Abstract
Efforts to identify the signal transduction pathways used by interleukin-10 (IL-10) have resulted in limited success. The anti-inflammatory effects elicited by IL-10, and the mechanisms by which these are mediated, are still relatively unknown. Understanding the signalling mechanisms behind the suppression of cytokine expression by IL-10 could be of potential therapeutic interest. Although the consensus is that the Janus kinase, Jak1, as well as the signal transducer and activator of transcription STAT3 are central, much controversy exists about the participation and roles of many other signalling pathways targeted by IL-10. The mechanisms of cytokine suppression proposed by various groups have included transcriptional, post-transcriptional and post-translational regulation of IL-10 target genes; nevertheless no unifying model has emerged thus far. Here we would like to highlight novel findings and discuss their implications in the context of current understanding of IL-10 signalling.
Keywords: signalling/signal transduction, cytokines/interleukins, inflammation/inflammatory mediators including eicosanoids
Introduction
The immune system's inflammatory response is essential to protect the host from infection, injury and neoplasia. The destruction of invading pathogens requires the production of powerful cytopathic factors; however, the immune response has to be of the appropriate amplitude and duration to prevent the unnecessary destruction of healthy tissue. Excessive production of these inflammatory factors can result in diseases such as rheumatoid arthritis, Crohn's disease and septic shock. The immune system has developed multiple anti-inflammatory mediators to prevent the inflammatory response from spiralling out of control. One of the most potent of these anti-inflammatory factors is interleukin-10 (IL-10). IL-10 is a pleiotropic cytokine that has an important role in regulating the immune response. It was originally described as a cytokine synthesis inhibitor factor produced by murine T helper 2 (Th2) cells.1 However, its expression profile has now widened and it has been shown to be expressed in various subsets of T cells, macrophages, monocytes, dendritic cells, mast cells, B cells, eosinophils, keratinocytes, epithelial cells and various tumour cell lines.2 Its main biological functions seem to be to limit and terminate the inflammatory responses, block pro-inflammatory cytokine secretion and regulate the differentiation and proliferation of several immune cells such as T cells, B cells, natural killer cells and mast cells.3 IL-10 gene homologues have been found in numerous viral genomes and it is thought they act as virulence factors to manipulate the immune response.4,5 In addition many tumours also acquire an IL-10-secreting phenotype that may permit malignant cells to evade cell-mediated immune defences.6–8 The convergence of these evolutionary distinct mechanisms for immune evasion further underscores the importance of IL-10 as central immune modulator. More recently, five novel cytokines that display structural similarity to IL-10 have been identified in the human genome: IL-19, IL-20, IL-22, IL-24 and IL-26.9–13 These, however, do not appear to display an anti-inflammatory function.
IL-10 potently inhibits macrophage activation. This results in reduced expression of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), IL-1, IL-12, IL-6 and granulocyte–macrophage colony-stimulating factor, inflammatory enzymes such as cyclo-oxygenase 2 and inducible nitric oxide synthase, chemokines such as regulated on activation, normal, T-cell expressed and secreted (RANTES), membrane inflammatory protein-1α (MIP1α), IL-8, and eotaxin, thus limiting the course of an inflammatory response by curtailing the activation and recruitment of a wide range of haematopoietic cells.2
IL-10 augments this inhibitory activity by enhancing the release of soluble TNF receptors and IL-1 receptor antagonist (IL-1RA).14,15 Similarly, the potentially destructive activities of matrix metalloproteinases (MMP) are limited by IL-10, as it not only inhibits the production of MMP2 and MMP9 but also induces the production of tissue inhibitor of MMPs (TIMP), TIMP1.16 Another key feature of IL-10's immunosuppressive capabilities is its effectiveness in disabling antigen presentation (AP)/T-cell activation by inhibiting expression of major histocompatibility complex (MHC) class II, CD80 (B7-1) and CD86 (B7-2) on macrophages and dendritic cells,17,18 thus effectively blocking antigen presentation to T cells. Besides these indirect effects on T cells via inhibition of APC function, IL-10 does have some direct effects on T cells. It shows inhibitory activities towards CD4+ T cells via suppressing IL-2, interferon-γ (IFN-γ), IL-4 and IL-5 production.19–21 It has also been suggested that IL-10 plays a direct role in the differentiation of regulatory T cells.22 In particular, the differentiation of Tr1 T cells might be controlled by tolerogenic dendritic cells that produce IL-10 and express tolerogenic costimulatory molecules.23–25
The effects of IL-10 on B cells are more stimulatory. IL-10 enhances B-cell survival, and is a potent cofactor for proliferation of human B-cell precursors and mature B cells activated by Staphylococcus aureus Cowan 1 (SAC), anti-immunoglobulin M (IgM) or CD40 ligation.26,27 IL-10 can affect B-cell differentiation and isotype switching.28 Long-term culture of B cells with anti-CD40 and IL-10 can result in the development of plasma cells.28 The effects of IL-10 on B cells could suggest that antagonism of IL-10 function could be useful in the treatment of antibody mediated autoimmune disease such as systemic lupus erythematosus (SLE). In support of this view, the spontaneous in vitro production of IgM, IgG, and IgA by peripheral blood mononuclear cells (PBMC) from SLE patients is strongly increased by IL-10. Conversely, anti-IL-10 antibodies strongly inhibited the production of autoantibodies in severe combined immunodeficiency mice injected with PBMC from SLE patients.29 Furthermore, anti-IL-10 treatment of SLE patients ameliorated disease in a limited trial.30
IL-10 deficient mice have highly polarized Th1 responses and develop a severe colitis which supports the essential role of IL-10 in balancing the cytokine network. Generally considered as a Th2 cytokine, IL-10 inhibits Th1-dominated responses. The potency of the anti-inflammatory effects of IL-10 has been demonstrated in animal models of inflammation such as sepsis,31 collagen-induced arthritis,32 inflammatory bowel disease,33 insulitis,34 and in some models of experimental autoimmune encephalomyelitis (EAE).35,36 In a clinical setting, encouraging data has emerged from phase II trials of systemic administration of IL-10 in the treatment of psoriatic skin lesions37 although similar data from Crohn's disease and rheumatoid arthritis produced only a mild amelioration of disease activity.38,39
The potent anti-inflammatory activity of IL-10 could be harnessed therapeutically, from a more comprehensive understanding of the signalling pathways involved. We will review the current literature regarding the signalling mechanisms employed by IL-10 focusing particularly upon its cytokine suppressing activities.
