Abstract
The productive activation of CD4+ T lymphocytes, leading to proliferation and cytokine secretion, requires precise temporal regulation of intracellular cyclic AMP concentrations. The major effector molecule activated by cyclic AMP in mammalian cells is the cyclic AMP-dependent protein kinase A (PKA). The type I PKA isozyme mediates the inhibitory effects of cyclic AMP on T-cell activation. Using laser scanning confocal microscopy, we demonstrated that the regulation of PKA type I activity involves spatial redistribution of PKA type I molecules following T-cell receptor (TCR) stimulation. In resting T cells, PKA type I was located in membrane proximal regions and distributed equally across the cell. Shortly after antigen engagement, T cells and antigen-presenting cells formed an area of intense contact, known as the immunological synapse. TCR concentrated at the synapse, whereas PKA type I molecules redistributed to the opposite cell pole within 10 min after T-cell stimulation. Type I PKA redistribution was solely dependent on TCR signalling, because we observed the same temporal and spatial distribution after antibody-mediated cross-linking of the TCR-associated CD3 complex. Segregation of TCR and PKA type I molecules was maintained for at least 20 min. Thirty minutes after stimulation, PKA type I partially colocalized with the TCR. After 60 min, PKA type I distribution again approached the resting state. Considering that initial TCR signals lead to increases in intracellular cyclic AMP, PKA type I molecules may be targeted towards localized cyclic AMP accumulations or transported away from these areas, depending on the requirements of the cellular response.
Keywords: cellular localization, confocal microscopy, signal transduction, T lymphocytes
Introduction
In mammalian cells, the major effector activated by cyclic adenosine monophosphate (cAMP) is the cAMP-dependent protein kinase A, PKA.1,2 In the absence of cAMP, PKA is an enzymatically inactive, tetrameric holoenzyme consisting of two catalytic (C) subunits and two regulatory (R) subunits. The co-operative binding of four cAMP molecules to two sites on each R subunit3,4 drastically decreases the binding affinity between R and C subunits5 and induces dissociation into dimeric R and two monomeric C subunits. Monomeric C subunits possess serine/threonine kinase activity.1,6,7
T cells express two isozymes of PKA; PKA type I (PKA I) and PKA II, which differ in their regulatory subunits, termed RI and RII. These two isozymes also have different subcellular localizations. PKA I is soluble and preferentially cytosolic in fibroblasts8 but in lymphocytes it has also been reported as being associated with the plasma membrane.9,10 In contrast, PKA II resides in various subcellular compartments including the nucleus, centrosome, the Golgi bodies, mitochondria and endoplasmic reticulum.11,12 Compartmentalization of PKA is mediated through binding of the R subunits to A-kinase-anchoring proteins (AKAPs) located within subcellular compartments.13–15
PKA I, but not PKA II, mediates the inhibitory effects of cAMP on T-cell proliferation and cytokine production following T-cell receptor (TCR) signalling.16–18 PKA I antagonizes T-cell activation at multiple levels. For example, PKA I activates the C-terminal Src kinase, Csk, which inhibits p56lck.19 PKA I may also phosphorylate Ser 43 of Raf1, blocking the MAP kinase pathway.20 In the nucleus, stable protein–DNA interactions at the NF-κB, NFAT and AP1 binding sites of the interleukin-2 enhancer are prevented by activation of PKA I.21 In addition, PKA I inhibits cyclin D3 expression and induces the cyclin-dependent kinase inhibitor p27kip1.22 Synthesis of D-type cyclins, including cyclin D3, during the G1 phase of the cell cycle is required for progression of T cells from G1 into S phase.23,24 The D-type cyclins bind cyclin-dependent kinase (Cdk), forming an active kinase complex that phosphorylates and inactivates retinoblastoma protein (pRb).25,26 Inactivation of pRb allows cells to pass through the late G1-phase restriction point and enter the S phase. However, cyclin D/Cdk complexes can associate with the Cdk inhibitor, p27kip1, leading to their inactivation.27,28 In addition to cyclin D induction, T-cell proliferation requires p27kip1 down-regulation.24,27,28 Thus, PKA I-mediated inhibition of cyclin D3 expression and induction of p27kip1 block T-cell cycle progression.
Because the activation of Csk by PKA I inhibits p56lck,19,29 PKA I blocks T-cell activation at an early stage. This suggests that productive T-cell activation requires the inhibition of PKA I activity. However, PKA I phosphotransferase activity increases within 5 min of T-cell activation, returning to the resting level after 60 min.30 In addition, an early, transient increase in intracellular cAMP is required for the successful activation of T cells.31,32 Therefore, PKA I activity must be stringently regulated.
