative of sulphydryl protein interaction and dimer formation. It is plausible that the observed higher molecular size for the ovine fusion protein reported in Fig. 1(a) is due to post-translational modification of the CTLA4eEGFP and may be attributed to an extra N-glycosylation site and three O-glycosylation motifs identified in the ovine gene sequence15 compared to the human. These structural differences did not appear to influence the ability of CTLA4eEGFP to bind to its target cells or to inhibit the MLR. The cross-species binding of ovCTLA4eEGFP to human DC is related to the highly conserved MYPPPY motif, which facilitates strong binding to CD80/86.27 Furthermore, the inhibitory activity of CTLA4eEGFP paralleled that reported for CTLA4-Ig28 at comparable concentrations of 1–10 µg/ml.
The confirmation of CTLA4eEGFP function will allow further studies on the direct genetic modification of DC. While dendritic cells transfected with adenoviral vectors containing the green fluorescent protein as a reporter molecule to monitor the transfection efficiency of DC have been used previously,29 our studies have combined EGFP to a functional immunomodulatory molecule and reported in vitro function. A similar study by Zawitkowski30 has shown in vitro function of the CD40 molecule fused in-frame with EGFP. In our studies the EGFP tag facilitated the flow cytometric detection of transfected DC expressing functional CTLA4 and routinely demonstrated a transduction efficiency of 70–90%. In addition, the potential of obtaining even higher percentages of CTLA4eEGFP expressing cells may be achieved by flow sorting the genetically modified DC. In our studies the CTLA4eEGFP expressing DC showed a significantly reduced capability of inducing the proliferation of allogeneic T cells compared to the unmodified or ADV-EGFP transfected DC, thus demonstrating potential for use in a transplant setting to modify allograft outcome.
In summary, this study verified that the ovine CTLA4 fusion protein functions in a similar manner to its human homologue and demonstrated cross-species reactivity. These results warrant further investigation into the effects of DC transduced with adenoviral CTLA4eEGFP in vivo in an ovine model of renal transplantation.
Acknowledgments
The authors wish to acknowledge the support of the Australian Kidney Foundation. Dr Chris Russell and Dr Geoff Dandie are gratefully acknowledged for cannulation of the ovine lymphatic vessels and the assistance of Adrian Hines in the management and care of animals. Ashley Newland is a recipient of the Australian Kidney Foundation Biomedical Research Scholarship.
Abbreviations
- DC
dendritic cells
- EGFP
enhanced green fluorescent protein
- PBMC
peripheral blood mononuclear cells
- CsA
cyclosporin A
- ov-
ovine
- hu-
human
References
- 1.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily-CTLA4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 3.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;5:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7–1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC classII+, CD80dim, CD86–) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WC, Zhong C, Qian S, et al. Phenotype, function, and in vivo migration and survival of allogeneic dendritic cell progenitors genetically engineered to express TGF-β. Transplantation. 1998;66:1810–7. doi: 10.1097/00007890-199812270-00040. [DOI] [PubMed] [Google Scholar]
- 9.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 10.Takayama T, Kaneko K, Morelli AE, Li W, Tahara H, Thomson AW. Retroviral delivery of transforming growth factor-beta1 to myeloid dendritic cells. inhibition of T-cell priming ability and influence on allograft survival. Transplantation. 2002;74:112–9. doi: 10.1097/00007890-200207150-00019. [DOI] [PubMed] [Google Scholar]
- 11.Coates PT, Krishnan R, Kireta S, Johnston J, Russ GR. Human myeloid dendritic cells transduced with an adenoviral interleukin-10 gene construct inhibit human skin graft rejection in humanized NOD-scid chimeric mice. Gene Ther. 2001;8:1224–33. doi: 10.1038/sj.gt.3301513. [DOI] [PubMed] [Google Scholar]