The il-10r: structure and function
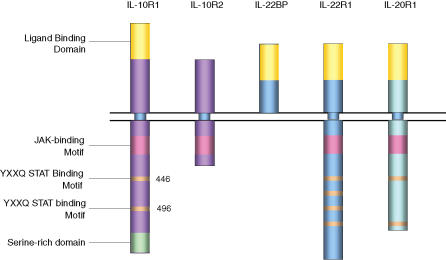
The IL-10R is a heterotetramer comprising of two molecules of the IL-10R1 and two molecules of the IL-10R2 (also known as CRF 2–4) chain. Janus kinase-1 (Jak1) and Tyk2 are permanently associated with the IL-10R1 and IL-10R2 chain, respectively.40–42 Knockout models of IL-10R242 and signal transducer and activator of transcription-3 (STAT3)43 have confirmed that these components of the IL-10 signalling machinery mediate IL-10 activity, as the phenotype of spontaneous enterocolitis in all these ablations is analogous to that found in IL-10-deficient mice. In contrast, the absence of Tyk2 did not have an effect on IL-10 activity nor did it result in a related phenotype.44 The functional relevance of several segments within the amino acid sequences of the human (578 residues)40 and murine (576 residues)45 IL-10R1 has been investigated through various deletion and substitution mutations of the cytoplasmic or signalling part of the receptor. This strategy has led to the identification of several active domains and residues. The tyrosine residues present in a cytokine receptor box 3 motif (YXXQ) at positions 446 and 496 in the human IL-10R1 chain were found to be critical for IL-10 function and highly conserved between murine and human sequences.46,47 This motif has been described as being common to all STAT3-activating cytokine receptors including IL-10R1 and gp13048 and more recently the IL-20R1 and IL-22R1.49,50 In addition, a stretch of 30 C-terminal amino acids, which was thought to contain at least one critical serine residue, was also implicated in mediating the anti-inflammatory activity of IL-10. It was this segment of 30 C-terminal amino acids that was said to be the unique receptor domain that conferred the specificity for the anti-inflammatory activity of IL-10.47 This finding has gained new relevance since the cloning and characterization of the receptors for the IL-10 homologues IL-22 and IL-26. The receptors for these IL-10 family cytokines have distinct receptor chains (IL-22R1 for IL-22 and IL-20R1 for IL-26) and the same accessory chain as the IL-10 receptor complex the IL-10R2/CRF2-4 chain and are thought to form multimeric receptors,50–54 as shown in Fig. 1. Despite these basic structural similarities, IL-22 has been shown to activate not only the Jak/STAT pathway, but all three mitogen-activated protein (MAP) kinase pathways (JNK, extracellular signal-related kinase and p38) in a rat hepatoma cell line and IL-26 activated STAT1 in human colon carcinoma cells.54–56 There is also data to suggest that IL-22 is capable of binding the IL-10R2 chain, which is unlike IL-10 and like IL-26, possibly activating additional pathways.49,51,54 More significantly though is the finding that both IL-22 and IL-26 are strong inducers of STAT3 phosphorylation in target cells expressing the relevant receptor complexes.54,57–60 The expression of the ligand-binding chains of the IL-22R1 and IL-20R1 of the complexes were predominantly present in non-haematopoietic cells including epithelial cells of the colon, as well as hepatocytes, stromal cells and fibroblasts of various other tissues.9,57–60 Despite the ability to induce STAT3 in target cells, IL-22 and IL-26 have been shown to have pro-inflammatory activities.56,57,61 This suggests that the presence of a box 3 YXXQ motif and the activation of STAT349 do not solely confer anti-inflammatory activity to a receptor complex as seen in the case of the IL-10R complex and other motifs within the IL-10R complex may be uniquely responsible for mediating the anti-inflammatory activity of IL-10. However, in the case of IL-22 more recent work has indicated that in a model of T-cell mediated murine viral hepatitis, IL-22 may have a protective role indicating that IL-22 may harbour some anti-inflammatory properties,60 thus warranting further structural comparisons of the IL-22R1 and the IL-10R1 chains in the context of inflammatory responses.
Figure 1.
Domains and binding motifs of the IL-10 receptor family which utilize the IL-10R2 chain. Shown in this figure are the receptor complexes involved in IL-10, IL-22 and IL-26 signalling which all utilize the IL-10R2 chain as part of the active receptor complex. IL-22 binding protein (BP) is believed to act as an inhibitor of IL-22 signalling by competition for ligand binding. The human IL-10R1 and IL-20R1 chains both contain two YXXQ STAT binding motifs while IL-22R1 has four.
The association of Jak1 to the IL-10R1 is dependent on a membrane-proximal part of the receptor (amino acids 269–274 in the human IL-10R1) containing a region designated a box 2B motif. This is characterized by a core of four hydrophobic residues flanked by a serine and charged residues.62 Mutation of the serine and the phenylalanine in the SVLLFKK sequence led to the loss of Jak1 binding to a human GST-IL-10R1 fusion protein. In a related study further mapping of the IL-10R1 and Jak1 interaction domains revealed significant binding of amino acids 300–598 of the human IL-10R1 to Jak1.63
Another investigation has characterized three major domains within the murine IL-10R1. Using stably transfected pro-B-cell lines, the activity of the three domains was assayed by the proliferative response (amino acids 459–559 and amino acids 401–432) and changes in the expression of cell surface antigens (amino acids 459–559), a mechanism that was related to differentiation of the cells. One region also had a potential negative repressor function (amino acids 282–389), as cells lacking this region showed enhanced sensitivity to IL-10.64 A polymorphism (glycine to arginine) in the human IL-10R1 region analogous to the mouse IL-10R1 region (amino acids 282–389) appears to confer altered sensitivity to IL-10 in vitro in cells from polymorphic carriers.65
Taken together, molecular events involving IL-10RI structures have only been partly elucidated. The current understanding of downstream steps following receptor activation will be discussed in more detail in the following section.