Following initial TCR stimulation, an area of intense contact forms between the T cell and the antigen-presenting cell (APC), termed the immunological synapse.33,34 A possible mechanism for the control of PKA I activation independent of the cAMP concentration in the vicinity of the immunological synapse could involve the physical separation between TCR/CD3 complexes and PKA I. Using laser scanning confocal microscopy, we tested this hypothesis.
Materials and methods
Cell lines and antibodies
All media and supplements were from Life Technologies (Gaithersburg, MD) and reagents were from Sigma (St Louis, MO) unless otherwise stated. Murine fibroblast L cells transfected with cDNAs encoding wild-type or mutant 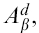 , wild-type
, wild-type 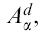 , intercellular adhesion molecule type 1 (ICAM-1), and B7-1 were cultured in complete Dulbecco's modified Eagle's minimum essential medium35 and used as APC.
, intercellular adhesion molecule type 1 (ICAM-1), and B7-1 were cultured in complete Dulbecco's modified Eagle's minimum essential medium35 and used as APC.
Monoclonal antibodies (mAbs) against mouse CD3 [clone 145-2C11; hamster immunoglobulin G (IgG)] were purified from hybridoma supernatants by protein G–Sepharose chromatography (Sigma). Monoclonal antibodies against PKA RIα and PKA C subunits (clone 18 and 5B, respectively; mouse IgG2b) and PKA RIIα (clone 45, mouse IgG1) were purchased from BD Biosciences (Franklin Lakes, NJ). Goat anti-mouse IgG2b-Alexa Fluor® 488, IgG1-Alexa Fluor® 488, and goat anti-hamster IgG-Alexa Fluor® 633 were obtained from Molecular Probes (Eugene, OR).
Immunofluorescence and confocal microscopy
For stimulation with antigen, APCs were first plated on coverslips to form a monolayer and were pulsed with 0·16 μm of peptide 323–339 from chicken ovalbumin (Ova323) for 2 hr. CD4+ T cells were prepared from the lymph nodes of DO.11.10 mice as described elsewhere36 and resuspended in phosphate-buffered saline (PBS) at 1 × 107 cells/ml. One hundred microlitres of the T-cell suspension was added to APCs, and incubated at 37° for the indicated lengths of time. The incubation was stopped by adding 3 ml ice-cold PBS. Coverslips were washed and stained with 70 μl of 10 μg/ml 145-2C11 for 30 min on ice. After four washes with PBS containing 0·5% bovine serum albumin (BSA), coverslips were stained with 70 μl of 6 μg/ml goat anti-hamster IgG-Alexa Fluor® 633 for 30 min on ice. After four washes with PBS containing 0·5% BSA, cells were fixed on coverslips with 100 μl paraformaldehyde in PBS pH 7·5 for 15 min at room temperature followed by incubation with 1·5 ml PBS containing 0·1 m glycine for 20 min at room temperature. Cells were then permeabilized in 100 μl PBS containing 0·1% Triton-X-100 for 10 min at room temperature followed by incubation with 1·5 ml PBS containing 0·5% BSA for 30 min at room temperature. Coverslips were stained with 70 μl of 1 μg/ml PKA RIα or PKA C subunit mAb for 30 min on ice. After four washes with PBS containing 0·5% BSA, coverslips were stained with 70 µl of 6 μg/ml goat anti-mouse IgG2b-Alexa Fluor® 488 for 30 min on ice. After four washes with PBS containing 0·5% BSA, coverslips were allowed to dry completely before being mounted on glass slides and covered with a small drop of VECTASHIELD (Vector Laboratories, Burlingame, CA). Staining for PKA RIIα was performed under identical conditions using the appropriate antibodies.
For antibody cross-linking experiments, T cells were first incubated on coverslips for 20 min at 4° to allow adherence to take place. The coverslips were incubated with 10 μg/ml of 145-2C11 for 30 min on ice. After four washes with ice-cold PBS containing 0·5% BSA, T cells were incubated with 6 μg/ml of goat anti-hamster IgG-Alexa Fluor® 633 for 20 min on ice. Then, coverslips were transferred to a 37° incubator and kept there for the indicated lengths of time. After four washes with ice-cold PBS containing 0·5% BSA, T cells were fixed, permeabilized, stained for PKA, and prepared for examination as described above.