- 12.O'Rourke RW, Kang SM, Lower JA, et al. A dendritic cell line genetically modified to express CTLA4-Ig as a means to prolong islet allograft survival. Transplantation. 2000;69:1440–6. doi: 10.1097/00007890-200004150-00039. [DOI] [PubMed] [Google Scholar]
- 13.Grooby W, Krishnan R, Johnston JK, Rao MM, Russ G. Combined anti-vascular cell adhesion molecule-1 and antileukocyte function associated molecule-1 monoclonal antibody therapy does not prolong allograft survival in an ovine model of renal transplanation. Transplantation. 1998;66:920–4. doi: 10.1097/00007890-199810150-00018. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Chaplin PJ, Pietrala LN, Scheerlinck JPY. Cloning and sequence comparison of sheep CD28 and CTLA-4. Immunogenetics. 1999;49:583–4. doi: 10.1007/s002510050542. [DOI] [PubMed] [Google Scholar]
- 16.Dariavach P, Mattei MG, Goldstein P, Lefranc MP. Human Ig superfamily CTLA4 gene: chromosomal localisation and identity of protein sequence between murine and human CTLA4 cytoplasmic domains. Eur J Immunol. 1988;18:1901–5. doi: 10.1002/eji.1830181206. [DOI] [PubMed] [Google Scholar]
- 17.He TC, Zhou S, DaCosta LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol. 1994;47:157–66. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- 19.Nyberg-Hoffman C, Shabram P, Li W, Giroux D, Aguilar-Cordova E. Sensitivity and reproducibility in adenoviral infectious titer determination. Nature Med. 1997;3:808–11. doi: 10.1038/nm0797-808. [DOI] [PubMed] [Google Scholar]
- 20.Matsudaira PT, Burgess DR. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978;87:386–96. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Tedder T. CD14 blood monocytes can differentiate into functionally mature CD83 dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandie GW, Watkins FY, Ragg SJ, Holloway PE, Muller HK. The migration of Langerhans' cells into and out of lymph nodes draining normal, carcinogen and antigen-treated sheep skin. Immunol Cell Biol. 1994;72:79–86. doi: 10.1038/icb.1994.12. [DOI] [PubMed] [Google Scholar]
- 23.Dietz AB, Vuk-Pavlovic S. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91:392–8. [PubMed] [Google Scholar]
- 24.Chan SS, McConnell I, Blacklaws BA. Generation and characterization of ovine dendritic cells derived from peripheral blood monocytes. Immunology. 2002;107:366–72. doi: 10.1046/j.1365-2567.2002.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragg SJ, Dandie GW, Woods GM, Muller HK. Abrogation of afferent lymph dendritic cell function after cutaneously applied chemical carcinogens. Cell Immunol. 1995;162:80–8. doi: 10.1006/cimm.1995.1054. [DOI] [PubMed] [Google Scholar]
- 26.Hock BD, Fearnley DB, Boyce A, et al. Human dendritic cells express a 95 kDa activation/differentiation antigen defined by CMRF-56. Tissue Antigens. 1999;53:320–34. doi: 10.1034/j.1399-0039.1999.530402.x. [DOI] [PubMed] [Google Scholar]
- 27.Peach RJ, Bajorath J, Brady W, et al. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7–1. J Exp Med. 1994;180:2049–58. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady JL, Lew AM. Additive efficacy of CTLA4Ig and OX40Ig secreted by genetically modified grafts. Transplantation. 2000;69:724–30. doi: 10.1097/00007890-200003150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Dietz AB, Bulur PA, Brown CA, Pankratz VS, Vuk-Pavlovic S. Maturation of dendritic cells infected by recombinant adenovirus can be delayed without impact on transgene expression. Gene Ther. 2001;8:419–23. doi: 10.1038/sj.gt.3301406. [DOI] [PubMed] [Google Scholar]
- 30.Zawitkowski M, Russ G, Krishnan R. Cloning and expression of the ovine CD40 molecule and the inhibition of the mixed lymphocyte reaction by the ov CD40e-EGFP fusion protein. Vet Immunol Immunopathol. 2002;89:37–45. doi: 10.1016/s0165-2427(02)00182-4. [DOI] [PubMed] [Google Scholar]