Stat proteins in il-10 signalling
Binding of IL-10 to its receptor causes the activation of the receptor-associated Janus tyrosine kinases, Jak1 and Tyk2.66 These kinases are responsible for the phosphorylation of tyrosine residues within the intracellular domain of IL-10R1 which serve as docking sites for STAT molecules.64 IL-10 was initially shown to activate STAT1 and STAT3.67,68 These molecules can form either STAT1 or STAT3 homodimers, or a heterodimer comprising of STAT1 and STAT3 and the authors suggest that the three different STAT complexes may induce different sets of genes according to the particular cell type involved.
The role STAT1 plays in IL-10 signalling still remains unclear. We and others observed a low level or even absent STAT1 phosphorylation in human macrophages in response to IL-10. Herrero et al. have shown that IL-10 induced STAT1 phosphorylation was only observed after a high dose of IFN-γ priming and the consequence of this alteration in STAT phosphorylation resulted in IL-10 no longer being able to suppress cytokine synthesis or down regulate MHC class II expression.69 Furthermore, macrophages isolated from STAT1 deficient mice show normal responses to IL-10 suggesting that STAT1 plays only a minor role in IL-10 signalling.70
The role STAT3 plays in IL-10 signalling has been studied to a much greater degree. IL-10 rapidly activates STAT3 and it remains phosphorylated over a sustained period. This is in contrast to IL-6 mediated STAT3 activation which is transient.71,72 This observation may be explained by the receptor coupling of suppressor of cytokine signalling-3 (SOCS3) to the IL-6 receptor. SOCS3 is rapidly induced by both IL-6 and IL-10, in a STAT3-dependent manner, and has recently been shown to specifically inhibit IL-6 but not IL-10 signalling. The SOCS family of proteins are thought to regulate the responses of immune cells to cytokines. They are rapidly induced after cytokine receptor engagement and can bind directly to either Jaks or cytokine receptors. Through the recruitment of ubiquitin transfer system, these proteins then mediate the degradation of proteins associating through the N-terminal region of the SOCS proteins. Neither of the IL-10 receptor chains contains a SOCS consensus motif (SOCS box), whereas the IL-6 gp130 signalling chain contains the necessary tyrosine motif which targets these receptor complexes for degradation. Pretreatment of macrophages with SOCS3-inducing cytokines prevents the activation of STAT3 in response to IL-6, but has no effect upon IL-10-induced STAT3 activation. Consistent with these findings are the recent papers describing the generation of SOCS3-deficient macrophages.73–75 In these cells, IL-6-induced STAT3 is sustained for many hours, the consequence of this is that it becomes strongly anti-inflammatory, whereas IL-10 signalling was not affected. This would suggest that even though IL-10 strongly induces SOCS3 it plays no role in the negative regulation of IL-10 signalling, although what role it does play remains to be resolved. The IL-10 receptor system may have simply evolved to escape this level of regulation which may account for ability to maintain a high level of STAT3 activation that may be necessary for it be an effective immunosuppressive cytokine.
Molecular mechanisms of cytokine inhibition by il-10
The mechanism by which IL-10 mediates the suppression of proinflammatory cytokine synthesis remains an area of contention and contradiction. Most research focuses upon lipopolysaccharide (LPS), the outer membrane component of Gram-negative bacteria, as an inducer of proinflammatory cytokine/chemokine release in macrophages. LPS induces a number of signalling cascades, many of which have been shown to play a critical role in the production of pro-inflammatory cytokine release. TNF-α is perhaps the most extensively studied cytokine in this context. LPS rapidly induces TNF-α, with mRNA levels detectable within 15 min of stimulation, and protein levels evident after 60 min of LPS stimulation.72 In human macrophages, IL-10 can inhibit TNF-α mRNA as soon as the mRNA is detected; however, the levels of suppression of mRNA are more profound at later time points (2 hr). In the murine system, IL-10 can also inhibit TNF-α mRNA accumulation but not to the same extent as in the human system.76 IL-10 potently inhibits TNF-α protein production, even when given up to 2 hr post LPS stimulation.77–79 However, in vivo IL-10 only effectively suppresses cytokine production when given before LPS, but not when given after LPS in experimental endotoxaemia.80
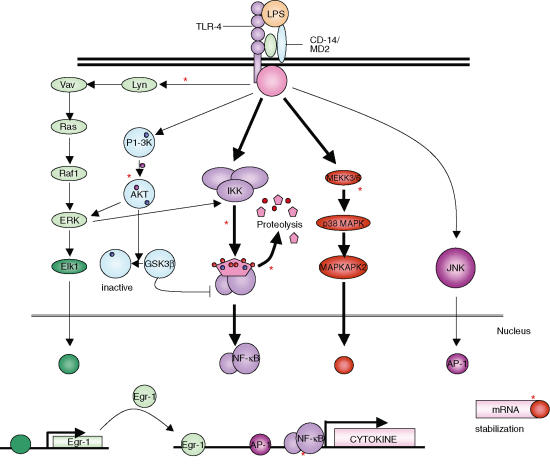
At what point in the LPS signalling cascade does IL-10 intervene? Fig. 2 shows the signalling cascades activated by LPS which have been shown to be critical to cytokine release and highlights the reports of IL-10 intervention. We will review this literature in terms of the LPS signalling cascade and what signalling mechanisms IL-10 employs to target these pathways.
Figure 2.