The samples were observed on an Oz Video Rate Laser Scanning Confocal Microscope made by Noran (Middleton, WI) mounted on an inverted microscope (Nikon TE300) and controlled by the intervision software package running on a silicon graphics (SGI) workstation. High-resolution confocal images were obtained using a Nikon ×60 oil, 1·4 NA Plan-Apo objective. The Alexa Fluor® 488 label was detected using the 488-nm line of an argon/krypton laser (excitation) and a 525/52 nm band-pass filter (emission). The Alexa Fluor® 633 signal was detected using the 633-nm line of a helium/neon laser in combination with a 660 nm long-pass filter. With these settings, no ‘bleed-through’ occurred between the two fluorescent signals. In general, a non-confocal transmission image was also obtained for each imaging field as a reference. All images were the result of 64 frame-averages. The zoom factor was adjusted for optimal sampling rate considering the lateral resolution of the objective used. For tri-dimensional studies, series of images were obtained at regular intervals along the z-axis using a motorized focus control.
For each slide, three or four areas containing CD3-capped T cells were randomly chosen for examination. Pseudo-colours were assigned to CD3 (green) and PKA (red). The acquired images were converted to tiff format and transferred to a PC for analysis using metamorph (version 4·67, Universal Imaging, Downingtown, PA).
Results
Cellular distribution of PKA I after antigenic activation
We first examined the intracellular distribution of PKA I in naïve CD4+ lymph node T cells from DO.11.10 TCR transgenic mice. The isozymes PKA I and II differ only in their regulatory subunits. Therefore, we initially used an antibody against the PKA RIα subunit to detect PKA I localization. PKA RI appeared to be distributed throughout the resting T cells (Fig. 1). Figure 1 also depicts specificity controls to demonstrate the specific staining of CD3 and PKA RIα. Confocal image analysis was consistent with plasma membrane association of PKA RIα(Figs 2b,c). Importantly, at this stage, PKA RI was equally distributed across the membrane proximal regions of the cell and was not localized to specific areas (Figs 1 and 2b,c). The TCR-associated CD3 complex was diffusely distributed on the cell surface (Figs 2a,c).
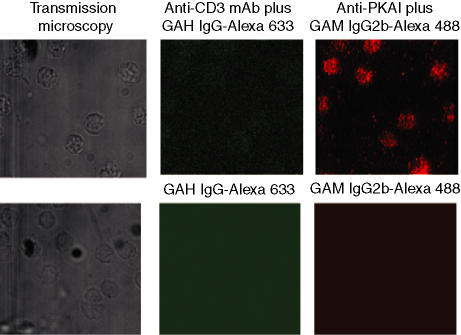
Figure 1.
Distribution of PKA RIα in resting CD4+ T cells and specificity controls. The left panels show transmission micrographs of the same cells as depicted in the middle and right panels. The cells in the upper panels were incubated with anti-CD3 mAb 145-2C11 followed by secondary antibody [goat anti-hamster (GAH) IgG-Alexa Fluor® 633]. The cells were then permeabilized and incubated with anti-PKA I mAb and secondary antibody [goat anti-mouse (GAM) IgG2b-Alexa Fluor® 488]. The incubations were performed at 4° to prevent the activation of the cells. The upper right panel shows distribution of PKA RIα throughout the cell. The cells in the lower panels were incubated with irrelevant control mAbs followed by secondary antibody.
Figure 2.
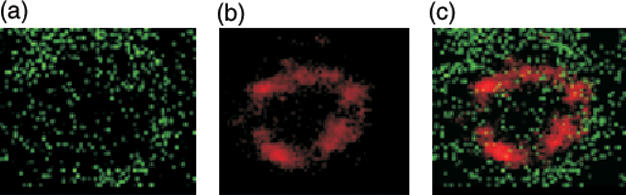
Distribution of PKA RIα in unstimulated CD4+ T cells. Cells were incubated on ice with antibodies against CD3 (green) and PKA RIα (red). CD3 staining indicates diffuse distribution of TCR/CD3 complexes on the cell surface. Images taken from sections through the centre of the cell reveal equal distribution of PKA RIα beneath the plasma membrane. Combined staining of CD3 and PKA RIα. A confocal image reconstruction from 0·5-μm sections showing simultaneously the distribution of CD3 and PKA RIα can be seen in Figure 3. The images are representative of multiple cells from five independently performed experiments.