Signalling cascades of the LPS pathway and reported sites of IL-10 intervention. LPS activates numerous signalling cascades that are critical for the production of cytokines by macrophages. The two dominant pathways of cytokine production, the NF-κB and the p38 MAPK pathway have both been cited as being inhibited by IL-10. The NF-κB pathway has been suggested to be regulated at several levels, with IL-10 inhibiting the activation of IKK and thereby the degradation of IκB by the proteosome and also preventing the binding of the NF-κB subunits to its binding motif. Other reports have suggested IL-10 prevents translation in murine systems by inhibiting p38-MAPK. In addition 3′ UTR of the TNF-α gene was found to be critical to IL-10 activity. With the exception of JNK, the other pathways activated by LPS have also demonstrated sensitivity to the effects of IL-10. *, Suggested site of IL-10 inhibition.
Nuclear factor (NF)-κB
A body of work exists in support of this transcriptional control of TNF-α by IL-10 and focuses on the inhibition of the NF-κB pathway. NF-κB is a transcription factor utilized by different cytokines and LPS for the induction of a large array of pro-inflammatory cytokines.81,82 The NF-κB complex is maintained in the cytoplasm in an inactive state through association with the inhibitor of NF-κB (IκB).83 Following stimulation, IκB kinases (IKK) phosphorylate IκB, targeting it for ubiquitination and subsequent degradation. The NF-κB protein complex is then free to translocate to the nucleus and bind to target DNA sequences.84 Cytokines such as TNF-α have numerous κB consensus sites within the promoter region which are engaged upon LPS stimulation with a heterodimer comprised predominantly of p65 and p50 subunits of NF-κB.85–87 Over-expression of the inhibitor IκB markedly suppresses the expression of cytokines such as TNF-α, IL-6 and vascular endothelial growth factor in human macrophages, and along with the generation of knockout mice deficient in components of the NF-κB pathway has identified this pathway, as a key effector of cytokine production in response to LPS.88,89
Numerous reports have shown an effect of IL-10 upon the NF-κB pathway. The first report by Wang et al. proposed that IL-10 acted through the regulation of transcription. In peripheral blood mononuclear cells (PBMC) IL-10 had no effect on cytokine mRNA accumulation during the first hour of IL-10 stimulation but after 2·5 hr stimulation substantial inhibition was observed and this was then reflected in the levels of TNF-α protein produced.90 This work was extended to propose that this control was exerted through the inhibition of LPS induced NF-κB binding to its motif and thereby inhibiting cytokine production in PBMCs. Two further papers also supported this observation.91,92 Shames et al. demonstrated that pretreatment with IL-10 prevented the degradation of IκBα and thereby the translocation of NF-κB to the nucleus and its activation of cytokine production. Schottelius et al. demonstrated substantial inhibition by IL-10 of NF-κB activity using a reporter construct with three tandem MHC class I NF-κB binding motifs, this inhibition by IL-10 was proposed to act by blocking the activity of the IKK molecule, thereby preventing the degradation of IκB. This inhibition by IL-10 resulted in a 60% inhibition of NF-κB inducible genes. However, this blockage of IκB degradation was a transient effect of IL-10, IKK activity was inhibited at 5–10 min post IL-10 stimulation, and therefore IL-10 inhibition of NF-κB binding at 1 hr post stimulation was independent of IκB activity. and finally, using the same MHC class I NF-κB binding motifs, a recent study has shown that with pretreatment of immature dendritic cells with IL-10 results in a reduction of LPS induced NF-κB activation characterized by reduced degradation of IκBα and inhibition of serine phosphorylation of p65.93 However, in primary human macrophages the effects of IL-10 upon the NF-κB pathway have been shown to be negligible at concentrations where the effects of IL-10 are saturating.79 Supporting this observation, Zhou et al. have shown that rather than IL-10 affecting the recruitment of NF-κB to the IL-12p40 gene, IL-10 abolished the recruitment of RNA polymerase II to the promoter and the authors suggested that several mechanisms were employed by IL-10; including, reduced C/EBPβ binding to the promoter and reduced nucleosome remodelling.94 Moreover, Clarke et al. only see an inhibitory effect of IL-10 on NF-κB activity, in the murine macrophage cell line, RAW 264.7, at concentrations much higher than those required to suppress cytokine production.95 Clearly, this field remains contentious and requires clarification. The heterogeneity of results may be a consequence of different systems used by different laboratories, in particular the different NF-κB consensus sequences used may be a key factor in the apparent conflicting results obtained.
P42/44 MAP kinase (MAPK)
LPS also rapidly induces numerous tyrosine kinases including the src family tyrosine kinases. Small molecular weight inhibitors of tyrosine kinases such as genistein, herbimycin A and PP2 have shown that tyrosine kinases play a key role in the regulation of cytokine production from LPS stimulated monocytes,96,97 as have inhibitors of p42/p44 MAPK activation.98 The src family member lyn, associates with the guanine exchange factor Vav. IL-10 has been shown to inhibit LPS-induced activation of lyn and subsequent activation of downstream events in this pathway such as Ras activation and p42/44 MAPK kinase activation.99 However this early study has never been repeated and other researchers have even shown that IL-10 actually induces p42/44 MAPK activation.71
P38 MAPK
p38 MAPK is a stress response kinase rapidly activated by LPS. The generation of inhibitors of p38 MAP kinase such as the pyridyl imidozole compound SB203580 have shown that this kinase plays a pivotal role in the control of TNF-α and many other pro-inflammatory cytokines.100 p38 MAPK is thought to fulfil an important role in the stabilization of cytokine mRNA by targeting adenosine-uracil (AU) rich elements (ARE) in the 3′ untranslated regions (UTR) of these genes.101 In the case of TNF-α, removal of the 3′ UTR results in an elevated level of expression of this cytokine. In transgenic mice engineered to express the human TNF-α transgene, an excessive production of TNF-α is observed which leads to the development of an inflammatory arthritis.102 More specifically, the removal of a 69 bp ARE from the murine TNF-α-3′ UTR also leads to an overproduction of TNF-α.103 Interestingly, in the TNF-α-3′ UTR modified transgenic mice, IL-10 is no longer capable of inhibiting TNF-α production, implying this region of gene as a critical target. Further support for such a conclusion has been provided by the generation of TNF-α luciferase constructs by Denys et al.79 In these experiments, when IL-10 was given simultaneously with LPS, IL-10 was only capable of inhibiting constructs containing the 3′ UTR, as constructs carrying the TNF promoter alone were only modified by IL-10 when the cells were pretreated for 24 hr prior to LPS stimulation. Given that p38 MAP kinase plays such an important role in cytokine mRNA stability, it has been an attractive candidate target for the action of IL-10 intervention. Kontoyannis et al. have proposed that the main effect of IL-10 signalling is the inhibition of the p38 MAPK pathway and have demonstrated in murine macrophage cells a 90% inhibition of p38 MAPK phosphorylation and suggest that the inhibition of MAP KAP K2 (a downstream target of p38 MAPK) prevents the polysomal coupling of cytokine mRNA without affecting mRNA stability but rather disrupting mRNA–peptide translation. However, we and others have failed to show any affect of IL-10 on p38 MAPK activity in human mononuclear cells and would suggest that the effects of IL-10 are independent of p38 MAPK and are at the level of post-transcriptional control.79,104 It is difficult to reconcile such opposing views, however, work done by Biswas et al.105 support the post-transcriptional control hypothesis. Although IL-10 has no direct effect on mRNA decay it was demonstrated, using in vitro systems, that the effects of IL-10 on the chemokine KC mRNA stability were to antagonize the stabilizing effects of LPS. Kishore et al. also support this hypothesis as IL-10-mediated inhibition of a KC reporter construct required the AU-rich elements in the 3′ UTR, a characteristic of post-transcriptional control.106
STAT3
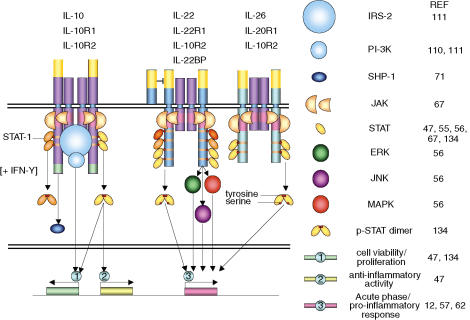
The literature sites only two main signalling cascades that are initiated by IL-10: the Jak/STAT pathway; and the phosphatidylinositol-3 (PI-3) kinase pathway, as summarized in Fig. 3. Of these, only the Jak/STAT pathway has been shown to have an anti-inflammatory role in monocytes/macrophages. Studies using the Jak1 and macrophage conditional STAT3 knockout mice have shown that these two molecules are absolutely required for IL-10 mediated cytokine suppression.47,107,108 More recently, it has been shown that it is the STAT3α isoform, rather than the β isoform that specifically mediates IL-10 inhibition of TNF-α and IL-6 production in murine macrophages.109 Further studies in murine cell lines have deleted the STAT3 binding sites within the IL-10 receptor and shown an ablation of IL-10's ability to suppress TNF-α production in response to LPS.46,47 Finally, the use of a STAT3 dominant-negative (Y705F) suggests that STAT3 is the dominant effector molecule mediating the majority of IL-10 s anti-inflammatory effects in human macrophages such as inhibition of cytokine production, induction of soluble TNF-receptor production and TIMP-1 production and down regulation of MHC class II expression. However, expression of the dominant negative was unable to inhibit early phases of IL-10 signalling suggesting STAT3-independent signalling.72
Figure 3.
IL-10, IL-22 and IL-22 receptor signalling. Binding of IL-10, IL-22 and IL-26 to their respective receptor complex results in the initiation of various signalling cascades. In all cases the JAK/STAT pathway is the primary pathway known to be activated by ligand binding. IL-10 also activates the PI-3K pathway via the binding of the IRS-2 adaptor molecule to the IL-10R complex. STAT-1 and SHP-1 have also been shown to be activated by IL-10, however, the former is only induced with IFN-γ; preincubation. IL-22 and IL-26, like IL-10, initiate the dimerization of STAT-3, however, IL-22 also activates the JNK, ERK and MAPK pathways as well as STAT1 and -5. IL-22 and -26 also activate the acute phase/pro-inflammatory responses in cells rather than de-activation of the inflammatory response.
PI-3 kinase
IL-10 activates PI-3 kinase, and its downstream effectors, AKT and p70 S6 kinase.110–112 LPS also induces PI 3 kinase and AKT,113 and it has been suggested that PI-3 kinase limits LPS signalling pathways in vitro114 and in vivo,115 potentially via AKT mediated regulation of NF-κB. However in human monocytes, the inhibition of IL-10 induced PI-3 kinase pathway had no effect on anti-inflammatory activity of IL-10 and the authors suggest that activation of this pathway promotes cell viability/cell proliferation.110 This is supported by Zhou et al. who have shown that in myeloid precursors, IL-10 promotes cell survival via the activation of the PI-3 kinase pathway. PI-3 kinase is activated by IL-10 via its association with the adaptor molecule IRS-2, and proceeds to then activate AKT.111
Conversely, Bhattacharya et al. have suggested that pretreatment of bone-marrow-derived dendritic cells with IL-10 resulted in the inhibition of LPS induced PI-3 kinase pathway, which in turn prevented the activation of IKK and the subsequent degradation of IκB.93 Once again, contradiction plagues this field. The key factor in this case may be the requirement for pretreatment, clearly suggesting that IL-10 is inducing a gene whose actions are central to the suppression of LPS signalling pathways leading to inhibition of cytokine synthesis.