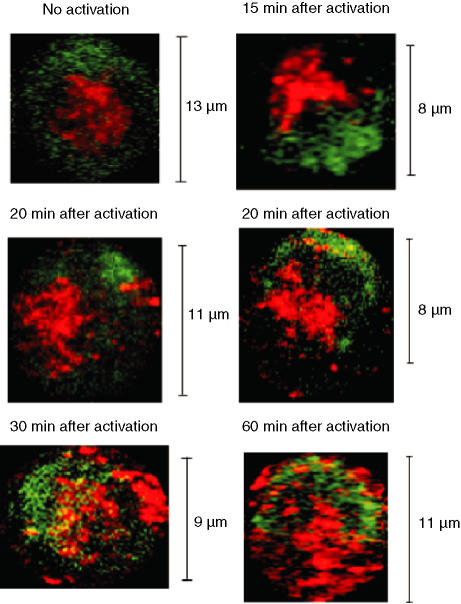
The T cells from DO.11.10 mice are restricted by I-Ad and specific for Ova323. To stimulate the DO.11.10 T cells via the TCR, we plated APC onto microscope coverslips, and incubated the cells with Ova323 for 2 hr. Stably transfected fibroblasts that expressed I-Ad, B7-1, and ICAM-1 were used as APCs.35 Following stimulation by APCs presenting Ova323, the distribution of CD3 and PKA RI changed. The TCR-associated CD3 complex rapidly formed a cap at the tight area of interaction between the APC and the T cell, also known as the immunological synapse. Fifteen minutes after combining APCs and T cells, PKA RI was concentrated at the cell pole opposite the synapse. This area may be identical to the cell structure that has been termed the distal cell pole.37 The concentrated distribution pattern of PKA RI persisted until, at 30 min, the PKA RI partially colocalized with the TCR/CD3 complex. After 60 min, PKA RI was again distributed equally across membrane proximal regions (Fig. 3). As a control, APCs were incubated under identical conditions with a peptide the composition of which was identical to that of Ova323 in scrambled sequence.36 Thus, during early T-cell stimulation, the cellular distribution of PKA RI changed from an equal distribution across the membrane proximal regions of the cell to a concentration at the distal cell pole, followed by partial colocalization with the TCR at the immunological synapse and again equal distribution across membrane proximal regions.
Figure 3.
Distribution of PKA RIα in antigen-activated T cells. CD4+ lymph node T cells from TCR transgenic DO.11.10 mice were incubated with APC loaded with Ova323 for the indicated lengths of time. The first panel shows an unstimulated T cell. After incubation, cells were stained for CD3 (green) and PKA RIα (red). During confocal microscopical examination, three or four fields containing CD3-capped T cells were randomly chosen for each slide. Image analyses were performed only on CD3-capped T cells. Confocal image reconstructions from 0·5-μm sections from one representative experiment of three independently performed experiments are shown.
To determine whether the redistribution of PKA RI depended on the engagement of CD4 by major histocompatibility complex (MHC) class II molecules, we compared temporal redistribution events of PKA RI in DO.11.10 T cells that were stimulated with Ova323 presented by APC expressing either wild-type I-Ad or a mutant I-Ad form incapable of interacting with CD4. The mutation lies in the I-Adβ-chain, and substitutes alanine for glutamic acid at position 137 and valine at position 142. This mutation eliminates the ability of the I-Adαβ heterodimer to engage CD4, but does not affect peptide binding or the interaction with the TCR.38 Antigen presented by wild-type and mutant MHC class II-expressing APC induced identical redistribution of TCR and PKA RI in DO.11.10 T cells (data not shown).
Control of cellular PKA I distribution by TCR stimulation
Because the cellular redistribution of PKA RI following antigenic stimulation of DO.11.10 T cells was independent of CD4 engagement by MHC class II, we investigated whether TCR/CD3 signals alone induced PKA RI movement, or whether other cellular interactions between APC and T cells contributed. To address this question, we examined the distribution of PKA RI in T cells that were stimulated by antibody-mediated CD3 cross-linking.
Purified CD4+ lymph node T cells from DO.11.10 TCR transgenic mice or BALB/c mice were allowed to adhere to coverslips. Lymphocytes were then incubated with a limiting concentration of anti-CD3 antibody (i.e. a concentration that induced CD3 capping in only a fraction of the T cells) followed by incubation with a secondary antibody. The initial CD3 cross-linking was performed at 4°. The T cells were then transferred to 37° for various lengths of time.