Il-10 inducible genes
A number of studies have suggested that protein synthesis is required for IL-10 to mediate its cytokine suppressive effects, as many groups report a requirement for pretreatment of IL-10 to inhibit many aspects of LPS signalling.72,93,116,117 The identity of the gene(s) mediating these effects still remains unclear. Until the advent of expression profiling using microarrays there was only a limited number of genes known to be regulated by IL-10 in mononuclear cells: these included FcR1 (CD64),67 TIMP-1,16 monocyte chemoattractant protein-1 (MCP-1),118 CCR5,119 CD163,120 IL-1ra,15 TNF-R2121 and SOCS3.122 In the last 2 years a number of profiling studies have greatly expanded our knowledge of IL-10 inducible genes in both human and murine mononuclear cells.69,123–127 In the most extensive study of Jung et al. up to 500 genes were shown to be induced by IL-10 over multiple time points reflecting coherent IL-10 responses in human PBMCs. However, when the data generated from the human studies are compared to the murine studies only a limited number of genes are common to both (Table 1). It seems surprising that only 21 genes are common to both species. This may be a reflection of the human and murine Affymatrix chips used containing a differential set of genes or it could be due to the fact that the murine studies were all performed in mature macrophages where as most of the human studies were performed in monocytes. We found that as human monocytes matured into macrophages, many genes become insensitive to IL-10 regulation.123
Table 1.
IL-10 inducible genes observed in both human and murine mononuclear cells
| Name | Function |
|---|---|
| B-ATF | Transcriptional regulator |
| GADD45β | Signal transduction |
| SOCS3 | |
| Vav-1 | |
| Tyrosine phosphatase | |
| IL-10 | Cytokine/chemokines |
| MIP-1β | |
| MCP2 | |
| MCP5 | |
| IL-1ra | Cytokine antagonist |
| IL-4Rα | Cytokine/chemokine receptors |
| CCR1 | |
| CCR5 | |
| FcRII | Immunoglobulin receptor |
| CD163 | Scavenger receptor |
| Arginase II | Enzymes |
| Glycerol kinase | |
| Chondroitin sulphate | Miscellaneous |
| Proteoglycan-2 (versican) | |
| Pentraxin | |
| Latent TGF-β binding protein | |
| Metallothionein-l |
B-ATF, B-activating transcription factor; GADD, growth arrest and DNA damage; TGF-β, transforming growth factor-β.
One notable group of IL-10-inducible genes absent from Table 1 are the leucocyte immunoglobulin-like receptor (LIR) family. This family comprises 10 family members, related to the natural killer cell immunoglobulin receptors for human leucocyte antigen class I molecules.128 Distinct LIR family members are differentially expressed in lymphocytes, monocytes, macrophages, DC cells and granulocytes. These receptors can either be activating or inhibitory, the cytoplasmic domain of the later contain immunoreceptor tyrosine-based inhibitory receptor motifs which recruit SH2 containing protein tyrosine phosphatase-1 (SHP-1) to the receptor complex. At the RNA level, IL-10 has been shown to induce LIR-1, -2, -3, -4, -6a, and -7.125 At the protein level LIR-3 and -4 (both inhibitory) are up-regulated by IL-10 in dendritic cells.129 These two LIR have been shown to play a crucial role in the tolerization of dendritic cells.130 Up-regulation of these two LIR by IL-10 may represent an additional mechanism by which IL-10 treatment of dendritic cells renders these cells tolerogenic.
Bcl-3, a member of the IκB protein family, was shown to be strongly up-regulated by IL-10 in murine macrophages in two profiling studies124,126 but absent from any of the human studies. Kuwata et al. were able to demonstrate that IL-10 also up-regulated Bcl-3 at the protein level in a STAT3-dependent manner. Using a combination of approaches they show convincing data for a role of Bcl-3 in the suppression of LPS-induced TNF-α, but not IL-6, production via antagonism of the NF-κB signalling pathway. Bcl-3 preferentially associates with the p50 subunit of NF-κB and the authors suggest that Bcl-3 acts as a negative regulator of NF-κB and enhances p50-mediated inhibition of TNF-α promoter activity by antagonizing the formation of a functional NF-κB p50/p65 heterodimer
Another recently described IL-10 inducible gene is heme oxygenase 1. This protein has been shown to be regulated at the protein level in both human and murine macrophages.131,132 In the murine system this protein has been shown to be critical to the anti-inflammatory activities of IL-10 but it does not appear to have the same role in human systems.125,133
Conclusion
The IL-10 field is still centred round the Jak1/STAT3 pathway as a central mediator of IL-10 anti-inflammatory effects. However, it is still unclear how STAT3 mediates these effects, given that many other cytokines also activate this factor. Possibly other signalling moieties may be involved or alternatively, the duration of the STAT3 response induced by IL-10 may be the critical factor, as unlike other cytokines, IL-10 induction of STAT3 has evolved to escape the control mechanism such as SOCS3 to limit STAT3 activation. What is also unclear is whether STAT3 is acting solely as a transcription factor or whether it fulfils another role in IL-10 signalling. If STAT3 is acting as a transcription factor, the identity of the key gene it induces to suppress cytokine synthesis still remains elusive. Although candidate genes such as Bcl-3 exist, no one mechanism has emerged that encompasses all the data published regarding both the NF-κB transcriptional control and the control of mRNA stability via the 3′ UTR. It may simply be that IL-10 employs multiple mechanisms within different target cells and that this puzzle still remains to be solved.
References
- 1.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2003;55:241–69. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 4.Hsu DH, de Waal Malefyt R, Fiorentino DF, et al. Expression of interleukin-10 activity by Epstein–Barr virus protein BCRF1. Science. 1990;250:830–2. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 5.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci USA. 2000;97:1695–700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastl GA, Abrams JS, Nanus DM, et al. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993;55:96–101. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–7. [PubMed] [Google Scholar]
- 8.Mocellin S, Wang E, Marincola FM. Cytokines and immune response in the tumor microenvironment. J Immunother. 2001;24:392–407. [PubMed] [Google Scholar]
- 9.Blumberg H, Conklin D, Xu WF, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher G, Dickensheets H, Eskdale J, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–50. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 11.Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–5. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 13.Knappe A, Hor S, Wittmann S, Fickenscher H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J Virol. 2000;74:3881–7. doi: 10.1128/jvi.74.8.3881-3887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce DA, Gibbons DP, Green P, Steer JH, Feldmann M, Brennan FM. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol. 1994;24:2699–705. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994;13:47–54. [PubMed] [Google Scholar]
- 16.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–10. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CH, Furue M, Tamaki K. B7-1 expression of Langerhans cells is up-regulated by proinflammatory cytokines, and is down-regulated by interferon-gamma or by interleukin-10. Eur J Immunol. 1995;25:394–8. doi: 10.1002/eji.1830250213. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 19.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 20.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–71. [PubMed] [Google Scholar]
- 21.Schandene L, Alonso-Vega C, Willems F, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–74. [PubMed] [Google Scholar]
- 22.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 23.Curiel TJ, Wei S, Dong H, et al. Blockade of B7–H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 24.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–17. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 25.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 26.Saeland S, Duvert V, Moreau I, Banchereau J. Human B cell precursors proliferate and express CD23 after CD40 ligation. J Exp Med. 1993;178:113–20. doi: 10.1084/jem.178.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdin N, Van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol. 1995;154:2533–44. [PubMed] [Google Scholar]
- 29.Llorente L, Zou W, Levy Y, et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llorente L, Richaud-Patin Y, Garcia-Padilla C, et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walmsley M, Katsikis PD, Abney E, Parry S, Williams RO, Maini RN, Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay JO, Ciesielski CJ, Scheinin T, Brennan FM, Hodgson HJ. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52:363–9. doi: 10.1136/gut.52.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb H, Worz-Pagenstert U, Kleemann R, Rothe H, Rowsell P, Rastegar S, Scott FW. Insulin therapy of prediabetes suppresses TH1 associated gene expression in BB rat pancreas. Autoimmunity. 1997;26:1–6. doi: 10.3109/08916939709009544. [DOI] [PubMed] [Google Scholar]
- 35.Cua DJ, Coffman RL, Stohlman SA. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157:2830–6. [PubMed] [Google Scholar]
- 36.Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–10. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Docke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–94. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473–82. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 39.Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:629–39. doi: 10.1016/s0889-857x(05)70030-2. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–9. [PubMed] [Google Scholar]
- 41.Kotenko S, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer SD, Di Marco F, Hooley J, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–8. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welte T, Zhang SS, Wang T, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–84. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–60. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 45.Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci U S A. 1993;90:11267–71. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms. evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–21. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 48.Lai CF, Ripperger J, Morella KK, et al. Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. J Biol Chem. 1996;271:13968–75. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- 49.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex. The IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–32. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 50.Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–40. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 51.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL) -22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 52.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int Immunopharmacol. 2004;4:593–608. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–21. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- 54.Sheikh F, Baurin VV, Lewis-Antes A, et al. Cutting edge. IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol. 2004;172:2006–10. doi: 10.4049/jimmunol.172.4.2006. [DOI] [PubMed] [Google Scholar]
- 55.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 56.Hor S, Pirzer H, Dumoutier L, et al. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem. 2004;279:33343–51. doi: 10.1074/jbc.M405000200. [DOI] [PubMed] [Google Scholar]
- 57.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA. 2000;97:10144–9. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol. 2004;4:577–92. doi: 10.1016/j.intimp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–91. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–53. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 62.Usacheva A, Kotenko S, Witte MM, Colamonici OR. Two distinct domains within the N-terminal region of Janus kinase 1 interact with cytokine receptors. J Immunol. 2002;169:1302–8. doi: 10.4049/jimmunol.169.3.1302. [DOI] [PubMed] [Google Scholar]
- 63.Usacheva A, Sandoval R, Domanski P, Kotenko SV, Nelms K, Goldsmith MA, Colamonici OR. Contribution of the Box 1 and Box 2 motifs of cytokine receptors to Jak1 association and activation. J Biol Chem. 2002;277:48220–6. doi: 10.1074/jbc.M205757200. [DOI] [PubMed] [Google Scholar]
- 64.Ho AS, Wei SH, Mui AL, Miyajima A, Moore KW. Functional regions of the mouse interleukin-10 receptor cytoplasmic domain. Mol Cell Biol. 1995;15:5043–53. doi: 10.1128/mcb.15.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gasche C, Grundtner P, Zwirn P, et al. Novel variants of the IL-10 receptor 1 affect inhibition of monocyte TNF-alpha production. J Immunol. 2003;170:5578–82. doi: 10.4049/jimmunol.170.11.5578. [DOI] [PubMed] [Google Scholar]
- 66.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of Tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–90. [PubMed] [Google Scholar]
- 67.Larner A, David M, Feldman GM, et al. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993;261:1730–3. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- 68.Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996;394:365–70. doi: 10.1016/0014-5793(96)00990-8. [DOI] [PubMed] [Google Scholar]
- 69.Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, Ivashkiv LB. Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol. 2003;171:5034–41. doi: 10.4049/jimmunol.171.10.5034. [DOI] [PubMed] [Google Scholar]