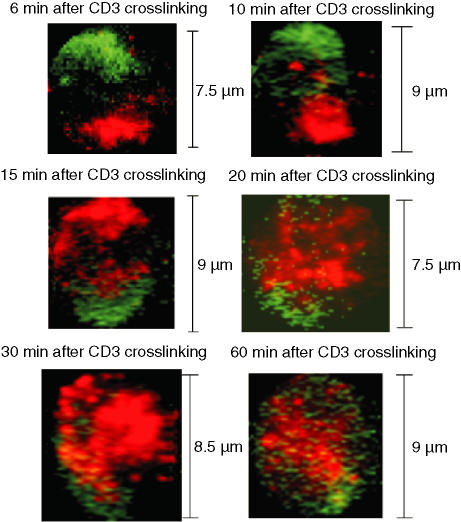
Six minutes after CD3 cross-linking, PKA RI redistributed to the distal cell pole opposite the CD3 cap (Fig. 4 and Table 1). Concentration of PKA RI at the distal cell pole and separation from CD3 persisted at least until 10 min after CD3 cross-linking. Fifteen minutes after CD3 cross-linking, PKA RI redistributed again, and 30 min after cross-linking, PKA RI colocalized with CD3 at the immunological synapse. After 60 min, the cellular distribution of PKA RI was similar to that in resting T cells (Fig. 4). We did not observe differences in the temporal distribution of CD3 or PKA RI between DO.11.10 and BALB/c mice (data not shown), indicating that PKA RI redistribution during T-cell activation is a general process rather than unique to a specific TCR.
Figure 4.
Distribution of PKA RIα in CD3-cross-linked T cells. CD4+ lymph node T cells from TCR transgenic DO.11.10 mice were either left untreated or cross-linked with anti-CD3 antibody plus goat anti-hamster secondary antibody (green) for the indicated length of time. Then, T cells were stained for PKA RIα (red). During confocal microscopical examination, three or four fields containing CD3-capped T cells were randomly chosen for each slide. Image analyses were performed only on CD3-capped T cells. The images are from one representative experiment of four independently performed experiments in which two to three slides per time point were analysed.
Table 1.
Relationship between CD3 capping and PKA type I subunit distribution 6 min after CD3 cross-linking
| Total CD4+ T cells analysed for PKA RIα or PKA C | CD4+ T cells with CD3 cap (percentage of all CD4+ T cells) | % CD3-capped T cells with PKA I condensed on the opposite cell pole | % Non-capped T cells with PKA I condensed |
|---|---|---|---|
| PKA RIα: 191 | 33 (17·3%) | 69·7% | 1·9% |
| PKA C: 89 | 19 (21·3%) | 73% | 0% |
Because PKA I is activated via dissociation of regulatory subunits from catalytic subunits, the movement of PKA RIα away from the TCR could be the result of either the movement of the PKA I holoenzyme, or the movement of regulatory subunits dissociated from catalytic subunits. If the PKA I holoenzyme translocated to the distal cell pole, TCR/CD3 complexes and PKA I would be physically separated. Therefore, PKA I activity would not affect signalling events that occurred near the immunological synapse. On the other hand, if regulatory subunits were translocated separately from catalytic subunits, PKA I activity might be temporarily increased during early T-cell activation. To determine whether the PKA I holoenzyme, or only the regulatory subunit relocated to areas of the cell distant from the TCR/CD3 cap, we examined the distribution of PKA C following antibody-mediated CD3 cross-linking. Confocal microscopy of cells after 6 min of cross-linking revealed that PKA C condensed at the distal cell pole, showing a similar distribution to PKA RIα (Table 1). Compared to PKA RIα, however, a diffuse background distribution of PKA C staining remained visible throughout the experimental time–course. The remaining, diffusely distributed PKA C was presumably the result of staining of PKA II because staining of the cells with anti-PKA RIIα antibody showed the same diffuse cellular distribution throughout the whole course of the experiment (not shown). Staining of PKA RIIα remained unaffected by TCR/CD3 signalling.