- 70.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–50. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 71.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Muller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 72.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 73.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–50. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 74.Croker BA, Krebs DL, Zhang JG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 75.Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 76.Kontoyiannis D, Kotlyarov A, Carballo E, et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 2001;20:3760–70. doi: 10.1093/emboj/20.14.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 78.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes. an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denys A, Udalova IA, Smith C, Williams LM, Campbell J, Andrews C, Kwaitkowski D, Foxwell BM. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–45. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 80.Pajkrt D, Camoglio L, Tiel-van Buul MC, et al. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J Immunol. 1997;158:3971–7. [PubMed] [Google Scholar]
- 81.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 82.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 83.Finco TS, Baldwin AS. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–72. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 84.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 85.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Udalova IA, Knight JC, Vidal V, Nedospasov SA, Kwiatkowski D. Complex NF-kappaB interactions at the distal tumor necrosis factor promoter region in human monocytes. J Biol Chem. 1998;273:21178–86. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- 87.Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045. [PubMed] [Google Scholar]
- 88.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bondeson J, Browne KA, Brennan FM, Foxwell BM, Feldmann M. Selective regulation of cytokine induction by adenoviral gene transfer of IkappaBalpha into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-kappaB independent. J Immunol. 1999;162:2939–45. [PubMed] [Google Scholar]
- 90.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–63. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 91.Shames BD, Selzman CH, Meldrum DR, Pulido EJ, Barton HA, Meng X, Harken AH, McIntyre RC., Jr Interleukin-10 stabilizes inhibitory kappaB-alpha in human monocytes. Shock. 1998;10:389–94. [PubMed] [Google Scholar]
- 92.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–74. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and I{kappa}B kinase activity. Blood. 2004;10:1100–9. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- 94.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–96. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clarke CJ, Hales A, Hunt A, Foxwell BM. IL-10-mediated suppression of TNF-alpha production is independent of its ability to inhibit NF kappa B activity. Eur J Immunol. 1998;28:1719–26. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 96.Geng Y, Zhang B, Lotz M. Protein tyrosine kinase activation is required for lipopolysaccharide induction of cytokines in human blood monocytes. J Immunol. 1993;151:6692–700. [PubMed] [Google Scholar]
- 97.Beaty CD, Franklin TL, Uehara Y, Wilson CB. Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur J Immunol. 1994;24:1278–84. doi: 10.1002/eji.1830240606. [DOI] [PubMed] [Google Scholar]
- 98.Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BMJ, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-α: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–8. [PubMed] [Google Scholar]
- 99.Geng Y, Gulbins E, Altman A, Lotz M. Monocyte deactivation by interleukin 10 via inhibition of tyrosine kinase activity and the Ras signaling pathway. Proc Natl Acad Sci USA. 1994;91:8602–6. doi: 10.1073/pnas.91.18.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee JC, Laydon JT, McDonnell PC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 101.Clark AR, Dean JL, Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546:37–44. doi: 10.1016/s0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]
- 102.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor. a predictive genetic model of arthritis. EMBO J. 1991;10:4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–88. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 104.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 105.Biswas R, Datta S, Gupta JD, Novotny M, Tebo J, Hamilton TA. Regulation of chemokine mRNA stability by lipopolysaccharide and IL-10. J Immunol. 2003;170:6202–8. doi: 10.4049/jimmunol.170.12.6202. [DOI] [PubMed] [Google Scholar]
- 106.Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge. clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162:2457–61. [PubMed] [Google Scholar]
- 107.Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 108.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 109.Maritano D, Sugrue ML, Tininini S, et al. The STAT3 isoforms alpha and beta have unique and specific functions. Nat Immunol. 2004;5:401–9. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 110.Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70, S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J Biol Chem. 1996;271:16357–62. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- 111.Zhou JH, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW. IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3′-kinase. J Immunol. 2001;167:4436–42. doi: 10.4049/jimmunol.167.8.4436. [DOI] [PubMed] [Google Scholar]
- 112.Pahan K, Khan M, Singh I. Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J Neurochem. 2000;75:576–82. doi: 10.1046/j.1471-4159.2000.0750576.x. [DOI] [PubMed] [Google Scholar]
- 113.Herrera-Velit P, Reiner NE. Bacterial lipopolysaccharide induces the association and coordinate activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J Immunol. 1996;156:1157–65. [PubMed] [Google Scholar]
- 114.Guha M, Mackman N. The Phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccheride activation of signalling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 115.Williams DL, Li C, Ha T, et al. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–56. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 116.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–44. [PubMed] [Google Scholar]
- 117.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin 10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 118.Yano S, Yanagawa H, Nishioka Y, Mukaida N, Matsushima K, Sone S. T helper 2 cytokines differently regulate monocyte chemoattractant protein-1 production by human peripheral blood monocytes and alveolar macrophages. J Immunol. 1996;157:2660–5. [PubMed] [Google Scholar]
- 119.Sozzani S, Ghezzi S, Iannolo G, et al. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–44. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G. The scavenger receptor CD163: regulation, promoter structure and genomic organization. Pathobiology. 1999;67:257–61. doi: 10.1159/000028105. [DOI] [PubMed] [Google Scholar]
- 121.Dickensheets HL, Freeman SL, Smith MF, Donnelly RP. Interleukin-10 upregulates tumor necrosis factor receptor type-II (p75) gene expression in endotoxin-stimulated human monocytes. Blood. 1997;90:4162–71. [PubMed] [Google Scholar]
- 122.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–63. [PubMed] [Google Scholar]
- 123.Williams L, Jarai G, Smith A, Finan P. IL-10 expression profiling in human monocytes. J Leukoc Biol. 2002;72:800–9. [PubMed] [Google Scholar]
- 124.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 125.Jung M, Sabat R, Kratzschmar J, et al. Expression profiling of IL-10-regulated genes in human monocytes and peripheral blood mononuclear cells from psoriatic patients during IL-10 therapy. Eur J Immunol. 2004;34:481–93. doi: 10.1002/eji.200324323. [DOI] [PubMed] [Google Scholar]
- 126.Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, Akira S. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–9. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 127.Perrier P, Martinez FO, Locati M, et al. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–42. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 128.Colonna M, Nakajima H, Navarro F, Lopez-Botet M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J Leukoc Biol. 1999;66:375–81. doi: 10.1002/jlb.66.3.375. [DOI] [PubMed] [Google Scholar]
- 129.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 130.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T (S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 131.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of IL-10 in mice. Nat Med. 2002;8:240–6. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 132.Petit-Bertron AF, Fitting C, Cavaillon JM, Adib-Conquy M. Adherence influences monocyte responsiveness to interleukin-10. J Leukoc Biol. 2003;73:145–54. doi: 10.1189/jlb.0802388. [DOI] [PubMed] [Google Scholar]
- 133.Ricchetti G, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–26. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]