Discussion
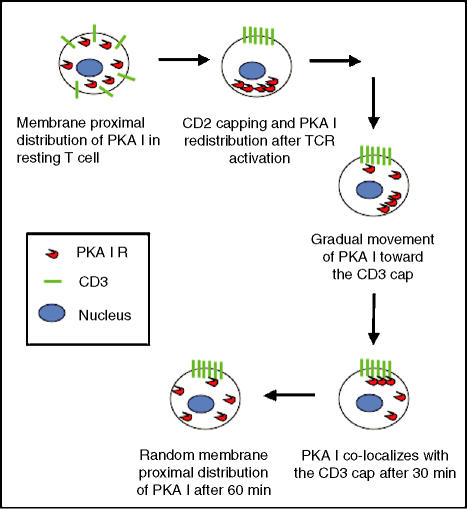
The distribution of PKA I in activated T cells during activation with antigen indicated that TCR-mediated signals trigger movement of PKA I regulatory subunits. In resting T cells, PKA I was equally distributed throughout the membrane proximal regions of the cell. Shortly (6–15 min) after engagement of TCR/CD3, PKA I condensed at the distal cell pole opposite the CD3 cap. Later, PKA I colocalized with CD3 (30 min). Sixty minutes after activation, PKA I distribution was approaching that of the resting state (Fig. 5).
Figure 5.
Model for the kinetics of the movement of PKA I in antigen-activated T cells.
PKA I, but not PKA II, mediates the inhibitory role of cAMP on T-cell proliferation that is induced by TCR signalling.16,17 PKA I antagonizes T-cell activation at multiple levels,19,22 one of which is to activate the protein tyrosine kinase Csk.19 Csk inhibits Lck activity.39,40 By inhibiting Lck, PKA I can diminish T-cell activation at the initiation stage. This suggests that during T-cell activation, PKA I activity must be tightly regulated. We have recently reported that TCR-independent signals induced via CD4 regulate cAMP levels in activated T cells.32 The CD4-mediated signals activate cAMP phosphodiesterases and inhibit adenylyl cyclase to counteract the initial rise in intracellular cAMP that is induced by TCR/CD3 stimulation.32 The TCR-induced redistribution of PKA I in combination with the CD4-mediated regulation of cAMP levels in the vicinity of the emerging signal transduction complex may represent a powerful mechanism with which to fine-tune the effects of this important second messenger system.
Previously, very little was known about the distribution of PKA I in activated T cells. Skållheg and colleagues demonstrated colocalization of the PKA I holoenzyme with the TCR/CD3 complex in human peripheral blood T cells after cross-linking with anti-CD3 mAb for 30 min.10 We also observed colocalization of PKA I and CD3 30 min after activation of T cells with either antigen/APCs or anti-CD3 mAb cross-linking. However, a more dynamic distribution pattern of PKA I was revealed by our study.
In neutrophils, cAMP accumulates at signal initiation sites during phagocytosis, implying that cAMP is involved in the regulation of phagocytosis.41 In both neutrophils and monocytes, PKA accumulates at nascent phagosomes.42 PKA may phosphorylate the proteins associated with pseudopod formation and phagosome internalization. Thus, depending on the requirements of the cellular response to signals induced by cell surface receptors, cAMP effector molecules may be targeted towards localized cAMP accumulations, or transported away from these areas.
Activation of T cells induces the recruitment of many membrane proteins such as TCR/CD3, CD2, CD4 and CD28 to the immunological synapse.33,43 Large membrane proteins, such as CD43, are excluded from the immunological synapse and aggregate at the distal cell pole.37,44 However, the movement of soluble, cytoplasmic signalling proteins is less well understood. The PKC isozyme, PKC-θ, translocates to the c-SMAC region of the immunological synapse, where it colocalizes with the TCR/CD3 complex.33,45 The relocation of PKA I reported here provides an example of the intracellular movement of soluble signalling proteins involved in T-cell activation, and emphasizes the importance of microcompartmentalization for the regulation of signalling pathways. To our knowledge, this is the first report demonstrating the exclusion of soluble inhibitors of TCR signalling from the vicinity of the immunological synapse. The kinetics of PKA I redistribution, and its transient concentration at the cell pole opposite the immunological synapse are reminiscent of the formation of the distal pole complex, a structure that forms after TCR engagement and includes CD43.37,44
PKA II is targeted to different subcellular compartments including the nucleus, the Golgi, and mitochondria. This distribution is mediated through binding of PKA II regulatory subunits to AKAPs located within the subcellular compartments. The regulation of PKA I distribution in the cytoplasm may also depend on AKAPs.46 However, no PKA I-binding AKAPs have been identified in lymphocytes.47 Our results indicate that TCR/CD3 signalling may control PKA I-specific AKAPs. Furthermore, the movement of PKA I regulatory subunits occurred within minutes after TCR/CD3 engagement. This suggests that proximal signals induced by TCR engagement, possibly protein phosphorylation, are responsible for the control of PKA I-specific AKAPs. Depending on the requirements of the cellular response to signals induced by the TCR, PKA I molecules may be targeted towards localized cAMP accumulations or transported away from these areas.
Acknowledgments
This work was supported by the American Heart Association grant 9750717 N, the National Science Foundation grant MCB-9630187, and the National Institute of Environmental Health Sciences grant ES06676. W.Z. was supported by a fellowship from the McLaughlin Fellowship Fund and the American Foundation for Aging Research. W.Z. also acknowledges support from the Graduate School of Biological Sciences at UTMB.
Abbreviations
- Ag
antigen
- AKAP
A-kinase-anchoring protein
- APC
antigen-presenting cell
- cAMP
cyclic AMP
- GAH
goat anti-hamster
- GAM
goat anti-mouse
- mAb
monoclonal antibody
- Ova323
peptide 323–339 from chicken ovalbumin
- PKA I
protein kinase A type I
- C subunit
catalytic subunit
- R subunit
regulatory subunit
- TCR
T-cell receptor
References
- 1.Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–5. [PubMed] [Google Scholar]
- 2.Walsh DA, Van Patten SM. Multiple pathway signal transduction by the cAMP-dependent protein kinase. Faseb J. 1994;8:1227–36. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- 3.Corbin JD, Sugden PH, West L, Flockhart DA, Lincoln TM, McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3′,5′-monophosphate-dependent protein kinase. J Biol Chem. 1978;253:3997–4003. [PubMed] [Google Scholar]
- 4.Doskeland SO, Ogreid D. Binding proteins for cyclic AMP in mammalian tissues. Int J Biochem. 1981;13:1–19. doi: 10.1016/0020-711x(81)90131-2. [DOI] [PubMed] [Google Scholar]
- 5.Doskeland SO, Maronde E, Gjertsen BT. The genetic subtypes of cAMP-dependent protein kinase – functionally different or redundant? Biochim Biophys Acta. 1993;1178:249–58. doi: 10.1016/0167-4889(93)90201-y. [DOI] [PubMed] [Google Scholar]
- 6.Robinson-Steiner AM, Corbin JD. Probable involvement of both intrachain cAMP binding sites in activation of protein kinase. J Biol Chem. 1983;258:1032–40. [PubMed] [Google Scholar]
- 7.Houge G, Steinberg RA, Ogreid D, Doskeland SO. The rate of recombination of the subunits (RI and C) of cAMP-dependent protein kinase depends on whether one or two cAMP molecules are bound per RI monomer. J Biol Chem. 1990;265:19507–16. [PubMed] [Google Scholar]
- 8.Meinkoth JL, Ji Y, Taylor SS, Feramisco JR. Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells. Proc Natl Acad Sci USA. 1990;87:9595–9. doi: 10.1073/pnas.87.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler P, Moore JJ, Kammer GM. Human T lymphocyte cAMP-dependent protein kinase. subcellular distributions and activity ranges of type I and type II isozymes. Faseb J. 1992;6:2735–41. doi: 10.1096/fasebj.6.9.1319361. [DOI] [PubMed] [Google Scholar]
- 10.Skålhegg BS, Taskén K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–7. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 11.Johnson LR, Foster JA, Haig-Ladewig L, VanScoy H, Rubin CS, Moss SB, Gerton GL. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Dev Biol. 1997;192:340–50. doi: 10.1006/dbio.1997.8767. [DOI] [PubMed] [Google Scholar]
- 12.Reinton N, Collas P, Haugen TB, Skålhegg BS, Hansson V, Jahnsen T, Taskén K. Localization of a novel human A-kinase-anchoring protein, hAKAP220, during spermatogenesis. Dev Biol. 2000;223:194–204. doi: 10.1006/dbio.2000.9725. [DOI] [PubMed] [Google Scholar]
- 13.Scott JD, Stofko RE, McDonald JR, Comer JD, Vitalis EA, Mangili JA. Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J Biol Chem. 1990;265:21561–6. [PubMed] [Google Scholar]
- 14.Scott JD, McCartney S. Localization of A-kinase through anchoring proteins. Mol Endocrinol. 1994;8:5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- 15.Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982;257:3284–90. [PubMed] [Google Scholar]
- 16.Skålhegg BS, Landmark BF, Doskeland SO, Hansson V, Lea T, Jahnsen T. Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J Biol Chem. 1992;267:15707–14. [PubMed] [Google Scholar]
- 17.Aukrust P, Aandahl EM, Skålhegg BS, Nordoy I, Hansson V, Taskén K, Froland SS, Muller F. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol. 1999;162:1178–85. [PubMed] [Google Scholar]
- 18.Aandahl EM, Moretto WJ, Haslett PA, Vang T, Bryn T, Taskén K, Nixon DF. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J Immunol. 2002;169:802–8. doi: 10.4049/jimmunol.169.2.802. [DOI] [PubMed] [Google Scholar]
- 19.Vang T, Torgersen KM, Sundvold V, et al. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramstad C, Sundvold V, Johansen HK, Lea T. cAMP-dependent protein kinase (PKA) inhibits T cell activation by phosphorylating ser-43 of raf-1 in the MAPK/ERK pathway. Cell Signal. 2000;12:557–63. doi: 10.1016/s0898-6568(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Rothenberg EV. Interleukin 2 transcription factors as molecular targets of cAMP inhibition: delayed inhibition kinetics and combinatorial transcription roles. J Exp Med. 1994;179:931–42. doi: 10.1084/jem.179.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oirschot BA, Stahl M, Lens SM, Medema RH. Protein kinase A regulates the expression of p27 (kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem. 2001;276:33854–60. doi: 10.1074/jbc.M104395200. [DOI] [PubMed] [Google Scholar]
- 23.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–13. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 24.Boonen GJ, van Dijk AM, Verdonck LF, van Lier RA, Rijksen G, Medema RH. CD28 induces cell cycle progression by IL-2-independent down-regulation of p27kip1 expression in human peripheral T lymphocytes. Eur J Immunol. 1999;29:789–98. doi: 10.1002/(SICI)1521-4141(199903)29:03<789::AID-IMMU789>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–84. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 27.Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 28.Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–990. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vang T, Abrahamsen H, Myklebust S, Horejsi V, Taskén K. Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase a inhibits T cell receptor signaling. J Biol Chem. 2003;278:17597–600. doi: 10.1074/jbc.C300077200. [DOI] [PubMed] [Google Scholar]
- 30.Laxminarayana D, Berrada A, Kammer GM. Early events of human T lymphocyte activation are associated with type I protein kinase A activity. J Clin Invest. 1993;92:2207–14. doi: 10.1172/JCI116823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz E, Zubiaga AM, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W, König R. T cell receptor-independent CD4 signalling. CD4–MHC class II interactions regulate intracellular calcium and cyclic AMP. Cell Signal. 2003;15:751–62. doi: 10.1016/s0898-6568(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 33.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 34.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 35.Gilfillan S, Shen X, König R. Selection and function of CD4+ T lymphocytes in transgenic mice expressing mutant MHC class II molecules deficient in their interaction with CD4. J Immunol. 1998;161:6629–37. [PubMed] [Google Scholar]
- 36.Shen X, Hu B, McPhie P, Wu X, Fox A, Germain RN, König R. Peptides corresponding to CD4-interacting regions of murine MHC class II molecules modulate immune responses of CD4+ T lymphocytes in vitro and in vivo. J Immunol. 1996;157:87–100. [PubMed] [Google Scholar]
- 37.Allenspach EJ, Cullinan P, Tong J, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–50. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 38.König R, Huang LY, Germain RN. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–8. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 39.Marth JD, Cooper JA, King CS, Ziegler SF, Tinker DA, Overell RW, Krebs EG, Perlmutter RM. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol Cell Biol. 1988;8:540–50. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990;10:5197–206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pryzwansky KB, Steiner AL, Spitznagel JK, Kapoor CL. Compartmentalization of cyclic AMP during phagocytosis by human neutrophilic granulocytes. Science. 1981;211:407–10. doi: 10.1126/science.6261328. [DOI] [PubMed] [Google Scholar]
- 42.Pryzwansky KB, Kidao S, Merricks EP. Compartmentalization of PDE-4 and cAMP-dependent protein kinase in neutrophils and macrophages during phagocytosis. Cell Biochem Biophys. 1998;28:251–75. doi: 10.1007/BF02737813. [DOI] [PubMed] [Google Scholar]
- 43.Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3zeta during T cell recognition. Science. 2000;289:1349–52. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 44.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 45.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–6. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 46.Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–64. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 47.Skålhegg BS, Taskén K. Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–93. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]